1. Atherosclerosis and oxidized low-density
lipoproteins
Atherosclerosis is a chronic inflammatory disease
involving vascular cells, immune cells and their released
cytokines, including interferon-α and chemokine C-C ligand 5
(1-3).
The vascular endothelium, which becomes dysfunctional during
atherosclerosis, is considered a key factor in the initiation of
the disease. This can lead to the subendothelial accumulation of
the oxidized forms of low-density lipoproteins (LDLs) and prompt
the mechanism of arterial remodeling and the thickening of the
arterial wall (4). Among the
factors associated with this mechanism, the oxidation of LDLs
remains of principal interest to scientists, since it has been
demonstrated that macrophages preferably accumulate oxidized LDLs
(but not native LDLs) leading to the formation of foam cells and
progression of atherosclerotic plaque (5,6).
2. The major type(s) of oxidized low-density
lipoproteins
There is an ongoing debate as regards the mechanisms
through which an LDL is oxidized in the system, and the exact
mechanisms of LDL modification in vivo remain controversial
(7). Similarly, multiple
approaches have assisted in deciphering this conundrum more
effectively by describing LDL oxidation through metal ions,
reactive oxygen species (ROS) and enzymes, such as myeloperoxidase
(MPO) (8-12).
MPO is an enzyme that belongs to the mammalian peroxidase family
and is secreted mainly by neutrophils, monocytes and macrophages,
where it contributes to their bactericidal activity by producing
ROS (mainly hypochlorous acid). LDL is among the biomolecular
targets of MPO, which is currently proposed as a major sponsor for
the formation of oxidized LDLs in vivo (13). Multiple clinical studies have also
indicated that a solid association exists between serum MPO levels
and acute symptoms of coronary artery disease have been found in
patients with atherosclerosis (14,15).
Furthermore, it has been shown that MPO is highly expressed in
atheroma plaques. As regards LDL modification, it appears that
hypochlorous acid produced by MPO mainly targets the protein moiety
(APOB-100) of LDLs (16). As a
result, oxidized LDLs are unable to bind to the LDL receptor; thus,
they can be alternatively recognized by scavenger receptors. This
will trigger an inflammatory process in the interacting cell,
regardless of whether it is a monocyte, a smooth muscle cell, or an
endothelial cell (11,17).
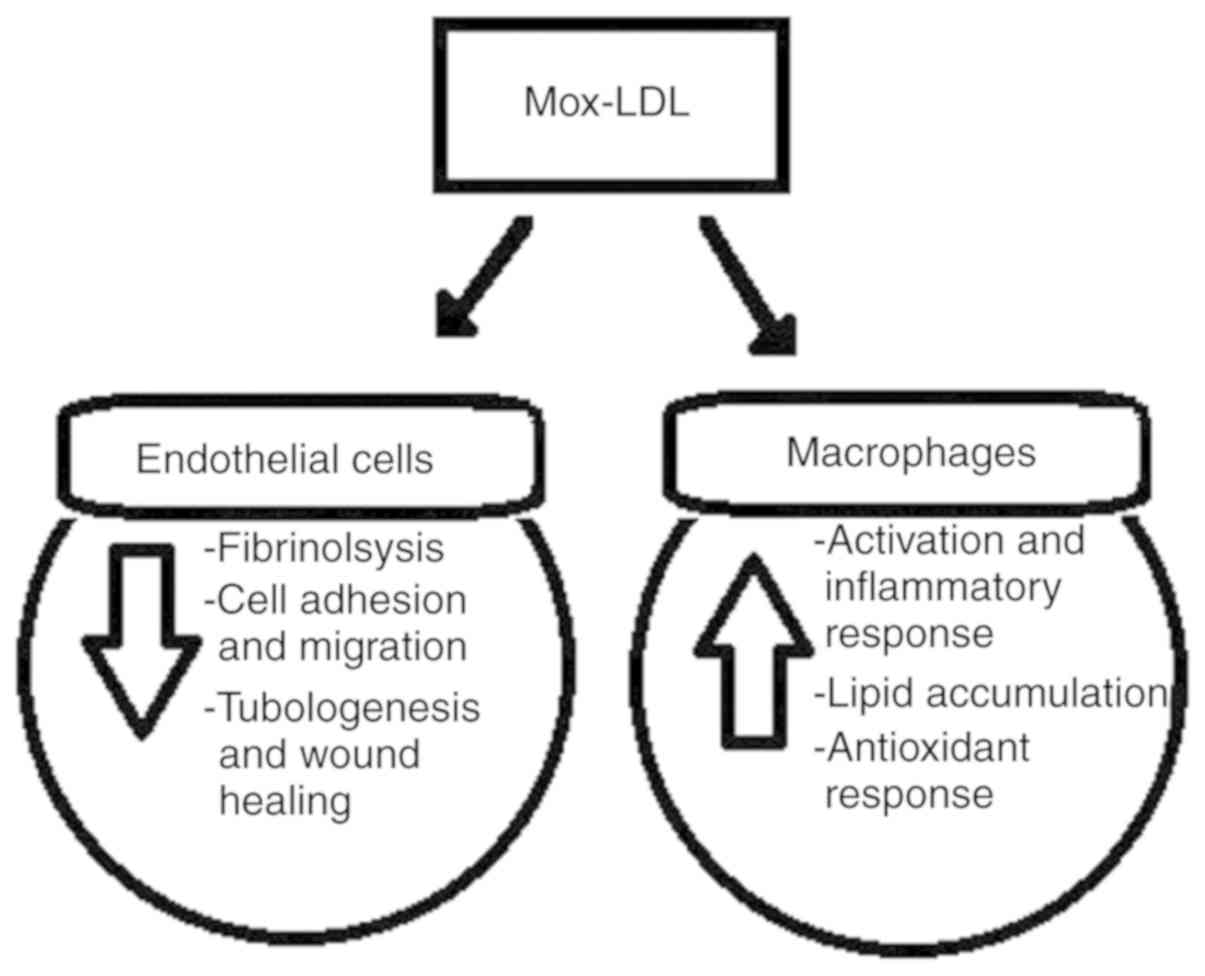
3. In vitro effects of
myeloperoxidase oxidized low-density lipoproteins
Previously, it was reported that LDLs modified by
MPO cause dysfunction in several macrophage and endothelial cell
models of atherosclerosis. Notably, these effects differ from those
in LDLs modified by other systems, namely by the copper metal ion.
It has been demonstrated that an MPO-oxidized LDL (Mox-LDL) does
not trigger the apoptosis of THP-1 monocytes as opposed to a
copper-oxidized LDL (CuOx-LDL), which achieves this by inducing a
caspase-dependent programmed cell death pathway (18). The author has also described the
same analogy in human umbilical vein endothelial cells (HUVECs),
where CuOx-LDLs are known to trigger cell death, which was not
observed with Mox-LDLs (19).
Similarly, the author demonstrated that Mox-LDLs decrease cell
migration, wound healing and tubulogenesis in human umbilical vein
endothelial cells, while the opposite has been recently
demonstrated using the other model of LDL oxidation in human
coronary artery smooth muscle cells (19,20).
The treatment of HUVECs with physiological serum concentrations of
Mox-LDL was shown to significantly decrease (up to 50%) the ability
of the cells to form tubules on Matrigel (19).
The author also previously investigated the in
vitro effect of Mox-LDL on endothelial cells angiogenic
properties by studying its outcome on the expression of
angiogenesis-related genes. It was reported that Mox-LDL decreased
endothelial cell motility and tubulogenesis through an increase in
the expression of genes that are related to angiogenic mechanisms,
namely micro RNA-22 and heme oxygenase 1(19). At the ROS production level, the
author observed that Mox-LDLs did not increase ROS production in
human aortic endothelial cells possibly via the upregulation of
LOX-1 scavenger receptors, which is the case for CuOx-LDLs
(21). On that same note, it was
previously demonstrated that Mox-LDLs increase ROS production and
antioxidant responses in macrophages to a greater extent than with
CuOx-LDLs by using different signaling pathways (16). Finally, the author described that
Mox-LDLs decrease pericellular fibrinolysis in EA.hy926 endothelial
cells, which would increase blood coagulation and thrombus
formation in patients with atherosclerosis. The treatment of
EA.hy926 endothelial cells with physiological serum concentrations
of Mox-LDL was shown to significantly decrease the pro-fibrinolytic
capacity of the cells by up to 15% (22).
4. Future challenges
In summary, several clinical studies (in addition to
the ongoing in vitro experiments by the author) support the
key role that Mox-LDLs may play in the pathology of
atherosclerosis. As indicated, this form of oxidized LDL induces
endothelial cell dysfunction and monocyte activation (Fig. 1), and the effects differ compared
to those of the other CuOx-LDL model. This may be due to the
oxidation process and as the MPO enzyme mainly targets the protein
part of the LDL molecule. In vivo, it has been shown that
MPO is present in atherosclerotic lesions, as well as in the
circulation of patients with cardiovascular complications.
Therefore, Mox-LDLs should be considered a more pathophysiological
model of LDL oxidation than that involving copper ions.
Furthermore, this exact type of modified LDL should now be
considered in prospective studies. In the future, a number of
challenges remain with regard to deciphering the molecular pathways
that are promoted by Mox-LDLs in endothelial cell dysfunction and
macrophage activation. This may aid scientists to reveal the
cellular pathobiology of this type of oxidized LDL in more detail,
which could have a tremendous impact on the understanding of
atherosclerosis and may aid scientists to conceive and develop more
effective treatment methods.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Author's contribution
JD conceived and designed the present review
article, and also wrote, edited and revised the manuscript. The
author has read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lind L: Circulating markers of
inflammation and atherosclerosis. Atherosclerosis. 169:203–214.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Noels H and Weber C: Editorial comment:
Catching up with important players in atherosclerosis: Type 1
interferons and neutrophils. Curr Opin Lipidol. 22:144–145.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Heinecke JW: Oxidants and antioxidants in
the pathogenesis of atherosclerosis: Implications for the oxidized
low density lipoprotein hypothesis. Atherosclerosis. 141:1–15.
1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Steinberg D, Parthasarathy S, Carew TE,
Khoo JC and Witztum JL: Beyond cholesterol. Modifications of
low-density lipoprotein that increase its atherogenicity. N Engl J
Med. 320:915–924. 1989.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yoshida H and Kisugi R: Mechanisms of LDL
oxidation. Clin Chim Acta. 411:1875–1882. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen K, Thomas SR and Keaney JF Jr: Beyond
LDL oxidation: ROS in vascular signal transduction. Free Radic Biol
Med. 35:117–132. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Obama T, Kato R, Masuda Y, Takahashi K,
Aiuchi T and Itabe H: Analysis of modified apolipoprotein B-100
structures formed in oxidized low-density lipoprotein using
LC-MS/MS. Proteomics. 7:2132–2141. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sparrow CP, Parthasarathy S and Steinberg
D: Enzymatic modification of low density lipoprotein by purified
lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative
modification. J Lipid Res. 29:745–753. 1988.PubMed/NCBI
|
|
10
|
Malle E, Waeg G, Schreiber R, Gröne EF,
Sattler W and Gröne HJ: Immunohistochemical evidence for the
myeloperoxidase/H2O2/halide system in human atherosclerotic
lesions: Colocalization of myeloperoxidase and hypoch. Eur J
Biochem. 267:4495–4503. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hazell LJ, Arnold L, Flowers D, Waeg G,
Malle E and Stocker R: Presence of hypochlorite-modified proteins
in human atherosclerotic lesions. J Clin Invest. 97:1535–1544.
1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hansson GK: Regulation of immune
mechanisms in atherosclerosis. Ann N Y Acad Sci. 947:157–166.
2001.PubMed/NCBI
|
|
13
|
Vanhamme L, Zouaoui Boudjeltia K, Van
Antwerpen P and Delporte C: The other myeloperoxidase: Emerging
functions. Arch Biochem Biophys. 649:1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brennan ML, Penn MS, Van Lente F, Nambi V,
Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES,
Topol EJ, et al: Prognostic value of myeloperoxidase in patients
with chest pain. N Engl J Med. 349:1595–1604. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Baldus S, Heeschen C, Meinertz T, Zeiher
AM, Eiserich JP, Munzel T, Simmons ML and Hamm CW: CAPTURE
Investigators: Myeloperoxidase serum levels predict risk in
patients with acute coronary syndromes. Circulation. 108:1440–1445.
2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Delporte C, Van Antwerpen P, Vanhamme L,
Roumeguère T and Zouaoui Boudjeltia K: Low-density lipoprotein
modified by myeloperoxidase in inflammatory pathways and clinical
studies. Mediators Inflamm. 2013(971579)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kruth HS, Huang W, Ishii I and Zhang WY:
Macrophage foam cell formation with native low density lipoprotein.
J Biol Chem. 277:34573–34580. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vicca S, Hennequin C, Nguyen-Khoa T, Massy
ZA, Descamps-Latscha B, Drüeke TB and Lacour B: Caspase-dependent
apoptosis in THP-1 cells exposed to oxidized low-density
lipoproteins. Biochem Biophys Res Commun. 273:948–954.
2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Daher J, Martin M, Rousseau A, Nuyens V,
Fayyad-Kazan H, Van Antwerpen P, Courbebaisse G, Martiat P, Badran
B, Dequiedt F, et al: Myeloperoxidase oxidized LDL interferes with
endothelial cell motility through miR-22 and heme oxygenase 1
induction: Possible involvement in reendothelialization of vascular
injuries. Mediators Inflamm. 2014(134635)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chellan B, Rojas E, Zhang C and Hofmann
Bowman MA: Enzyme-modified non-oxidized LDL (ELDL) induces human
coronary artery smooth muscle cell transformation to a migratory
and osteoblast-like phenotype. Sci Rep. 8(11954)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
El Samad G, Bazzi S, Karam M, Boudjeltia
KZ, Vanhamme L and Daher J: Effect of myeloperoxidase modified LDL
on bovine and human aortic endothelial cells. Exp Ther Med.
18:4567–4574. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zouaoui Boudjeltia K, Daher J, Van
Antwerpen P, Moguilevsky N, Delree P, Ducobu J, Raes M, Badran B,
Vanhaeverbeek M, Brohee D, et al: Exposure of endothelial cells to
physiological levels of myeloperoxidase-modified LDL delays
pericellular fibrinolysis. PLoS One. 7(e38810)2012.PubMed/NCBI View Article : Google Scholar
|