1. Introduction
Celiac disease (CD) is an unusual malabsorption
syndrome, an autoimmune enteropathy among genetically susceptible
individuals. CD has been known as an abdominal disorder and has
long been listed in the medical lexicon. It was first described in
the first century A.D. by Aretaeus Cappadocia, a contemporary of
the Roman physician, Galen, using the Greek term ‘koeliakos’
(suffering of the bowels) (1). At
a later date, Samuel Gee (1880), a British physician, defined CD as
a type of chronic indigestion in humans (2). The cause of the disease, specifically
distinct from other digestive disorders and their symptoms, has
been studied over the past 4 decades (3). Initially, it was reported to be
prevalent in western part of the world, although its distribution
is found globally. This disease has become a very common lifetime
disorder among individuals with a prevalence of 0.5 to 1% worldwide
(4). However, the World
Gastroenterology Organization (WGO) suggests that the female to
male for CD is 2:1. Serological studies using anti-gliadin,
anti-endomysium or anti-transglutaminase antibody assays, which are
hallmark tests for CD, have demonstrated a high prevalence of CD
noted in the Middle East, North Africa and India (5,6). In
India, the number of cases with CD has exhibited an increasing
trend recently and perhaps coincides with a large intake of
gluten-rich foods (6). Currently,
due to the prevalence of diabetes, Southern Indians also prefer
wheat as a staple food, unlike previously. This may also be one of
the reasons for the observed increase in the number of cases of CD
in India. Therefore, the Indian Task Force for Celiac Disease
directed and encouraged research on the prevalence and diagnosis of
CD. It has also made the regulations for the marking of
gluten-free/reduced products and subsidies on these foods, and has
stated that these foods should be sold at a reasonable price in
order to be widely available to patients with CD (7,8).
Moreover, there is a great demand for gluten-free/reduced food for
the prevention and management of CD in India. In this regard, the
present review article highlights the exploration of
gluten-hydrolyzing probiotics as another important management
strategy for patients with CD.
2. Implications of celiac disease on human
health
CD, is referred to by various terms, such as celiac
sprue, non-tropical sprue, idiopathic sprue, idiopathic steatorrhea
and gluten enteropathy. CD is observed as a permanent intolerance
to the storage proteins of wheat, rye and barley among human
leucocyte antigen (HLA)-DQ2/DQ8-positive individuals (9). This condition has been characterized
by complex adaptive and innate immune responses that yield
characteristic chronic inflammation and villous atrophy in the
small intestine, as well as systemic inflammation with the
deposition of disease-specific auto-induced antibodies in various
parts of the body (10). CD can
manifest in individuals with a previously unexpected range of
clinical symptoms consisting of malabsorption syndrome with chronic
diarrhea, weight loss and abdominal distention, affecting the
intestine as a primary site of the disease, leading to the
destruction of any organ and/or the digestive system of the body
and consequent multisystemic disorder (3,11).
In addition, CD has been associated with a number of complications,
mainly including malignancy and autoimmune disorders (12), which can lead to an improper
diagnosis with tropical sprue, particularly due to overlapping or
with atypical symptoms. CD can be diagnosed by the detection of
autoantibodies generated upon the ingestion of gluten or by a bowel
biopsy examination. In addition to this, the incidence of CD has
been found to be associated with diabetes or hypothyroidism, and or
chronic liver disease when compared to tropical sprue (7). Typical disease symptoms include
metabolic bone disease, malnutrition, iron-deficient anemia,
chronic diarrhea, abdominal bloating and distention, weight loss,
damage to the jejuna mucosa and others (13). Conventionally, anemic patients are
generally examined for CD. Typical asymptomatic patients with iron
deficiency anemia have been evaluated by serological testing and
have been found to exhibit a prevalence of CD ranging from 2.3 to
5.0% (14), and in some cases even
between 10.3 to 15% (15). Further
research has also indicated the prevalence of CD among
premenopausal women with iron deficiency anemia (16). It has also been associated with
liver disease, hypertransaminasemia and with a high risk of
neuro-psychiatric disorders, such as peripheral neuropathy, mood
swings, psychosis and epilepsy (17).
Gluten is rich in proline and glutamine residues,
and therefore escapes proteolysis by human digestive enzymes, which
lack post-prolyl endopeptidase activity. Thus, partially digested
gluten peptides are deposited in the intestinal epithelial lumen
and thus increase permeability by binding to CXCR3 receptors, and
enter the lumen (18). Upon this
entry process, the peptides undergo deamidation at glutamine
residues by tissue transglutaminase (tTG) in the lamina propria
region. The deamidated peptides bind to HLA-DQ2/DQ8 molecules with
a greater affinity to gluten reactive CD4+ T cells,
activating the immune response (18,19).
Notably, although one third of the Western population carries these
HLA alleles (20), fortunately,
only 3% develops CD, indicating that HLA-DQ2/DQ8 is necessary, but
not sufficient for the development of the disease (4). Noticeably, 90 to 95% of individuals
with CD express HLA-DQ2, and only 5 to 10% express HLA-DQ8(19).
Although, the significant link between CD and
HLA-DQ2/DQ8 has been established, CD is not present at the time of
birth or before the consumption of gluten in diet (4) and generally does not appear before
the age of 2 years, even in individuals expressing HLA-DQ2/DQ8
(21,22). In recent years, the prevalence of
CD seems to have doubled and has been attributed to the
environment, such as the administration of a gluten-rich diet to
infants and the occurrence of certain gastrointestinal infections
and immunological factors. It has been reported that breast-feeding
for a long period of time and the delay in the ingestion of
gluten-based food can perhaps postpone the onset of CD,
particularly in young children (8). The occurrence of CD among children
aged up to 4 months has not been observed upon the consumption of a
gluten-rich diet; however, children at 7 months old have been found
with autoimmunity (8,19).
3. Role of peptides in the development of
celiac disease
The development of CD may be attributed to HLA. Two
peptides that present HLA-DQ molecules, DQ (α1*0501, β1*02)/DQ2 or
sometimes often DQ (α1*03, β1*0302)/DQ8, present on
antigen-presenting cells (APCs), are the major genetic factors
responsible for CD. HLA-DQ2, a heterodimer, found in 90% of
patients with CD, is generally expressed in a cis/trans form
(23). However, DR3 haplotypes
exist in cis form; where HLA-DQ alleles HLA-DQA1*05 encode α
chain and HLA-DQB1*0201 encodes a β chain of the dimer. A1-B8-DR3
is a classical Caucasian haplotype, whereas A26-B8-DR3 and
Ax-B21-DR3 are typical haplotypes of CD in India (24). In trans form, DR7 haplotypes
are related to the DQB1*0202 allele which encodes the β chain. The
DR11, DR12 or DR 13 haplotype with the HLA-DQA1*05 allele on other
chromosomes encode the α chain, and the α and β chains then unite
to form CD-associated dimer. The α and β chains are encoded by
HLA-DQA1*03 and HLA-DQB1*0302, respectively, in the case of
HLA-DQ8(25). In cis form,
the α and β chains of DQ2 have been shown to be expressed by the
HLA DQA1*0501 and HLA DQB1*0201 alleles of the DR3 haplotype.
However, in trans form, the HLA DQA1*0501 allele of the DR5
haplotype encodes the α chain and DR7 haplotype with the DQB1*0202
allele on other chromosomes that encodes the β chain. Furthermore,
these two chains are united to form the HLA DQ2 molecule on APCs
(18).
Role of gluten and tTG in CD
Glutenin and gliadin are major protein fractions of
wheat gluten. Gliadin fractions are more immunogenic than glutenin
as they have more glutamine and proline residues. In addition,
glutenin peptide epitopes are capable of activating DQ8-restricted
T cell proliferation with QGYYPTSPQQS residues (26). Based on these amino acid sequences,
are gliadins grouped into α, γ and ω. These gliadins contain
epitopes that exhibit an intense affinity towards DQ2/DQ8 molecules
on APCs and are selectively accepted by gliadin-reactive T cells,
which are only observed in the intestinal mucosa of CD-prone
individuals (27). Three
gliadin-derived DQ2-restricted epitopes, such as DQ2-α-I-gliadin,
DQ2-α-II-gliadin and DQ2-γ-I-gliadin, and 2 DQ8-restricted
epitopes, DQ8-α-I-gliadin and DQ8-I-glutenin, are recognized by gut
T cells (26,28).
Gluten peptides become resistant to gastric,
pancreatic and intestinal protease activity due to the high proline
content and therefore enhance their retention in the small
intestine. Furthermore, through epithelial transcytosis otherwise
increases epithelial tight junction permeability, these gluten
peptides reach the lamina propria and stimulate the tTG-mediated
deamidation process (11,29). In this context, tTG catalyzes
selective crosslinking or the deamidation of protein-bound specific
glutamine residues; in addition, acidic pH in the stomach leads to
the random deamidation of a number of peptides (29).
When genetically susceptible individuals ingest
proline-rich gluten, the generation of gluten peptides occurs.
These gluten peptides are not catalyzed by proteases and enter the
lamina propria to form the crosslinking tTG that leads to
deamidation. Deamidated peptides contain more immunostimulatory
epitopes and are presented to gluten-reactive CD4 T-cells by
HLA-DQ2/DQ8. Subsequently, these activated T-cells produce
autoantibodies and other immunological mediators, which may lead to
tissue damage [increased permeability, the dysfunction of
intestinal tight junctions, infiltration of intraepithelial
lymphocytes (IELs), the flattening of villi, and inflammation and
malabsorption, as in late phase of the pathogenesis of CD]
(18).
Fate of deamidated peptides and
HLA-DQ2/DQ8
Deamidated gluten peptides with more negatively
charged residues bind to HLA-DQ2 or HLA-DQ8 molecules with a high
intensity. T cells that recognize the majority of DQ2-specific
gliadin epitopes are tTG-targeted residues (26). A higher amount of glutamine and
proline present in glutenins and gliadins of wheat gluten function
as ideal substrates for TG2. The conversion of glutamine to
glutamic acid residues through the deamidation process perhaps
leads to a relatively large number of negatively charged residues
of gliadin peptides. However, the affinity between gliadin epitopes
and the peptide binding motif of HLA DQ2/DQ8 is crucial and leads
to T cell proliferation (18,19).
In corroboration, deamidated peptide-specific T cell proliferation
has been clearly observed when T cells are mixed with deamidated
peptide and incubated with monocyte-derived dendritic cells
(30).
Moreover, T cells trigger the humoral-mediated
immune response (HMIR) and thus stimulate B cells to produce
corresponding antibodies for gluten peptides and tTG, and also
produce groups of cytokines such as interferon (INF)-γ, interleukin
(IL)-1β, tumor necrosis factor (TNF)-α, IL-6 and IL-8 at increased
levels (31). These cytokines
promote the development of enteric lymphocytes as cytotoxic cells
and result in local inflammation. However, gluten-induced T cells
trigger the immune system, affecting the synthesis of suitable
immune components and decrease the production of IL-17 and IL-22,
resulting in an adverse or abnormal mucosal structure. However, a
gluten-reduced or -free food intake augments the secretion of IL-17
and IL-22 with better mucosal composition (32). Molecular approaches open up
numerous strategies for the effective treatment of CD (19).
4. Diagnosis of celiac disease
The diagnostic criteria for CD from the European
Society for Pediatric Gastroenterology, Hepatology, and Nutrition
(ESPGHAN) were published during the 1990s (33). According to these criteria, the
diagnosis was based on the morphological assessment of the small
intestinal mucosa obtained at 3 distinct conditions, namely: i)
Initial flat mucosa when the patient has ingested gluten; ii)
improvement in the small intestinal mucosa upon the withdrawal of
gluten from the diet; iii) deterioration of the mucosa with gluten
challenge. In 1990, the criteria were revised for both childhood
and adult CD when an individual is on a gluten diet, based on a
small intestinal biopsy and typical histopathological morphology
(34 and refs. therein). Furthermore, upon the complete restriction
on gluten from the diet, there should be a full clinical response.
However serological and biopsy analysis are gold standard tests
(17). Histopathologically, CD
presents a range in severity. Based on the severity of intestinal
mucosal damage, several scoring systems for histological evaluation
have been suggested (34).
Furthermore, antibodies, such as endomysial
antibodies (EMA), tTG antibodies (tTGA) and antibiodies against
gliadin (AGA) of the IgA-class are also significant diagnostic
tools for CD, among which EMA and tTGA are widely used. There is a
high occurrence of CD among individuals with IgA deficiency (8%).
In addition, antibodies against gliadin can be measured by enzyme
linked immunosorbent assay (ELISA) (15). Endomysium is a connective tissue
protein found in the collagenous matrix of mucosal cells.
Antibodies to endomysium can be measured using an immunochemical
assay with monkey esophagus or human umbilical cord as a substrate
in the diagnosis of CD. However, this test is costly and requires
skilled personnel to perform. On the contrary, the measurement of
tTGA using ELISA with guinea pig liver or human recombinant tTG as
a substrate is less costly and more feasible than EMA (15).
The critical role of CD in the pathogenesis of CD is
played by the HLA system; in particular, the role of HLA-DQ2/DQ8 in
the development of CD has been well documented. As HLA-DQ2/DQ8 are
heterodimers, in the majority of cases, carriers do not develop CD
(40%) and therefore, genetic tests for the diagnosis of CD have
limited applications (19), but
can be used to rectify the uncertain diagnosis.
5. Possible therapies for celiac
disease
Non-dietary strategies
A number of non-dietary strategies, that include
decreasing intestinal tight junction (TJ) permeability using TJ
regulators, such as larazotide acetate, the inhibition of tTG
activity, the use of corticosteroids, such as budesonide, and
altering the structure of gliadin using sequestering polymers are
preferred for the treatment of CD (35,36).
Such innovative options pave the way for alternative or adjunctive
therapy. However, the effectiveness of this strategy perhaps is
uncertain in terms of safety, efficacy and longer duration,
rendering monitoring difficult. Furthermore, the follow-up of
patients with CD with these therapeutic measures did not appear
feasible (18). Intestinal TJ
dysfunction leads to the increased permeability of intestinal
barriers to gliadin peptides and exposes submucosal cells to
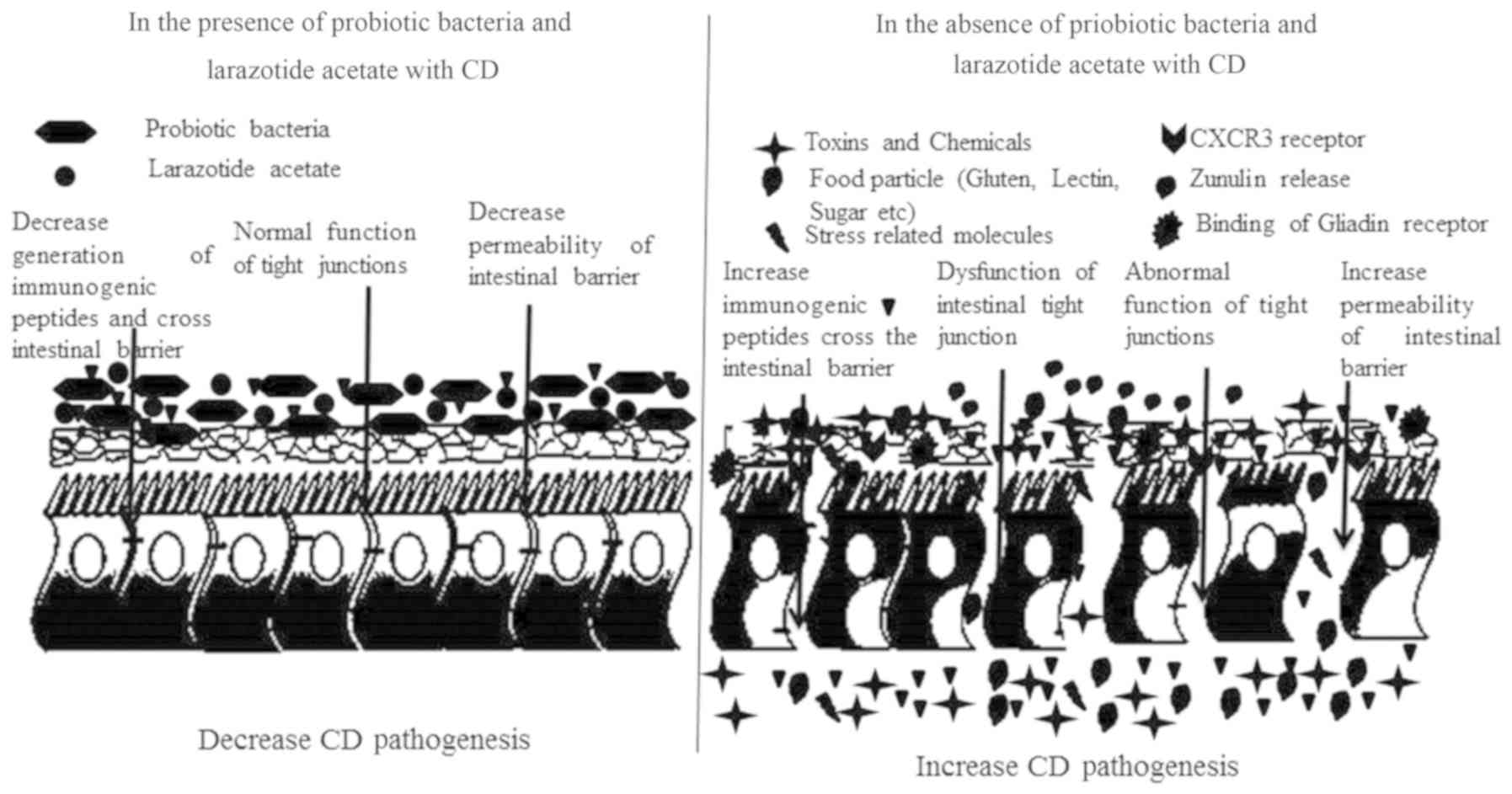
immunogenic peptide-induced toxic effects. Larazotide acetate, a
peptide regulating TJs, prevents the opening of intestinal
epithelial TJs and has exhibited no severe adverse effects in
clinical trials (Fig. 1) (36). The oral administration of this drug
prior to each gluten intake perhaps helps to include the regulation
of gluten-based food, alleviating the uncomfortable symptoms of CD.
This perhaps requires further validation for the improvement of the
efficacy of the drug to reduce gastrointestinal symptoms.
tTG is one of the endomysial auto-antigens which
block the catalytic activity against auto-immunization and reduces
the deamidation of glutamine-rich content. In an in vitro
and in situ study, IgG and IgA classes of antibodies from
patients with CD inhibited tTG activity in a dose-dependent manner
(35). By contrast, tTG activities
are inhibited by anti-tTG antibodies, and when targeted to active
sites with relatively higher concentrations, residual enzyme
activity would be sufficient to induce pathogenesis (37). Budesonide is one of the
corticosteroid drugs which is often used to reduce looseness of the
bowels, inflammation and intestinal tissue damage. Furthermore,
budesonide effectiveness was previously assessed for the treatment
of adults with CD; a group of patients with malabsorption was
administered a only gluten-free diet only and the other group was
administered a gluten-free diet with budesonide on a daily basis;
after 28 days, the subjects treated with both the gluten-free diet
and budesonide exhibited better health compared to the group that
was treated only with the gluten-free diet (35). Furthermore, Goerres et al
(38) reported that the
combination of immunomodulatory medications, such as the steroids,
azathioprine and prednisone, can be used with corticosteroids for
the treatment of a number of autoimmune diseases associated with
CD. In addition, polyhydroxy ethyl methacrylate-co-styrene sodium
sulfonate [P(HEMA-co-SS)] is a sequestering polymer
(non-absorbable). This polymer at gastric pH 1.2 and at intestinal
pH 6.8 reacts with α gliadin peptide and induces changes in
configuration and thus forms larger complex particles; therefore,
tTG fails to recognize structurally altered peptides. Sequestering
the gliadins with the polymer prevents the enzymatic action and the
progression of disease halts (36). Instead, it has been proposed that
this polymer-sequestered peptide would be discharged from the body
prior to entering the blood (18,39).
Dietary strategies
Dietary therapy opted for the treatment of CD should
be safe, effective and feasible with marginal or without
side-effects. Nutritional dietary therapy involves a diet devoid of
wheat, rye and barley. The presence of gluten in food could be
reduced by biotechnological strategies using proteases produced by
microbial cells, that hydrolyze immunogenic gluten peptides
(40,41).
These ideal strategies perhaps offer a potential
alternative or adjunctive treatment options; however, they raise
important questions of safety, efficacy and monitoring during
long-term treatment. However, gluten-free dietary therapy has been
found to be safe, and has become the mainstay of CD management.
Gluten-reduced diet. It is possible to reduce
the gluten content by producing less immunogenic varieties of wheat
or related crop and or other biotechnological approaches to
hydrolyze immunogenic gluten peptides using microbial proteases.
Several studies have revealed that the follow-up of a strict
gluten-free diet reduces the pathogenicity of CD (8). However, this type of diet is
difficult to adhere to by patients, as it requires the lifetime
exclusion of gluten-rich food from their regular diet. In addition,
the FAO/WHO-recommended standards specify that the quality factors
for gluten-free foods should not exceed 20 mg/kg. This consists of
foods with ingredients from wheat (Triticum species), rye,
barley, oats or other varieties. ‘Gluten-free’ foodstuffs
substitute the original foodstuffs with the replacement of
important basic nutrients with the same content of vitamins and
minerals, and are produced under Good Manufacturing Practices (GMP)
to avoid cross-contamination with prolamines. For this reason,
patients with CD following a gluten-free diet, are recommended to
take regular vitamin supplementation. Gluten-free fruits,
vegetables and other food stuffs would also be consumed as a
vitamin source or for micro nutrients. However, the availability of
gluten-free food products according to dietary guidelines is
limited in developing countries, and when available, these products
are costly. Therefore, it is of utmost importance to develop
gluten-reduced wheat foods using microorganisms. Moreover, the
in vivo efficacy of microbial proteases or enzyme
preparations, systematic delivery against gastric acid pH in the
stomach, formulation and dosage are the main challenges that need
to be met (42). Over the past
decade, research in probiotic lactic acid bacteria has proven to be
efficient in the treatment of metabolic disorders and several types
of cancer (40,43,44).
6. Importance of microorganisms in the
treatment of celiac disease
The gut microflora has recently attracted attention
again due to its critical role in health management and new
concepts have been put forth regarding this by medical researchers
(45). Consequently, the
association between human health and the gut microbiota is
significantly acknowledged and confirms that a healthy gut
microflora is crucial for the comprehensive health of an individual
(46). Over the period of
host-microbial co-evolution, the intestine adjusts to bidirectional
host-microbial exchange and also harbors a diverse microbiota which
is separated by a single layer of epithelial cells. The interaction
of the gut with its commensal microorganisms plays a crucial role
in promoting homeostatic functions, such as immunomodulation, the
upregulation of cytoprotective genes, the prevention and regulation
of apoptosis, and the maintenance of barrier function, among others
(46).
Several studies have reported that, at the
epithelial level, a number of factors, such as the masking or
modification of microbial-associated molecular patterns (MAPS) that
are generally recognized by host receptors, as well as the
inhibition of the NF-κB inflammatory pathway allow host cells to
tolerate commensal microorganisms (47). Furthermore, some gut bacteria
produce anti-inflammatory compounds, which result in a controlled
inflammatory response, conferring protection against pathogens.
Sometimes, the generation of a low-grade inflammatory response from
commensal bacteria could be realized to boost the immune system
against the pathogen (48). In
addition, some gut bacteria produce a variety of metabolites
ranging from relatively non-specific fatty acids, proteases with
antimicrobial property and peroxides to highly specific
bacteriocins (49). The gut
microbiota, through these and other related mechanisms, have been
found to play a crucial role in protecting the host from invading
pathogens. These observations suggest that increasing the number of
beneficial bacteria in the gut may be helpful in maintaining gut
health and this could be achieved by the application of probiotics
(19).
Supplementation of probiotics as an
alternative treatment for CD
Microbiologists in the late 18th century identified
that the gut microbiome of healthy individuals differed from that
of infected individuals. The beneficial microorganisms found in the
gut were termed as probiotics. The term probiotic means ‘for life’
and it currently refers to the beneficial effects on humans and
animals. As per FAO/WHO (2001; http://www.fao.org/3/a-a0512e.pdf), probiotics were
defined as living bacteria and when administered in an adequate
quantity, confer health benefits to the host. However, the history
of probiotics dates back to late 18th century. The credit for first
observation made on the positive role of some selected bacteria was
attributed to Eli Metchnikoff (1908). According to their findings,
the bacterial community inhabiting the large intestine of humans
was a source of toxic substances that were detrimental to the host,
intoxicating the blood and contributing to the ageing process,
leading to autointoxication (50).
In 1907, Metchnikoff had postulated that the natural
fermentation of milk by lactic acid producing bacteria, i.e.,
Lactobacillus bulgaricus and Streptococcus
thermophilus, prevented the growth of proteolytic species and
therefore, lactic acid bacteria were used for the implantation of
beneficial microbiota in the gastrointestinal tract. Furthermore,
Fuller's (51) definition for
probiotics reads as ‘live microbial feed supplements which
beneficially affect the host animal by improving its intestinal
microbial balance’. Subsequently, Havenaar (52) corresponded to the probiotic
definition with the following description: A viable mono or mixed
culture of microorganisms which, applied to animals or man,
beneficially affects the host by improving the properties of the
indigenous microflora. At the end of the millennium, Salminen et
al (53) proposed the
following definition: ‘Probiotics are microbial cell preparations
or components of microbial cells that have a beneficial effect on
the health and well-being of host’.
The probiotic microorganisms consist mostly of the
strains of the genera Lactobacillus and
Bifidobacterium, Bacillus, Pediococcus and
others (54). Of note, the
probiotic effect is strain-specific; i.e., different strains of the
same species are always unique. Furthermore, they may differ in
their adherence sites (site-specific) and may also exert specific
immunological effects. Consequently, their action on a healthy and
an inflamed mucosal milieu differs (55). Proposed mechanisms of probiosis
include effects on the composition and function of the intestinal
microbiome. Probiotics produce antimicrobial agents or metabolic
compounds that suppress the growth of other microorganisms or
compete for receptors and binding sites with other intestinal
microorganisms on the intestinal mucosa (56), and thereby prevent the pathogen
colonization. Probiotics strengthens the intestinal barrier, which
may result in the maintenance of immune tolerance, and the
decreased translocation of bacteria across the intestinal
mucosa.
In addition, probiotics can modulate intestinal
immunity and alter the responsiveness of the intestinal epithelia
and immune cells to microorganisms in the intestinal lumen
(57). Furthermore, the regulation
of apoptosis and inflammatory action, the inhibition of
procarcinogenic enzyme activity, and the induction of enzymatic
activity that aids digestion and nutrient absorption enhances host
health. These probiotic functions are the result of very complex
mechanisms of consortia of microorganisms (58). Probiotics can also be found in
dairy and non-dairy products. Probiotics are administered for the
prevention and management of several diseases and disorders that
mainly include traveler's diarrhea, rotavirus gastroenteritis,
pouchitis, vaginosis, cirrhosis, hyperlipidemia, Helicobacter
pylori infection, colitis, acute and chronic gastroenteritis,
irritable bowel syndrome, inflammatory bowel disease and neonatal
enterocolitis (59). In addition,
maldigestion-related conditions, such as lactose intolerance, cow's
milk protein allergy, soy protein allergy and gluten intolerance
can also be treated and managed using probiotics (19).
Detoxification of gluten peptides by
probiotics and their proteases
There are two alternative gluten peptide hydrolysis
strategies as follows: i) The medical approach which the
hydrolyzation of immunogenic gluten peptides following ingestion in
the gastrointestinal tract; and ii) the food technological approach
which involves the to hydrolyzation of gluten peptide prior to the
gluten ingestion during food processing (60).
i) Medical approach. Gluten degradation can
be performed by prolyl endopeptidases of microbial sources that
lend themselves to large-scale production (61). Prolyl endopeptidases (PEP), an
endoproteolytic enzyme of microbial origin, can readily cleave
proline-rich gluten epitopes in contrast to human digestive enzymes
(62). Several researchers have
reported the use of probiotics or their enzymes for gluten
reduction in wheat foods. For example, on long-term wheat flour
fermentation, VSL#3 probiotic bacterial preparation has been shown
to effectively reduce gluten toxicity; surprisingly, no increase in
the infiltration of CD3+ intraepithelial lymphocytes was
observed and moreover, a reduced zonulin release was observed when
the jejuna of patients with CD was exposed to peptic-tryptic digest
from VSL#3(40).
Gluten-detoxifying gelatin-encapsulated capsules of Myxococcus
xanthus prolyl endopeptidase (MX PEP) were characterized and
developed to protect the gastric environment, with safe release
into the duodenal region and a reduction in gluten-induced
inflammation (41). An in
vivo study reported that following the ingestion of gluten
pre-treated with ALV003 to patients with CD, the gluten-specific T
cell response was reduced compared to the placebo group (63). These studies clearly portrayed that
microbial or probiotic proteases perhaps, used as an efficient tool
to combat CD either by or other means.
Numerous studies have revealed the ability of prolyl
endopeptidase from Flavobacterium meningosepticum in
hydrolyzing the 33-mer gliadin peptide. Shan et al (64) also recommended the use of this
enzyme for oral therapy for patients with CD. Furthermore, these
findings were supported by an in vivo study on rats, where
PEP perfusion together with gluten peptides into the rat intestine
accelerated the digestion of gluten by approximately 50 to 100%
(61). In addition, Pyle et
al (65) reported that the
pre-treatment of gluten with PEP from F. meningosepticum,
prevented the development of fat and carbohydrate malabsorption.
PEP from Myxococcus xanthus, Sphingomonas capsulata
(41,64) and Lactobacillus helveticus
(66), Bacillus sp.
(67,68) also supported gluten detoxification
properties. However, Shan et al (64) mentioned that PEP were inactivated
by pepsin and acidic conditions in the host stomach. Similarly,
results were reported by Stepniak et al (69) for Aspergillus niger. This
enzyme can be produced on a large scale at food-grade quality in
industries at a reasonable cost and can be used as an oral
supplementation in patients with CD to reduce the burden of
ingested gluten (70).
ii) Food technological approach. Proteolysis
by sourdough starter culture usage has become a novel approach with
which to reduce gluten toxicity during food processing for patients
with CD (71,72). In several studies, wheat flour
fermentation with lactobacilli has been shown to decrease the
CD-inducing effect of gluten (72). This observation has been
extrapolated by Di Cagno et al (73) to produce sourdoughs that contain
30% of wheat flour and the remaining 70% of non-gluten flour with
selected lactobacilli. The mixed starter (Lactobacillus
alimentarius, L. brevis, L. sanfranciscensis and L.
hilgardii), was able to hydrolyze gliadin fractions and the
bread prepared from that sourdough was tolerated by patients with
CD, which was proven by an intestinal permeability challenge.
Furthermore, Di cagno et al (73) followed the same approach for
preparing pasta for patients with CD. In another study, the
combination of Lactobacillus alimentarius, L. brevis,
L. sanfranciscensis and L. hilgardii were used as
starter culture for pre-fermenting durum wheat semolina. The dough
was then freeze-dried and mixed with buck wheat flour at a ratio of
3:7 and the pasta was prepared. The immunological assay of this
sample has shown that the concentration of gluten has decreased
from 6,280 to 1,045 ppm. However, this level of gluten was higher
than the threshold levels as per the Codex Alimentarius Commission
of WHO. According to Codex Alimentarius, foods containing gluten
<20 ppm can be labeled as ‘gluten-free’, while products
containing gluten >20 and up to 100 ppm can be labeled as
‘gluten-reduced’. However, the combination of lactobacilli with two
fungal proteases from Aspergillus niger and A. oryzae
has decreased the gluten concentration of wheat flour below 10 ppm
during fermentation (74).
Corroborating this, Gobbetti et al (72) observed that functional probiotics
contribute to food tolerance through their array of enzymes. The
probiotic VSL#3 preparation containing Streptococcus
thermophilus, L. plantarum, L. casei, L.
delbrueckii spp. bulgaricus, Bifidobacterium breve,
B. longum and B. infantis were the starters in
baking. During fermentation, there was a marked degradation of
wheat gluten (12). Upon the
exposure of peptic-tryptic digest of VSL#3 fermented dough to
celiac jejunal biopsies, there was no increase in the infiltration
of CD3+ intraepithelial lymphocytes (40). In the food industry, to improve the
quality and other food parameters, microbial transglutaminases
(mTG) are being used (75). The
mTG formulated food could able to generate T cell reactive gluten
epitopes by deamidation. Therefore, it is recommended not to use
mTG in food formulations for celiacs. Based on these functions, it
has been hypothesized that probiotics are distinctly involved in
the dietetic management of CD (76).
7. Mechanisms of action of probiotics in
celiac disease
Strengthening of intestinal epithelial
barrier by probiotics
The intestinal epithelium is in constant interaction
with the luminal contents, as well as the enteric microbiota. The
major function of the intestinal epithelium is the maintenance of
epithelial integrity. Generally, the intestinal mucous layer,
antimicrobial peptides, secretory IgA-a dimer antibody, and the
epithelial junction adhesion complex constitutes the defense system
of the intestinal barrier (77).
The disruption of this barrier facilitates the invasion of
bacterial and food antigens into the submucosa, which further
induces inflammatory responses, resulting in intestinal disorders,
such as inflammatory bowel disease (IBD) (78). Furthermore, probiotic bacteria have
been extensively studied for their beneficial role in the
maintenance of the intestinal epithelial barrier. It has been
reported that probiotics enhance the expression of genes that
participate in tight junction signaling and possibly thereby
reinforce the intestinal barrier integrity (79). Lactic acid bacteria (LAB) modulate
the regulation of genes encoding adherence junction proteins in a
T84 cell barrier model, which include E-cadherin and β-catenin. The
incubation of intestinal cells with LAB differentially enhances the
phosphorylation of adherence junction proteins and results in the
formation of protein kinase C (PKC) isoforms, thereby positively
regulating epithelial barrier function (80).
Studies have demonstrated that probiotics mediate
the restoration of the impaired barrier function. In addition to
the prevention of the enteropathogenic Escherichia coli
(EPEC)-mediated disruption of the mucosal barrier, E. coli
also restores the mucosal integrity in T84 and Caco-2 cells. This
effect was found to be mediated by the enhanced expression and
redistribution of tight junction proteins of zonula occludens 2
(ZO-2) and PKCl, resulting in the reconstruction of the TJ complex
(81). Moreover, Lactobacillus
casei isolates have been shown to sustain intestinal barrier
function through similar mechanisms; they have also been shown to
protect the epithelial barrier and to increase tight junction
protein expression through the activation of the p38 and
extracellular regulated kinase signaling pathways in in vivo
and in vitro experiments (82). These probiotics enhance the
strength of the intestinal epithelial barrier indirectly; thus,
they may prove to be an effective therapy for CD (19).
Adhesion to intestinal mucosa by
probiotics
The ability to adhere to the intestinal mucosa is
considered as one of the main selection criteria for potential
probiotics as it prolongs their persistence in the intestine and
thus allows the probiotics to exert their beneficial effects
(83). Several probiotic bacterial
surface proteins have also been proven to promote mucous adhesion.
The majority of the probiotic bacterial species are Gram-positive
strains consisting of a thick peptidoglycan layer, polysaccharides
such as teichoic acid and lipoteichoic acid, and various
cell-surface proteins, including S-layer proteins. These typical
cell surface structures are in direct contact with the environment
and may function as adhesion factors, antigens, or receptors. In
addition, they are also known to take part in various physiological
functions (47). Based on the wide
variation of molecular structures, lactobacilli exhibit various
adhesive properties on mucin and mucin carbohydrate chains. Based
on these observations, it has been suggested that
Lactobacillus adapt to the constantly changing intestinal
environment of the host, and further indicate that the adhesion
factors of Lactobacillus, exhibiting specific binding
affinities, allow them to selectively colonize inside the host
while concurrently avoiding competition with other bacteria. This
process is mediated by a variety of proteins, saccharide moieties
and lipoteichoic acids (84).
Lactobacillus reuteri produced mucus-binding protein (MUB)
is the most studied example of mucus-targeting bacterial adhesins
(85). The proteins accounting for
the mucous adhesion phenotype of probiotics are mainly secreted and
are surface-associated proteins, which are either anchored to the
membrane through a lipid moiety or are embedded in the cell wall.
Under certain conditions, these MUB play a crucial role in
promoting gut colonization through the degradation of colonocytes
extracellular matrix and or by establishing close contact with the
epithelium (86). In an example,
MapA (mucous adhesion-promoting protein) mediates binding of few
LAB, such as L. reuteri and L. fermentum to mucus.
L. plantarum, has also been shown to induce MUC2 and MUC3
mucins and inhibited the adherence of EPEC (83).
Furthermore, VSL#3, a probiotic mixture, has been
reported to enhance the expression of cell surface mucin genes
(87). In addition, probiotics
also lead to modifications in the intestinal mucins that prevent
pathogen binding. Of note, the binding protein cleaved into an
antimicrobial peptide confers an anti-pathogenic effect to the
host, emphasizing the pleiotropic effect of probiotic surface
proteins (88). Moreover, adhesion
properties of gluten-hydrolyzing probiotics promise to improve the
overall health of patients with CD (12).
Competitive exclusion of pathogens by
probiotics
The concept of ‘competitive exclusion’ was first
proposed by Greenberg (89), in
which one particular bacterial species strongly competes for the
receptor binding site in the intestinal tract with other species of
bacteria. The mechanisms adapted by the bacterial species to
exclude the competitive species vary and mainly include the
establishment of a hostile environment, competing for available
receptor sites, the production of antimicrobial compounds, and the
competitive depletion of available nutrients. To elaborate further,
in order to gain a competitive advantage, bacteria modify their
environment unfavorable for the survival of their competitors by
producing metabolites, such as organic acids (90). In general, probiotic cells are
capable of inhibiting the attachment of pathogenic bacteria by
means of steric hindrance at enterocyte pathogen receptors
(88). Several studies have
reported the effect of probiotics on the competitive exclusion of
pathogens in vitro as well as in vivo. In the case of
Lactobacillus rhamnosus, it was found to eliminate herpes
simplex virus type I by activating macrophages (91). In addition, probiotics in the gut
may also help to eradicate Helicobacter pylori, mitigating
the frequency of epigastric pain, vomiting, nausea and diarrhea
(92).
Effect of antimicrobial compounds
produced from probiotics
The production of low molecular weight compounds
(<1,000 kDa), such as organic acids, and antibacterial
substances called bacteriocins (>1,000 kDa) are majorly
responsible for the health benefits conferred by probiotics in the
host (93). The organic acids
produced by probiotics mainly lactic and acetic acids exert potent
inhibitory effects on Gram-negative bacteria. These organic acids
enter the bacterial cells in their un-dissociated form and in the
cytoplasm they undergo dissociation. Furthermore, the accumulation
of ionized form of organic acids and/or the lowering of the
intracellular pH results in the death of target bacteria (83). A number of lactic acid bacteria
produce bacteriocins, such as lactacin B (L. acidophilus),
plantaricin (L. plantarum) and nisin (Lactococcus
lactis) (94). Bacteriocins
destruct the target cells by forming pores or inhibiting the cell
wall synthesis. To consider, nisin forms a complex with lipid II
and thereby inhibit the biosynthesis of cell wall (95). Bacteriocin production perhaps
encourages the establishment and increases the prevalence of
bacteriocin-producing strains, thus directly inhibiting pathogens
in the gastrointestinal tract (12).
Furthermore, probiotics producing anti-metabolic
substances inhibit the growth of fungi and other species of
bacteria (96). Furthermore, the
production of antifungal substances, such as benzoic acid,
methylhydantoin, mevalonolactone and short-chain fatty acids by
Lactobacillus spp. are quite evident (97). Another study reported the
production of proteinaceous compounds exhibiting antifungal
properties by Lactobacillus coryniformis (98). In addition, Dal Bello et al
(99) identified and chemically
characterized the 4 antifungal substances produced by L.
plantarum FST 1.7, including lactic acid, phenyl lactic acid
and 2 cyclic dipeptides, [cyclo(L-Leu-L-Pro) and
cyclo(L-Phe-L-Pro)]. A similar study reported the production of the
antifungal cyclic dipeptides, cyclo (L-Phe-L-Pro) and cyclo
(L-Phe-traps-4-OH-L-Pro), by lactic acid bacteria, which inhibited
the growth of food borne fungi (100).
Immunomodulatory effect of
probiotics
Probiotics interact with intestinal epithelial
cells, macrophages, lymphocytes and dendritic cells (101). In the adaptive immune response, B
and T cells specific for pathogens play an important role, while
the innate immune system responds to pathogen-associated molecular
patterns (PAMPs) which are shared by the majority of pathogens. The
pattern recognition receptors (PRP) that bind to PAMP trigger
primary immune response to pathogens (47). Furthermore, Toll-like receptors
(TLRs) are transmembrane proteins that are expressed on various
immune, as well as non-immune cells. In humans, there are 11
classes of TLRs that have been identified thus far. Amongst these,
TLR1, TLR2, TLR4, TLR5, TLR6 and TLR10 are associated with the
outer membrane and primarily respond to bacterial
surface-associated PAMPs. On the other hand, TLR3, TLR7, TLR8 and
TLR9 are found on the surface of endosomes, and they respond
primarily to nucleic acid-based PAMPs from viruses and other
bacteria (101). In addition, the
TLR-mediated signaling has been shown to regulate the maturation of
dendritic cells, upregulating various maturation markers such as
CD80, CD83 and CD86, as well as CCR7 chemokine receptor. Moreover,
it has been observed that following activation by commensal and
probiotic microorganisms, dendritic cells initiate an appropriate
response, such as the differentiation of Th0 to
Treg, which exhibit an inhibitory effect on the
Th1, Th2 and Th17 inflammatory
response (102).
Probiotics reduce intestinal inflammation via the
downregulation of TLR expression. The secretary metabolites from
probiotics prevent the entry of TNF-α into blood mononuclear cells
and also arrest NF-κB signaling in enterocytes (101). Furthermore, TLRs recognize
peptidoglycan, a major component of Gram-positive bacteria. Several
studies have demonstrated the necessity of TLRs for lactobacilli to
exert their immunomodulatory effects. It has been shown that the
cell wall components of lactobacilli mainly diacylated membrane
anchors of lipoproteins and lipoteichoic acids take part in
signaling upon concurrent binding to TLR2 and TLR6. The activation
of TLR2 enhances the production of cytokines and also increases
transepithelial resistance to invading pathogens (12,103). This evidence supports
immunomodulatory functions of probiotic bacteria in their host and
reduce the CD with managing the inflammation inducing transmembrane
signals (19,104).
Safety and efficacy of probiotics for
human use
It is mandatory that any given probiotic strain
should not be at any significant risk with regard to transferable
antibiotic resistance (105).
Furthermore, if any strain is under evaluation belonging to a
particular species, it needs to be examined for toxin production
and/or hemolytic activity (106).
The assessment of a lack of infectivity by a probiotic strain in
immune compromised individuals would be an additional advantage to
human use (107). The outcome of
efficacy studies on probiotics are required to be proven
significantly with benefits in human trials, such as an improvement
in conditions, symptoms, signs, wellbeing/quality of life, a
reduced risk of disease or longer time to next occurrence or faster
recovery from illness (19,104).
Each of the parameters should have a proven association with
probiotics and may be helpful for CD therapy (108).
8. Commonly available commercial
probiotics
The indigenous microbiota of infants is dominated
by bifidobacteria, which are recognized shortly after birth. Their
proliferation is stimulated by the glycoprotein components of
k-casein in human colostrum and, to a lesser extent, human milk.
The extent of bifidobacterial population decreases with the
increasing age of the human subject and eventually becomes the
third most abundant genus (accounting for approximately 25% of the
total adult gut microbiota) after the genera Bacteroides and
Eubacterium (109). The
commonly available probiotics are from the strains belonging to the
genera, Lactobacillus, Streptococcus,
Bifidobacterium and Bacillus spp. The commercially
available probiotic products in the market (110,111) are listed in Table I. Bifidobacteria are microorganisms
of paramount importance in the active and complex ecosystem of the
intestinal tract of humans and human gastrointestinal and
genitourinary tracts, the exact ratio of which is determined mainly
by age and diet (19).
 | Table IList of commonly available commercial
probiotics for human consumption (110,111). |
Table I
List of commonly available commercial
probiotics for human consumption (110,111).
| Microorganism | Company | Microorganism | Company |
|---|
| Bifidobacterium
adolescentis ATCC 15703 | Chr. Hansen | Bacillus
cereus strain IP 5832 (ATCC 14893) | Marion Merrell Dow
Laboratories |
| Bifidobacterium
animalis Bb-12 | Chr. Hansen | Bacillus
subtilis | Tendiphar
Corporation |
| Bifidobacterium
essencis | Danone®
(Activia) | B.
subtilis | Bidiphar-BinhDinh
Pharmaceutical |
| Bifidobacterium
infantis | Yakult
Danone® | B. subtilis
2335 and B. licheniformis 2336 | Biofarm |
| Bifidobacterium
lactis | DSM | B. subtilis
and Lactobacillus acidophilus | IVAC |
| Bacillus
lactis DR10 | Danisco
(Howaru™) | B.
pumilus | Biophar
Company |
| Lactobacillus
acidophilus | Chr. Hansen | B. cereus
strain GM | Geyer Medicamentos
S. |
| LA-1/LA-5 | Rhodia | | |
| NCFM | Nebraska
Cultures | | |
| DDS-1 | Snow Brand
Milk | | |
| SBT-2062 | Products | | |
| Lactobacillus
casei | Yakult
(Yakult®), Danone® | B.
polyfermenticus SCD | Binex Co.,
Ltd. |
| Lactobacillus
fermentum RC-14 | Urex Biotech | B. subtilis
strain RO179 Enterococcus faecium | Hanmi
Pharmaceutical Co., Ltd. |
| Lactobacillus
lactis L1A | Essum AB | B. subtilis,
B. polymyxa, B. pumilus | Nature’s First
Law |
| | | B.
laterosporus | |
| Lactobacillus
rhamnosus | Valio | B.
subtilis | Pasteur Institute
of Ho |
| GG | Urex Biotech | | Chi Minh City |
| GR-1 | Essum AB | | |
| LB21 | Probi AB | | |
| 271 | | | |
| Lactobacillus
plantarum 299v Lp01 | Probi AB | B.
cereus | Mekophar,
Pharmaceutical Factory |
Bacillus spp. have been used as a probiotic
for at least 50 years in an Italian product commercialized as
Enterogermina® (2X109 spores). Among this
group, some species that have been evaluated for their probiotic
potential, which include Bacillus subtilis, Bacillus
clausii, Bacillus cereus, Bacillus coagulans and Bacillus
licheniformis (112).
Currently, in the market, probiotic products containing GRAS
isolates of Bacillus are increasingly available, including
Bacillus subtilis, Bacillus cereus, Bacillus licheniformis,
Bacillus pumilus, Bacillus clausii and Bacillus
coagulans (111). The members
of Bacillus have been proven to form dormant forms as
endospores, a protective mechanism to overcome the unfavorable
conditions, such as nutrient deprivation and other factors of
environmental stress. The tough coat of endospores confers
resistance to high temperatures, low pH and low moisture conditions
(67,68,113). Taking into account the advantage
of this property, unlike Lactobacillus,
Bifidobacteria and other commonly used probiotic lactic acid
bacteria, Bacillus probiotics can be used in the form of spores,
which has an indefinite shelf life and does not require
refrigeration (114).
Furthermore, studies have demonstrated the potential probiotic
attributes of the Bacillus spp. and their efficacy in
gastrointestinal disorders (112,113). Consequently, GRAS isolates
of Bacillus spp. have attracted the attention of the
probiotic industry, having more advantages over conventional
probiotic lactic acid bacteria.
Other probiotic bacteria include Leuconostoc
mesenteroides, Leuconostoc lactis, Streptococcus
thermophilus, and Pediococcus spp. (115). Among the probiotic yeasts, the
most common genus, Saccharomyces cerevisiae has been used as
a potential probiotic. The potential probiotic effect of S.
cerevisiae and S. cerevisiae var. boulardii has
been demonstrated since they are able to tolerate low pH and bile
and protect against bacterial infections through the reduction of
the intestinal pro-inflammatory response (116) and have been used worldwide as a
therapeutic agent for diarrhea and other gastrointestinal
disturbances caused by the administration of antimicrobial agents
(117). Although lactic acid
bacteria are beneficial in alleviating or managing various health
issues, in recent times, these bacteria are used in alleviating a
high intolerance of gluten allergy leading to CD (12,19,104); therefore, they have become a food
of choice by patients with CD.
9. Conclusion
Current probiotic research aims at the use of the
normal, healthy gut microbiota as a therapy for CD and their
implications on human health. From all these aspects, probiotics
are generally safe and cost-effective compared to the drugs
available in the market. Bifidobacterium spp. and
Lactobacilli spp. are the promising agents for probiotic
therapy for patients with CD. Further studies should emphasize on
microbiota characterization with potential benefits to gut health of
patients with CD. The molecular mechanisms of probiotic action are
in a tranquil state and require to be characterized. The future
metabolomic approach would provide insight into the knowledge of
the mechanisms of the microbiota for CD therapy.
Acknowledgements
The authors sincerely acknowledge the support
rendered by Davangere University, Davangere, Karnataka, India
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DG conceived the study, drafted the manuscript, and
was also involved in editing, reviewing and revising the
manuscript, and also communicated the manuscript to the journal. AR
was involved in the conception of the study, as well as in the
processing of the data acquired for this review, the drafting of
the manuscript and processing of the figure. AR was also involved
in the study design and editing of the manuscript. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Losowsky MS: A history of coeliac disease.
Dig Dis. 26:112–120. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dowd B and Walker-Smith J: Samuel Gee,
Aretaeus, and the coeliac affection. BMJ. 2:45–47. 1974.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fasano A: Surprises from celiac disease.
Sci Am. 301:54–61. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dubé C, Rostom A, Sy R, Cranney A,
Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V,
et al: The prevalence of celiac disease in average-risk and at-risk
Western European populations: A systematic review.
Gastroenterology. 128 (Suppl 1):S57–S67. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Barada K, Bitar A, Mokadem MA, Hashash JG
and Green P: Celiac disease in Middle Eastern and North African
countries: A new burden? World J Gastroenterol. 16:1449–1457.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yadav P, Das P, Mirdha BR, Gupta SD,
Bhatnagar S, Pandey RM and Makharia GK: Current spectrum of
malabsorption syndrome in adults in India. Indian J Gastroenterol.
30:22–28. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fasano A, Berti I, Gerarduzzi T, Not T,
Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID,
et al: Prevalence of celiac disease in at-risk and not-at-risk
groups in the United States: A large multicenter study. Arch Intern
Med. 163:286–292. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mohindra S, Yachha SK, Srivastava A,
Krishnani N, Aggarwal R, Ghoshal UC, Prasad KK and Naik SR: Coeliac
disease in Indian children: Assessment of clinical, nutritional and
pathologic characteristics. J Health Popul Nutr. 19:204–208.
2001.PubMed/NCBI
|
|
9
|
Sollid LM: Molecular basis of celiac
disease. Annu Rev Immunol. 18:53–81. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Korponay-Szabó IR, Halttunen T, Szalai Z,
Laurila K, Király R, Kovács JB, Fésüs L and Mäki M: In vivo
targeting of intestinal and extraintestinal transglutaminase 2 by
coeliac autoantibodies. Gut. 53:641–648. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ivarsson A, Hernell O, Stenlund H and
Persson LA: Breast-feeding protects against celiac disease. Am J
Clin Nutr. 75:914–921. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chander AM, Yadav H, Jain S, Bhadada SK
and Dhawan DK: Cross-talk between gluten, intestinal microbiota and
intestinal mucosa in celiac disease: Recent advances and basis of
autoimmunity. Front Microbiol. 9(2597)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rastogi A, Bhadada SK, Bhansali A, Kochhar
R and Santosh R: Celiac disease: A missed cause of metabolic bone
disease. Indian J Endocrinol Metab. 16:780–785. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Howard MR, Turnbull AJ, Morley P, Hollier
P, Webb R and Clarke A: A prospective study of the prevalence of
undiagnosed coeliac disease in laboratory defined iron and folate
deficiency. J Clin Pathol. 55:754–757. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rostom A, Murray JA and Kagnoff MF:
American Gastroenterological Association (AGA) Institute technical
review on the diagnosis and management of celiac disease.
Gastroenterology. 131:1981–2002. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Annibale B, Lahner E, Chistolini A,
Gailucci C, Di Giulio E, Capurso G, Luana O, Monarca B and Delle
Fave G: Endoscopic evaluation of the upper gastrointestinal tract
is worthwhile in premenopausal women with iron deficiency anaemia
irrespective of menstrual flow. Scand J Gastroenterol. 38:239–245.
2003.PubMed/NCBI
|
|
17
|
Ludvigsson JF, Osby U, Ekbom A and
Montgomery SM: Coeliac disease and risk of schizophrenia and other
psychosis: A general population cohort study. Scand J
Gastroenterol. 42:179–185. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gayathri D and Rashmi BS: Development of
celiac disease; pathogenesis and strategies to control: A molecular
approach. J Nutr Food Sci. 4(310)2014.
|
|
19
|
Coqueiro AY, Bonvini A, Tirapegui J and
Rogero MM: Probiotics supplementation as an alternative method for
celiac disease treatment. Int J Probiotics Prebiotics. 12:23–32.
2017.
|
|
20
|
Högberg L, Fälth-Magnusson K, Grodzinsky E
and Stenhammar L: Familial prevalence of coeliac disease: A
twenty-year follow-up study. Scand J Gastroenterol. 38:61–65.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hill ID: Management of celiac disease in
childhood and adolescence: Unique challenges and strategies. Curr
Treat Options Gastroenterol. 9:399–408. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ludvigsson JF, Fälth-Magnusson K and
Ludvigsson J: Tissue transglutaminase auto-antibodies in cord blood
from children to become celiacs. Scand J Gastroenterol.
36:1279–1283. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sollid LM, Markussen G, Ek J, Gjerde H,
Vartdal F and Thorsby E: Evidence for a primary association of
celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp
Med. 169:345–350. 1989.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kaur G, Sarkar N, Bhatnagar S, Kumar S,
Rapthap CC, Bhan MK and Mehra NK: Pediatric celiac disease in India
is associated with multiple DR3-DQ2 haplotypes. Hum Immunol.
63:677–682. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Megiorni F and Pizzuti A: HLA-DQA1 and
HLA-DQB1 in Celiac disease predisposition: Practical implications
of the HLA molecular typing. J Biomed Sci. 19(88)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
van de Wal Y, Kooy YM, van Veelen P, Vader
W, August SA, Drijfhout JW, Peña SA and Koning F: Glutenin is
involved in the gluten-driven mucosal T cell response. Eur J
Immunol. 29:3133–3139. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Molberg O, Kett K, Scott H, Thorsby E,
Sollid LM and Lundin KE: Gliadin specific, HLA DQ2-restricted T
cells are commonly found in small intestinal biopsies from coeliac
disease patients, but not from controls. Scand J Immunol.
46:103–109. 1997.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Arentz-Hansen H, Körner R, Molberg O,
Quarsten H, Vader W, Kooy YM, Lundin KE, Koning F, Roepstorff P,
Sollid LM, et al: The intestinal T cell response to alpha-gliadin
in adult celiac disease is focused on a single deamidated glutamine
targeted by tissue transglutaminase. J Exp Med. 191:603–612.
2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Visser J, Rozing J, Sapone A, Lammers K
and Fasano A: Tight junctions, intestinal permeability, and
autoimmunity: Celiac disease and type 1 diabetes paradigms. Ann N Y
Acad Sci. 1165:195–205. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ráki M, Schjetne KW, Stamnaes J, Molberg
Ø, Jahnsen FL, Issekutz TB, Bogen B and Sollid LM: Surface
expression of transglutaminase 2 by dendritic cells and its
potential role for uptake and presentation of gluten peptides to T
cells. Scand J Immunol. 65:213–220. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Manavalan JS, Hernandez L, Shah JG,
Konikkara J, Naiyer AJ, Lee AR, Ciaccio E, Minaya MT, Green PH and
Bhagat G: Serum cytokine elevations in celiac disease: Association
with disease presentation. Hum Immunol. 71:50–57. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu H, Feely SL, Wang X, Liu DX, Borda JT,
Dufour J, Li W, Aye PP, Doxiadis GG, Khosla C, et al:
Gluten-sensitive enteropathy coincides with decreased capability of
intestinal T cells to secrete IL-17 and IL-22 in a macaque model
for celiac disease. Clin Immunol. 147:40–49. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Husby S, Koletzko S, Korponay-Szabó IR,
Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski
D, Catassi C, et al: ESPGHAN Working Group on Coeliac Disease
Diagnosis; ESPGHAN Gastroenterology Committee; European Society for
Pediatric Gastroenterology, Hepatology, and Nutrition: European
Society for Pediatric Gastroenterology, Hepatology, and Nutrition
guidelines for the diagnosis of coeliac disease. J Pediatr
Gastroenterol Nutr. 54:136–160. 2012.
|
|
34
|
Fasano A, Araya M, Bhatnagar S, Cameron D,
Catassi C, Dirks M, Mearin ML, Ortigosa L and Phillips A: Celiac
Disease Working Group, FISPGHAN. Federation of International
Societies of Pediatric Gastroenterology, Hepatology, and Nutrition
consensus report on celiac disease. J Pediatr Gastroenterol Nutr.
47:214–219. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ciacci C, Maiuri L, Russo I, Tortora R,
Bucci C, Cappello C, Santonicola A, Luciani A, Passananti V and
Iovino P: Efficacy of budesonide therapy in the early phase of
treatment of adult coeliac disease patients with malabsorption: An
in vivo/in vitro pilot study. Clin Exp Pharmacol Physiol.
36:1170–1176. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liang L, Pinier M, Leroux JC and Subirade
M: Interaction of alpha-gliadin with poly(HEMA-co-SS): Structural
characterization and biological implication. Biopolymers.
91:169–178. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dieterich W, Trapp D, Esslinger B,
Leidenberger M, Piper J, Hahn E and Schuppan D: Autoantibodies of
patients with coeliac disease are insufficient to block tissue
transglutaminase activity. Gut. 52:1562–1566. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Goerres MS, Meijer JW, Wahab PJ,
Kerckhaert JA, Groenen PJ, Van Krieken JH and Mulder CJ:
Azathioprine and prednisone combination therapy in refractory
coeliac disease. Aliment Pharmacol Ther. 18:487–494.
2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pinier M, Fuhrmann G, Galipeau HJ, Rivard
N, Murray JA, David CS, Drasarova H, Tuckova L, Leroux JC and Verdu
EF: The copolymer P(HEMA-co-SS) binds gluten and reduces immune
response in gluten-sensitized mice and human tissues.
Gastroenterology. 142:316–25.e1, 12. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
De Angelis M, Cassone A, Rizzello CG,
Gagliardi F, Minervini F, Calasso M, Di Cagno R, Francavilla R and
Gobbetti M: Mechanism of degradation of immunogenic gluten epitopes
from Triticum turgidum L. var. durum by sourdough
lactobacilli and fungal proteases. Appl Environ Microbiol.
76:508–518. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gass J, Ehren J, Strohmeier G, Isaacs I
and Khosla C: Fermentation, purification, formulation, and
pharmacological evaluation of a prolyl endopeptidase from
Myxococcus xanthus: Implications for Celiac Sprue therapy.
Biotechnol Bioeng. 92:674–684. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hallert C, Grant C, Grehn S, Grännö C,
Hultén S, Midhagen G, Ström M, Svensson H and Valdimarsson T:
Evidence of poor vitamin status in coeliac patients on a
gluten-free diet for 10 years. Aliment Pharmacol Ther.
16:1333–1339. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Asha and Gayathri D: Synergistic
impact of Lactobacillus fermentum, Lactobacillus
plantarum and vincristine on 1,2-dimethylhydrazine-induced
colorectal carcinogenesis in mice. Exp Ther Med. 3:1049–1054.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gayathri D, Asha and Devaraja TN:
Lactobacillus sp. as probiotics for human health with
special emphasis on colorectal cancer. Indian J Sci Technol.
4:1008–1014. 2011.
|
|
45
|
Quigley EMM: Gut bacteria in health and
disease. Gastroenterol Hepatol (N Y). 9:560–569. 2013.PubMed/NCBI
|
|
46
|
Patel RM and Lin PW: Developmental biology
of gut-probiotic interaction. Gut Microbes. 1:186–195.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lebeer S, Vanderleyden J and De
Keersmaecker SC: Host interactions of probiotic bacterial surface
molecules: Comparison with commensals and pathogens. Nat Rev
Microbiol. 8:171–184. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pagnini C, Saeed R, Bamias G, Arseneau KO,
Pizarro TT and Cominelli F: Probiotics promote gut health through
stimulation of epithelial innate immunity. Proc Natl Acad Sci USA.
107:454–459. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Corr SC, Li Y, Riedel CU, O'Toole PW, Hill
C and Gahan CG: Bacteriocin production as a mechanism for the
antiinfective activity of Lactobacillus salivarius UCC118.
Proc Natl Acad Sci USA. 104:7617–7621. 2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Metchnikoff E: The nature of man. Studies
in optimistic philosophy. William Heinemann, London, 1908.
|
|
51
|
Fuller R: Probiotics in man and animals. J
Appl Bacteriol. 66:365–378. 1989.PubMed/NCBI
|
|
52
|
Havenaar R, Brink T and Huis in't Veld
JHJ: Selection of Strains for Probiotic Use. In: Probiotics: The
Scientific Basis. Fuller R (ed). Chapman & Hall, London,
pp151-170, 1992.
|
|
53
|
Salminen S, Ouwehand A, Benno Y and Lee
YK: Probiotics: How should they be defined. Trends Food Sci
Technol. 10:107–110. 1999.
|
|
54
|
Khatri I, Sharma S, Ramya TNC and
Subramanian S: Complete genomes of Bacillus coagulans S-lac
and Bacillus subtilis TO-A JPC, two phylogenetically
distinct probiotics. PLoS One. 11(e0156745)2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Isolauri E, Salminen S and Ouwehand AC:
Microbial-gut interactions in health and diseasein infective
diarrhoea and inflammatory bowel diseases. J Gastroenterol Hepatol.
15:489–493. 2000.
|
|
56
|
Loponen J: Prolamin degradation in
sourdoughs(unpublished PhD thesis). University of Helsinki,
2006.
|
|
57
|
Piper JL, Gray GM and Khosla C: Effect of
prolyl endopeptidase on digestive-resistant gliadin peptides in
vivo. J Pharmacol Exp Ther. 311:213–219. 2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hausch F, Shan L, Santiago NA, Gray GM and
Khosla C: Intestinal digestive resistance of immunodominant gliadin
peptides. Am J Physiol Gastrointest Liver Physiol. 283:G996–G1003.
2002.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tye-Din JA, Anderson RP, Ffrench RA, Brown
GJ, Hodsman P, Siegel M, Botwick W and Shreeniwas R: The effects of
ALV003 pre-digestion of gluten on immune response and symptoms in
celiac disease in vivo. Clin Immunol. 134:289–295. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Shan L, Marti T, Sollid LM, Gray GM and
Khosla C: Comparative biochemical analysis of three bacterial
prolyl endopeptidases: Implications for coeliac sprue. Biochem J.
383:311–318. 2004.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pyle GG, Paaso B, Anderson BE, Allen DD,
Marti T, Li Q, Siegel M, Khosla C and Gray GM: Effect of
pretreatment of food gluten with prolyl endopeptidase on
gluten-induced malabsorption in celiac sprue. Clin Gastroenterol
Hepatol. 3:687–694. 2005.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen YS, Christensen JE, Broadbent JR and
Steele JL: Identification and characterization of Lactobacillus
helveticus PepO2, an endopeptidase with post-proline
specificity. Appl Environ Microbiol. 69:1276–1282. 2003.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Rashmi BS and Gayathri D: Draft genome
sequence of gluten hydrolysing bacterium Bacillus subtilis
GS 188, Isolated from Wheat Sourdough. Genome Announc.
5(e00952)2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Rashmi BS and Gayathri D: Molecular
characterization of gluten hydrolysing Bacillus sp and their
efficacy and biotherapeutic potential as probiotics using Caco-2
cell line. J Appl Microbiol. 123:759–772. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Stepniak D, Spaenij-Dekking L, Mitea C,
Moester M, de Ru A, Baak-Pablo R, van Veelen P, Edens L and Koning
F: Highly efficient gluten degradation with a newly identified
prolyl endoprotease: Implications for celiac disease. Am J Physiol
Gastrointest Liver Physiol. 291:G621–G629. 2006.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Edens L, Dekker P, van der Hoeven R, Deen
F, de Roos A and Floris R: Extracellular prolyl endoprotease from
Aspergillus niger and its use in the debittering of protein
hydrolysates. J Agric Food Chem. 53:7950–7957. 2005.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Di Cagno R, De Angelis M, Lavermicocca P,
De Vincenzi M, Giovannini C, Faccia M and Gobbetti M: Proteolysis
by sourdough lactic acid bacteria: Effects on wheat flour protein
fractions and gliadin peptides involved in human cereal
intolerance. Appl Environ Microbiol. 68:623–633. 2002.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Gobbetti M, Cagno RD and De Angelis M:
Functional microorganisms for functional food quality. Crit Rev
Food Sci Nutr. 50:716–727. 2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
di Cagno R, de Angelis M, Alfonsi G, de
Vincenzi M, Silano M, Vincentini O and Gobbetti M: Pasta made from
durum wheat semolina fermented with selected lactobacilli as a tool
for a potential decrease of the gluten intolerance. J Agric Food
Chem. 53:4393–4402. 2005.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Rizzello CG, De Angelis M, Di Cagno R,
Camarca A, Silano M, Losito I, De Vincenzi M, De Bari MD, Palmisano
F, Maurano F, et al: Highly efficient gluten degradation by
lactobacilli and fungal proteases during food processing: New
perspectives for celiac disease. Appl Environ Microbiol.
73:4499–4507. 2007.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Dekking EHA, Veelen PAV, de Ru A,
Kooy-Winkelaar EMC and Groneveld T: Microbial transglutaminases
generate T cell stimulatory epitopes involved in celiac disease. J
Cereal Sci. 47:339–346. 2008.
|
|
72
|
de Sousa Moraes LF, Grzeskowiak LM, de
Sales Teixeira TF and Gouveia Peluzio MC: Intestinal microbiota and
probiotics in celiac disease. Clin Microbiol Rev. 27:482–489.
2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ohland CL and Macnaughton WK: Probiotic
bacteria and intestinal epithelial barrier function. Am J Physiol
Gastrointest Liver Physiol. 298:G807–G819. 2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Sartor RB: Mechanisms of disease:
Pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin
Pract Gastroenterol Hepatol. 3:390–407. 2006.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Anderson RC, Cookson AL, McNabb WC, Park
Z, McCann MJ, Kelly WJ and Roy NC: Lactobacillus plantarum
MB452 enhances the function of the intestinal barrier by increasing
the expression l. Probiotics. Best Pract Res Clin Gastroenterol.
18:299–313. 2004.
|
|
76
|
O'Shea EF, Cotter PD, Stanton C, Ross RP
and Hill C: Production of bioactive substances by intestinal
bacteria as a basis for explaining probiotic mechanisms:
Bacteriocins and conjugated linoleic acid. Int J Food Microbiol.
152:189–205. 2012.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Thomas CM and Versalovic J:
Probiotics-host communication: Modulation of signaling pathways in
the intestine. Gut Microbes. 1:148–163. 2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Boirivant M and Strober W: The mechanism
of action of probiotics. Curr Opin Gastroenterol. 23:679–692.
2007.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Gionchetti P, Rizzello F, Venturi A and
Campieri M: Probiotics evels of genes involved in tight junction
formation. BMC Microbiol. 10(316)2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hummel S, Veltman K, Cichon C, Sonnenborn
U and Schmidt MA: Differential targeting of the
E-Cadherin/β-Catenin complex by gram-positive probiotic
lactobacilli improves epithelial barrier function. Appl Environ
Microbiol. 78:1140–1147. 2012.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Zyrek AA, Cichon C, Helms S, Enders C,
Sonnenborn U and Schmidt MA: Molecular mechanisms underlying the
probiotic effects of Escherichia coli Nissle 1917 involve
ZO-2 and PKCzeta redistribution resulting in tight junction and
epithelial barrier repair. Cell Microbiol. 9:804–816.
2007.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Dai C, Zhao DH and Jiang M: VSL#3
probiotics regulate the intestinal epithelial barrier in
vivo and in vitro via the p38 and ERK signaling
pathways. Int J Mol Med. 29:202–208. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ouwehand AC, Salminen S, Tölkkö S, Roberts
P, Ovaska J and Salminen E: Resected human colonic tissue: New
model for characterizing adhesion of lactic acid bacteria. Clin
Diagn Lab Immunol. 9:184–186. 2002.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Vélez MP, De Keersmaecker SC and
Vanderleyden J: Adherence factors of Lactobacillus in the
human gastrointestinal tract. FEMS Microbiol Lett. 276:140–148.
2007.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Buck BL, Altermann E, Svingerud T and
Klaenhammer TR: Functional analysis of putative adhesion factors in
Lactobacillus acidophilus NCFM. Appl Environ Microbiol.
71:8344–8351. 2005.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Sánchez B, Urdaci MC and Margolles A:
Extracellular proteins secreted by probiotic bacteria as mediators
of effects that promote mucosa-bacteria interactions. Microbiology.
156:3232–3242. 2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Juntunen M, Kirjavainen PV, Ouwehand AC,
Salminen SJ and Isolauri E: Adherence of probiotic bacteria to
human intestinal mucus in healthy infants and during rotavirus
infection. Clin Diagn Lab Immunol. 8:293–296. 2001.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Coconnier MH, Bernet MF, Chauvière G and
Servin AL: Adhering heat-killed human Lactobacillus
acidophilus, strain LB, inhibits the process of pathogenicity
of diarrhoeagenic bacteria in cultured human intestinal cells. J
Diarrhoeal Dis Res. 11:235–242. 1993.PubMed/NCBI
|
|
89
|
Greenberg B: Salmonella suppression
by known populations of bacteria in flies. J Bacteriol. 99:629–635.
1969.PubMed/NCBI
|
|
90
|
Schiffrin EJ and Blum S: Interactions
between the microbiota and the intestinal mucosa. Eur J Clin Nutr.
56 (Suppl 3):S60–S64. 2002.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Khani S, Motamedifar M, Golmoghaddam H,
Hosseini HM and Hashemizadeh Z: In vitro study of the effect of a
probiotic bacterium Lactobacillus rhamnosus against herpes
simplex virus type 1. Braz J Infect Dis. 16:129–135.
2012.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ahmad K, Fatemeh F, Mehri N and Maryam S:
Probiotics for the treatment of pediatric Helicobacter
pylori infection: A randomized double blind clinical trial.
Iran J Pediatr. 23:79–84. 2013.PubMed/NCBI
|
|
93
|
De Keersmaecker SC, Verhoeven TL, Desair
J, Marchal K, Vanderleyden J and Nagy I: Strong antimicrobial
activity of Lactobacillus rhamnosus GG against Salmonella
typhimurium is due to accumulation of lactic acid. FEMS
Microbiol Lett. 259:89–96. 2006.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Hassan M, Kjos M, Nes IF, Diep DB and
Lotfipour F: Natural antimicrobial peptides from bacteria:
Characteristics and potential applications to fight against
antibiotic resistance. J Appl Microbiol. 113:723–736.
2012.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Bierbaum G and Sahl HG: Lantibiotics: Mode
of action, biosynthesis and bioengineering. Curr Pharm Biotechnol.
10:2–18. 2009.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Coloretti F, Carri S, Armaforte E,
Chiavari C, Grazia L and Zambonelli C: Antifungal activity of
lactobacilli isolated from salami. FEMS Microbiol Lett.
271:245–250. 2007.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Prema P, Smila D, Palavesam A and Immanuel
G: Production and characterization of an antifungal compound
(3-phenyllactic acid) produced by Lactobacillus plantarum
strain. Food Bioprocess Technol. 3:379–386. 2008.
|
|
98
|
Magnusson J and Schnürer J: Lactobacillus
coryniformis subsp. coryniformis strain Si3 produces a
broad-spectrum proteinaceous antifungal compound. Appl Environ
Microbiol. 67:1–5. 2001.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Dal Bello F, Clarke CI, Ryan LAM, Ulmer H,
Schober TJ, Ström K, Sjögren J, van Sinderen D, Schnürer J and
Arendt EK: Improvement of the quality and shelf life of wheat bread
by fermentation with the antifungal strain Lactobacillus
plantarum FST 1.7. J Cereal Sci. 45:309–318. 2007.
|
|
100
|
Ström K, Sjögren J, Broberg A and Schnürer
J: Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic
dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and
3-phenyllactic acid. Appl Environ Microbiol. 68:4322–4327.
2002.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Gómez-Llorente C, Muñoz S and Gil A: Role
of Toll-like receptors in the development of immunotolerance
mediated by probiotics. Proc Nutr Soc. 69:381–389. 2010.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wells JM: Immunomodulatory mechanisms of
lactobacilli. Microb Cell Fact. 10 (Suppl 1)(S17)2011.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Vizoso Pinto MG, Rodriguez Gómez M,
Seifert S, Watzl B, Holzapfel WH and Franz CM: Lactobacilli
stimulate the innate immune response and modulate the TLR
expression of HT29 intestinal epithelial cells in vitro. Int J Food
Microbiol. 133:86–93. 2009.PubMed/NCBI View Article : Google Scholar
|
|
104
|
D'Angelo C, Reale M and Costantini E:
Microbiota and Lnd HIV infection. Nutrients. 9(615)2017.
|
|
105
|
Sharma P, Tomar SK, Goswami P, Sangwan SR
and Singh R: Antibiotic resistance among commercially available
probiotics. Food Res Int. 57:176–195. 2014.
|
|
106
|
Sorokulova IB, Pinchuk IV, Denayrolles M,
Osipova IG, Huang JM, Cutting SM and Urdaci MC: The safety of two
Bacillus probiotic strains for human use. Dig Dis Sci. 53:954–963.
2008.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Sanders ME, Akkermans LM, Haller D,
Hammerman C, Heimbach J, Hörmannsperger G, Huys G, Levy DD,
Lutgendorff F, Mack D, et al: Safety assessment of probiotics for
human use. Gut Microbes. 1:164–185. 2010.PubMed/NCBI View Article : Google Scholar
|
|
108
|
McFarland LV and Dublin S: Meta-analysis
of probiotics for the treatment of irritable bowel syndrome. World
J Gastroenterol. 14:2650–2661. 2008.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Finegold SM, Sutter VL and Mathisen GE:
Normal indigenous intestinal flora. In: Human Intestinal Microflora
in Health and Disease. Hentiges DJ (ed). Academic Press, New York,
NY, 1983.
|
|
110
|
Didari T, Solki S, Mozaffari S, Nikfar S
and Abdollahi M: A systematic review of the safety of probiotics.
Expert Opin Drug Saf. 13:227–239. 2014.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Hong HA, Duc H and Cutting SM: The use of
bacterial spore formers as probiotics. FEMS Microbiol Rev.
29:813–835. 2005.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Cutting SM: Bacillus probiotics. Food
Microbiol. 28:214–220. 2011.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Hoa NT, Baccigalupi L, Huxham A, Smertenko
A, Van PH, Ammendola S, Ricca E and Cutting AS: Characterization of
Bacillus species used for oral bacteriotherapy and
bacterioprophylaxis of gastrointestinal disorders. Appl Environ
Microbiol. 66:5241–5247. 2000.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Sonenshein AL, Hoch JA and Losick R:
Bacillus subtilis and other gram-positive bacteria:
biochemistry, physiology, and molecular genetics. American Society
for Microbiology, Washington, DC, 1993.
|
|
115
|
Zhang JL, Xie QM, Ji J, Yang WH, Wu YB, Li
C, Ma JY and Bi YZ: Different combinations of probiotics improve
the production performance, egg quality, and immune response of
layer hens. Poult Sci. 91:2755–2760. 2012.PubMed/NCBI View Article : Google Scholar
|
|
116
|
van der Aa Kühle A, Skovgaard K and
Jespersen L: In vitro screening of probiotic properties of
Saccharomyces cerevisiae var. boulardii and
food-borne Saccharomyces cerevisiae strains. Int J Food
Microbiol. 101:29–39. 2005.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Czerucka D, Piche T and Rampal P: Review
article: Yeast as probiotics -Saccharomyces boulardii.
Aliment Pharmacol Ther. 26:767–778. 2007.PubMed/NCBI View Article : Google Scholar
|