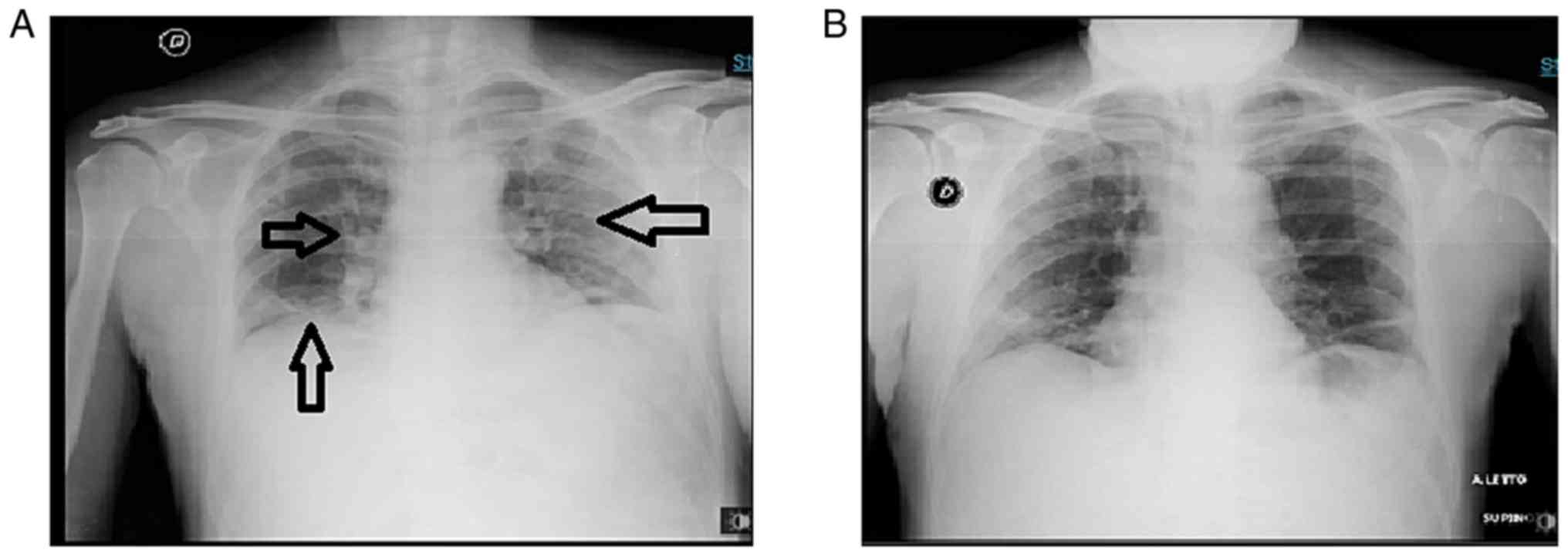
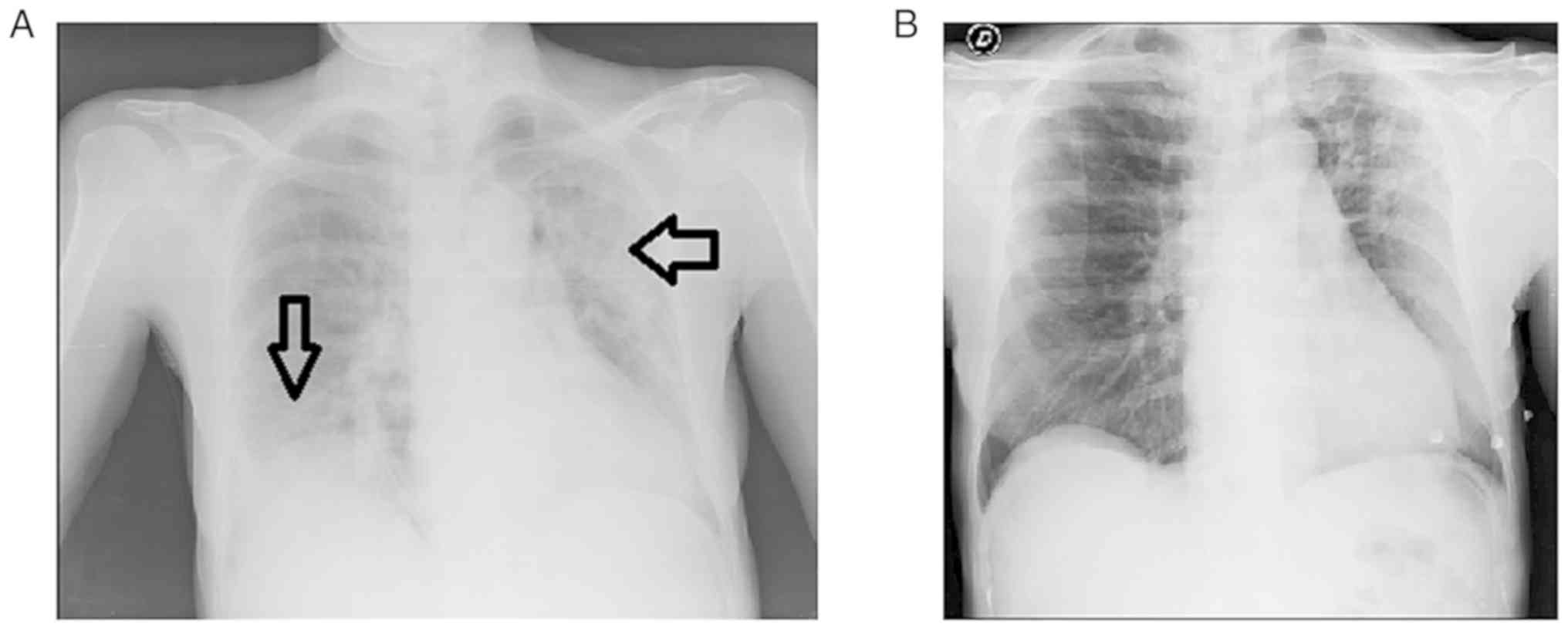
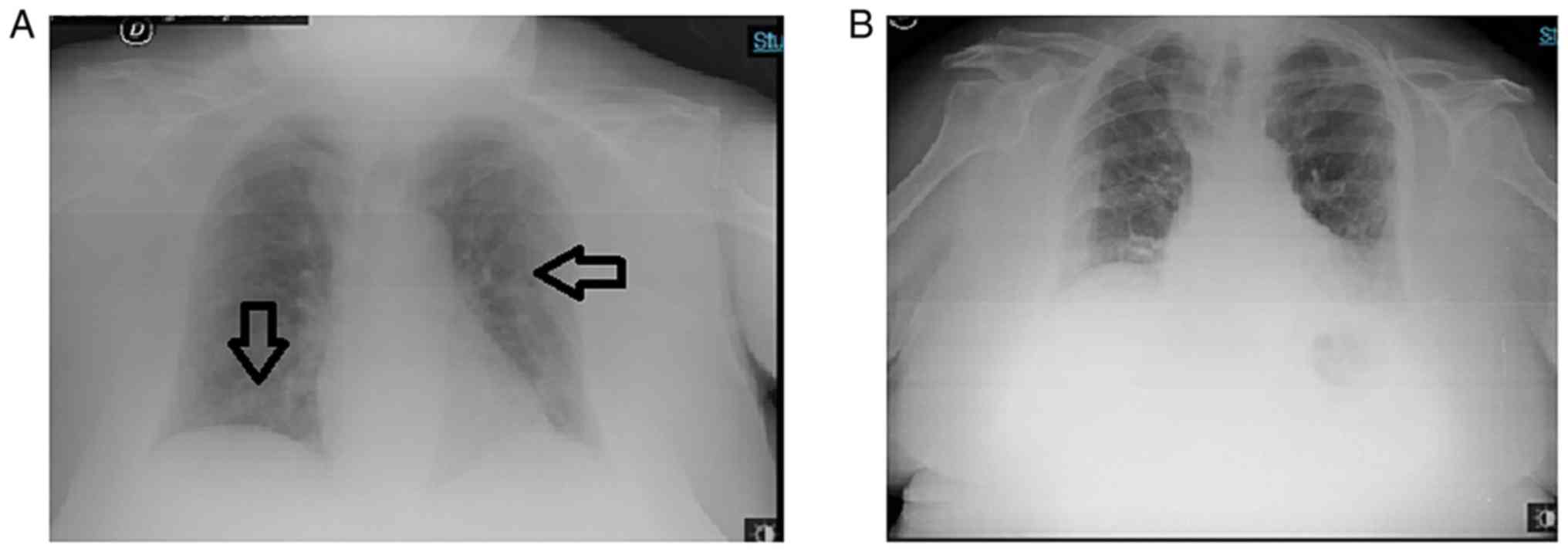
|
1
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
WHO. Coronavirus (COVID-19) events as they
happen. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
2020. Access date, April 26, 2020.
|
|
3
|
WHO: Events as they happen. Rolling
updates on coronavirus disease (COVID-19). WHO, 2020.
|
|
4
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Riccardo F, Ajelli M, Andrianou X, Bella
A, Del Manso M, Fabiani M, Bellino S, Boros S, Urdiales AM, et al:
Epidemiological characteristics of COVID-19 cases in Italy and
estimates of the reproductive numbers one month into the epidemic.
medRxiv, 2020. doi: https://doi.org/10.1101/2020.04.08.20056861.
|
|
6
|
Bhimraj A, Morgan RL, Shumaker AH,
Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ,
O'Horo JC, et al: Infectious diseases society of America Guidelines
on the Treatment and Management of Patients with COVID-19. Clin
Infect Dis Apr: Apr 27 doi: 10.1093/cid/ciaa478 (Epub ahead of
print).
|
|
7
|
Whittle JS, Pavlov I, Sacchetti AD, Atwood
C and Rosenberg MS: Respiratory support for adult patients with
COVID-19. J Am Coll Emerg Physicians Open: Apr 2020 doi:
10.1002/emp2.12071.
|
|
8
|
Shi Y, Wang Y, Shao C, Huang J, Gan J,
Huang X, Bucci E, Piacentini M, Ippolito G and Melino G: COVID-19
infection: The perspectives on immune responses. Cell Death Differ.
27:1451–1454. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Marino A, Cosentino F, Pampaloni A,
Scuderi D, Moscatt V, Gussio M, Onorante A, Zagami A, Torrisi S,
Grasso S, et al: Role of tocilizumab and high flow nasal cannula in
the clinical management of severe Covid-19. J Clin Trials.
10(427)2020.
|
|
10
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A Trial of
lopinavir-ritonavir in adults hospitalized with severe covid-19. N
Engl J Med. 382:1787–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Taccone FS, Gorham J and Vincent JL:
Hydroxychloroquine in the management of critically ill patients
with COVID-19: The need for an evidence base. Lancet Respir Med.
8:539–541. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Felsenstein S, Herbert JA, McNamara PS and
Hedrich CM: COVID-19: Immunology and treatment options. Clin
Immunol [Internet]. 2020/04/27. 2020 Jun; 215:108448. Available
from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7185015/.
|
|
13
|
Cattaneo M, Bertinato EM, Birocchi S,
Brizio C, Malavolta D, Manzoni M, Muscarella G and Orlandi M:
Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the
recommendation to use high-dose heparin for thromboprophylaxis
justified? Thromb Haemost. 120:1230–1232. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aziz M, Aziz M, Fatima R and Assaly R:
Elevated Interleukin-6 and Severe COVID-19: A meta-analysis. Med
Virol: 28 April, 2020 https://doi.org/10.1002/jmv.25948.
|
|
15
|
Zhang C, Wu Z, Li JW, Zhao H and Wang GQ:
The cytokine release syndrome (CRS) of severe COVID-19 and
Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the
key to reduce the mortality. Int J Antimicrob Agents.
55(105954)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Luo P, Liu Y, Qiu L, Liu X, Liu D and Li
J: Tocilizumab treatment in COVID-19: A single center experience. J
Med Virol: 6 April, 2020 https://doi.org/10.1002/jmv.25801.
|
|
17
|
Fu B, Xu X and Wei H: Why tocilizumab
could be an effective treatment for severe COVID-19? J Trans Med.
18(164)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nishimura M: High-flow nasal cannula
oxygen therapy in adults. J Intensive Care. 3(15)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Frat JP, Thille AW, Mercat A, Girault C,
Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, et
al: High-flow oxygen through nasal cannula in acute hypoxemic
respiratory failure. N Engl J Med. 372:2185–2196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rochwerg B, Granton D, Wang DX, Helviz Y,
Einav S, Frat JP, Mekontso-Dessap A, Schreiber A, Azoulay E, Mercat
A, et al: High flow nasal cannula compared with conventional oxygen
therapy for acute hypoxemic respiratory failure: A systematic
review and meta-analysis. Intensive Care Med. 45:563–572.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nagata K, Morimoto T, Fujimoto D, Otoshi
T, Nakagawa A, Otsuka K, Seo R, Atsumi T and Tomii K: Efficacy of
high-flow nasal Cannula therapy in acute hypoxemic respiratory
failure: Decreased use of mechanical ventilation. Respir Care.
60:1390–1396. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koga Y, Kaneda K, Fujii N, Tanaka R,
Miyauchi T, Fujita M, Hidaka K, Oda Y and Tsuruta R: Comparison of
high-flow nasal cannula oxygen therapy and non-invasive ventilation
as first-line therapy in respiratory failure: A multicenter
retrospective study. Acute Med Surg. 7(e461)2019.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Wang K, Zhao W, Li J, Shu W and Duan J:
The experience of high-flow nasal cannula in hospitalized patients
with 2019 novel coronavirus-infected pneumonia in two hospitals of
Chongqing, China. Ann Intensive Care. 10(37)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li J, Fink JB and Ehrmann S: High-flow
nasal cannula for COVID-19 patients: Low risk of bio-aerosol
dispersion. Eur Respir J. 55(2000892)2020.PubMed/NCBI View Article : Google Scholar
|