Introduction
In late 2019, a type of pneumonia of unknown origin
was reported in Wuhan, China (1).
The causative pathogen was then identified as a novel
β-coronavirus, and was subsequently named severe acute respiratory
syndrome coronavirus 2 (SARS-CoV2) (1,2).
Since then, there has been a rapid spread of the virus worldwide,
leading the World Health Organization (WHO) to declare SARS-CoV2
outbreak a global pandemic on March 11, 2020(3).
The majority of infected individuals who develop
disease from SARS-CoV2 infection (COVID-19) exhibit a
mild-to-moderate illness (80%); however, 14% suffer from serious
disease and in 6% of cases, this evolves towards a severe acute
respiratory distress syndrome (ARDS), requiring intensive care
support (4).
Up to the May 23, 2020, >5 million cases of
SARS-CoV2 infections were reported and 340,260 individuals
succumbed to the disease worldwide. To date, a total of 228,658
cases have been reported in Italy, 35,616 of which have not
survived (5).
There are no proven specific antiviral agents for
the treatment of COVID-19; nevertheless, several new and old
molecules have been used in the context of clinical trials, while
waiting for solid evidences to render drug administration safer and
more precise (6).
A role has been claimed for the monoclonal antibody,
tocilizumab, that blocks the cellular receptor of interleukin
(IL)-6, playing a crucial role in the development and maintenance
of inflammation. In addition, oxygen support plays a key role in
the management of severe cases of COVID-19(7). In that context, high-flow nasal
cannula (HFNC) oxygenation may represent a promising therapeutic
support option in the governance of these critically ill
patients.
The present study reports the cases of 3 patients
critically ill with COVID-19, confirmed by positive results of
SARS-CoV2 RT-PCR on nasopharyngeal swab; the conditions of the
patients markedly improved and they were successfully discharged
following tocilizumab administration and HFNC treatment.
Case report
First patient
Upon admission, the patient was feverish
(temperature, 37.5˚C), with a blood pressure of 110/70 mmHg, a
heart rate (HR) of 92 bpm, oxygen saturation rate of 89% in room
air and a respiratory rate (RR) of 23/min. Blood tests revealed
elevated levels of inflammatory markers along with lymphopenia
(Table I). Arterial blood analysis
in room air revealed a partial pressure of oxygen (PO2)
rate of 60 mmHg, a partial pressure of carbon dioxide
(PCO2) rate of 36 mmHg, pH 7.46, and an arterial partial
pressure of oxygen (PaO2)/fractional inspired oxygen
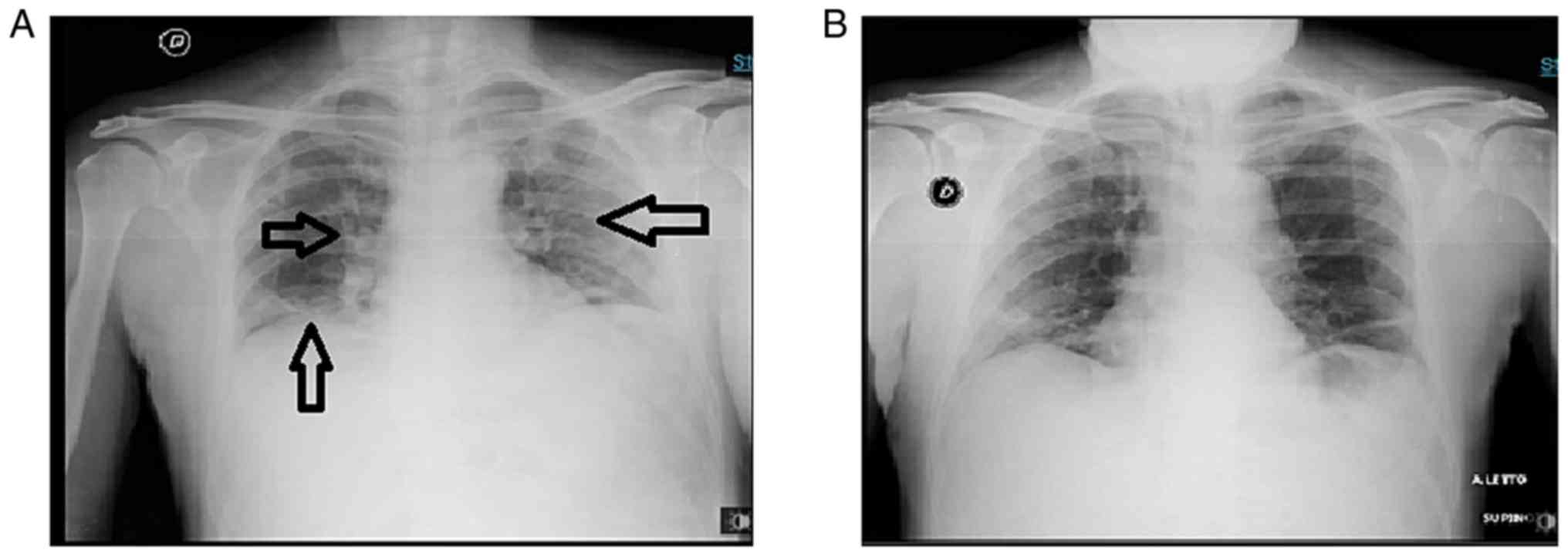
(FiO2) ratio of 285. A chest X-ray revealed bilateral
interstitial pneumonia (Fig.
1).
 | Table IDemographics, clinical characteristics
at the time of admission, treatment and outcomes of the 3 patients
with COVID-19. |
Table I
Demographics, clinical characteristics
at the time of admission, treatment and outcomes of the 3 patients
with COVID-19.
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
|---|
| Age, years | 63 | 55 | 77 |
| Sex | Male | Female | Female |
| Comorbidities | None | None | COPD, hypertension,
hypothyroidism, hypercholesterolemia |
| Home therapy | None | None |
Perindopril/indapamide, bisoprolol,
statin, levothyroxine |
| Chest X-ray
findings | Bilateral
interstitial pneumonia | Bilateral ground
glass area | Bilateral
interstitial pneumonia |
| Days between the
onset of symptoms and hospital admission | 8 | 5 | 5 |
| Symptoms on
admission | Dyspnea, fever cough,
headache | Dyspnea, fever
cough | Dyspnea, fever
cough |
| Laboratory findings,
unit (reference range) | | | |
|
WBC,
cells/mmc (4,000-10,000) | 6,800 | 8,300 | 9,500 |
|
Neutrophils,
% (40-75) | 78 | 83 | 74 |
|
Lymphocytes,
% (25-50) | 15 | 8 | 14, 2 |
|
Monocytes, %
(2-10) | 5, 8 | 8 | 1, 1 |
|
Platelets,
cells/mmc x103 (150-400) | 323 | 201 | 126 |
|
Hemoglobin,
g/dl (12-16) | 13, 6 | 11, 6 | 13, 4 |
|
AST, UI/l
(15-35) | 44 | 22 | 47 |
|
ALT, UI/l
(15-35) | 40 | 11 | 32 |
|
LDH, UI/l
(80-250) | 348 | 279 | 325 |
|
Creatinine,
mg/dl (0,8-1,2) | 0, 8 | 1, 2 | 0, 75 |
|
CRP, mg/dl
(0-0.5) | 6, 3 | 4 | 9 |
|
ESR, mm/h
(0-10) | 67 | 75 | 50 |
|
IL-6, pg/ml
(<20) | 450 | 1,500 | 343 |
|
Ddimer,
ng/ml (<250) | 1,708 | 900 | 1,675 |
|
Ferritin,
ng/ml (20-200) | 1,300 | 2,600 | 1,572 |
|
Lowest
PaO2/FiO2 ratio (126) | 133 | 120 | |
| Antiviral therapy
(duration) |
Darunavir/cobicistat (5 days) | Lopinavir/ritonavir
(5 days) |
Darunavir/cobicistat (5 days) |
| Antibiotic therapy
(duration) | Azythromicin (10
days) | Azythromicin (7
days) Ceftriaxone (7 days) | Azythromicin (5
days) Ceftriaxone (5 days) |
| Other therapies
(duration) | Hydroxycloroquine
(10 days), enoxaparin 6,000 UI s.c. (24 days) | Hydroxycloroquine
(7 days), enoxaparin 6,000 UI s.c. (22 days) | Hydroxycloroquine
(7 days), enoxaparin 6,000 UI s.c. (18 days) |
| Days on HFNC | 8 | 11 | 10 |
| Tocilizumab
dose | 8 mg/kg (2
doses) | 8 mg/kg (2
doses) | 8 mg/kg (2
doses) |
| Days from admission
to tocilizumab | 3 | 2 | 3 |
| Time to hospital
discharge (days) | 24 | 22 | 18 |
He was administered darunavir/cobicistat (800+150
mg/day), hydroxychloroquine (400 mg/day, after loading dose),
azithromycin (500 mg/day), enoxaparin (6,000 UI/day). Moreover, he
was administered oxygen with a Venturi mask at 14 l/min
(FiO2 60%).
On the 3rd day from the time of admission, due to
the worsening of RR (32/min; PaO2 75 mmHg;
PCO2 37 mmHg; pH 7.46; PaO2/FiO2
126), he was administered oxygen ventilation with HFNC (Optiflow™
Nasal High Flow Therapy delivered by AIRVO™ 2), 50 l/min,
FiO2 50%. On the same day, the patient was administered
tocilizumab 8 mg/kg i.v., at 2 consecutive doses within 12 h.
Within 2 days from tocilizumab administration, the
clinical status and respiratory performances of the patient
markedly improved. HFNC ventilation was continued for a further 6
days. Subsequently, HFNC treatment was slowly reduced by
interchanging it with a Venturi mask for another 2 days, at which
time high-flow ventilation was definitively terminated. Both
arterial blood analysis and chest X-rays revealed progressive
amelioration.
Second patient
Upon admission, the patient was feverish
(temperature, 38.5˚C), blood pressure was 140/80 mmHg, HR was 100
bpm, oxygen saturation was 91% in room air and the RR was 24/min.
Blood tests revealed high levels of inflammatory markers along with
lymphopenia (Table I). Arterial
blood analysis in room air revealed PaO2 50 mmHg,
PCO2 35 mmHg, and pH 7.44; the PaO2/FiO2 ratio was 238.
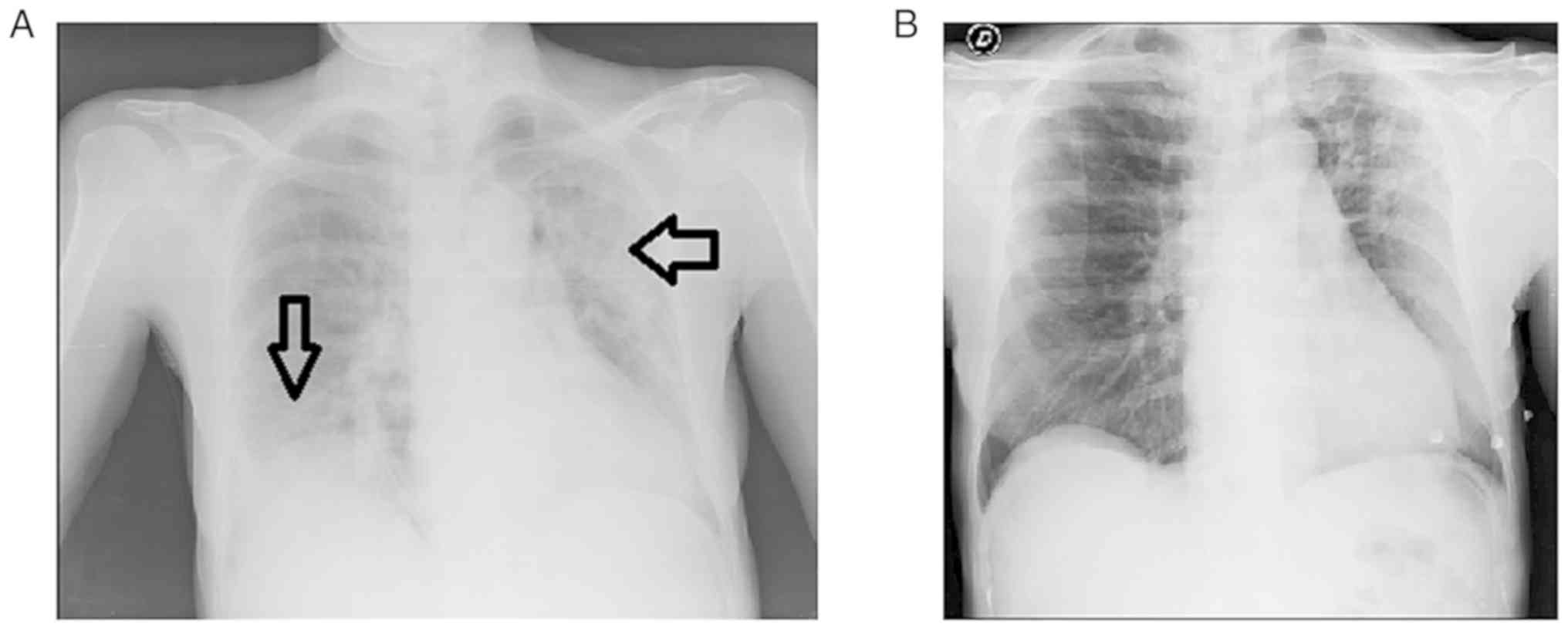
Her chest X-ray revealed bilateral ground glass areas without
consolidations (Fig. 2).
The patient was administered lopinavir/ritonavir
(200 mg/50 mg 4 tabs/day per os), hydroxychloroquine (400
mg/day per os, after loading dose), azithromycin (500 mg/day
per os), ceftriaxone (2 gr/day, i.v.) and enoxaparin (6,000
UI/day). Furthermore, she was administered oxygen support therapy
with a Venturi mask (12 l/min, FiO2 60%).
On day 2 from the time of admission, her clinical
conditions began to deteriorate, with chest X-ray results
worsening. She became dyspneic (RR was 30/min) and arterial blood
analysis during oxygen ventilation revealed a PaO2 of 80
mmHg, PCO2 of 33.4 mmHg, pH 7.38 (PaO2/FiO2 ratio was
133). She was administered HFNC ventilation (50 l/min,
FiO2 60%) and tocilizumab was also administered
intravenously, at 2 doses of 8 mg/kg, 12 h apart.
Within 24 h, her clinical condition began to improve
with a marked improvement in the chest X-ray results. The levels of
serum inflammatory markers also decreased. At 6 days following the
administration of tocilizumab, HFNC treatment was slowly reduced,
interchanging it with a Venturi mask every 6 h. After 11 days, HFNC
was terminated and treatment with a Venturi mask was continued
until discharge.
Third patient
Upon admission, the patient was feverish (37.8˚C),
with a blood pressure of 140/70 mmHg, HR of 100 bpm, RR of 25/min
and an oxygen saturation of 90% in room air. Blood tests revealed
high levels of inflammatory markers (Table I). Arterial blood analysis in room
air revealed a PO2 of 53 mmHg, PCO2 of 37
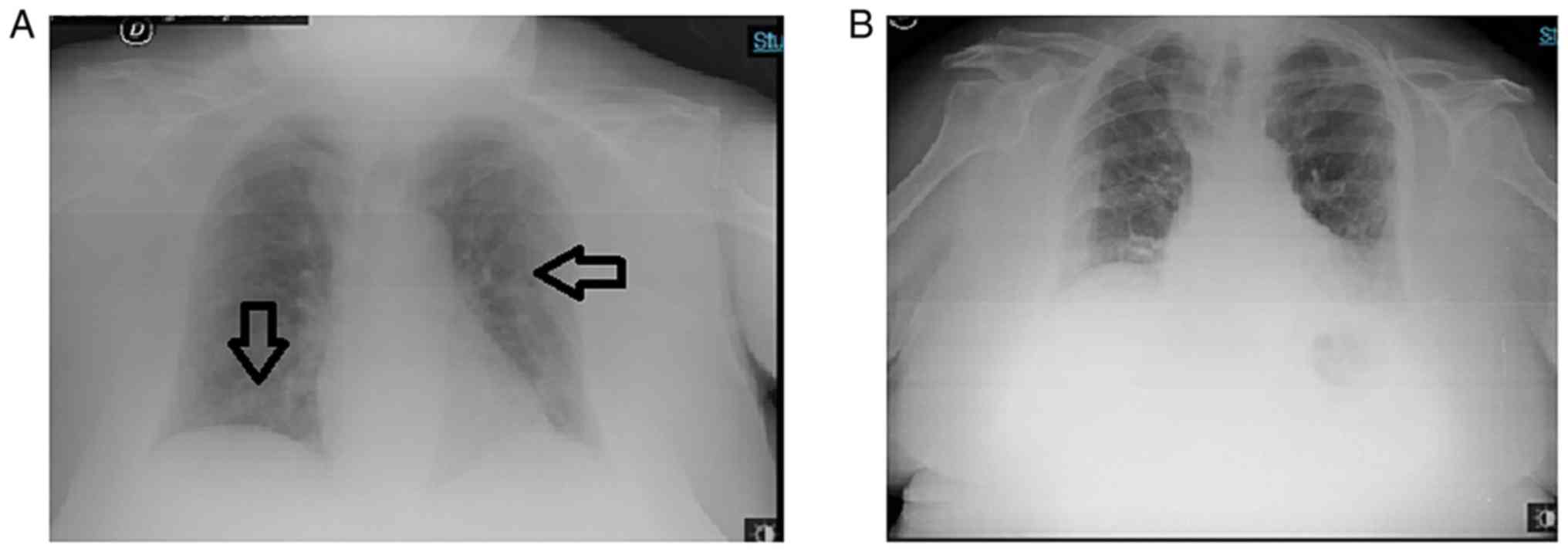
mmHg, pH 7.45 and a PaO2/FiO2 of 250. A chest
X-ray revealed bilateral interstitial pneumonia without
consolidation (Fig. 3).
The patient was administered darunavir/cobicistat
(800+150 mg/day), hydroxychloroquine (400 mg/day after loading
dose), azithromycin (500 mg/day), ceftriaxone (2 g/day) and
enoxaparin (6,000 UI/day). Furthermore, she was administered oxygen
therapy with a Venturi mask at 14 l/min (FiO2 60%).
In spite of treatment, the clinical status of the
patient deteriorated (on the 3rd day from the time of admission)
together with a deterioration in the RR (34/min) and an extension
of bilateral infiltrates on chest X-rays. Since the arterial gas
analysis values worsened (PaO2 72 mmHg, PCO2
37 mmHg, pH 7.47, PaO2/FiO2 120), HFNC
ventilation was commenced (50 l/min, FiO2 60%) and 2
doses of tocilizumab (8 mg/kg) were administered intravenously
within 12 h.
At 36 h following the commencement of HFNC and
tocilizumab administration, the clinical condition of the patient
began to progressively improve. HFNC was terminated within 10 days,
and a Venturi mask was used for 4 days. Two consecutive Chest
X-rays revealed a clear-cut improvement of the bilateral
interstitial infiltrative lesions.
Discussion
There are two main pathogenetic stages in the
development of COVID-19. Namely, the ‘viral phase’ due to viral
intracellular replication, including of mild symptoms (8); and an ‘inflammatory phase’ due to the
host immune response, including severe respiratory symptoms and
even ARDS with a marked increase in levels of serum inflammatory
markers, known as the so-called ‘cytokine storm’ (8,9). In
each of these phases, in the cases presented herein, successful
treatment intervention was achieved with different treatments.
Treatment for the viral phase has been based on the
uncertain antiviral activity of certain molecules, such as
lopinavir/ritonavir or darunavir/cobicistat (10), hydroxychloroquine (11) and azithromycin (12), along with enoxaparin, in
prophylactic or therapeutic dosage, to treat the ipercoagulative
status (13).
The Infectious Diseases Society of America (IDSA)
guidelines (6) recommend the use
of antiviral drugs only in the context of clinical trials, with
accurate caution to the collateral effects, which may be
particularly pernicious (such as QT prolongation for azithromycin
and hydroxychloroquine as well as important bleeding for enoxaparin
or diarrhea with lopinavir/ritonavir).
According to the clinical conditions of the patients
presented herein, it was decided that they should be administered a
short course of antiviral drugs and a prophylactic dose of
enoxaparin. They exhibited no adverse drug reactions. Moreover, QTc
daily was assessed without any evidence of prolongation.
IL-6 is a relevant marker of inflammation implicated
in the COVID-19 cytokine burst. The IL-6 level can assist
clinicians in recognizing patients with a major risk of severe
disease progression (14).
Tocilizumab, which is a recombinant monoclonal antibody targeting
the IL-6 receptor, has been already used in the treatment of
rheumatoid arthritis and Crohn's disease (15). To date, only a small group of
patients or simple studies have reported the use of tocilizumab in
the treatment of patients with severe COVID-19 infection, achieving
promising clinical results (15,16).
Tocilizumab can be used in patients with extensive
bilateral lung involvements or in patients with severe/critical
illness, with elevated levels of serum IL-6. The dose is 8 mg/kg
i.v., diluted in 100 ml of 0.9% saline solution. For patients with
a poor clinical response, a second dose could be administered after
8-12 h (17).
In the cases presented herein, two 8 mg/kg
tocilizumab doses were administered intravenously, 12 h apart. The
patients did not exhibit any adverse drug reactions. Prior to the
tocilizumab administration, the hepatitis B virus (HBV) status of
the patients was assessed and latent tuberculosis infection was
excluded by specific interferon (IFN)-γ assay. Repeated chest
X-rays revealed the progressive reabsorption of interstitial
exudation in all cases.
High-flow oxygen systems (such as HFNC) provide
heated, oxygen-rich, humidified gas to the patient at flow levels
sufficient to deliver a constant, precisely set high
FiO2 (7). HFNC flow
rates reach up to 60 l/min, reducing the pulmonary dead space,
providing low levels of positive end-expiratory pressure (PEEP),
and decreasing breathing frequency and effort (18). The use of HFNC is associated with a
lower mortality rate in hypoxemic respiratory failure (19). Compared to non-invasive ventilation
(NIV) oxygen therapy, HFNC is associated with a decreased need of
subsequent intubation and ICU admission (20,21),
and with a lower risk of 30-day mortality in patients with
pneumonia (22).
Moreover, patients have found HFNC to be more
comfortable and better tolerated than NIV and the management of
HFNC is relatively easier (23).
In a retrospective study of 610 COVID19-positive patients from
China, where 10% of the affected patients required critical care,
an early use of HFNC was associated with a reduced necessity of
mechanical ventilation and a lower mortality rate (24). Furthermore, HFNC has been shown to
be associated with a significantly lower risk of bioaerosol
dispersion, reducing the risk of hospital-acquired infections for
health workers (25).
Following HFNC ventilation, the patients in the
present study achieved a marked improvement in respiratory
function, as well as lower respiratory fatigue, with better results
on arterial gas analysis. Teh initial approach included 24-h HFNC,
and this was then interchanged with a Venturi mask (every 6 h) to
avoid sudden interruption.
It should be noted that it is probable that the
cases presented herein were successfully influenced by the age of
the patients (they were not that elderly) and as regards the first
2 patients, by the absence of comorbidities. Moreover, their
admission occurred only a few days following the onset of symptoms
and the level of lymphopenia was not so severe, whereas their IL-6
levels were considerably elevated. All patients were discharged
with 2 negative results RT-PCR for SARS-CoV2 on a nasopharyngeal
swab.
In conclusion, SARS-CoV2 infection arrived ‘out of
the blue’ for the entire world, even in Italy. In Sicily, in South
Italy, inhabitants were warned by what had already occurred in
North Italy. Moreover, lockdown measures and therapeutic
experiences coming from more affected areas greatly assisted the
condition.
On the whole, as demonstrated herein,
COVID-19-postitive patients progressing towards a more severe
course may benefit from the synergistic effects of treatment with
intravenously administered tocilizumab and oxygen ventilation with
HFNC. Herein, 3 cases are reported, which progressed towards a
marked amelioration and resolution of the disease following
treatment with such a combination.
As far as was currently known up to the time of the
preparation of this manuscript, to the best of our knowledge, the
presently reported case series is the first to focus on the
synergistic efficacy of the combined use of tocilizumab and HFNC.
However, extensive randomized controlled trials (RCTs) are
warranted in order to confirm the beneficial effects of such
treatments and to standardize indications and timing.
Acknowledgements
The authors would like to thank Dr Pietro Leanza for
his kind English revision of the manuscript.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article as no
datasets were generated or analyzed during the current study.
Authors' contributions
All authors (AM, AP, DS, FC, VM, MC, MG, BMC, RB,
SB, GN and BC) contributed to the study conception and design. AM
wrote the manuscript. AP, DS, FC, VM and SB revised the literature
and references. MG, BMC and MC provided clinical assistance to the
patients. RB was responsible for the laboratory tests and
pharmacological treatments. GN and BC revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all individual
participants included in the present case series.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
WHO. Coronavirus (COVID-19) events as they
happen. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
2020. Access date, April 26, 2020.
|
|
3
|
WHO: Events as they happen. Rolling
updates on coronavirus disease (COVID-19). WHO, 2020.
|
|
4
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Riccardo F, Ajelli M, Andrianou X, Bella
A, Del Manso M, Fabiani M, Bellino S, Boros S, Urdiales AM, et al:
Epidemiological characteristics of COVID-19 cases in Italy and
estimates of the reproductive numbers one month into the epidemic.
medRxiv, 2020. doi: https://doi.org/10.1101/2020.04.08.20056861.
|
|
6
|
Bhimraj A, Morgan RL, Shumaker AH,
Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ,
O'Horo JC, et al: Infectious diseases society of America Guidelines
on the Treatment and Management of Patients with COVID-19. Clin
Infect Dis Apr: Apr 27 doi: 10.1093/cid/ciaa478 (Epub ahead of
print).
|
|
7
|
Whittle JS, Pavlov I, Sacchetti AD, Atwood
C and Rosenberg MS: Respiratory support for adult patients with
COVID-19. J Am Coll Emerg Physicians Open: Apr 2020 doi:
10.1002/emp2.12071.
|
|
8
|
Shi Y, Wang Y, Shao C, Huang J, Gan J,
Huang X, Bucci E, Piacentini M, Ippolito G and Melino G: COVID-19
infection: The perspectives on immune responses. Cell Death Differ.
27:1451–1454. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Marino A, Cosentino F, Pampaloni A,
Scuderi D, Moscatt V, Gussio M, Onorante A, Zagami A, Torrisi S,
Grasso S, et al: Role of tocilizumab and high flow nasal cannula in
the clinical management of severe Covid-19. J Clin Trials.
10(427)2020.
|
|
10
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A Trial of
lopinavir-ritonavir in adults hospitalized with severe covid-19. N
Engl J Med. 382:1787–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Taccone FS, Gorham J and Vincent JL:
Hydroxychloroquine in the management of critically ill patients
with COVID-19: The need for an evidence base. Lancet Respir Med.
8:539–541. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Felsenstein S, Herbert JA, McNamara PS and
Hedrich CM: COVID-19: Immunology and treatment options. Clin
Immunol [Internet]. 2020/04/27. 2020 Jun; 215:108448. Available
from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7185015/.
|
|
13
|
Cattaneo M, Bertinato EM, Birocchi S,
Brizio C, Malavolta D, Manzoni M, Muscarella G and Orlandi M:
Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the
recommendation to use high-dose heparin for thromboprophylaxis
justified? Thromb Haemost. 120:1230–1232. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aziz M, Aziz M, Fatima R and Assaly R:
Elevated Interleukin-6 and Severe COVID-19: A meta-analysis. Med
Virol: 28 April, 2020 https://doi.org/10.1002/jmv.25948.
|
|
15
|
Zhang C, Wu Z, Li JW, Zhao H and Wang GQ:
The cytokine release syndrome (CRS) of severe COVID-19 and
Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the
key to reduce the mortality. Int J Antimicrob Agents.
55(105954)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Luo P, Liu Y, Qiu L, Liu X, Liu D and Li
J: Tocilizumab treatment in COVID-19: A single center experience. J
Med Virol: 6 April, 2020 https://doi.org/10.1002/jmv.25801.
|
|
17
|
Fu B, Xu X and Wei H: Why tocilizumab
could be an effective treatment for severe COVID-19? J Trans Med.
18(164)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nishimura M: High-flow nasal cannula
oxygen therapy in adults. J Intensive Care. 3(15)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Frat JP, Thille AW, Mercat A, Girault C,
Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, et
al: High-flow oxygen through nasal cannula in acute hypoxemic
respiratory failure. N Engl J Med. 372:2185–2196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rochwerg B, Granton D, Wang DX, Helviz Y,
Einav S, Frat JP, Mekontso-Dessap A, Schreiber A, Azoulay E, Mercat
A, et al: High flow nasal cannula compared with conventional oxygen
therapy for acute hypoxemic respiratory failure: A systematic
review and meta-analysis. Intensive Care Med. 45:563–572.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nagata K, Morimoto T, Fujimoto D, Otoshi
T, Nakagawa A, Otsuka K, Seo R, Atsumi T and Tomii K: Efficacy of
high-flow nasal Cannula therapy in acute hypoxemic respiratory
failure: Decreased use of mechanical ventilation. Respir Care.
60:1390–1396. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koga Y, Kaneda K, Fujii N, Tanaka R,
Miyauchi T, Fujita M, Hidaka K, Oda Y and Tsuruta R: Comparison of
high-flow nasal cannula oxygen therapy and non-invasive ventilation
as first-line therapy in respiratory failure: A multicenter
retrospective study. Acute Med Surg. 7(e461)2019.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Wang K, Zhao W, Li J, Shu W and Duan J:
The experience of high-flow nasal cannula in hospitalized patients
with 2019 novel coronavirus-infected pneumonia in two hospitals of
Chongqing, China. Ann Intensive Care. 10(37)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li J, Fink JB and Ehrmann S: High-flow
nasal cannula for COVID-19 patients: Low risk of bio-aerosol
dispersion. Eur Respir J. 55(2000892)2020.PubMed/NCBI View Article : Google Scholar
|