Introduction
Streptokinase is a protein harvested from β-hemolytic Streptococci (1). It has been established that streptokinase possesses fibrinolytic properties through the activation of plasma plasminogen via fibrin-dependent and fibrin-independent pathways (2). Therefore, streptokinase has been used as one of the therapeutic modalities in the treatment of thrombo-embolic diseases, such as pulmonary embolism and acute myocardial infarction. Despite its proven efficacy, hypotension is a common side-effect observed with intravenous streptokinase therapy in patients with acute myocardial infarction.
Hypotension with streptokinase therapy is commonly attributed to the non-specific effect of streptokinase, as it not only activates the clot-derived plasminogen, but also the circulating plasma plasminogen, resulting in systemic fibrinolysis and fibrinogenolysis (3); thus, its uses is associated with the occurrence of hypotension and bleeding tendencies (4-6). Streptokinase-induced hypotension has been the subject of interest since the 1970s. To date, the exact mechanisms responsible for its development remain obscure (7).
Therefore, the present study aimed to predict the occurrence of streptokinase-induced hypotension from selected baseline clinical parameters in order to aid the identification of patients with acute myocardial infarction who are at risk of developing hypotension during streptokinase therapy.
Patients and methods
Patients
This cross-sectional study involved data transcription from hospital National Indicator Approach (NIA) records for acute myocardial infarction (AMI) cases recorded at the Emergency and Trauma Department of Hospital Tuanku Fauziah (HTF), Perlis, Malaysia from 2015 to 2018. Acute myocardial infarction was diagnosed in the presence of typical signs and symptoms, the presence of suggestive electrocardiographic changes, and positive cardiac markers (including, but not limited to troponin test).
ST-elevation acute myocardial infarction cases receiving intravenous streptokinase were included in the present study. On the other hand, patients requiring prior hemodynamic stabilisation prior to streptokinase administration (i.e., requiring inotropic support or glyceryl trinitrate infusion prior to streptokinase), or prior cardiopulmonary resuscitation were excluded from the study. Information on demographic details (personal identifier, age, sex, race) and acute management details (time of streptokinase delivery and completion, side-effects observed during streptokinase and management, and vital signs (serial blood pressure and heart rate recording during streptokinase) were transcribed into a separate data sheet. The Killip classification quantifies the severity of heart failure following acute myocardial infarction as follows: Killip class I refers to no evidence of heart failure; class II refers to signs of suggestive of mild-to-moderate heart failure, such as the presence of S3 gallop and distended jugular veins; class III refers to overt pulmonary edema, and class IV refers to cardiogenic shock (8).
Laboratory results obtained at pre- and immediately post-streptokinase delivery were traced from the digital records of the hospital database. Laboratory data included data for baseline cardiac enzymes, such as creatine kinase (CK)-MB (CKMB), full blood count and renal profile.
Ethical clearance
The present study was registered with the National Medical Research Registry of Ministry of Health, Malaysia NMRR-19-2871-50953 and received ethical clearance from the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia [KKM/NIHSEC/P19-2421(5)]. Informed consent related to the present study was waived by the Medical Research and Ethics Committee of the Ministry of Health Malaysia. The study only utilized secondary data source and did not involve any form of physical interaction with the patients.
Statistical analyses
Statistical analyses were conducted using IBM SPSS software version 23.0 (IBM Corp.). Descriptive data, such as sex and race were described using percentages and distribution. Numerical data (such as age and baseline hemoglobin) were described as the mean ± standard deviation; however, skewed data, such as the Troponin-I level, baseline total white blood cell count, baseline platelet count, baseline neutrophil count, baseline urea and baseline creatinine level were described as the median and interquartile range (IQR).
Continuous variables between two groups (hypotensive vs. normotensive) were compared using an independent t-test in data with normal distribution (i.e., age), and with the Mann-Whitney U test in data with skewed distribution (i.e., Troponin-I level, baseline total white blood cell count, baseline platelet count, baseline neutrophil count, baseline urea and baseline creatinine levels).
Pearson's Chi-squared test of independence was performed to determine the association between categorical variables (sex, location myocardial infarction, baseline creatine kinase levels), between patients with hypotension vs. those with no hypotension. For categorical associations not eligible for Pearson's Chi-squared test (by having >20% of the expected counts of <5), a corresponding Fisher's exact test was performed (i.e., baseline urea/creatinine ratio).
Repeated measures analysis of variance (ANOVA) with the Greenhouse-Geisser correction was performed to examine the effect of time on systolic and diastolic blood pressure. The Greenhouse-Geisser correction was applied to adjust for the lack of sphericity in repeated measures ANOVA. Multivariate analysis was performed to identify predictive factors contributing to systemic hypotension through simple logistic regression (SLR) and multiple logistic regression (MLR) analyses. MLR was performed using the ENTER method whereby all the variables were included in the modelling regardless of their independent statistical significance. The ENTER method was selected in view of its default option for regression analysis in the majority of statistical software (9). Nagelkerke R squares provides the indication of the amount of variation present in the dependent variable able to be explained by the model. The value ranges from zero (0) to one (1), and commonly described in percentage (10). A probability value (P-value) <0.05 was deemed as statistically significant.
Results
Patients with treatment-related complications
There were 412 patients enrolled in the present study based on the pre-defined criteria. The majority of the patients (n=258, 62.6%) did not develop any complications from the therapy, whereas the most common adverse drug reactions observed among patients who were treated was hypotension (n=109, 26.5%), hypertension (n=16, 14.7%), allergic reaction (n=11, 2.7%) and bleeding tendencies (n=11, 2.7%). The less common complications included bradycardia (n=5, 1.2%) and arrhythmia (n=2, 0.4%).
The mean age of the study participants was 59.3±12.61 years. The majority were male (n=303, 73.5%) and of Malay descent (n=328, 79.6%). Anterior myocardial infarction was more common vs. inferior myocardial infarction (50.1 vs. 41.7%).
Baseline study parameters
The demographic characteristics and comparison of baseline parameters between patients with streptokinase-induced hypotension and patients with no hypotension from streptokinase therapy are presented in Table I. Only complete datasets with regards to individual variable were included in this descriptive analysis; hence, the cumulative numbers may not add up to the total of 412 patients.
 |
Table I
Baseline characteristics and parameters of study population.
|
Table I
Baseline characteristics and parameters of study population.
| |
Hypotension |
No hypotension |
|
| Variable(s) |
Mean (SD) |
n (%) |
Mean (SD) |
n (%) |
P-value |
| Agea |
60.6 (10.79) |
106 (29.2) |
58.7 (13.27) |
257 (70.8) |
0.158 |
| Sexb |
|
|
|
|
0.100 |
| Male |
|
84 (27.7) |
|
219 (72.3) |
|
| Female |
|
24 (38.1) |
|
39 (61.9) |
|
| Racec |
|
|
|
|
0.103 |
| Malay |
|
99 (30.2) |
|
229 (69.8) |
|
| Chinese |
|
3 (12.5) |
|
21 (87.5) |
|
| Indian |
|
3 (33.3) |
|
6 (66.7) |
|
| Others |
|
3 (60.0) |
|
2 (40.0) |
|
| Location MIb |
|
|
|
|
0.090 |
| Anterior |
|
47 (25.5) |
|
137 (74.5) |
|
| Inferior |
|
52 (34.0) |
|
101 (66.0) |
|
| Baseline CKb (U/l) |
|
|
|
|
0.970 |
| Normal (<171) |
|
52 (31.1) |
|
115 (68.9) |
|
| Raised (≥171) |
|
43 (30.9) |
|
96 (69.1) |
|
| Baseline SBP |
131.6 (22.91) |
|
138.1 (21.09) |
|
0.011f |
| Baseline DBP |
80.4 (15.16) |
|
86.7 (15.33) |
|
0.001f |
| Baseline Trop-Id (ng/ml) |
2.0 (0.96) |
18 (32.1) |
2.3 (1.10) |
38 (67.9) |
0.329 |
| Baseline TWBCd (x103/µl) |
12.1 (4.20) |
95 (28.5) |
11.5 (5.51) |
238 (71.5) |
0.465 |
| Baseline Hba (g/dl) |
13.8 (1.86) |
95 (28.5) |
14.2 (2.02) |
238 (71.5) |
0.069 |
| Baseline Plt countd (x103/µl) |
277.0 (93.00) |
95 (28.7) |
258.5 (95.0) |
236 (71.3) |
0.240 |
| Baseline neutrophil countd (x103/µl) |
7.4 (5.19) |
95 (28.5) |
6.9 (4.69) |
238 (71.5) |
0.601 |
| Baseline uread (mmol/l) |
5.2 (3.20) |
94 (30.7) |
4.6 (2.40) |
212 (69.3) |
0.107 |
| Baseline creatinined (µmol/l) |
107.0 (40.0) |
94 (30.7) |
96.0 (32.00) |
212 (69.3) |
0.142 |
| Baseline Ur/Creat ratioc,e |
|
|
|
|
0.793 |
| Pre-renal |
|
4 (40.0) |
|
6 (60.0) |
|
| Renal |
|
11 (30.6) |
|
25 (69.4) |
|
| Post-renal |
|
5 (38.5) |
|
8 (61.5) |
|
Systolic and diastolic blood pressure
The mean systolic blood pressure recorded at baseline was 138.1±21.09 mmHg in patients with no hypotension and 131.6±22.91 mmHg in patients with streptokinase-induced hypotension, t(357)=2.56, P=0.011.
The mean diastolic blood pressure recorded at baseline was 86.7±15.33 mmHg in patients with no hypotension and 80.4±15.16 mmHg in patients with streptokinase-induced hypotension, t(356)=3.46, P=0.001.
Fig. 1 presents a graphical comparison of the systolic and diastolic blood pressure variations with time between patients with streptokinase-induced hypotension and no hypotension.
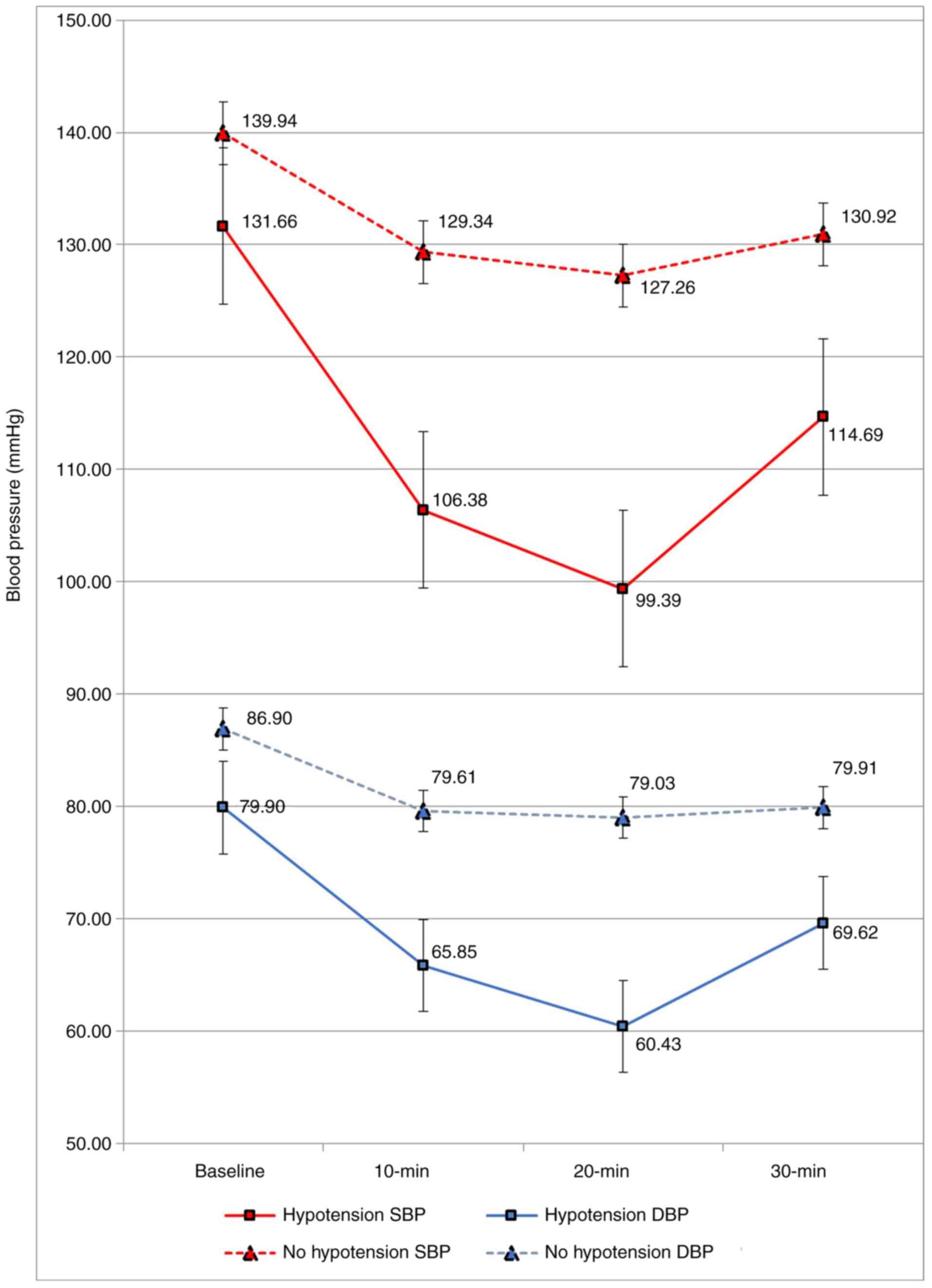 |
Figure 1
Systolic and diastolic blood pressure variations with time in patients with hypotension vs. those with no hypotension. SBP, systolic blood pressure; DBP, diastolic blood pressure.
|
Sub-analysis in patients with streptokinase-induced hypotension
Sub analyses in patients with streptokinase-induced hypotension were performed on 109 subjects. The median duration for patients to develop streptokinase-induced hypotension was at 18.5 min (IQR, 10.00 min) from the initiation of intravenous streptokinase infusion therapy (range, 2.0-60.0 min).
The lowest mean arterial pressure recorded among patients with streptokinase-induced hypotension was 54.6±9.72 mmHg. The lowest recorded mean systolic blood pressure among patients with streptokinase-induced hypotension was 75.8±10.81 mmHg, whereas the lowest recorded mean diastolic blood pressure was 42.9±10.37 mmHg, and the mean heart rate recorded at the lowest blood pressure drop among this group of patients was 76.5±20.74 beats per minute.
Similarly, both groups of patients (with and without hypotension) received the median cumulative dosage of 33.9 ml (IQR, 18.30 ml; range, 3.7-109.8 ml) of intravenous streptokinase, which equates to 508,500 IU of streptokinase.
The results of repeated measures ANOVA between the systolic blood pressure, diastolic blood pressure and heart rate at baseline, and at 10, 20 and 30 min during streptokinase therapy in patients with streptokinase-induced hypotension are presented in Table II.
 |
Table II
Changes in blood pressure in patients with hypotension receiving streptokinase.
|
Table II
Changes in blood pressure in patients with hypotension receiving streptokinase.
| |
Pre-STK |
During STK therapy |
|
| Variable(s) |
Baseline mean (SEM) |
10-min mean (SEM) |
20-min mean (SEM) |
30-min mean (SEM) |
P-value |
| SBPa (mmHg) |
130.7 (2.33) |
108.5 (3.05) |
100.0 (2.75) |
114.5 (2.24) |
<0.001b |
| DBPa (mmHg) |
80.0 (1.58) |
66.7 (2.24) |
59.9 (1.97) |
69.0 (1.41) |
<0.001b |
| HRa (bpm) |
78.4 (1.96) |
77.6 (1.91) |
77.8 (2.08) |
79.8 (2.12) |
0.441 |
Predictors of streptokinase-induced hypotension
SLR was conducted to determine the odds of each selected variable in predicting streptokinase-induced hypotension independently, whereas MLR was performed done to determine the optimal predictive model with a combination of variables that could positively predict the occurrence of streptokinase-induced hypotension (Table III). Only complete datasets with regards to individual variable were included in this descriptive analysis, hence the cumulative numbers may not add up to the total of 412 patients.
 |
Table III
Simple and multiple logistic regressions for potential predictors to streptokinase-induced hypotension.
|
Table III
Simple and multiple logistic regressions for potential predictors to streptokinase-induced hypotension.
| |
Hypotension |
No hypotension |
Simple logistic regression |
|
Multiple logistic regression |
|
| Variable(s) |
Mean (SD) |
n (%) |
Mean (SD) |
n (%) |
Crude OR (95% CI) |
P-valuea |
Adj. OR (95% CI) |
B value |
P-valueb |
| Age |
60.6 (10.79) |
60 (25.6) |
58.7 (13.27) |
174 (74.4) |
1.0 (0.99, 1.03) |
0.914 |
- |
|
- |
| Sex |
|
|
|
|
|
0.102 |
- |
|
- |
| Male |
|
84 (77.8) |
|
219 (84.9) |
0.6 (0.35, 1.10) |
|
|
|
|
| Female |
|
24 (22.2) |
|
39 (15.1) |
1.0 (Ref.) |
|
|
|
|
| Killip |
|
|
|
|
|
0.011d |
|
- |
0.013c |
| I |
|
30 (46.9) |
|
82 (60.3) |
1.0 (Ref.) |
|
1.0 (Ref.) |
|
|
| II |
|
21 (32.8) |
|
46 (33.8) |
1.3 (0.64, 2.43) |
|
1.4 (0.69, 2.81) |
|
|
| III and IV |
|
13 (20.3) |
|
8 (5.9) |
4.4 (1.68, 11.78) |
|
4.9 (1.71, 14.24) |
|
|
| Baseline CK |
|
|
|
|
|
0.970 |
- |
|
- |
| Not raised |
|
52 (54.7) |
|
115 (54.5) |
1.0 (0.62, 1.64) |
|
|
|
|
| Raised |
|
43 (45.3) |
|
96 (45.5) |
1.0 (Ref.) |
|
|
|
|
| Location MI |
|
|
|
|
|
0.091 |
- |
|
- |
| Anterior |
|
47 (47.5) |
|
137 (57.6) |
0.7 (0.42, 1.07) |
|
|
|
|
| Inferior |
|
52 (52.5) |
|
101 (42.4) |
1.0 (Ref.) |
|
|
|
|
| Baseline SBP (mmHg) |
131.6 (22.91) |
60 (25.6) |
138.1 (21.09) |
174 (74.4) |
0.99 (0.976, 0.997) |
0.012d |
- |
|
- |
| Baseline DBP (mmHg) |
80.5 (15.16) |
60 (25.6) |
86.7 (15.33) |
174 (74.4) |
0.97 (0.959, 0.989) |
0.001d |
- |
|
- |
| Baseline HR (mmHg) |
78.4 (19.84) |
60 (25.6) |
85.0 (21.17) |
174 (74.4) |
0.98 (0.973, 0.996) |
0.008d |
0.975 (0.958, 0.991) |
-0.026 |
0.003c |
| Baseline Hb (g/dl) |
13.8 (1.86) |
60 (25.6) |
14.2 (2.02) |
174 (74.4) |
0.90 (0.796, 1.009) |
0.070 |
- |
|
- |
MLR using the ENTER method revealed that Killip categories and baseline heart rate were predictive to the development of hypotension after adjusting for age, sex, baseline CK and baseline systolic and diastolic blood pressures. The Nagelkerke R-squared measures determined that 12.9% of the variation in the dependent variable can be explained by the model.
It was found that with every one-unit increment in the heart rate, the risk of hypotension was reduced by 2.6%. Additionally, patients classified as Killip III and IV had increased odds for the development of hypotension by ~5-fold as compared to those classified as Killip I.
Discussion
Fibrinolytic therapy is secondary to the gold standard percutaneous coronary intervention (PCI) for the management of patients with acute myocardial infarction (11). Despite being the gold standard treatment approach for myocardial infarction, the availability of PCI service is limited to cardiac referral centers, particularly in developing countries. The majority of public healthcare institutes without cardiac facilities still utilize first generation thrombolytics, such as streptokinase, as the primary mode of management in patients with acute myocardial infarction.
The present study determined that the hypotension observed during the intravenous infusion of streptokinase was a specific side-effect of the therapy in view of the comparable baseline demographic data and pertinent cardiac biomarkers, such as CKMB and Troponin-I, between patients with hypotension and without hypotension.
It was found that the onset of streptokinase-induced hypotension at occurred 18.5 min (IQR, 10.00 min) from the initiation of intravenous streptokinase therapy, synonymous to other studies that have reported a mean of 15-30 min (4,12-14).
Previous studies have also suggested that streptokinase-induced hypotension is a dose-related phenomenon (15-17); however, this hypothesis could not be supported, as the present study found no association between streptokinase cumulative dosages with the lowest documented mean arterial pressure. This finding was also shared by other previous studies that have suggested that an accelerated streptokinase delivery >20-30 min, instead of the conventional 60-min infusion is equally safe, apart from offering higher coronary reperfusion rates (17-19).
As regards the predictive factors for streptokinase-induced hypotension, the present study found that there were no significant differences in the baseline cardiac-specific biomarkers, such as the CKMB and Troponin-I levels between patients with hypotension and patients with no hypotension. Hence, it could not be proved that the occurrence of hypotension during streptokinase therapy is related to the extent of myocardial injury, as similarly observed by in the study by Pachaï et al (20). Other baseline clinical components that were considered as potential confounding factors to the development of hypotension during intravenous streptokinase therapy include a poor hydration status (15) and inferior infarcts (21). In the present study, the state of hydration was determined from the disproportionate increase in the urea to creatinine ratio, which was insignificant between the two groups in the study population. Additionally, neither anterior nor inferior infarcts, predicted the occurrence of hypotension during streptokinase therapy in the present study; a similar observation was reported in the study by Chau and Choi (21).
The present study did not use other underlying comorbidities in patients, such as diabetes or hypertension as factors in the predictive model, as the present study focused on the immediate side-effects of streptokinase therapy during the acute onset of myocardial necrosis. The presence and level of control of these underlying medical problems were indeed significant risk factors for the development of acute myocardial infarction; however, they have not been proven to independently influence the development of hypotension during streptokinase therapy. However, the present study examined all the measurable determinants of blood pressure, including factors that affect cardiac output and systemic vascular resistance. This included the measurements of heart rate, calculated whole blood viscosity and the assessment of hydration.
To the best of our knowledge, no previous study to date has investigated the potential baseline clinical parameters for the prediction of the development of streptokinase-induced hypotension. The present study revealed two baseline parameters that significantly predicted the occurrence of hypotension in patients with acute myocardial infarction receiving streptokinase therapy, which are the baseline heart rate prior to streptokinase therapy and the Killip classification. The Killip classification was first introduced in 1967(22) as a tool in clinical practice to assess the severity of heart failure following myocardial infarction. The four stages of Killip classification ranges from no clinical signs of heart failure to overt cardiogenic shock. Secondly, the lower baseline heart rate was also determined to be predictive of hypotension during streptokinase therapy. The heart rate is part of the hemodynamic markers and reflects the autonomic function of the heart and residual myocardial reserve following myocardial injury. Therefore, it may also affect blood pressure through the modulation in cardiac output. This finding is in agreement with that of the study by Lew et al (12) who found that patients with a compromised hemodynamic status had a higher risk of developing hypotension during streptokinase therapy.
Therefore, these predictive factors may assist clinicians in identifying susceptible individuals and may encourage vigilance when delivering streptokinase in patients with acute myocardial infarction.
In conclusion, the findings of the present study underscore the importance of clinical vigilance during streptokinase therapy. The anticipation of hypotension in susceptible individuals prompts caution during clinical management to prevent further hypoperfusion in the acutely ischemic myocardium. A hypotensive episode during acute myocardial infarction could further extend the rate of myocardial necrosis and affect the non-ischemic myocardium as well, particularly if the areas are supplied by collaterals of stenotic coronary arteries.
Acknowledgements
The authors would like to thank the Director General of Health Malaysia for his permission to publish this article.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
The study was conceptualized by KK, REA, SYL and IZZA. KK and SYL performed the data collection and statistical analysis, prepared the tables and drafted the manuscript. REA, SYL and IZZA commented on the manuscript and tables. KK and SYL confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was registered with the National Medical Research Registry of Ministry of Health, Malaysia (NMRR-19-2871-50953 and received ethical clearance from the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia [KKM/NIHSEC/P19-2421(5)]. Informed consent related to the present study was waived by the Medical Research and Ethics Committee of the Ministry of Health Malaysia. The study only utilized secondary data source and did not involve any form of physical interaction with the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare there that they have no competing interests.
References
|
1
|
Adivitiya and Khasa YP: The evolution of recombinant thrombolytics: Current status and future directions. Bioengineered. 8:331–358. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Banerjee A, Chisti Y and Banerjee UC: Streptokinase-a clinically useful thrombolytic agent. Biotechnol Adv. 22:287–307. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grierson DS: Clinical pharmacology of streptokinase. In: Acute Coronary Care. Springer, New York, NY, pp67-74, 1985.
|
|
4
|
Gemmill JD, Hogg KJ, Douglas JT, Dunn FG, Lowe GD, Rae AP and Hillis WS: The incidence and mechanism of hypotension following thrombolytic therapy for acute myocardial infarction with streptokinase-containing agents-lack of relationship to pretreatment streptokinase resistance. Eur Heart J. 14:819–825. 1993.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kunamneni A, Abdelghani TT and Ellaiah P: Streptokinase-The drug of choice for thrombolytic therapy. J Thromb Thrombolysis. 23:9–23. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aslanabadi N, Safaie N, Talebi F, Dousti S and Entezari-Maleki T: The streptokinase therapy complications and its associated risk factors in patients with acute ST elevation myocardial infarction. Iran J Pharm Res. 17 (Suppl 1):S53–S63. 2018.PubMed/NCBI
|
|
7
|
Kumolosasi E, Wei WS and Wee CE: The use of thrombolytic agents in acute myocardial infarction (AMI) patients. Int J Pharm Pharm Sci. 5:63–67. 2013.
|
|
8
|
El-Menyar A, Zubaid M, Almahmeed W, Sulaiman K, Alnabti A, Singh R and Al Suwaidi J: Killip classification in patients with acute coronary syndrome: Insight from a multicenter registry. Am J Emerg Med. 30:97–103. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ranganathan P, Pramesh CS and Aggarwal R: Common pitfalls in statistical analysis: Logistic regression. Perspect Clin Res. 8:148–151. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nagelkerke NJD: A note on a general definition of the coefficient of determination. Biometrika. 78:691–692. 1991.
|
|
11
|
Nakamura M: Angiography is the gold standard and objective evidence of myocardial ischemia is mandatory if lesion severity is questionable. -Indication of PCI for angiographically significant coronary artery stenosis without objective evidence of myocardial ischemia (Pro)-. Circ J. 75204–210. (217)2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lew AS, Laramee P, Cercek B, Shah PK and Ganz W: The hypotensive effect of intravenous streptokinase in patients with acute myocardial infarction. Circulation. 72:1321–1326. 1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Battershill PE, Benfield P and Goa KL: Streptokinase. A review of its pharmacology and therapeutic efficacy in acute myocardial infarction in older patients. Drugs Aging. 4:63–86. 1994.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Carnemolla R and Muzykantov VR: Vascular targeting of antithrombotic agents. IUBMB Life. 63:632–639. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ghaffari S, Kazemi B and Golzari IG: Efficacy of a new accelerated streptokinase regime in acute myocardial infarction: A double blind randomized clinical trial. Cardiovasc Ther. 31:53–59. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Srimahachota S, Sangwatanaroj S, Boonyaratavej S, Suitichaiyakul T and Ngarmukos P: Efficacy of rapid infusion of streptokinase in patients with acute myocardial infarction. J Med Assoc Thai. 83:8–12. 2000.PubMed/NCBI
|
|
17
|
Tatu-Chiţoiu G, Dorobanţu M, Teodorescu C, Craiu E, Vintilǎ M, Minescu B, Burghină D, Stamate S, Serban L, Protopopescu T, et al: Accelerated streptokinase in ST-elevation myocardial infarction-a romanian (ASK-ROMANIA) multicenter registry. Int J Cardiol. 122:216–223. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Herlitz J, Hartford M, Aune S and Karlsson T: Occurrence of hypotension during streptokinase infusion in suspected acute myocardial infarction, and its relation to prognosis and metoprolol therapy. Am J Cardiol. 71:1021–1024. 1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Koren G, Weiss AT, Ben-David Y, Hasin Y, Luria MH and Gotsman MS: Bradycardia and hypotension following reperfusion with streptokinase (Bezold-Jarisch reflex): A sign of coronary thrombolysis and myocardial salvage. Am Heart J. 112:468–471. 1986.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pachaï A, Erlendsson AK and Brandslund I: Streptokinase, complement activation and hypotension. APMIS. 105:650–654. 1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chau HW and Choi KK: Efficacy and safety of tenectaplase versus streptokinase in treating st-elevation myocardial infarction patients in Hong Kong: A four-year retrospective review in Queen Elizabeth Hospital. Hong Kong J Emerg Med. 20:359–363. 2013.
|
|
22
|
Killip T III and Kimball JT: Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 20:457–464. 1967.PubMed/NCBI View Article : Google Scholar
|















