Introduction
Sleep is pivotal for the maintenance of mental and
physical health, and globally, research interests into sleep
disorders are increasing. Some patients with chronic liver diseases
(CLDs) complain of sleep disorders. Iwasa et al reported
that out of 1,788 patients with CLD, 4.0% experienced severe sleep
disorders, and 33.4% had moderate sleep disorders (1). Ghabril et al reported that 81%
of patients with advanced cirrhosis had a disturbed sleep (2). One of the reasons for this is the
disturbance of the sleep-regulating hormone (i.e., melatonin) and
the appetite-regulating hormone (i.e., leptin) during the day.
Itchy skin due to CLDs and anxiety regarding the illness can also
cause insomnia (3-5).
Shigiyama et al demonstrated that sleep disturbance was
associated with fat accumulation in the liver and glucose
intolerance in mice (6). Thus,
sleep disorders can be a critical issue for patients with CLD. The
Pittsburgh Sleep Quality Index (PSQI) is a widely used and
well-validated patient-reported sleep questionnaire (7-9).
Skeletal muscle is also an endocrine organ that
secretes myokines that regulate systemic glucose and lipid
homeostasis, and regulate protein synthesis in muscle tissue
(10). Sarcopenia is a condition
accompanied by a decrease in skeletal muscle mass and strength or
physical function (11). Primary
sarcopenia is a condition in which skeletal muscle mass and
strength or physical function decline with aging. Secondary
sarcopenia is defined as a condition in which skeletal muscle mass
and strength or physical function are impaired due to underlying
diseases, such as respiratory diseases, heart diseases,
inflammatory diseases, malignancies, renal diseases and liver
diseases (12). As regards the
mechanisms of the development of sarcopenia in patients with CLD,
the involvement of various factors (aging, protein energy
malnutrition, signal transduction related to protein synthesis and
degradation, myokines and sex hormones, etc.) has been reported
(13-15).
Sarcopenia can result in a decreased quality of life (QOL) of
affected patients and be associated with unfavorable outcomes in
patients with CLD (13,16-18).
In a previous cross-sectional study, the authors
demonstrated the close association between sarcopenia and sleep
disorders in patients with CLD (19). There are several reports regarding
the association between sarcopenia and sleep disorders (19-22).
However, the causal association between sarcopenia and sleep
disorders in patients with CLD is unclear. To clarify this
association, the present study sought to examine the influence of
sarcopenia-related factors (i.e., muscle strength and muscle mass)
on the progression of sleep disorders in patients with CLD.
Patients and methods
Patients
Using a retrospective computerized database, a total
of 182 individuals with CLD who visited the Hyogo College of
Medicine Hospital between December, 2013 and April, 2018 were
retrospectively analyzed. Clinical features, the Japanese version
of PSQI (PSQI-J) scores and laboratory data recorded at baseline
were collated. Diagnosis for cirrhosis was determined according to
the current guidelines (23). In
all analyzed patients, evaluation using PSQI-J questionnaire was
performed twice or more during the observation period. The time
interval from the date of baseline PSQI-J and the first confirmed
date of the elevation of PSQI-J score was calculated in each
subject. The most suitable intervention for each underlying liver
disease was performed (23-26).
The study protocol rigorously conformed to the 1975 Helsinki
Declaration, and approval of ethics was obtained from the
institutional review board in Hyogo College of Medicine Hospital.
An opt out method was employed.
PSQI-J score and the present study
cohort
Sleep quality was evaluated by PSQI-J, which is a
screening tool for sleep disorders (7-9).
PSQI-J consists of 7 categories (a total of 10 questions) as
follows: i) Subjective sleep quality; ii) sleep latency; iii) sleep
duration; iv) habitual sleep efficiency; v) sleep disorders; vi)
use of sleep medications; and vii) daytime sleep disturbance. Each
category was scored on a scale of 0 to 3, and the sum of PSQI-J
scores for all categories was 21 points. Higher PSQI-J scores
indicate a poorer sleep quality. Favorable sensitivity and
specificity were reported to be found when the sum of PSQI-J scores
exceeded 6 points (8). The
patients in the present study was categorized as have normal skeep
(0-5 points), mild sleep disorders (6-8 points), moderate to mild
sleep disorders (9-11 points) and mild to severe sleep disorder (12
or more points) (7-9).
Muscle strength and muscle mass
measurement
Muscle strength [grip strength (GS) in the present
study] measurement and muscle mass measurements were performed
based on previous findings (12).
For the evaluation of muscle mass, bioelectrical impedance analysis
(BIA) was performed using InBody 720 to calculate appendicular
muscle mass. Skeletal muscle index (SMI) was calculated as sum of
muscle mass in the upper and lower extremities divided by height
squared (kg/m2). Based on the criteria of the Japanese
Society of Hepatology (JSH), muscle strength weakness was diagnosed
as a GS of <26 kg for males and <18 kg for females. Likewise,
the loss of muscle mass was diagnosed by a SMI of <7.0
kg/m2 for males and <5.7 kg/m2 for females
on BIA (12).
Statistical analysis
Continuous variables are presented as median value
[interquartile range (IQR)] and compared using the Student's
t-test. The primary endpoint was the elevation of the PSQI-J score
compared to the baseline PSQI-J score. Cumulative elevation rates
of the PSQI-J score were calculated by the Kaplan-Meier method and
compared between groups using the log-rank test. Univariate and
multivariate Cox proportional hazard models were employed for
identifying significant factors associated with the elevation rates
of PSQI-J score, and the results are presented as hazard ratios
(HRs) and 95% confidence intervals (CIs) with corresponding
P-values. In the univariate analysis, the cohort was divided into 2
categories using each median value. Variables with P-values <0.1
were entered into the multivariate analysis. JMP version 14.0
software (SAS Institute) was employed to analyze data statistically
(significant level, P-value <0.05).
Results
Patient characteristics
Of the 182 patients with CLDs, 82 (45.1%) were males
[age, median, 64 years; IQR, 55-71 years]. There were 136 patients
(74.7%) with non-cirrhosis and 46 patients (25.3%) with cirrhosis.
No patients were found to have overt hepatic encephalopathy,
hepatocellular carcinoma, or severe ascites. The main liver disease
etiology was hepatitis C virus (HCV, 155 cases, 85.2%). The median
and IQR values for the PSQI-J score were as follows: Median, 5;
IQR, 3-7. A PSQI-J score of 0-5 (normal) was observed in 99 (54.4%)
patients, a score of 6-8 (mild sleep disorders) was found in 53
(29.1%) patients, a score of 9-11 (moderate sleep disorders) was
found in 19 (10.4%) patients, and a score of ≥12 (severe sleep
disorders) was observed in 11 (6.0%) patients. The median and IQR
values for the PSQI-J scores in cirrhotic patients and
non-cirrhotic patients were as follows: Cirrhotic patients: Media,
6; IQR, 4-9; non-cirrhotic patients: Median, 5; IQR, 3-7; P=0.0662.
A decline in GS as defined by the JSH criteria was observed in 9
male patients (11.0%) and 39 female patients (39.0%). A decline in
SMI as defined by the JSH criteria was observed in 25 male patients
(30.5%) and 39 female patients (39.0%). Sarcopenia as defined by
the JSH criteria was observed in 25 patients (13.7%). In patients
with any grade of sleep disorder at baseline (PSQI-J score >5,
n=83), 24 (28.9%) had a decline in GS, and 28 (33.7%) had a decline
in SMI. The baseline clinical characteristics and laboratory data
of all analyzed patients are summarized in Table I.
 | Table IPatient baseline characteristics
(n=182). |
Table I
Patient baseline characteristics
(n=182).
| Variables | All cases
(n=182) |
|---|
| Age (years) | 64 (55-71) |
| Sex,
male/female | 82/100 |
| Liver disease
etiology | |
| HCV/HBV/others | 155/13/14 |
| Presence of
sarcopenia, yes/no | 25/157 |
| PSQI-J score | 5 (3-7) |
| Presence of
cirrhosis, yes/no | 46/136 |
| Body mass index
(kg/m2) | 22.7
(20.4-25.425) |
| SMI
(kg/m2), male | 7.69
(7.0-8.07) |
| SMI
(kg/m2), female | 5.9
(5.36-6.34) |
| Grip strength (kg),
male | 35.1
(29.8-42.1) |
| Grip strength (kg),
female | 20.35
(17.2-22.825) |
| Total bilirubin
(mg/dl) | 0.8 (0.6-1.1) |
| Serum albumin
(g/dl) | 4.2
(3.975-4.4) |
| Prothrombin time
(INR) | 1.07
(1.02-1.13) |
| Platelet count
(x104/mm3) | 15.9
(11.8-20.05) |
| AST (IU/l) | 29.5
(22-45.25) |
| ALT (IU/l) | 27 (17-47) |
| ALP (IU/l) | 243.5
(202.75-322) |
| GGT (IU/l) | 26.5 (19-44) |
| eGFR
(ml/min/1.73m2) | 82 (71.75-95) |
Cumulative elevation rate of PSQI-J
score for all cases (n=182)
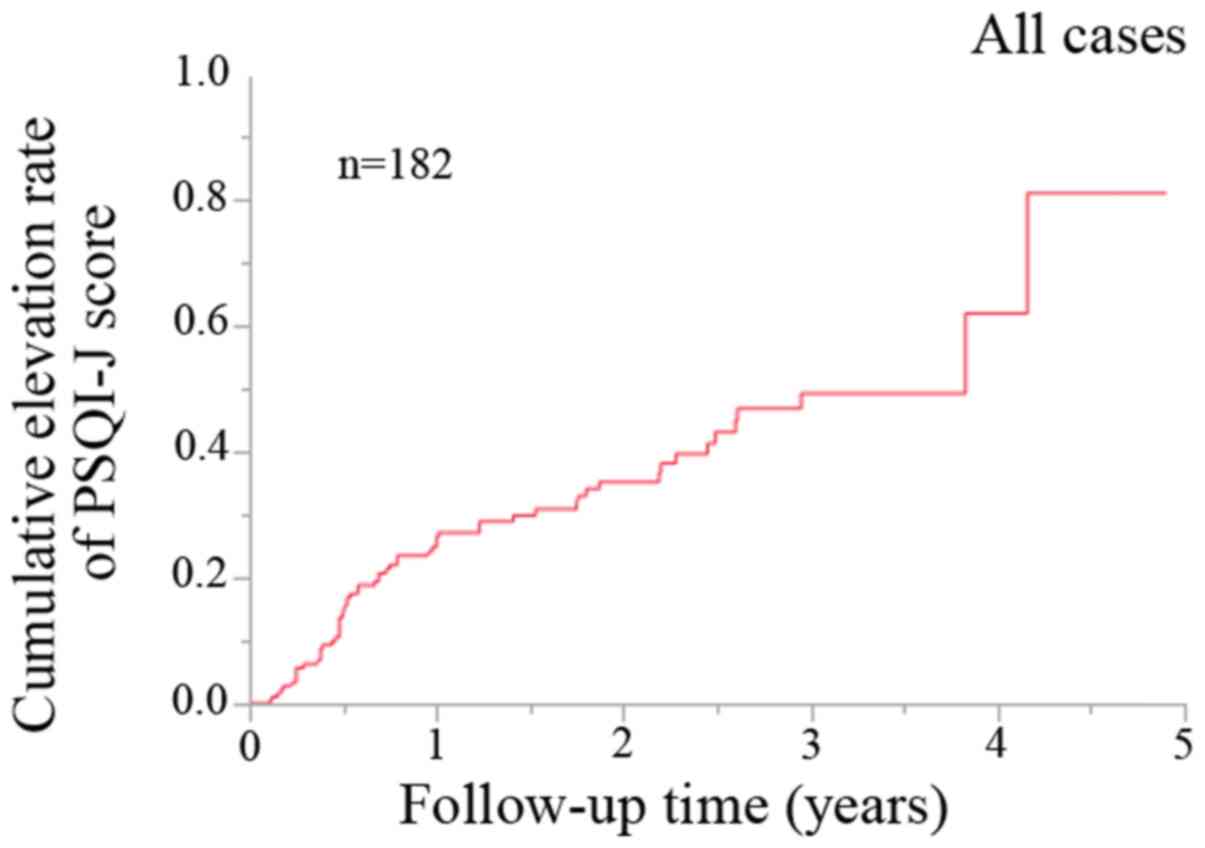
During the observation period, 61 patients (33.5%)
exhibited an elevation in the PSQI-J score. For all cases, the 1-,
2- and 3-year cumulative elevation rates of the PSQI-J score were
26.4, 35.2 and 49.3% (Fig. 1).
Cumulative elevation rates of the
PSQI-J score according to the GS and SMI values
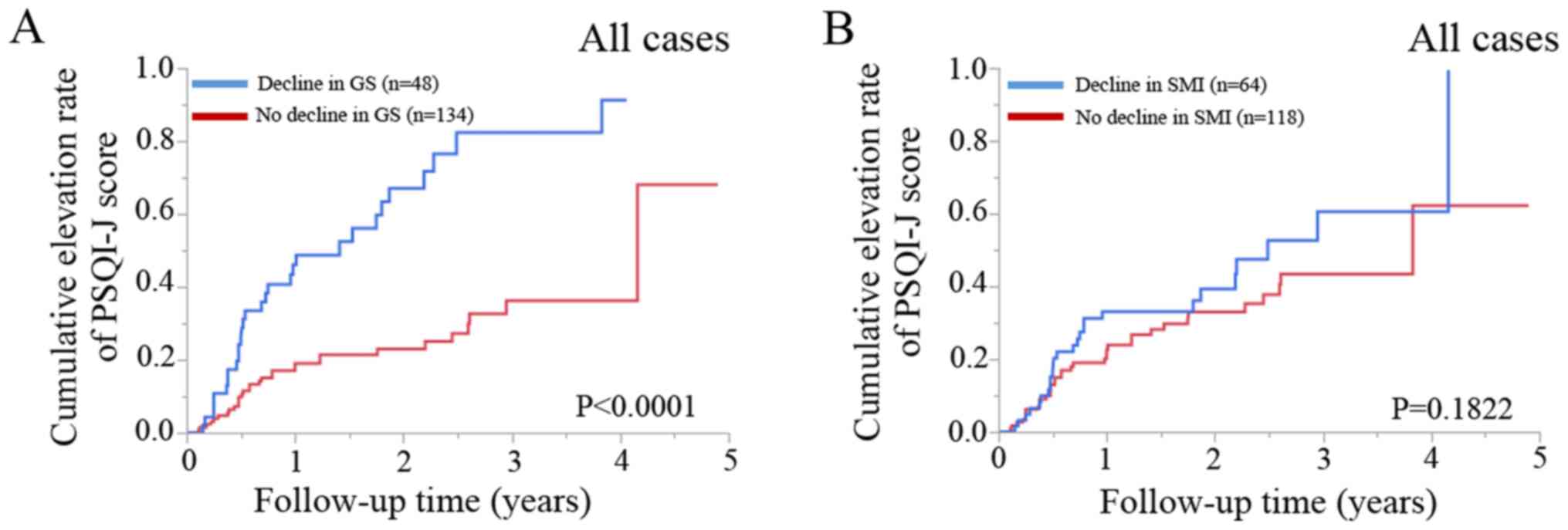
In patients with a decline in GS (n=48), the 1-, 2-
and 3-year cumulative elevation rates of the PSQI-J score were
46.0, 67.1 and 82.4%, while in patients with no decline in GS
(n=134), the 1-, 2- and 3-year cumulative elevation rates of the
PSQI-J score were 19.1, 22.9 and 36.2% (P<0.0001; Fig. 2A).
In patients with a decline in SMI (n=64), the 1-, 2-
and 3-year cumulative elevation rates of the PSQI-J score were
33.1, 39.3 and 60.6%, while in patients with no decline in SMI
(n=118), the 1-, 2- and 3-year cumulative elevation rates of the
PSQI-J score were 22.7, 33.0 and 43.4% (P=0.1822; Fig. 2B).
Predictors of the elevation of PSQI-J
score in all patients by univariate and multivariate analyses
As per the univariate analyses, age >64 years
(P=0.0095), sex (P=0.0292) and a lower GS (P<0.0001) were found
to be significantly associated with the elevation of the PSQI-J
score, while HCV or not (P=0.0632) and serum albumin ≤4.2 g/dl
(P=0.0887) tended to be significant (Table II). As per the multivariate
analyses, only a lower GS (P=0.0002) was identified to be a
significant factor associated with the elevation of PSQI-J score
(Table III). The HRs and 95% CIs
for age >64 years, sex, a lower GS, HCV or not and serum albumin
≤4.2 g/dl are shown in Table
III.
 | Table IIUnivariate analysis of factors linked
to the elevation of the PSQI-J score (n=182). |
Table II
Univariate analysis of factors linked
to the elevation of the PSQI-J score (n=182).
| Variables | Number of each
category | Univariate
P-value |
|---|
| Age (years) ≥64,
yes/no | 98/84 | 0.0095 |
| Sex,
male/female | 82/100 | 0.0292a |
| Cause of liver
diseases, HCV/non-HCV | 155/27 | 0.0632 |
| Grip strength,
high/low | 134/48 |
<0.0001a |
| Skeletal muscle
index, high/low | 118/64 | 0.1822 |
| Presence of
cirrhosis, yes/no | 46/136 | 0.1597 |
| Presence of sleep
disorder at baseline, yes/no | 83/99 | 0.8226 |
| AST ≥29.5 IU/l,
yes/no | 91/91 | 0.6134 |
| ALT ≥27 IU/l,
yes/no | 93/89 | 0.5078 |
| ALP ≥243.5 IU/l,
yes/no | 91/91 | 0.7334 |
| GGT ≥26.5 IU/l,
yes/no | 91/91 | 0.2278 |
| Serum albumin ≤4.2
g/dl, yes/no | 100/82 | 0.0887 |
| Total bilirubin
≥0.8 mg/dl, yes/no | 108/74 | 0.6928 |
| Prothrombin time
(INR) ≥1.07, yes/no | 93/89 | 0.4460 |
| Platelet count
≤15.9 x104/mm3, yes/no | 91/91 | 0.7827 |
| eGFR ≤82
ml/min/1.73m2, yes/no | 93/89 | 0.4019 |
| Body mass index
≥22.7 kg/m2, yes/no | 93/89 | 0.8695 |
 | Table IIIMultivariate analysis of factors
linked to the elevation of the PSQI-J score. |
Table III
Multivariate analysis of factors
linked to the elevation of the PSQI-J score.
| | Multivariate
analysis |
|---|
| Variables | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age, ≥64 years | 1.561 | 0.859-2.838 | 0.1443 |
| Low-GS | 2.984 | 1.685-5.285 | 0.0002a |
| Sex (female) | 1.041 | 0.561-1.932 | 0.8990 |
| Serum albumin ≤4.2
g/dl | 1.126 | 0.625-2.029 | 0.6929 |
| HCV | 2.458 | 0.868-6.959 | 0.0902 |
Cumulative elevation rates of the
PSQI-J score according to the GS and SMI values in cirrhotic
patients and non-cirrhotic patients
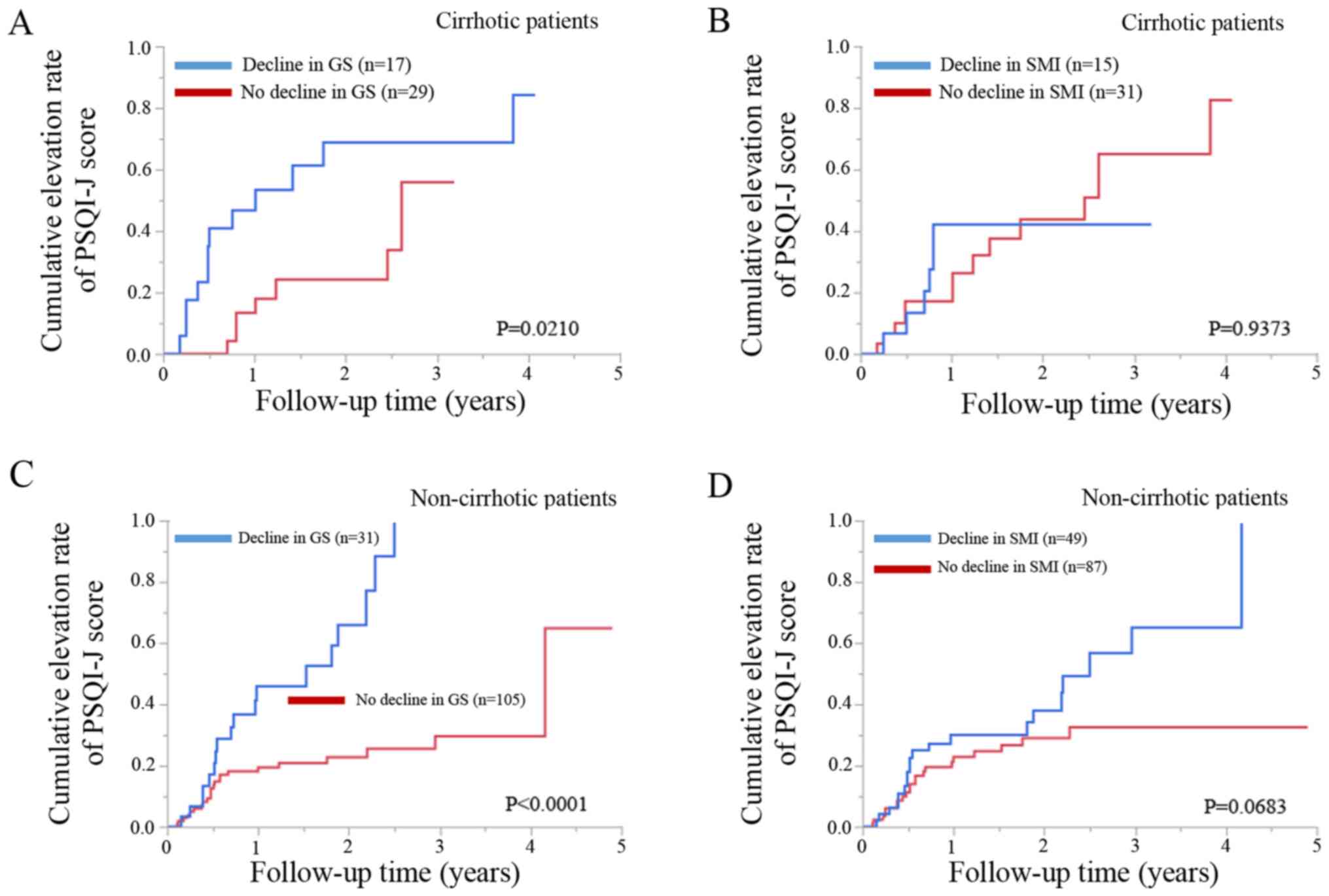
Cirrhotic patients with a decline in GS (n=17) had
significantly higher cumulative elevation rates of the PSQI-J score
compared to those with no decline in GS (n=29) (P=0.0210; Fig. 3A). Cirrhotic patients with a
decline in SMI (n=15) did not have a significantly higher
cumulative elevation rates of the PSQI-J score compared to those
with no decline in SMI (n=31) (P=0.9373; Fig. 3B).
Non-cirrhotic patients with a decline in GS (n=31)
had significantly higher cumulative elevation rates of the PSQI-J
score compared to those with no decline in GS (n=105) (P<0.0001;
Fig. 3C). Non-cirrhotic patients
with a decline in SMI (n=49) tended to have significantly higher
cumulative elevation rates of the PSQI-J score compared to those
with no decline in SMI (n=87) (P=0.0683; Fig. 3D).
Cumulative elevation rates of the
PSQI-J score according to the GS and SMI values in patients aged
≥64 years and patients aged <64 years
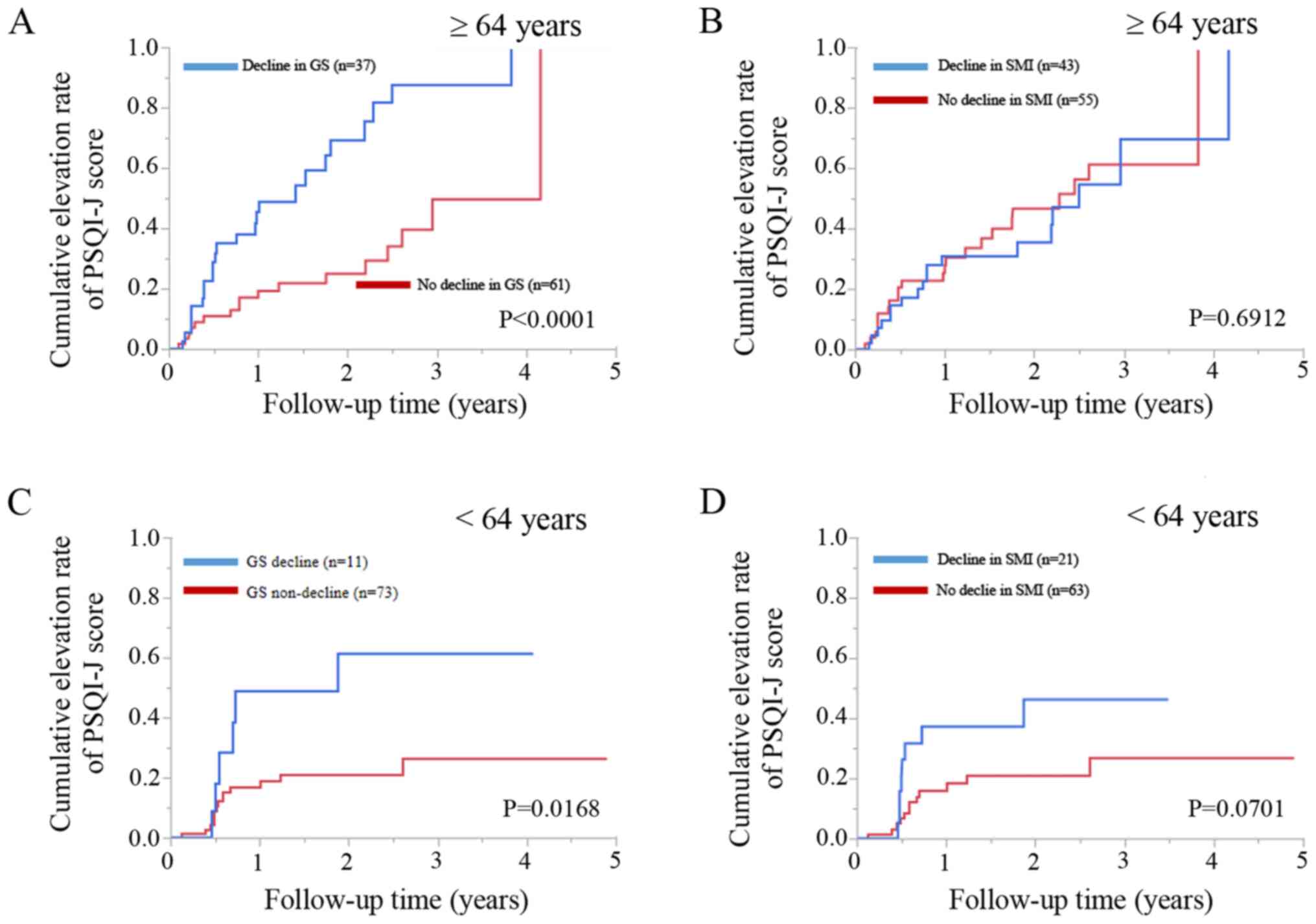
Patients aged ≥64 years (median age in the present
study) or with a decline in GS (n=37) had significantly higher
cumulative elevation rates of the PSQI-J score compared to those
with no decline in GS (n=61) (P<0.0001; Fig. 4A). However, patients aged ≥64 years
with a decline in SMI (n=43) did not have significantly higher
cumulative elevation rates of the PSQI-J score compared to patients
with no decline in SMI (n=55) (P=0.6912; Fig. 4B).
Patients aged <64 years with a decline in GS
(n=11) had significantly higher cumulative elevation rates of the
PSQI-J score compared to those with no decline in GS (n=73)
(P=0.0168; Fig. 4C). Patients aged
<64 years with a decline SMI (n=21) tended to have significantly
higher cumulative elevation rates of the PSQI score compared to
patients with no decline in SMI (n=63) (P=0.0701; Fig. 4D).
Cumulative elevation rates of the
PSQI-J score according to the GS and SMI values in male and female
patients
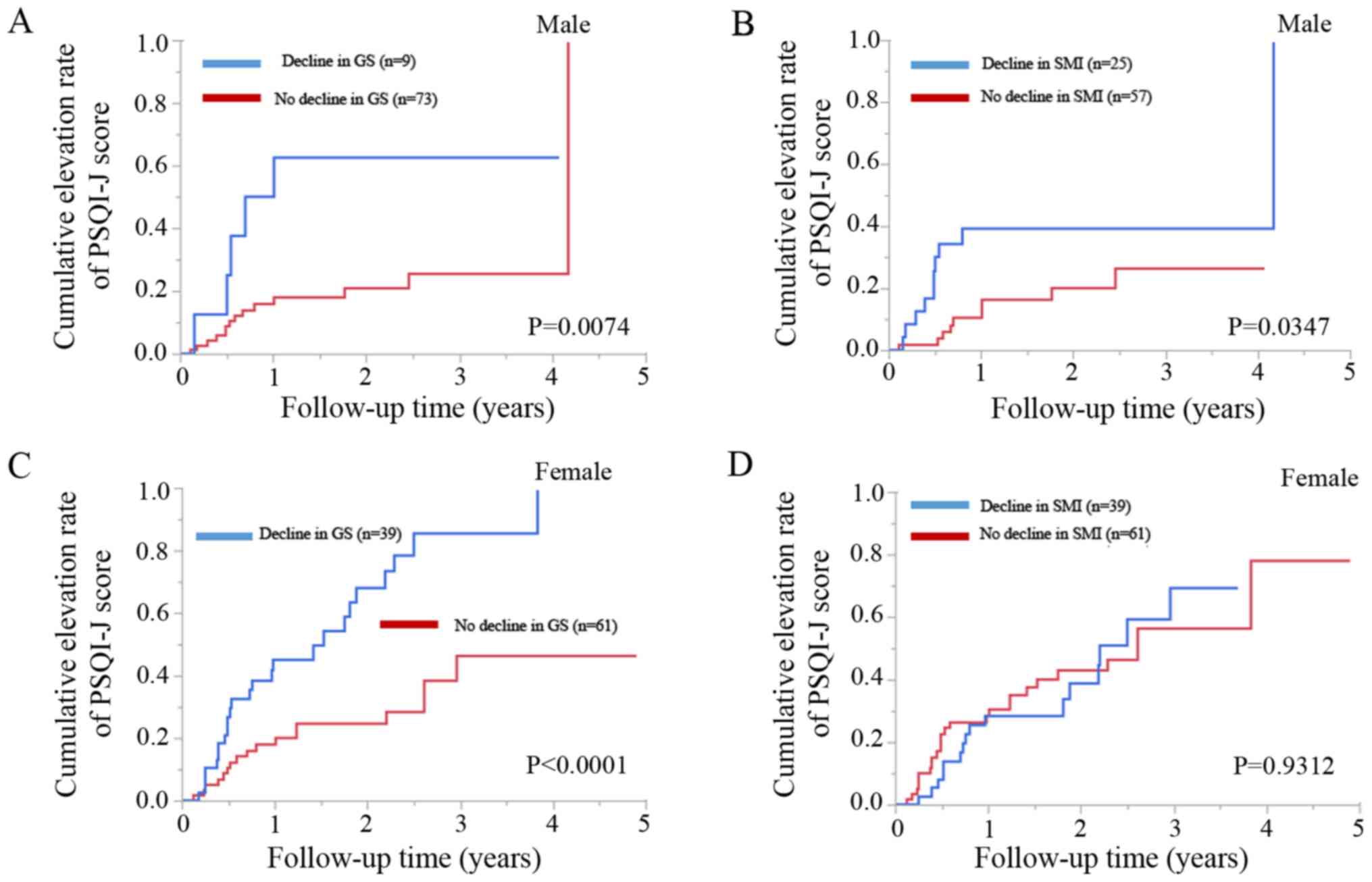
Male patients with a decline in GS (n=9) had
significantly higher cumulative elevation rates of PSQI-J score
compared to those with no decline in GS (n=73) (P=0.0074; Fig. 5A). Likewise, male patients with a
decline in SMI (n=25) had significantly higher cumulative elevation
rates of the PSQI-J score compared to those with no decline in SMI
(n=57) (P=0.0347; Fig. 5B).
Female patients with a decline in GS (n=39) had
significantly cumulative higher elevation rates of the PSQI-J score
compared to those with no decline in GS (n=61) (P<0.0001;
Fig. 5C). However, female patients
with a decline in SMI (n=39) did not have significantly higher
cumulative elevation rates of the PSQI-J score compared to those
with no decline in SMI (n=61) (P=0.9312; Fig. 5D).
Cumulative elevation rates of the
PSQI-J score according to the GS and SMI values in patients with
baseline PSQI score >5 (baseline) and baseline PSQI score <5
(baseline)
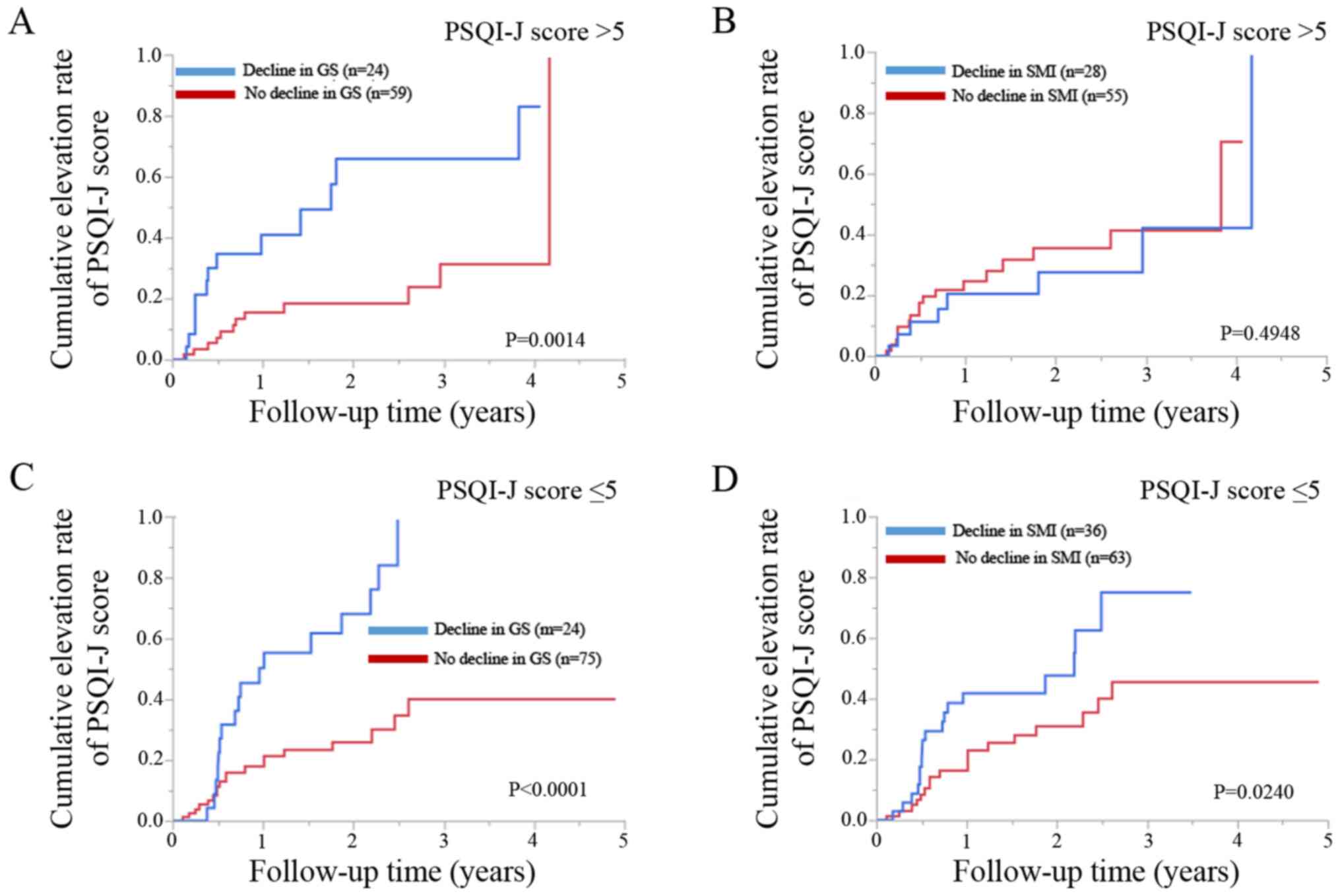
Patients with baseline PSQI-J score >5 with a
decline in GS (n=24) had significantly higher cumulative elevation
rates of the PSQI score compared to those with no decline in GS
(n=59) (P=0.0014; Fig. 6A).
However, patients with a baseline PSQI score >5 with a decline
in SMI (n=28) did not have significantly higher cumulative
elevation rates of PSQI-J score compared to those with no decline
in SMI (n=55) (P=0.4948; Fig.
6B).
Patients with baseline PSQI-J score ≤5 with a
decline in GS (n=24) had significantly higher cumulative elevation
rates of PSQI-J score compared to those with no decline in GS
(n=75) (P<0.0001; Fig. 6C).
Likewise, patients with baseline PSQI-J score ≤5 with a decline in
SMI (n=36) had significantly higher cumulative elevation rates of
PSQI-J score compared to those with no decline in SMI (n=63)
(P=0.0240; Fig. 6D).
Discussion
The causal association between sleep disorders and
sarcopenia-related factors in patients with CLD has not yet been
fully examined. In patients with CLD, hepatic events or severity of
liver fibrosis, as well as aging can be associated with a decline
in GS (27,28). In the present study, comprehensive
analyses regarding the influence of sarcopenia-related factors on
the elevation of PSQI-J score in patients with CLDs were performed.
Multivariate analysis identified only GS decline as a significant
adverse predictor associated with the elevation of PSQI-J score. To
conclude, reduced GS rather than muscle mass was associated with
the elevation of the PSQI-J score independent of age, cirrhosis
status, sex and baseline sleep condition. The causal association
between sleep disorders and sarcopenia-related factors in patients
with CLD was clarified to some extent through the present study. To
the best of our knowledge, this is the first report demonstrating
the impacts of sarcopenia-related factors on the progression of
sleep disorder in patients with CLDs.
It is unclear why the weakness of muscle strength
can better predict the exacerbation of sleep status in patients
with CLDs compared to muscle mass loss. One possible reason for
this is that a decline in muscle strength occurs 2-5-fold faster
than muscle mass loss, which can be linked to a decline in QOL,
resulting in the elevation of the PSQI-J score (29). Another possible reason is that
muscle strength decline is associated with hormonal changes, such
as insulin-like growth factors 1 and testosterone, potentially
leading to the exacerbation of sleep status (30). GS is representative of whole-body
muscle strength and has been shown to be an independent marker of
nutrition (31). However, in the
present study, in male patients and in patients with baseline
PSQI-J score ≤5, the group with a decline in SMI had significantly
higher cumulative elevation rates of PSQI-J score compared to the
SMI non-decline group. While the current study emphasizes the
significance of GS on the progression of sleep disorder, it does
not deny the significance of muscle mass on prognosis.
In the present study, 83 patients (45.6%) out of the
analyzed subjects had a baseline PSQI-J score >5. In patients
with cirrhosis (n=46), 27 patients (58.7%) had baseline PSQI-J
score >5. Samanta et al reported that 60 out of 100
cirrhotic patients (60%) had PSQI score >5, which was in
agreement with the present data (32). Clinicians should be aware of the
high prevalence of sleep disorder in CLDs. During the observation
period, 20 cirrhotic patients (43.5%) had the elevation of PSQI
score, while 41 non-cirrhotic patients (30.1%) had the elevation of
PSQI score, which was largely different from cirrhotic patients. In
addition, the median baseline PSQI-J score in cirrhotic patients
tended to be higher than that in non-cirrhotic patients in the
present study cohort (P=0.0662). Longer liver disease duration in
cirrhotic patients and anxiety about having cirrhosis may be linked
to the current results.
HCV tended to be significant in our multivariate
analysis (P=0.0902). In patients with HCV (n=155), 57 patients
(36.8%) had the elevation of PSQI-J score during the observation
period. Most of these 57 patients received antiviral therapies with
sustained virological response (SVR). SVR does not eliminate the
possibility of liver carcinogenesis (33). Similarly, SVR does not solve the
sleep problems in patients with HCV considering the current data.
Clinicians should be fully aware of these, and post SVR
surveillance in HCV patients will be needed. On the other hand,
obstructive sleep apnea is frequently seen in patients with
non-alcoholic fatty liver disease with obesity (34). In the present study, the median
body mass index (BMI) was 22.7 kg/m2 and the number of
patients with BMI ≥30 kg/m2 was only 4 (2.2%). The
PSQI-J score in these 4 obese patients were 0 or 1. Therefore, it
is likely that obstructive sleep apnea is not included in the
analyzed subjects, and sleep disorder shown in this study may be
due to disease itself or other causes than obstructive sleep
apnea.
PSQI-J question 8 is a question regarding the
frequency of falling asleep while driving, eating and social
activities. In the present study, 19 patients (10.4%) had a scale
of ≥1 (i.e., experience of drowsiness at least once a week) in the
question 8 of PSQI-J. Excessive drowsiness, particularly while
driving, can lead to major accidents, so caution should be
exercised for such patients (35,36).
On the other hand, QOL can be influenced by sex (37). The role of menopause in the risk of
a decline in GS or sleep disturbance warrants further
investigations (37).
The limitations of the present study must be
acknowledged. First, the retrospective nature of the study limits
the evaluation of factors influencing the sleep condition such as
life circumstances or sleep medications. Second, PSQI-J is a
subjective assessment tool, and not objective one. Third, the data
were derived from Japanese CLD patient data; further examinations
on other cohorts will be required to extend the application.
Finally, several interventions for patients with CLD during the
observation period were performed, making bias for the disease
progression. Thus, interpretation with caution to the results will
be needed.
In conclusion, the present study would like to
emphasize the significance of muscle strength on the sleep
condition in CLDs. The findings involve essential implications in
clinical practice as they highlight that a reduced GS rather than
the loss of muscle mass is independently associated with an
elevated risk for the progression of sleep disorder. Appropriate
interventions for patients with CLD with a decline in GS will be
necessary for improving patient QOL, including sleep
conditions.
Acknowledgements
The Authors would like to thank Yasuko Higuchi
(nutritional therapist) at Hyogo College of Medicine Hospital for
the anthropometry measurement.
Funding
The present study was partly supported by Hyogo
Innovative Challenge, Hyogo College of Medicine, Japan.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
All authors HN, KY, HE, TN, SN and HI were involved
in the conception and design of the study. HN, KY, HE and TN were
involved in data curation. HN was involved in the formal analysis.
SN and HI supervised the study. HN and KY were involved in the
writing of the original draft. HE, SN and HI were involved in the
writing, reviewing and editing of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol rigorously conformed to the 1975
Helsinki Declaration, and approval of ethics was obtained from the
institutional review board in Hyogo College of Medicine Hospital.
An opt out method was employed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iwasa M, Karino Y, Kawaguchi T, Nakanishi
H, Miyaaki H, Shiraki M, Nakajima T, Sawada Y, Yoshiji H, Okita K,
et al: Relationship of muscle cramps to quality of life and sleep
disturbance in patients with chronic liver diseases: A nationwide
study. Liver Int. 38:2309–2316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ghabril M, Jackson M, Gotur R, Weber R,
Orman E, Vuppalanchi R and Chalasani N: Most individuals with
advanced cirrhosis have sleep disturbances, which are associated
with poor quality of life. Clin Gastroenterol Hepatol.
15:1271–1278.e6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Córdoba J, Cabrera J, Lataif L, Penev P,
Zee P and Blei AT: High prevalence of sleep disturbance in
cirrhosis. Hepatology. 27:339–345. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Montagnese S, Middleton B, Mani AR, Skene
DJ and Morgan MY: Sleep and circadian abnormalities in patients
with cirrhosis: Features of delayed sleep phase syndrome? Metab
Brain Dis. 24:427–439. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tordjman S, Chokron S, Delorme R, Charrier
A, Bellissant E, Jaafari N and Fougerou C: Melatonin: Pharmacology,
functions and therapeutic benefits. Curr Neuropharmacol.
15:434–443. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shigiyama F, Kumashiro N, Tsuneoka Y,
Igarashi H, Yoshikawa F, Kakehi S, Funato H and Hirose T:
Mechanisms of sleep deprivation-induced hepatic steatosis and
insulin resistance in mice. Am J Physiol Endocrinol Metab.
315:E848–E858. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mollayeva T, Thurairajah P, Burton K,
Mollayeva S, Shapiro CM and Colantonio A: The Pittsburgh sleep
quality index as a screening tool for sleep dysfunction in clinical
and non-clinical samples: A systematic review and meta-analysis.
Sleep Med Rev. 25:52–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Buysse DJ, Reynolds CF III, Monk TH,
Berman SR and Kupfer DJ: The Pittsburgh Sleep Quality Index: A new
instrument for psychiatric practice and research. Psychiatry Res.
28:193–213. 1989.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Doi Y, Minowa M, Uchiyama M, Okawa M, Kim
K, Shibui K and Kamei Y: Psychometric assessment of subjective
sleep quality using the Japanese version of the Pittsburgh Sleep
Quality Index (PSQI-J) in psychiatric disordered and control
subjects. Psychiatry Res. 97:165–172. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li F, Li Y, Duan Y, Hu CAA, Tang Y and Yin
Y: Myokines and adipokines: Involvement in the crosstalk between
skeletal muscle and adipose tissue. Cytokine Growth Factor Rev.
33:73–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie
Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA,
et al: Writing Group for the European Working Group on Sarcopenia
in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2:
Sarcopenia: Revised European consensus on definition and diagnosis.
Age Ageing. 48:16–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan Society of Hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sinclair M, Gow PJ, Grossmann M and Angus
PW: Review article: Sarcopenia in cirrhosis - aetiology,
implications and potential therapeutic interventions. Aliment
Pharmacol Ther. 43:765–777. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nishikawa H, Enomoto H, Ishii A, Iwata Y,
Miyamoto Y, Ishii N, Yuri Y, Hasegawa K, Nakano C, Nishimura T, et
al: Elevated serum myostatin level is associated with worse
survival in patients with liver cirrhosis. J Cachexia Sarcopenia
Muscle. 8:915–925. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dasarathy S: Myostatin and beyond in
cirrhosis: All roads lead to sarcopenia. J Cachexia Sarcopenia
Muscle. 8:864–869. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hsu CS and Kao JH: Sarcopenia and chronic
liver diseases. Expert Rev Gastroenterol Hepatol. 12:1229–1244.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nishikawa H, Enomoto H, Nishiguchi S and
Iijima H: Liver cirrhosis and sarcopenia from the viewpoint of
dysbiosis. Int J Mol Sci. 21(5254)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bunchorntavakul C and Reddy KR: Review
article: Malnutrition/sarcopenia and frailty in patients with
cirrhosis. Aliment Pharmacol Ther. 51:64–77. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nishikawa H, Enomoto H, Yoh K, Iwata Y,
Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, Takata R, et
al: Effect of sarcopenia on sleep disturbance in patients with
chronic liver diseases. J Clin Med. 8(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ida S, Kaneko R, Nagata H, Noguchi Y,
Araki Y, Nakai M, Ito S, Ishihara Y, Imataka K and Murata K:
Association between sarcopenia and sleep disorder in older patients
with diabetes. Geriatr Gerontol Int. 19:399–403. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tan X, Titova OE, Lindberg E, Elmståhl S,
Lind L, Schiöth HB and Benedict C: Association between
self-reported sleep duration and body composition in middle-aged
and older adults. J Clin Sleep Med. 15:431–435. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Piovezan RD, Abucham J, Dos Santos RV,
Mello MT, Tufik S and Poyares D: The impact of sleep on age-related
sarcopenia: Possible connections and clinical implications. Ageing
Res Rev. 23:210–220. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fukui H, Saito H, Ueno Y, Uto H, Obara K,
Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M, et al:
Evidence-based clinical practice guidelines for liver cirrhosis
2015. J Gastroenterol. 51:629–650. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
European Association for the Study of the
Liver: Electronic address: simpleEasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL
Recommendations on Treatment of Hepatitis C 2018. J Hepatol.
69:461–511. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kokudo N, Takemura N, Hasegawa K, Takayama
T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, et al:
Clinical practice guidelines for hepatocellular carcinoma: The
Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019
update. Hepatol Res. 49:1109–1113. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Drafting Committee for Hepatitis
Management Guidelines: the Japan Society of Hepatology. Japan
Society of Hepatology Guidelines for the Management of Hepatitis B
Virus Infection: 2019 update. Hepatol Res. 50:892–923.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nishikawa H, Enomoto H, Yoh K, Iwata Y,
Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, Takata R, et
al: Significant Correlation Between Grip Strength and m2bpgi in
Patients with Chronic Liver Diseases. J Clin Med.
8(1359)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yoh K, Nishikawa H, Enomoto H, Iwata Y,
Ikeda N, Aizawa N, Nishimura T, Iijima H and Nishiguchi S: Grip
strength: A useful marker for composite hepatic events in patients
with chronic liver diseases. Diagnostics (Basel).
10(238)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hanai T, Shiraki M, Imai K, Suetsugu A,
Takai K, Moriwaki H and Shimizu M: Reduced handgrip strength is
predictive of poor survival among patients with liver cirrhosis: A
sex-stratified analysis. Hepatol Res. 49:1414–1426. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dattilo M, Antunes HK, Medeiros A, Mônico
Neto M, Souza HS, Tufik S and de Mello MT: Sleep and muscle
recovery: Endocrinological and molecular basis for a new and
promising hypothesis. Med Hypotheses. 77:220–222. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Flood A, Chung A, Parker H, Kearns V and
O'Sullivan TA: The use of hand grip strength as a predictor of
nutrition status in hospital patients. Clin Nutr. 33:106–114.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Samanta J, Dhiman RK, Khatri A, Thumburu
KK, Grover S, Duseja A and Chawla Y: Correlation between degree and
quality of sleep disturbance and the level of neuropsychiatric
impairment in patients with liver cirrhosis. Metab Brain Dis.
28:249–259. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Manthravadi S, Paleti S and Pandya P:
Impact of sustained viral response postcurative therapy of
hepatitis C-related hepatocellular carcinoma: A systematic review
and meta-analysis. Int J Cancer. 140:1042–1049. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Parikh MP, Gupta NM and McCullough AJ:
Obstructive sleep apnea and the liver. Clin Liver Dis. 23:363–382.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Weaver MD, Vetter C, Rajaratnam SMW,
O'Brien CS, Qadri S, Benca RM, Rogers AE, Leary EB, Walsh JK,
Czeisler CA, et al: Sleep disorders, depression and anxiety are
associated with adverse safety outcomes in healthcare workers: A
prospective cohort study. J Sleep Res. 27(e12722)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Philip P, Taillard J and Micoulaud-Franchi
JA: Sleep restriction, sleep hygiene, and driving safety: The
importance of situational sleepiness. Sleep Med Clin. 14:407–412.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Blanco JR, Barrio I, Ramalle-Gómara E,
Beltran MI, Ibarra V, Metola L, Sanz M, Oteo JA, Melús E and Antón
L: Gender differences for frailty in HIV-infected patients on
stable antiretroviral therapy and with an undetectable viral load.
PLoS One. 14(e0215764)2019.PubMed/NCBI View Article : Google Scholar
|