Introduction
Ulcerative colitis (UC) is a chronic inflammatory
bowel disease characterized by episodes of exacerbation and periods
remission (1). In this context,
the evaluation of disease severity is of importance for selecting
the suitable treatment. Therapeutic goals that focus on clinical
remission have failed to modify the natural course of UC (2). Therefore, the therapeutic goal of UC
has evolved beyond the control of symptoms towards the tight
control of intestinal inflammation (3). Over the past years, mucosal healing
(MH) has emerged as a major therapeutic goal for patients with UC,
as MH is associated with better outcomes for patients with
inflammatory bowel disease. Patients who achieve MH have been shown
to have a lower rate of relapse and a reduced risk of colectomy and
hospitalization (4-6).
However, the definition of MH in patients with UC has yet to be
formally established. An international organization of inflammatory
bowel disease task defines MH as the absence of friability, blood,
erosions, or ulcers in the colonic mucosa (7). Since the study by Colombel et
al (8), MH has been defined as
a Mayo endoscopy subscore (MES, 0/1), regardless of histological
findings. However, this definition of MH is associated with mild
friability and erythema in the colonic mucosa (9). Erythema and mild friability indicate
an inflammatory condition in the colonic mucosa. Moreover, some
studies have demonstrated that the relapse rate of patients who
achieved complete MH (MES=0) was lower than that of patients who
achieved MH (MES, 1) (10,11). It is a desired therapeutic endpoint
for patients with UC to achieve complete MH rather than MH.
Colonoscopy is considered the gold standard for the assessment of
mucosal inflammation, which is reliable and accurate (12). However, it is an invasive,
expensive and time-consuming procedure. In this regard, a reliable,
noninvasive biomarker to predict complete MH is of utmost
importance. Fecal markers for the status of intestinal mucosa have
been evaluated in some studies and have been shown to correlate
well with endoscopic activity (13,14).
The common fecal markers include fecal calprotectin (FC) and fecal
immunochemical test (FIT). FIT is a surrogate marker for detecting
stool hemoglobin derived from blood loss in mucosal ulceration. In
addition, the predictive utility of FIT has been evaluated in some
studies (15-18).
FC, which has been found in the cytosol of macrophages and
neutrophils, is a calcium and zinc binding protein of the S-100
protein family. It is noteworthy that FC is resistant to
degradation and stable. The amount of FC is proportional to the
amount of neutrophil migration into the gut lumen and can be used
as a sensitive biomarker of intestinal inflammation (19).
Although the utility of FC in UC has been evaluated
in some studies, the accuracy of FC for predicting complete MH have
yet to be clearly demonstrated (18,20-25),
at least to the best of our knowledge. The aim of the present study
was to evaluate the overall diagnostic accuracy of FC for
predicting complete MH in patients with UC.
Materials and methods
Literature search
The PRISMA guidelines for systematic reviews were
strictly followed. A systematic search was performed of the
databases, including PubMed and EMBASE for relevant studies from
1992 to October, 2020 that evaluated MH in UC by FC. Both medical
subject heading (MeSH) terms and free words were used. Suitable
search terms were used as follows: ‘inflammatory bowel disease’ OR
‘IBD’ OR ‘Crohn's enteritis’ OR ‘Crohn's disease’ OR ‘ulcerative
colitis’ OR ‘colitis’ OR ‘enteritis’ AND ‘fecal calprotectin’ OR
‘calprotectin’. The language was limited to English. Reviews and
references of related literature were searched manually.
Study selection
Articles were first screened by 2 independent
reviewers (W.P. and Z.C.) based on the title and abstract. The full
text of an eligible study was then assessed independently.
Disagreements were resolved by discussion. Studies were eligible if
they met the following inclusion criteria: i) All the patients
included had an established diagnosis of UC according to endoscopic
and histologic assessments; ii) the study evaluated FC for
predicting complete MH in patients with UC; iii) endoscopic
activity was evaluated by the MES; iv) colonoscopy was considered
the gold standard for the assessment of mucosal inflammation; and
v) the studies contained appropriate data to calculate
true-positive, false-positive, true-negative and false-negative
results.
Data extraction and quality
assessment
The 2 investigators, J.J. and Z.C., extracted the
relevant data independently. The data extracted from the articles
included the authors, country, the publication year, age, patient
characteristics, the criteria and the FC features (method and
cut-off). The true-positive, false-positive, false-negative and
true-negative values were calculated for each included study.
The methodological quality of the included articles
was assessed by 2 authors (Z.C. and L.L.) independently using the
quality assessment of diagnostic accuracy studies (QUADAS-2) tool
(26). The QUADAS-2 tool comprises
4 domains: Patient selection, reference standard, index test, and
flow and timing. Each domain is assessed in terms of the risk of
bias. This tool consisted of 14 predefined validated questions as
described in Table I.
Disagreements were resolved by discussion with the senior reviewer
(J.J.).
 | Table IQUADAS Results for assessment of
included studies |
Table I
QUADAS Results for assessment of
included studies
| |
Quality
assessment using the QUADAS tool |
|---|
| Author/(Refs.),
year | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Item 12 | Item 13 | Item 14 |
|---|
| Hiraoka et
al (21), 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes |
| Takashima et
al (18), 2015 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes |
| Mak et al
(24), 2018 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Lobatón et
al (22), 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Kristensen et
al (20), 2015 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes |
| Theede et al
(23), 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Ryu et al
(25), 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Unclear | Unclear | Yes | Yes | Yes |
Data synthesis and statistical
analysis
Standard methods were used in the current
meta-analysis, as recommended in the ‘Cochrane Handbook for
Systematic Reviews of Diagnostic Test Accuracy’ (https://methods.cochrane.org/sdt/handbook-dta-reviews).
Sensitivity, specificity, the positive likelihood ratio (PLR), the
negative likelihood ratio (NLR) and the diagnostic odds ratio
(DOR), were calculated for each study, respectively. For the data
analysis, summary receiver operating characteristic (SROC) curves
and average operating points were estimated with each commonly
applied cut-off value. An SROC curve with 95% confidence region and
95% prediction region was performed to examine the interaction
between sensitivity and specificity. DOR and the area under the
SROC curve were calculated to evaluate the diagnostic performance
of FC for complete mucosal healing in patients with UC. Area under
the curve of 0.5 indicates a completely uninformative test and 1 a
perfect test. Pooled sensitivity, specificity and their 95%
confidence intervals (CIs) were calculated using a random-effects
model at each threshold. The heterogeneity was evaluated by a
Chi-squared test or Q-statistic and Higgins I-squared statistic
(I2). A P-value <0.1 was considered statistically
significant heterogeneity for the Chi-squared or Q-statistics. The
percentage of I2 represented the degree of
heterogeneity. I2 percentages of 25, 50 and 75%
indicated a low, moderate and high degree of heterogeneity,
respectively. Potential sources of heterogeneity investigated were
age, sample size, race and study type. Heterogeneity was evaluated
by including all potential covariates into a regression model.
Publication bias was assessed using Deeks' test. P<0.05 was
considered to indicate statistically significant publication bias.
Statistical analysis was performed on META-DISC (version 1.4 for
Windows), REVIEW MANAGER (version 5.3) and STATA (version 15).
Results
Study characteristics
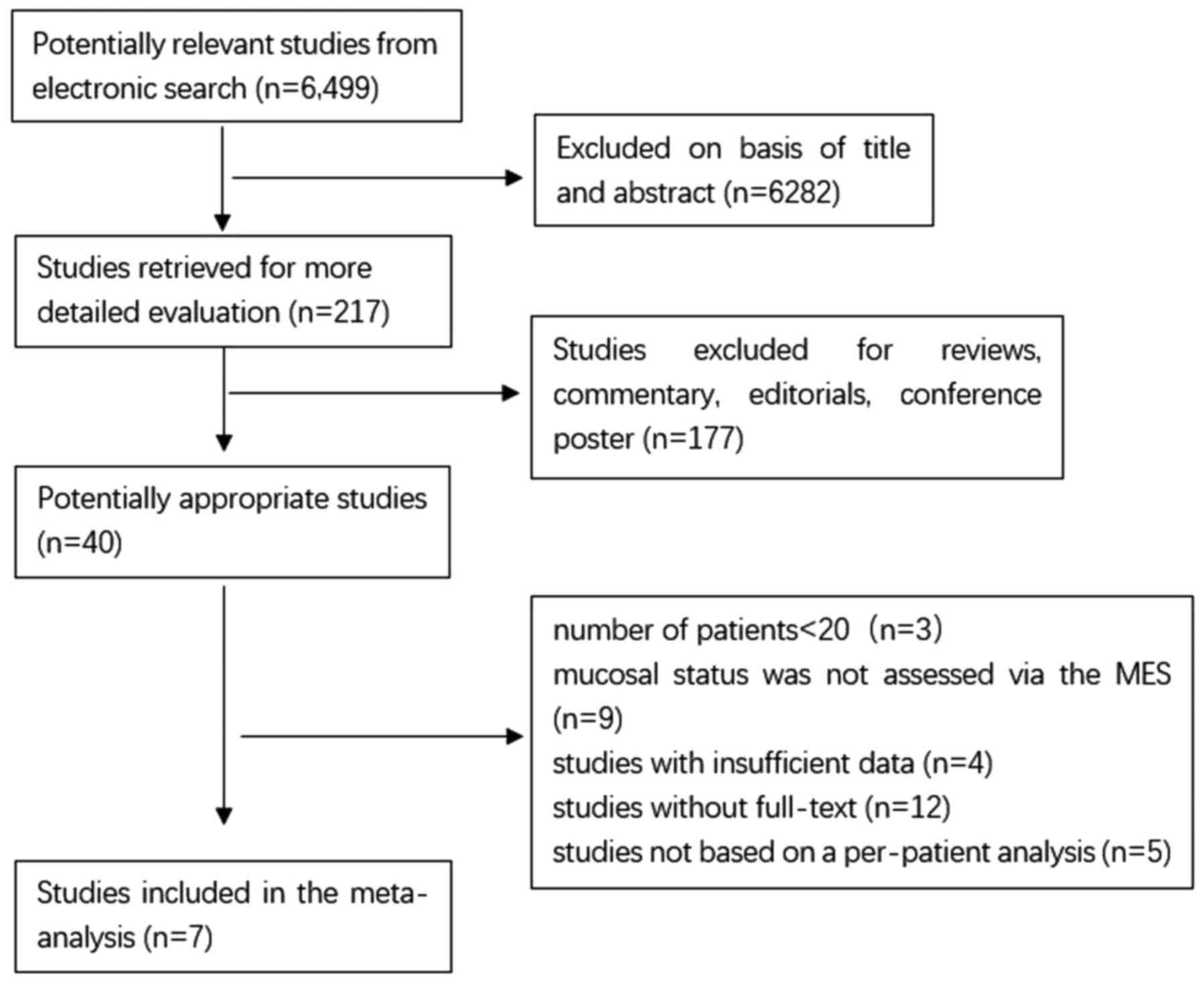
As shown in Fig. 1,
6,499 publications are available after the initial search. After
reading the titles and abstracts and reviewing the full texts, 7
publications, including 820 patients with UC were included in the
analysis. The clinical characteristics of the included studies are
listed in Table II. All studies
enrolled patients diagnosed with UC. In total, 3 of the studies
were conducted in Europe [1 study in Spain (22), 1 study in Denmark (23) and 1 study in Norway (20)]. In addition, 3 of the studies were
conducted in Asia [1 study in Korea (25) and 2 in Japan (18,21)]. Furthermore, 1 study was conducted
in the USA (24). In the USA or
Europe, FC is widely used in monitoring the disease activity and MH
in UC. The gold standard of the included studies was based on
endoscopy. The MES was used to assess the mucosal status of
patients with UC. Complete MH was defined as a MES of 0.
 | Table IICharacteristics of the included
studies. |
Table II
Characteristics of the included
studies.
| Author/(Refs.),
year | Country | Mean age
(years) | Design | Criteria | No. of
patients | Cut-off (µg/g) | TP | FP | FN | TN | SEN | SPE | PPV | NPV |
|---|
| Theede et al
(23), 2015 | Denmark | 36.6 |
Cross-sectional | MES=0 | 120 | 192 | 24 | 11 | 8 | 77 | 0.75 | 0.88 | 0.71 | 0.90 |
| Ryu et al
(25), 2019 | Korea | 47.2 |
Retrospectively | MES=0 | 174 | 170 | 40 | 31 | 11 | 92 | 0.78 | 0.75 | 0.56 | 0.89 |
| Takashima et
al (18), 2015 | Japan | 35.5 | Prospectively | MES=0 | 105 | 200 | 34 | 17 | 10 | 44 | 0.77 | 0.72 | 0.67 | 0.81 |
| Mak et al
(24), 2018 | USA | 29.3 | Prospectively | MES=0 | 61 | 200 | 4 | 11 | 1 | 45 | 0.75 | 0.80 | 0.22 | 0.98 |
| Lobatón et
al (22), 2013 | Spain | 47 | Prospectively | MES=0 | 146 | 160 | 24 | 17 | 12 | 93 | 0.67 | 0.85 | 0.59 | 0.89 |
| Hiraoka et
al (21), 2018 | Japan | 44 | No statement | MES=0 | 152 | 224 | 62 | 16 | 16 | 58 | 0.79 | 0.78 | 0.79 | 0.78 |
| Kristensen et
al (20), 2015 | Norway | 35.5 | Prospectively | MES=0 | 62 | 96 | 16 | 7 | 2 | 37 | 0.91 | 0.83 | 0.93 | 0.79 |
Methodological quality assessment
The methodological quality was assessed by 2 authors
independently, using the QUADAS-2 tool. All trials included in the
present study were of good quality, and the results are presented
in Table I. The scores of the
included studies were over 10 rated with a ‘yes’, indicating that
the included studies were of high quality. The weakness of the
majority of studies was the FC test lacking blinding from the
reference standard. The gold standard for evaluating complete MH
was based on endoscopy in all studies. All studies were deemed to
have a representative spectrum of patients. The clinical
characteristics of the included studies are listed in Table II. There was no evidence of
commercial funding in the included studies.
Diagnostic accuracy meta-analysis
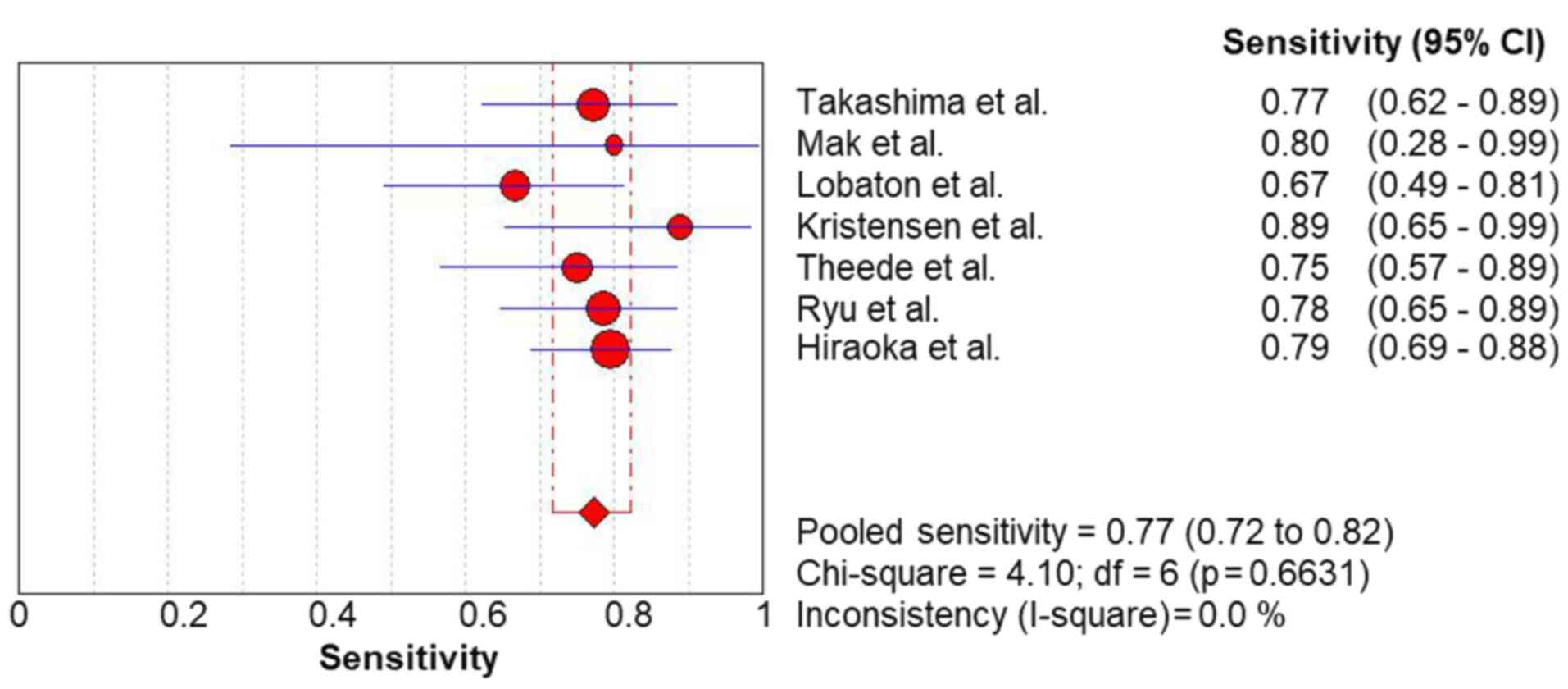
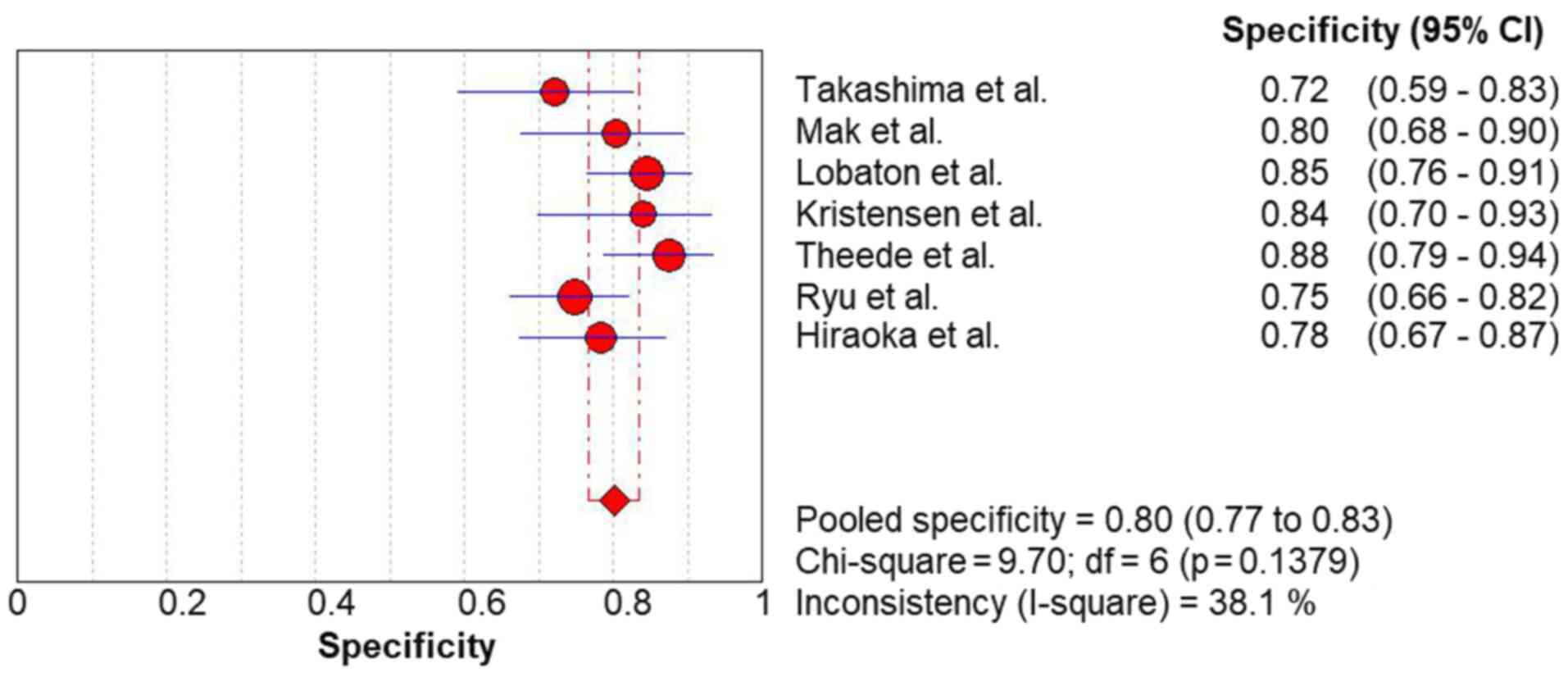
The pooled sensitivity (Fig. 2) and specificity (Fig. 3) values for predicting complete MH
in UC were 0.77 (95% CI, 0.72-0.82) and 0.80 (95% CI, 0.77-0.83),
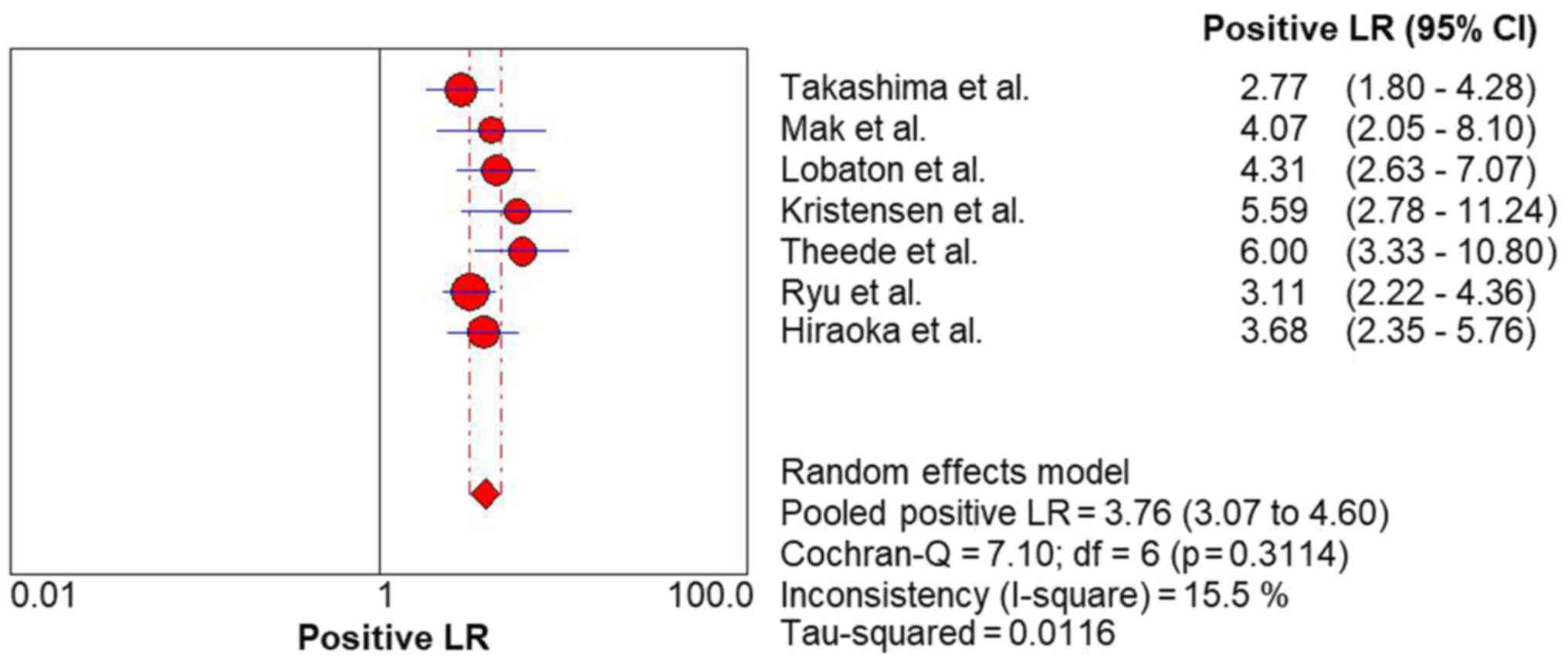
respectively. The FC level had a high rule-in value (PLR, 3.76; 95%
CI, 3.07-4.60) (Fig. 4) and a
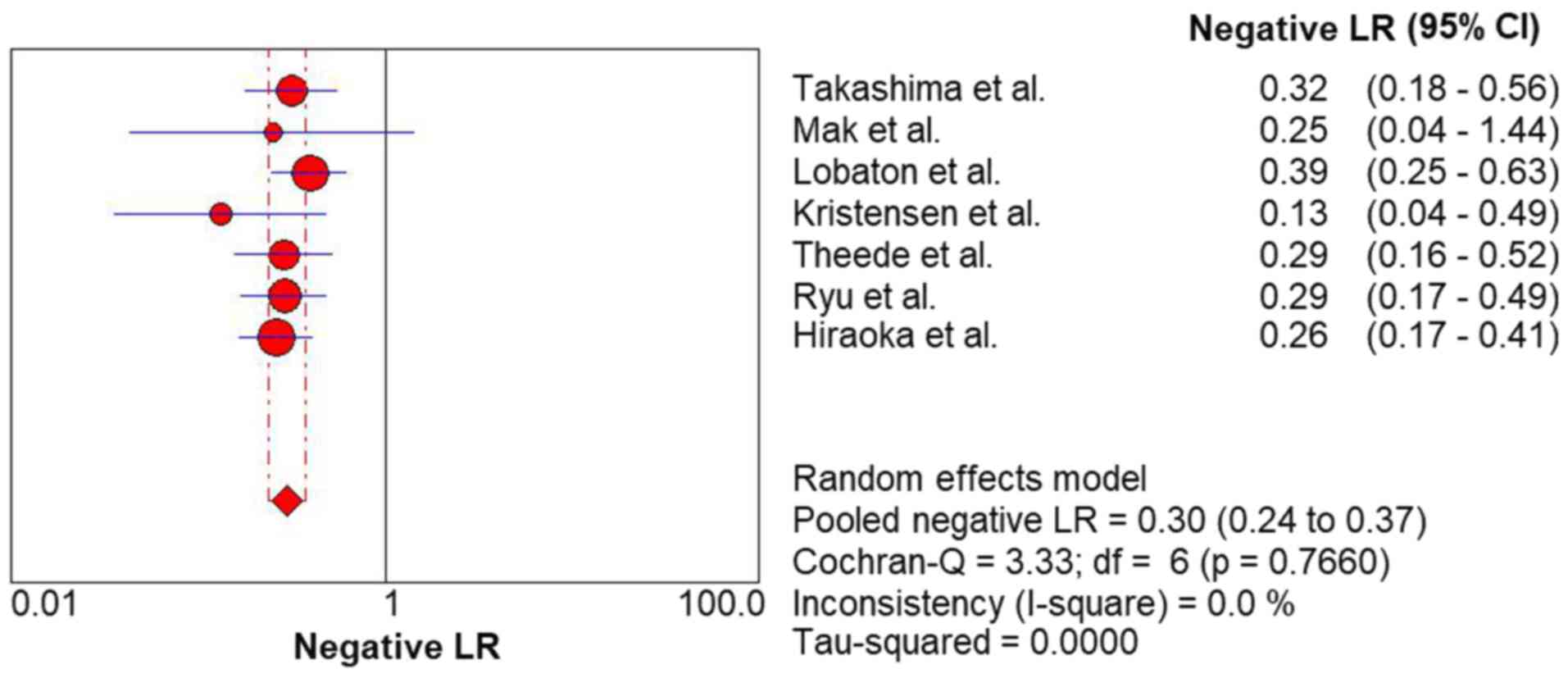
moderate rule-out value (NLR, 0.30; 95% CI, 0.24-0.37) (Fig. 5) for predicting complete MH in UC.
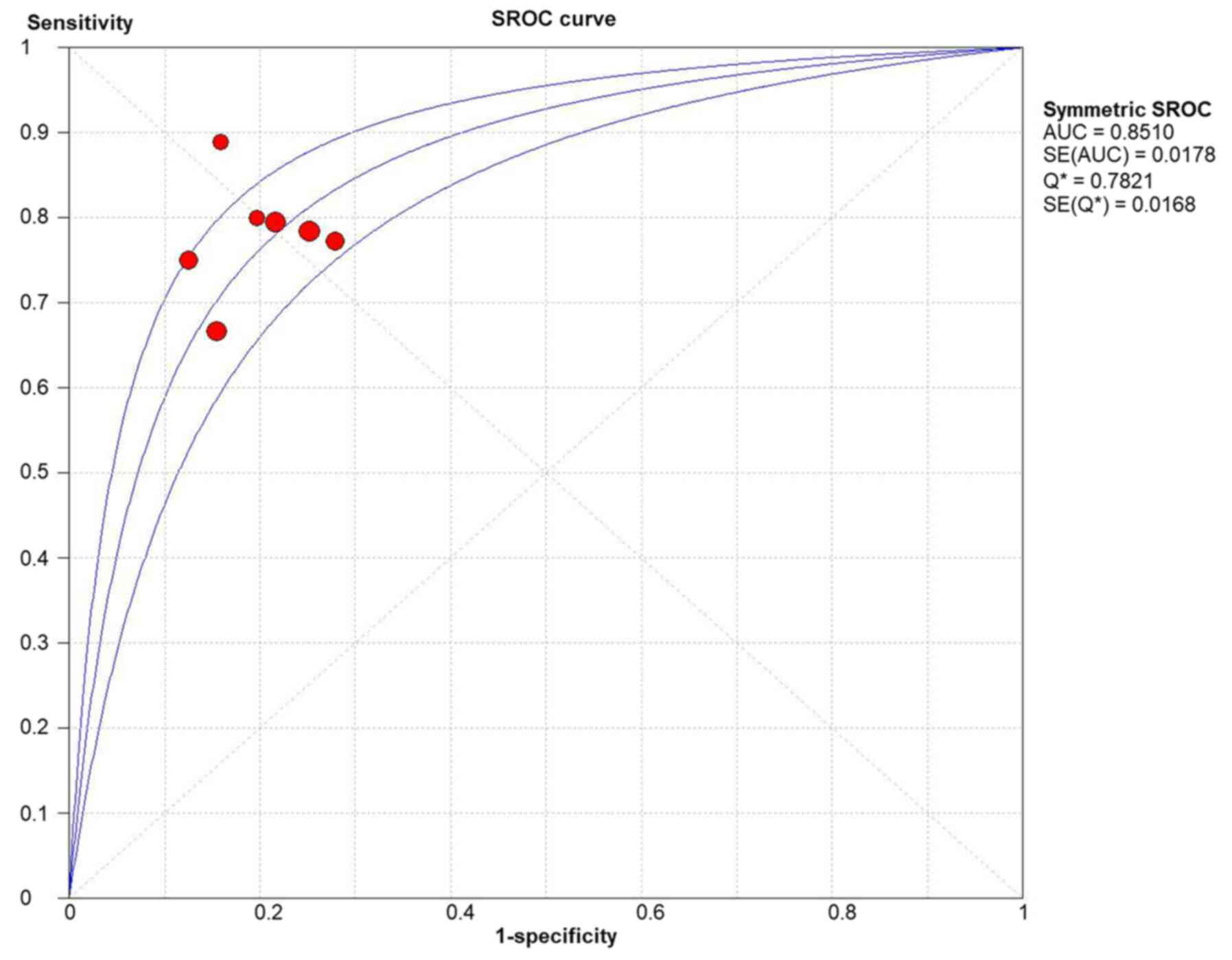
The results of the ROC curve analysis (area under the curve, 0.85;
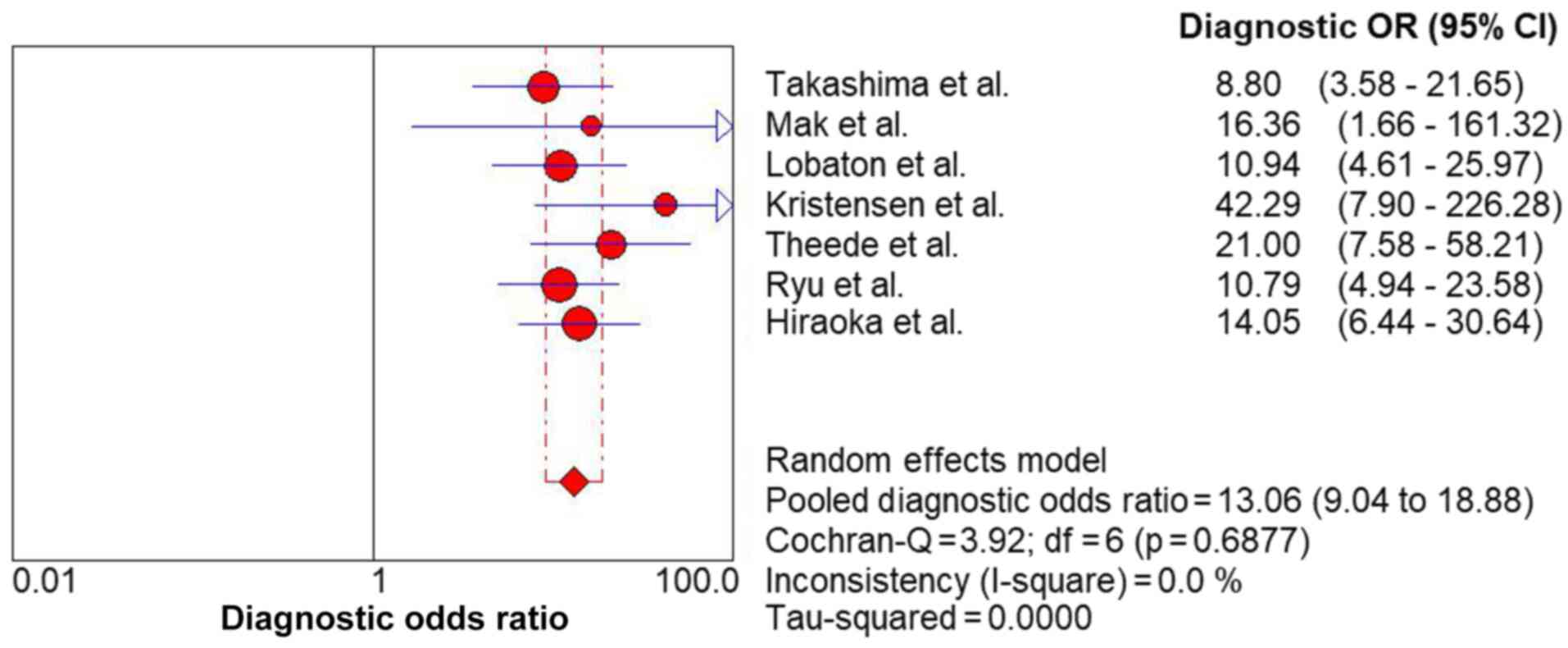
standard error of the mean, 0.02) (Fig. 6) and DOR (13.06; 95% CI,
9.04-18.88) (Fig. 7) also revealed
high discrimination for predicting complete MH in UC.
Heterogeneity and meta-regression
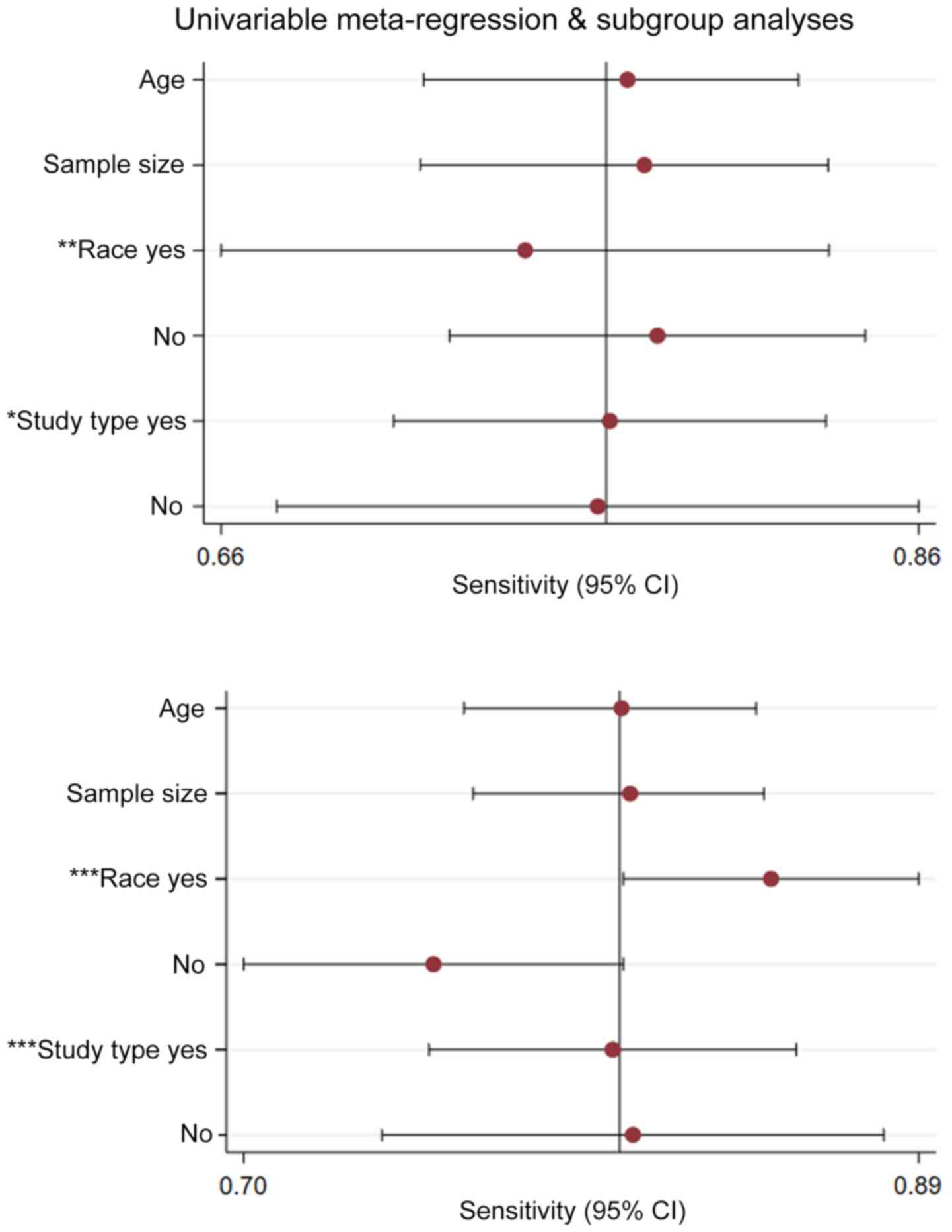
Results of the meta-regression examining the effect
of various parameters on study outcomes are shown in Fig. 8. Among the parameters included in
the meta-regression, study type and race appeared to contribute
significantly to heterogeneity in FC studies (P<0.05).
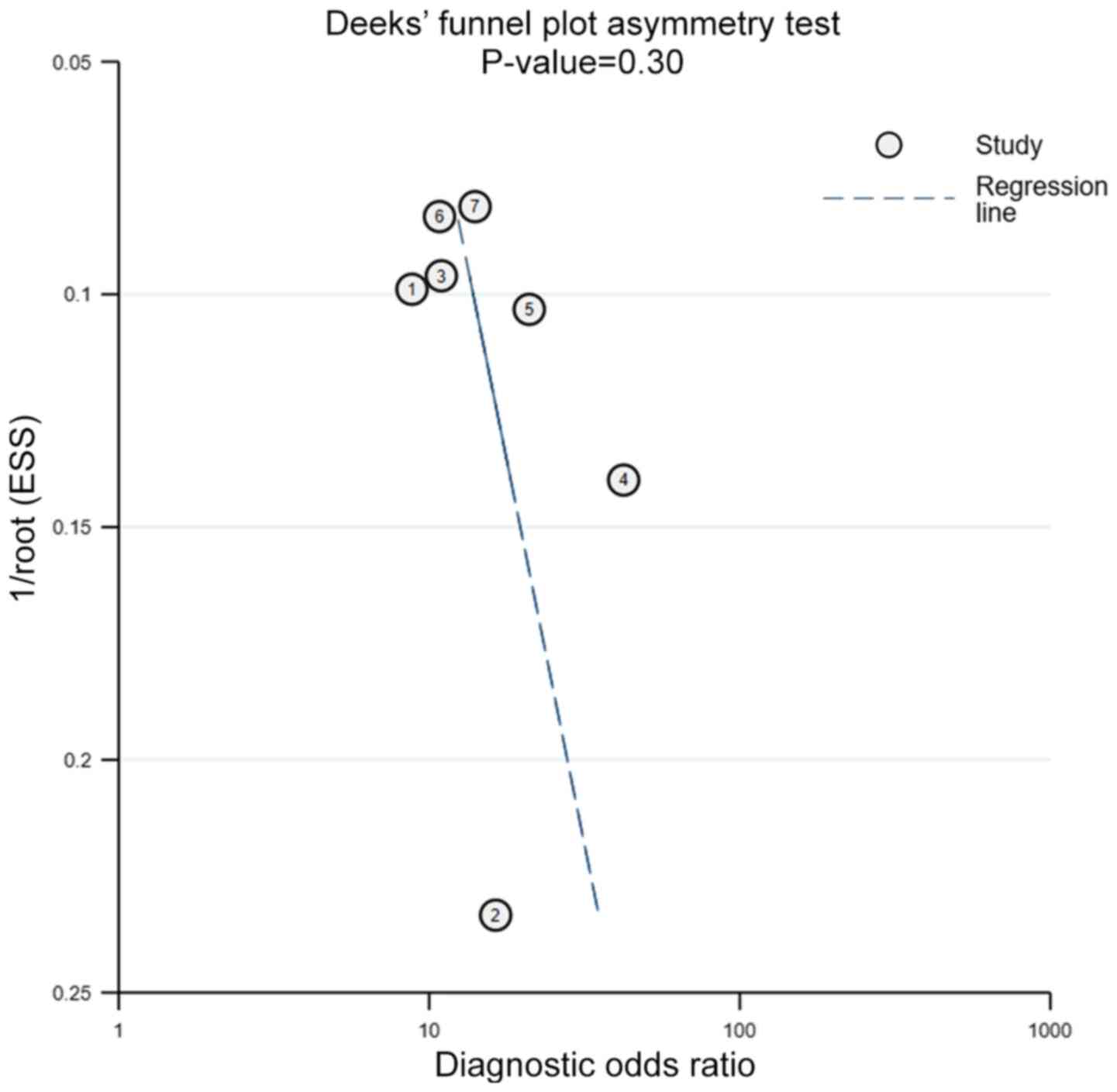
Publication bias
A funnel plot for the analysis of publication bias
was performed to compare the yield of FC levels for assessing
complete MH in UC. The Deeks' test revealed no evidence of
publication bias (P=0.30) (Fig.
9).
Discussion
Over the past years, MH has emerged as a major
therapeutic endpoint for patients with UC. Patients who achieve MH
have been shown to have a lower rate of relapse and a reduced risk
of colectomy and hospitalization (4-6).
Several scoring systems are used for evaluating endoscopic activity
of UC, among which MES is the most common one. MES is easy to use,
and has a demonstrated prognostic value. According to MES, MH is
often defined as a MES of 0/1, which includes mild friability and
erythema (9). Erythema and mild
friability indicate an inflammatory condition in the colonic
mucosa. Moreover, some studies have shown that the relapse rate of
patients who achieved complete MH was lower than that of patients
who achieved MH. Complete MH should be a desired therapeutic goal
for patients with UC to improve long-term outcomes (10,11).
Colonoscopy is considered the gold standard for assessment of
mucosal status. It is an invasive and costly procedure. Therefore,
the identification of a reliable, non-invasive marker to predict MH
is crucial. The common fecal markers in UC include FIT and FC. A
recent meta-analysis revealed that the sensitivity and specificity
of the FIT result for predicting MH in UC were 0.77 and 0.81,
respectively (27). FIT measures
the amount of blood from the damaged bowel mucosa, and it is used
for colorectal cancer screening. The level of FIT is also increased
in colorectal cancer (28).
Therefore, FIT is only used to evaluate IBD mucosal status, rather
than distinguishing between UC and other diseases. FC is a
neutrophil-derived protein of the S-100 protein family. The amount
of FC is proportional to the amount of neutrophil migration into
the gut lumen and can be used as a sensitive biomarker of
intestinal inflammation. The level of FC is related to endoscopic
severity (29), the prediction of
relapse (30) and the prediction
of mucosal healing. An early meta-analysis (31) comprising 1,471 patients with IBD
[UC, 744; Crohn's disease (CD), 727] evaluated the accuracy of FC
for differentiating between patients with active IBD and those in
remission. FC exhibited an AUROC value of 0.89 in distinguishing
between active and inactive IBD, being slightly higher for UC than
for CD. The pooled sensitivity and specificity values were 0.80 and
0.82, respectively. A later meta-analysis (32) comprising 2,102 patients with IBD
(UC, 1,069; CD, 1,033) compared 3 biomarkers (CRP, FC and fecal
lactoferrin) with endoscopic activity as the gold standard. FC
exhibited the highest combined values of pooled sensitivity and
specificity (0.88 and 0.73, respectively). Although the usefulness
of FC has been examined in some studies and meta-analyses in the
past, the accuracy of FC for predicting complete MH (MES, 0) has
yet to be clearly demonstrated, at least to the best of our
knowledge. The aim of the present study was to evaluate the overall
diagnostic accuracy of FC for predicting complete MH (MES, 0) in
patients with UC. In the present study, through a systematic review
and an appropriately performed meta-analysis, FC had a high
sensitivity (0.77; 95% CI, 0.72-0.82) and a high specificity (0.80;
95% CI, 0.77-0.83) for predicting complete MH in UC. The estimated
DOR for the FC in predicting complete MH of UC was 13.06 in the
present study. This indicates that for the FC, the odds for
positivity among subjects with complete MH of patients with UC is
13.06-fold higher than the odds for positivity among subjects
without complete MH in patients with UC. In the present study, the
pooled PLR and NLR were 3.76 and 0.30, respectively, suggesting
that patients with UC with complete MH are 3-fold more likely to
have lower FC levels. If the FC level is above the cut-off value,
the probability of non-complete MH is 30%.
The concentration of FC is usually measured by
quantitative enzyme-linked immunosorbent assay (ELISA). On the one
hand, ELISA is reliable and most frequently used. On the other
hand, ELISA has disadvantages, such as requiring a well-equipped
laboratory, being a costly and time-consuming process (19). Currently, a new
quantitative-point-of-care test (QPOCT) has been developed. The
QPOCT is simple, and able to provide an exact number as ELISA
(33). Some studies have explored
its ability to predict endoscopic activity in patients with UC. Lee
et al found that a cut-off value of 150.5 µg/g for the QPOCT
had a sensitivity of 85.7% and a specificity of 100% for the
prediction of endoscopic remission (19). Lobatón et al demonstrated
that the Spearman's correlation coefficient rank between QPOCT and
ELISA was 0.911 (P<0.001) (33). The good correlation between ELISA
and the QPOCT allows the use of FC more easily in clinical
practice. Recently, a new home test known as IBDOC has been
validated. Weber et al demonstrated that the performance of
the home testing system was comparable to laboratory-based ELISA
method (34). Wei et al
validated that the use of IBDOC to detect FC was feasible. The
IBDOC consists of a stool extraction device called CALEX Value and
an immunochromatographic rapid test (35). The test is simple and the results
rapidly available to the physician. The educational level of
patients with UC may be an obstacle. If the tool is implemented for
the home monitoring of patients, thorough instruction and guidance
are necessary.
The standardized measurement method and cut-off
value of FC test have not been established. The included studies
have used different assay kits, and the cut-off values used for
prediction have varied among these studies (18,20-25).
Thus, a standard measurement method of FC is warranted. Further
large-scale studies are required to determine the optimal cut-off
value of FC for predicting complete MH.
Through excluding studies that did not apply an
endoscopy index as a reference standard, the present meta-analysis
avoided the risk of partial verification bias. However, the present
study has several limitations. Firstly, due to the limitation of
the implementation conditions of FC assay, the majority of the
included studies were conducted in tertiary centers. The majority
of studies only recruited the patients with regular surveillance.
More multicenter large sample trials and strict patient access
systems are required to precisely investigate the diagnostic
accuracy of the FC for predicting complete MH in patients with UC.
Secondly, the standardized measurement method and cut-off value of
FC test have not been established, the non-uniform measurement
methods may be the reason for the heterogeneous data. Thirdly, the
majority of the corresponding authors could not be reached for
further information of the clinical characteristics of the
patients, restricting us from carrying out more analysis to
investigate the source of heterogeneity.
In conclusion, the present study demonstrated that
FC is a reliable non-invasive biomarker for predicting complete MH
in patients with UC. Further designed studies are required to
confirm such benefits and to find the best strategy of FC for
predicting complete MH in patients with UC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Medicine Key Scientific and Technology Project (grant no.
2018258924) and the Zhejiang Medicine Key Scientific and Technology
Project (grant no. 2019RC094).
Availability of data and materials
All data included in the present meta-analysis have
been previously published and referenced.
Authors' contributions
WP, JJ, ZC, LL and CY carried out the literature
search, selection, validity assessment, data abstraction and data
analysis. WP, JJ and ZC wrote the manuscript and incorporated the
comments from other authors and the peer reviewers. ZC, CY, LL, XG,
WP and JJ conceived the study and contributed to data abstraction
and analysis. All authors reviewed and approved the final draft of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
D'Haens G, Sandborn WJ, Feagan BG, Geboes
K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P,
Schölmerich J, et al: A review of activity indices and efficacy end
points for clinical trials of medical therapy in adults with
ulcerative colitis. Gastroenterology. 132:763–786. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Magro F, Rodrigues A, Vieira AI, Portela
F, Cremers I, Cotter J, Correia L, Duarte MA, Tavares ML, Lago P,
et al: Review of the disease course among adult ulcerative colitis
population-based longitudinal cohorts. Inflamm Bowel Dis.
18:573–583. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sandborn WJ, Hanauer S, Van Assche G,
Panés J, Wilson S, Petersson J and Panaccione R: Treating beyond
symptoms with a view to improving patient outcomes in inflammatory
bowel diseases. J Crohn's Colitis. 8:927–935. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ardizzone S, Cassinotti A, Duca P, Mazzali
C, Penati C, Manes G, Marmo R, Massari A, Molteni P, Maconi G, et
al: Mucosal healing predicts late outcomes after the first course
of corticosteroids for newly diagnosed ulcerative colitis. Clin
Gastroenterol Hepatol. 9:483–489.e3. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Frøslie KF, Jahnsen J, Moum BA and Vatn
MH: IBSEN Group. Mucosal healing in inflammatory bowel disease:
results from a Norwegian population-based cohort. Gastroenterology.
133:412–422. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Peyrin-Biroulet L, Ferrante M, Magro F,
Campbell S, Franchimont D, Fidder H, Strid H, Ardizzone S,
Veereman-Wauters G, Chevaux JB, et al: Scientific Committee of the
European Crohn's and Colitis Organization: Results from the 2nd
Scientific Workshop of the ECCO. I: Impact of mucosal healing on
the course of inflammatory bowel disease. J Crohns Colitis.
5:477–483. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Walsh A, Palmer R and Travis S: Mucosal
healing as a target of therapy for colonic inflammatory bowel
disease and methods to score disease activity. Gastrointest Endosc
Clin N Am. 24:367–378. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Colombel JF, Rutgeerts P, Reinisch W,
Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan
BG, et al: Early mucosal healing with infliximab is associated with
improved long-term clinical outcomes in ulcerative colitis.
Gastroenterology. 141:1194–1201. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mazzuoli S, Guglielmi FW, Antonelli E,
Salemme M, Bassotti G and Villanacci V: Definition and evaluation
of mucosal healing in clinical practice. Dig Liver Dis. 45:969–977.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nakarai A, Kato J, Hiraoka S, Inokuchi T,
Takei D, Moritou Y, Akita M, Takahashi S, Hori K, Harada K, et al:
Prognosis of ulcerative colitis differs between patients with
complete and partial mucosal healing, which can be predicted from
the platelet count. World J Gastroenterol. 20:18367–18374.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yokoyama K, Kobayashi K, Mukae M, Sada M
and Koizumi W: Clinical study of the relation between mucosal
healing and long-term outcomes in ulcerative colitis. Gastroenterol
Res Pract. 2013(192794)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oliva S, Di Nardo G, Hassan C, Spada C,
Aloi M, Ferrari F, Redler A, Costamagna G and Cucchiara S:
Second-generation colon capsule endoscopy vs. colonoscopy in
pediatric ulcerative colitis: A pilot study. Endoscopy. 46:485–492.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Judd TA, Day AS, Lemberg DA, Turner D and
Leach ST: Update of fecal markers of inflammation in inflammatory
bowel disease. J Gastroenterol Hepatol. 26:1493–1499.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wright EK, Kamm MA, De Cruz P, Hamilton
AL, Ritchie KJ, Keenan JI, Leach S, Burgess L, Aitchison A, Gorelik
A, et al: Comparison of fecal inflammatory markers in Crohn's
disease. Inflamm Bowel Dis. 22:1086–1094. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kato J, Hiraoka S, Nakarai A, Takashima S,
Inokuchi T and Ichinose M: Fecal immunochemical test as a biomarker
for inflammatory bowel diseases: Can it rival fecal calprotectin?
Intest Res. 14:5–14. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma C, Lumb R, Walker EV, Foshaug RR, Dang
TT, Verma S, Huang VW, Kroeker KI, Wong K, Dieleman LA, et al:
Noninvasive fecal immunochemical testing and fecal calprotectin
predict mucosal healing in inflammatory bowel disease: a
prospective cohort study. Inflamm Bowel Dis. 23:1643–1649.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakarai A, Kato J, Hiraoka S, Kuriyama M,
Akita M, Hirakawa T, Okada H and Yamamoto K: Evaluation of mucosal
healing of ulcerative colitis by a quantitative fecal
immunochemical test. Am J Gastroenterol. 108:83–89. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takashima S, Kato J, Hiraoka S, Nakarai A,
Takei D, Inokuchi T, Sugihara Y, Takahara M, Harada K, Okada H, et
al: Evaluation of mucosal healing in ulcerative colitis by fecal
calprotectin vs. fecal immunochemical test. Am J Gastroenterol.
110:873–880. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee YW, Lee KM, Lee JM, Chung YY, Kim DB,
Kim YJ, Chung WC and Paik CN: The usefulness of fecal calprotectin
in assessing inflammatory bowel disease activity. Korean J Intern
Med (Korean Assoc Intern Med). 34:72–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kristensen V, Klepp P, Cvancarova M,
Røseth A, Skar V and Moum B: Prediction of endoscopic disease
activity in ulcerative colitis by two different assays for fecal
calprotectin. J Crohn's Colitis. 9:164–169. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hiraoka S, Inokuchi T, Takashima S, Takei
D, Sugihara Y, Takahara M, Harada K, Seki Y, Watanabe K and Okada
H: P0824 A novel fecal calprotectin assay using the latex
agglutination turbidimetric immunoassay is useful for ulcerative
colitis patients in evaluation of mucosal healing. United European
Gastroenterol J. 4(A435)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lobatón T, Rodríguez-Moranta F, Lopez A,
Sánchez E, Rodríguez-Alonso L and Guardiola J: A new rapid
quantitative test for fecal calprotectin predicts endoscopic
activity in ulcerative colitis. Inflamm Bowel Dis. 19:1034–1042.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Theede K, Holck S, Ibsen P, Ladelund S,
Nordgaard-Lassen I and Nielsen AM: Level of fecal calprotectin
correlates with endoscopic and histologic inflammation and
identifies patients with mucosal healing in ulcerative colitis.
Clin Gastroenterol Hepatol. 13:1929–1936. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mak WY, Buisson A, Andersen MJ Jr, Lei D,
Pekow J, Cohen RD, Kahn SA, Pereira B and Rubin DT: Fecal
calprotectin in assessing endoscopic and histological remission in
patients with ulcerative colitis. Dig Dis Sci. 63:1294–1301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ryu DG, Kim HW, Park SB, Kang DH, Choi CW,
Kim SJ and Nam HS: Clinical implications of fecal calprotectin and
fecal immunochemical test on mucosal status in patients with
ulcerative colitis. Medicine (Baltimore). 98(e17080)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM: QUADAS-2 Group. QUADAS-2: A revised tool for the quality
assessment of diagnostic accuracy studies. Ann Intern Med.
155:529–536. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dai C, Jiang M, Sun MJ and Cao Q: Fecal
immunochemical test for predicting mucosal healing in ulcerative
colitis patients: A systematic review and meta-analysis. J
Gastroenterol Hepatol. 33:990–997. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Auge JM, Pellise M, Escudero JM, Hernandez
C, Andreu M, Grau J, Buron A, López-Cerón M, Bessa X,
Serradesanferm A, et al: PROCOLON Group: Risk stratification for
advanced colorectal neoplasia according to fecal hemoglobin
concentration in a colorectal cancer screening program.
Gastroenterology. 147:628–636.e1. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
D'Haens G, Ferrante M, Vermeire S, Baert
F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G,
et al: Fecal calprotectin is a surrogate marker for endoscopic
lesions in inflammatory bowel disease. Inflamm Bowel Dis.
18:2218–2224. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
García-Sánchez V, Iglesias-Flores E,
González R, Gisbert JP, Gallardo-Valverde JM, González-Galilea A,
Naranjo-Rodríguez A, de Dios-Vega JF, Muntané J and Gómez-Camacho
F: Does fecal calprotectin predict relapse in patients with Crohn's
disease and ulcerative colitis? J Crohn's Colitis. 4:144–152.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lin JF, Chen JM, Zuo JH, Yu A, Xiao ZJ,
Deng FH, Nie B and Jiang B: Meta-analysis: Fecal calprotectin for
assessment of inflammatory bowel disease activity. Inflamm Bowel
Dis. 20:1407–1415. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mosli MH, Zou G, Garg SK, Feagan SG,
MacDonald JK, Chande N, Sandborn WJ and Feagan BG: C-reactive
protein, fecal calprotectin, and stool lactoferrin for detection of
endoscopic activity in symptomatic inflammatory bowel disease
patients: a systematic review and meta-analysis. Am J
Gastroenterol. 110:802–819; quiz 820. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lobatón T, Rodríguez-Moranta F, Lopez A,
Sánchez E, Rodríguez-Alonso L and Guardiola J: A new rapid
quantitative test for fecal calprotectin predicts endoscopic
activity in ulcerative colitis. Inflamm Bowel Dis. 19:1034–1042.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Weber J, Ueberschlag ME, Prica M, Kräuchi
S, Reinhard C and Jermann T: Validation of a smartphone-based
patient monitoring system measuring Calprotectin as the therapy
follow-up marker. J Crohn's Colitis. 9 (Suppl 1):S212–S213.
2015.
|
|
35
|
Wei SC, Tung CC, Weng MT and Wong JM:
Experience of patients with inflammatory bowel disease in using a
home fecal calprotectin test as an objective reported outcome for
self-monitoring. Intest Res. 16:546–553. 2018.PubMed/NCBI View Article : Google Scholar
|