Introduction
Severe acute respiratory syndrome-coronavirus-2
(SARS-CoV-2) may cause extra-pulmonary illnesses in addition to a
broad spectrum of respiratory tract manifestations, ranging from
asymptomatic infections to severe pneumonia (1). In terms of severity, patients with
additional risk factors, including old age and chronic diseases,
are more susceptible to severe coronavirus disease 2019 (COVID-19)
infection, as compared to the general population (2).
Sickle cell disease (SCD) is an inherited
hemoglobinopathy affecting millions of individuals, particularly
among those of African descent. Patients with SCD may display
several clinical manifestations, the most common symptoms being
haemolytic anaemia, vaso-occlusive crisis (VOC), acute chest
syndrome (ACS), and fever, frequently requiring hospitalization
(3).
SCD can worsen the morbidity and mortality of
SARS-CoV-2 infection (4). On the
other hand, viral infections are significant contributors to
morbidity and mortality in patients with SCD, and COVID-19, along
with its clinical pattern (e.g., severe pneumonia with hypoxia),
may exacerbate or trigger SCD manifestations, including ACS and VOC
(5,6).
The SCD pathophysiology consists of endothelial
dysfunction, chronic inflammation, chronic anaemia,
immunocompromised status and hypercoagulability, all of which have
been recognized as risk factors for poorer outcomes in patients
with COVID-19. Furthermore, both patients with SCD (as well as
those with sickle cell trait) and patients with COVID-19 present
with a hypercoagulable state, being at higher risk of developing
severe complications and organ dysfunctions (7).
There are only a limited number of reports
concerning SARS-CoV-2 infection in patients with SCD. The present
study describes the case of a 33-year-old female patient of African
descent with SCD, who tested positive for COVID-19 infection and
developed a severe painful crisis within 5 days after
admission.
Case report
A 33-year-old female patient of Senegalese descent
was admitted to the Emergency Department of ARNAS Garibaldi
Hospital, Catania, Italy, with symptoms of 3 days of fever (up to a
temperature of 38.5˚C), fatigue and cough. A nasopharyngeal swab
resulted positive for SARS-COV-2 (both the antigenic
(Panbio™-COVID-19 Ag test Abbott) and molecular test results
(RT-qPCR targeting the E gene, RdRP gene and N gene of
SARS-CoV-2).
Her past medical history included SCD, with a
history of recurrent pain crisis (last crisis prior to admission
was reported 6 months earlier) and previous treatment with
hydroxyurea (HU), terminated 1 year prior. The patient did not take
any medication at the time of admission and had received a two-dose
vaccination against SARS-CoV-2 (BNT162b2 vaccine; BioNTech Pfizer,
Inc.). Upon admission, the patient was febrile (temperature, 38˚C);
blood pressure was measured at 130/70 mmHg, the pulse rate at 90
bpm, saturation at 97% in room air and the respiratory rate at 16
breaths/min.
Blood tests revealed mild anaemia with a reduced red
blood cell (RBC) count [haemoglobin (Hb), 9.8 g/dl; RBC,
3.34x106/mm3, normal white blood cell count
(WBC), increased C-reactive protein (CRP) levels (2.38 mg/dl) along
with high erythrocyte sedimentation rate levels (104 mm/h),
increased total bilirubin levels (2.85 mg/dl), aspartate
aminotransferase at 83 U/l and alanine aminotransferase at 56 U/l.
Creatinine levels and liver function marker (coagulation and
albumin) levels were normal (creatinine level, 0.56 mg/dl;
international normalized ratio, 0.98; and albumin, 3.8 g/dl).
Hepatitis B virus (HBV) and hepatitis C virus (HBC) along with
human immunodeficiency virus (HIV) tests were negative. A chest
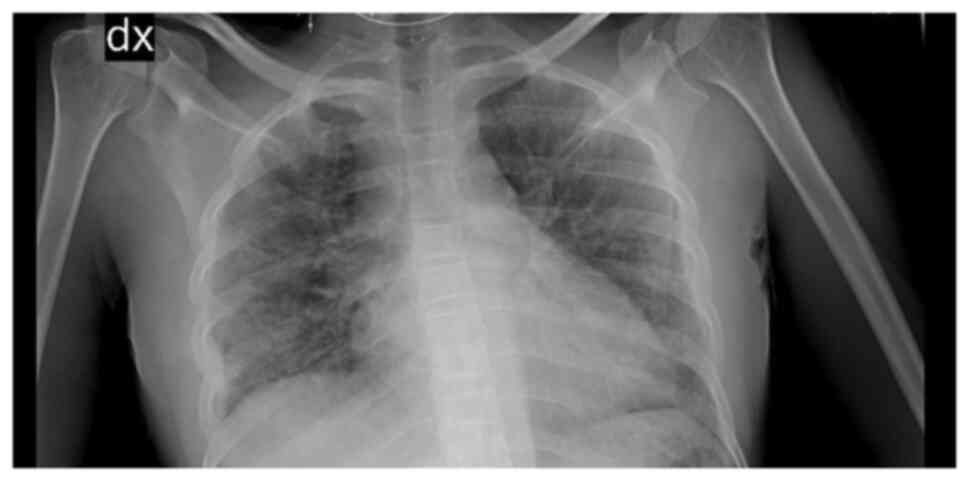
X-ray revealed right medio-basal lung consolidation with a
perivascular interstitial thickening (Fig. 1). Thorax computed tomography (CT)
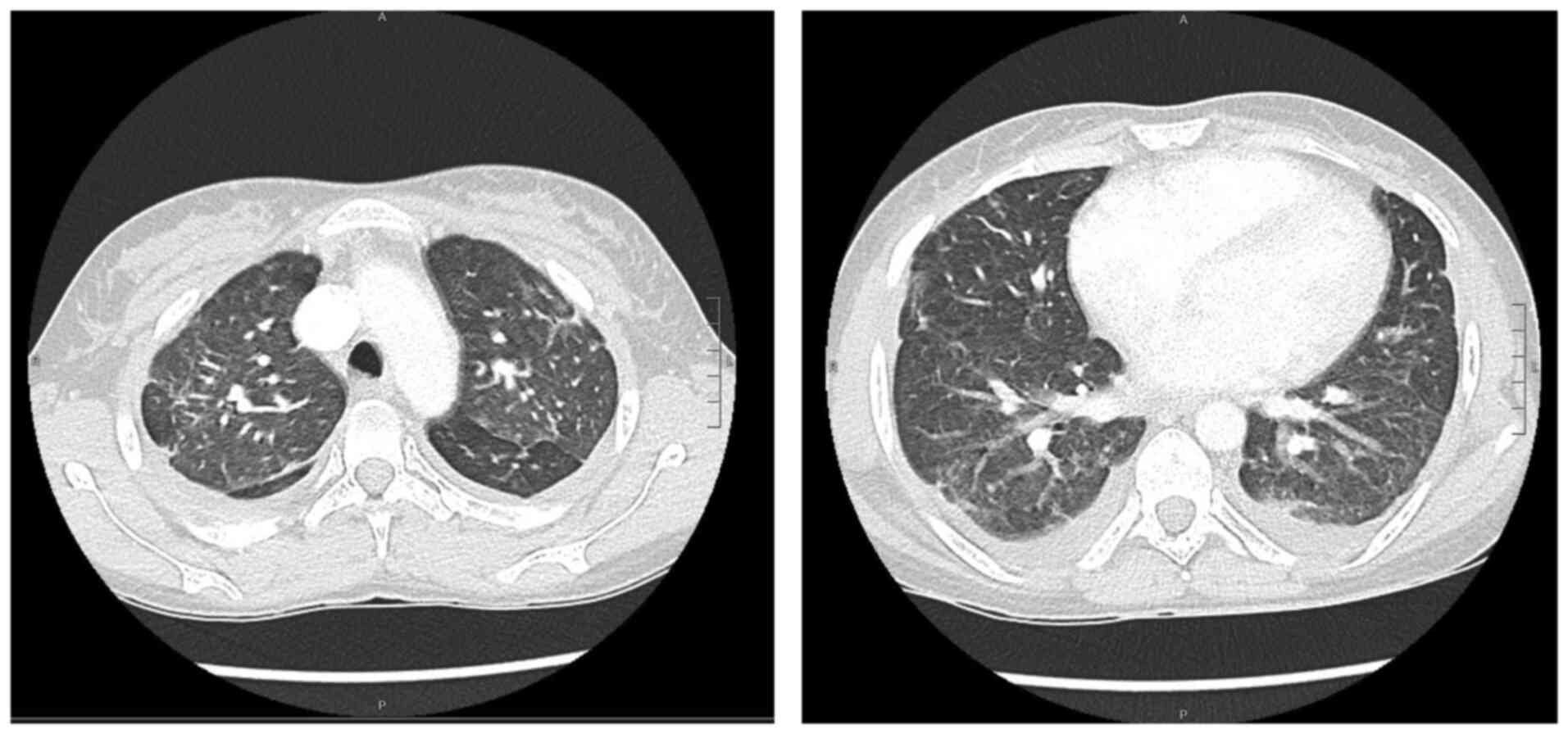
scan images (acquired before and after contrast administration,
using a SOMATOM® Definition Flash scanner; Xenetix, 350
mg/ml; Siemens AG) revealed bilateral ground glass opacities with
interlobular septal thickening, lower lobes consolidations and
bilateral pleural effusion (Fig.
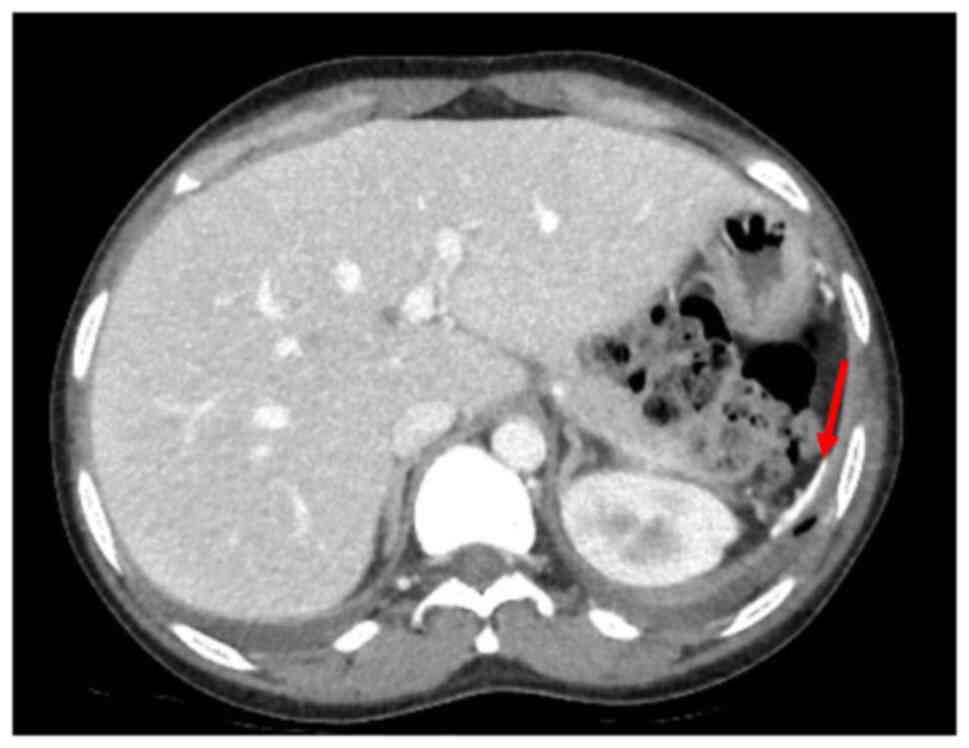
2), whereas an abdomen CT scan revealed homogeneous
hepatomegaly, and a 3 cm densely calcified spleen (Fig. 3).
Treatment with subcutaneous prophylactic enoxaparin
6,000 U/day, intravenous fluids (sodium chloride, 0.5%), and
amoxicillin-clavulanate (Fidia Farmaceutici S.p.A.; 1 g, three
times per day) was commenced. The fever dissipated in 48 h and the
clinical conditions ameliorated.
On the fifth day, the patient became febrile again
(temperature, 38.5˚C) and began to complain of intense pain
involving the chest, abdominal area and lower limbs [visual
analogue scale (VAS) score of 8)] (8); the response to non-steroidal
anti-inflammatory drugs or tramadol administration was poor. The
laboratory tests revealed lower Hb levels (8.8 g/dl) with
increasing WBC (19.800/mm3 75% neutrophils), higher
inflammatory markers levels (CRP, 17.46 mg/dl), procalcitonin at 2
ng/ml, higher lactate dehydrogenase (1,116 mU/ml) and bilirubin
levels (3.5 mg/dl) along with low haptoglobin levels (9 mg/dl).
Haemoglobin S (HbS) levels (high-performance liquid chromatography
technique) were 60.7%. In addition, two blood cultures and a urine
culture demonstrated no bacterial growth.
Due to the elevations in the levels of inflammatory
markers along with procalcitonin levels, in order to rule out
bacterial superinfection, antibiotic therapy was empirically
switched to meropenem (1 g, three times daily) plus teicoplanin
(400 mg/day, after a loading dose of 400 mg two times daily for
three administrations). In addition, an analgesic therapeutics
cocktail, including diclofenac, tramadol and metoclopramide was
administered in continuous infusion. Additionally, with the aid of
a haematologist consultant, suspecting a VOC, manual red cell
exchange transfusion was performed (venesection of 500 ml of blood
and transfusion of two units of packed red blood cells). Blood
exchange procedure was performed without adverse events, achieving
rapid pain relief.
Antibiotics therapy was terminated after 7 days, due
to the resolution of fever along with blood test normalization
(CRP, procalcitonin and WBC) and the patient was discharged after
having been tested negative to SARS-CoV-2 and addressed to the
haematological unit for follow-up.
Discussion
Several studies have highlighted that patients with
chronic comorbidities, such as diabetes, chronic obstructive
pulmonary disease and cardiovascular diseases are at an increased
risk of developing severe COVID-19 infection (9-11).
SCD is a common inherited blood cell disorder, caused by a valine
replacement of a single glutamic acid in the sixth residue of the
β-globin subunit, resulting in HbS. HbS is an abnormal haemoglobin
which, in specific circumstances, including hypoxia and infections,
may polymerize, shifting red blood cells into sickle-shaped cells.
Sickle cells cause microcirculatory occlusion, leading to tissue
ischemia with acute painful episodes and crises, and several
long-term complications (3).
Lee et al (5) reviewed the outcomes of patients with
COVID-19 with haemoglobinopathy, demonstrating that these patients
were more susceptible to severe SARS-CoV-2 infection and disease
compared to the general population, highlighting the need for
greater caution.
A higher fatality rate in patients with SCD with
COVID-19 has also been reported by Minniti et al (12); however, they revealed that the risk
of adverse outcomes in these patients may be reduced by
anticoagulant therapy. As a matter of fact, SARS-CoV-2 infection,
due to the resulting cytokine storm along with the hypercoagulable
state, culminates in an increased risk of thromboembolic events in
patients with SCD (13-15).
In addition, patients with SCD, due to hyposplenism
and vascular compromise, have an impaired immunity, particularly
against encapsulated bacteria (including Streptococcus
pneumoniae and Neisseria meningitidis), resulting in
worse outcomes and often requiring further antibiotic prophylaxis
(3). It has been highlighted in
previous studies that patients with SCD have a higher prevalence
for severe bacterial infections, in comparison with patients
without SCD (16). In addition,
infections may trigger SCD complications, including VOC (6).
VOC pathophysiology is a multifactorial process,
mainly characterized by high plasma viscosity due to a combination
of erythrocytes sickling, thrombosis, haemolysis, tissue damage and
inflammation. Those features may lead to blood vessel occlusion,
causing painful tissue ischemic injuries (6). All VOC characteristics have been also
observed in COVID-19 pathophysiology, resulting in ‘synergistic’
damage.
The association between SCD morbidity and
respiratory infections had been highlighted during the 2009 H1N1
pandemic (17), when those
patients faced both SCD and flu complications, leading to worse
outcomes compared to the general population.
The case series study conducted by Chakravorty et
al (18) explained the
benefits of early hospitalization for patients with SCD with
SARS-CoV-2 infection, even in asymptomatic or mild cases, since
acute complications appear to occur within 1 week following
infection.
There are several different strategies, including
pharmacological and non-pharmacological, for the treatment of VOC
in patients with SCD, depending on pain intensity, duration and
location (18,19). According to Italian guidelines
(20), in case of severe pain (VAS
score of 9-11) it is recommended to administer an initial analgesic
approach, consisting of a continuous intravenous infusion of weak
opioid (including codeine or tramadol) along with nonsteroidal
anti-inflammatory drugs (e.g., ketorolac) plus adjuvants (e.g.,
metoclopramide).
For patients with SCD, blood transfusion aims to
restore Hb levels in patients with acute anaemia (e.g., transient
aplastic anaemia or acute splenic sequestration) and to the rapid
reduction of the percentage of HbS, in order to prevent VOCs
deterioration, due to further sickle cell formation (19).
Transfusion therapy management during acute events
requires the monitoring of both total Hb levels and the percentage
of HbS. One of the goals of blood transfusion therapy is to
mitigate anaemia symptoms by avoiding hyperviscosity, without
exceeding post transfusion a Hb value of 10-11 g/dl. Another goal
is to treat or prevent acute events, including VOCs, decreasing the
percentage of HbS <40% (19).
Exchange transfusions can be performed manually or may be automated
according to Hb levels and possible complications are represented
by hyperviscosity and iron overload (19).
In the present case report study, the patient
developed acute painful VOC after 5 days of admission, probably
triggered by SARS-CoV-2 infection, despite being twice vaccinated,
demonstrating anaemia, leukocytosis and increasing levels of
anti-inflammatory markers and procalcitonin, along with high HbS
percentages. As suggested by guidelines and literature evidence
(19,20), an analgesic cocktail was
administered, along with RBC exchange to achieve prompt pain
control. Moreover, due to the presence of fever along with
increased CRP and procalcitonin levels, antibiotic therapy was
administered, and cultural tests were performed to rule out and
treat bacterial superinfections, whereas viral coinfections,
including HIV, HBV and HCV, were already excluded according to
protocols (21-27).
Previous research has highlighted the risk of delaying appropriate
management during VOCs in patients with SCD (12).
HU is an oral medication with disease-modifying
benefits for the treatment of SCD, preventing the onset and
reducing the frequency of SCD-related complications, reducing the
need for blood transfusions (28).
However, since it requires months to be effective, HU cannot be
used during acute episodes (29).
Indeed, it has been reported by Mucalo et al (4) that the use of HU by patients with SCD
may decrease the risk of pain occurrence during COVID-19 infection.
Therefore, the use of HU as a prophylaxis of SCD complications may
be a strategy with which to avoid analgesic medication and blood
transfusion in patients with SCD with SARS-CoV-2 infection;
however, further studies and additional solid evidence are required
to be collected.
In conclusion, case described in the present study
highlighted the association between SCD-related complications and
SARS-CoV-2 infection, which had already been pointed out by other
authors (17). Patients with SCD
and COVID-19 infection need to be critically evaluated by
clinicians, as a result of the worse outcomes predicted for this
group. Under the suspicion of the VOC, it is suggested that
patients with SCD be managed with analgesic therapy and to be
evaluated using RBC exchange therapy.
Acknowledgements
The authors would like to thank Dr Pietro Leanza,
University of Catania, Catania, Italy for assisting with the
language revisions.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
EC and AM contributed to the conceptualization and
design of the study. MC and MG acquired the patient data. FC, VM
and CM analysed the data. GN, BMC and BC critically interpreted the
data. EC and AM confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The patient signed a consent for the publication of
personal data and radiological imagines.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Esakandari H, Nabi-Afjadi M,
Fakkari-Afjadi J, Farahmandian N, Miresmaeili SM and Bahreini E: A
comprehensive review of COVID-19 characteristics. Biol Proced
Online. 22(19)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui
F, Yee NTS, Liu C, Nerurkar SN, Kai JCY, et al: Epidemiology of
COVID-19: A systematic review and meta-analysis of clinical
characteristics, risk factors, and outcomes. J Med Virol.
93:1449–1458. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kato GJ, Piel FB, Reid CD, Gaston MH,
Ohene-Frempong K, Krishnamurti L, Smith WR, Panepinto JA,
Weatherall DJ, Costa FF and Vichinsky EP: Sickle cell disease. Nat
Rev Dis Primers. 14(18010)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mucalo L, Brandow AM, Dasgupta M, Mason
SF, Simpson PM, Singh A, Taylor BW, Woods KJ, Yusuf FI and
Panepinto JA: Comorbidities are risk factors for hospitalization
and serious COVID-19 illness in children and adults with sickle
cell disease. Blood Adv. 5:2717–2724. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee JX, Chieng WK, Lau SCD and Tan CE:
COVID-19 and Hemoglobinopathies: A systematic review of clinical
presentations, investigations, and outcomes. Front Med (Lausanne).
8(1848)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Booth C, Inusa B and Obaro SK: Infection
in sickle cell disease: A review. Int J Infect Dis. 14:e2–e12.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hoogenboom WS, Alamuri TT, McMahon DM,
Balanchivadze N, Dabak V, Mitchell WB, Morrone KB, Manwani D and
Duong TQ: Clinical outcomes of COVID-19 in patients with sickle
cell disease and sickle cell trait: A critical appraisal of the
literature. Blood Rev: Nov 20, 2021 (Epub ahead of print).
|
|
8
|
McCormack HM, Horne DJ and Sheather S:
Clinical applications of visual analogue scales: A critical review.
Psychol Med. 18:1007–1019. 1988.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sanyaolu A, Okorie C, Marinkovic A,
Patidar R, Younis K, Desai P, Hosein Z, Padda I, Mangat J and Altaf
M: Comorbidity and its impact on patients with COVID-19. SN Compr
Clin Med. 2:1069–1076. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alyammahi SK, Abdin SM, Alhamad DW,
Elgendy SM, Altell AT and Omar HA: The dynamic association between
COVID-19 and chronic disorders: An updated insight into prevalence,
mechanisms and therapeutic modalities. Infect Genet Evol.
87(104647)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang B, Li R, Lu Z and Huang Y: Does
comorbidity increase the risk of patients with COVID-19: Evidence
from meta-analysis. Aging (Albany NY). 12:6049–6057.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Minniti CP, Zaidi AU, Nouraie M, Manwani
D, Crouch GD, Crouch AS, Callaghan MU, Carpenter S, Jacobs C, Han
J, et al: Clinical predictors of poor outcomes in patients with
sickle cell disease and COVID-19 infection. Blood Adv. 5:207–215.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Menapace LA and Thein SL: COVID-19 and
sickle cell disease. Haematologica. 105:2501–2504. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ceccarelli M, Marino A, Cosentino F,
Moscatt V, Celesia BM, Gussio M, Bruno R, Rullo EV, Nunnari G and
Cacopardo BS: Post-infectious ST elevation myocardial infarction
following a COVID-19 infection: A case report. Biomed Rep.
16(10)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Marino A, Pampaloni A, Scuderi D,
Cosentino F, Moscatt V, Ceccarelli M, Gussio M, Celesia BM, Bruno
R, Borraccino S, et al: High-flow nasal cannula oxygenation and
tocilizumab administration in patients critically ill with
COVID-19: A report of three cases and a literature review. World
Acad Sci J. 2(23)2020.
|
|
16
|
Cannas G, Merazga S and Virot E: Sickle
cell disease and infections in high- and low-income countries.
Mediterr J Hematol Infect Dis. 11(e2019042)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Strouse JJ, Reller ME, Bundy DG, Amoako M,
Cancio M, Han RN, Valsamakis A and Casella JF: Severe pandemic H1N1
and seasonal influenza in children and young adults with sickle
cell disease. Blood. 116:3431–3434. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chakravorty S, Padmore-Payne G, Ike F,
Tshibangu V, Graham C, Rees D and Stuart-Smith S: COVID-19 in
patients with sickle cell disease-A case series from a UK tertiary
hospital. Haematologica. 105:2691–2693. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brandow AM, Carroll CP, Creary S,
Edwards-Elliott R, Glassberg J, Hurley RW, Kutlar A, Seisa M,
Stinson J, Strouse JJ, et al: American society of hematology 2020
guidelines for sickle cell disease: Management of acute and chronic
pain. Blood Adv. 4:2656–2701. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Russo G, De Franceschi L, Colombatti R,
Rigano P, Perrotta S, Voi V, Palazzi G, Fidone C, Quota A,
Graziadei G, et al: Current challenges in the management of
patients with sickle cell disease-A report of the Italian
experience. Orphanet J Rare Dis. 14(120)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
El-Sokkary R, Uysal S, Erdem H, Kullar R,
Pekok AU, Amer F, Grgić S, Carevic B, El-Kholy A, Liskova A, et al:
Profiles of multidrug-resistant organisms among patients with
bacteremia in intensive care units: An international ID-IRI survey.
Eur J Clin Microbiol Infect Dis. 40:2323–2334. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Erdem H, Hargreaves S, Ankarali H,
Caskurlu H, Ceviker SA, Bahar-Kacmaz A, Meric-Koc M, Altindis M,
Yildiz-Kirazaldi Y, Kizilates F, et al: Managing adult patients
with infectious diseases in emergency departments: International
ID-IRI study. J Chemother. 33:302–318. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Marino A, Zafarana G, Ceccarelli M,
Cosentino F, Moscatt V, Bruno G, Bruno R, Benanti F, Cacopardo B
and Celesia BM: Immunological and clinical impact of DAA-Mediated
HCV Eradication in a Cohort of HIV/HCV Coinfected Patients:
Monocentric Italian Experience. Diagnostics (Basel).
11(2336)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Celesia BM, Marino A, Borracino S,
Arcadipane AF, Pantò G, Gussio M, Coniglio S, Pennisi A, Cacopardo
B and Panarello G: Successful extracorporeal membrane oxygenation
treatment in an acquired immune deficiency syndrome (AIDS) patient
with acute respiratory distress syndrome (ARDS) complicating
pneumocystis jirovecii pneumonia: A challenging case. Am J Case
Rep. 21(e919570)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ceccarelli M, Venanzi Rullo E, Marino MA,
D'Aleo F, Pellicanò GF, D'Andrea F, Marino A, Cacopardo B, Celesia
BM, La Rocca G, et al: Non-AIDS defining cancers: A comprehensive
update on diagnosis and management. Eur Rev Med Pharmacol Sci.
24:3849–3875. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Celesia BM, Marino A, Del Vecchio RF,
Bruno R, Palermo F, Gussio M, Nunnari G and Cacopardo B: Is it safe
and cost saving to defer the CD4+ cell count monitoring in stable
patients on art with more than 350 or 500 cells/µl? Mediterr J
Hematol Infect Dis. 11(e2019063)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Marino A, Cosentino F, Ceccarelli M,
Moscatt V, Pampaloni A, Scuderi D, D'Andrea F, Rullo EV, Nunnari G,
Benanti F, et al: Entecavir resistance in a patient with
treatment-naïve HBV: A case report. Mol Clin Oncol.
14(113)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McGann PT and Ware RE: Hydroxyurea therapy
for sickle cell anemia. Expert Opin Drug Saf. 14:1749–1758.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yasara N, Premawardhena A and Mettananda
S: A comprehensive review of hydroxyurea for β-haemoglobinopathies:
The role revisited during COVID-19 pandemic Orphanet J Rare. Dis.
16(114)2021.PubMed/NCBI View Article : Google Scholar
|