Introduction
Stenotrophomonas maltophilia (S.
maltophilia) is a glucose non-fermenting, aerobic,
Gram-negative bacillus that is ubiquitously found in aquatic
environments and soil (1).
Although it is considered a low virulence pathogen, it is
increasingly recognised as a main cause of nosocomial infections
(2,3).
S. maltophilia, due to his high virulence
determinants, including biofilm production, can cause various type
of infections, such as skin manifestations and bacteraemia
(1), mainly those involving the
respiratory tract (4). Major
concerns particularly involve vulnerable hosts, such as patients in
intensive care units (ICUs) or patients with cystic fibrosis,
haematological malignancies, and other significant underlying
illnesses (5,6). Moreover, its intrinsic multidrug
resistance to several classes of antibiotics poses a major clinical
and therapeutic challenge (7).
Historically, trimethoprim/sulfamethoxazole
(TMP-SMX) has represented the drug of choice used in the treatment
of S. maltophilia infections, playing a key role in
antibiotic management. However, the emergence of TMP-SMX resistance
has already been reported. Other treatment options may include
minocycline, tigecycline, levofloxacin, cefiderocol and
ceftazidime/avibactam plus aztreonam (8).
The differentiation between S. maltophilia
infections and colonisation remains challenging, particularly in
patients with ventilator devices (1,9).
Over the past years, researchers have assessed S.
maltophilia infections in ICUs, including bloodstream
infections, skin and soft tissues infections, urinary tract
infections and ventilator-associated pneumonia (VAP), reporting
high mortality rates (5,10). Despite its undeniable clinical
impact, solid data on S. maltophilia are limited compared
with other Gram-negative bacteria (11). Moreover, S. maltophilia
infections are often combined with other bacteria, rendering the
clinical picture even more complex (1,11).
The present study, describes the case of a patient
with VAP caused by S. maltophilia infection, highlighting
the challenges in the management of this condition and the
importance of prompt recognition and aggressive treatment.
Furthermore, the present study highlights the need to consider
alternative, effective antimicrobial agent in patients with VAP who
are not responsive to broad-spectrum β-lactam antibiotics.
Case report
A 66-year-old male was admitted to the Emergency
Department of ARNAS Garibaldi Hospital due to the onset of seizures
along with a profoundly altered mental status and fever (maximum
temperature, 38˚C). His medical history included type 2 diabetes
mellitus, hypertension, benign prostatic hyperplasia,
dyslipidaemia, and a history of smoking. The patient was taking
ertugliflozin, ramipril, alfuzosin and atorvastatin.
Upon admission, the patient was transferred to the
ICU and was intubated due to a severely impaired consciousness
(Glasgow Coma Scale 8). A brain computed tomography (CT) scan did
not reveal any abnormalities.
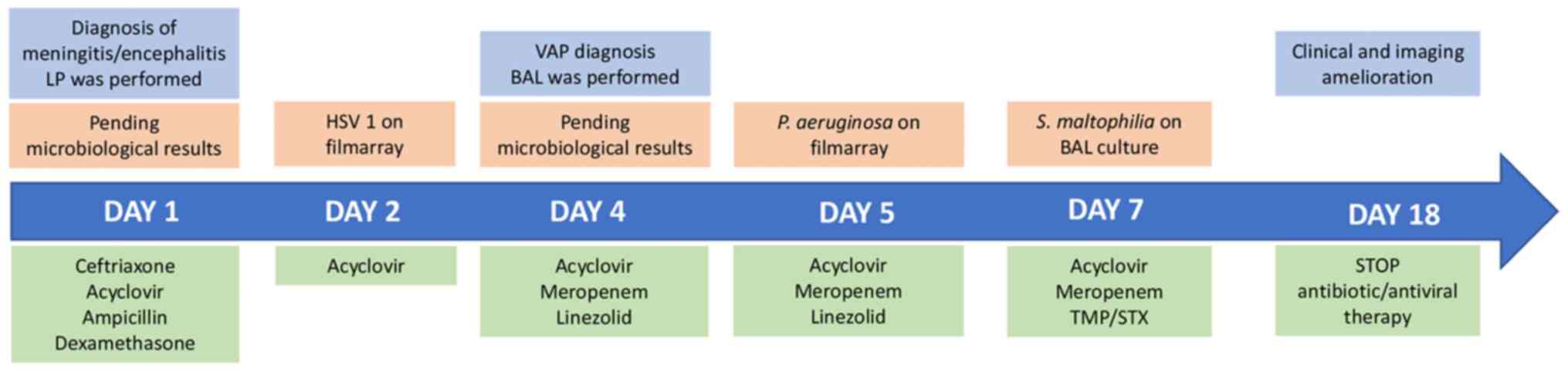
Lumbar puncture (LP) was performed and empirical
antibiotic treatment with intravenous ceftriaxone, acyclovir,
ampicillin, and dexamethasone was commenced. The examination of
cerebrospinal fluid (CSF) revealed lymphocytic pleocytosis, normal
glucose levels and a higher protein concentration. The analysis of
CSF using the BIOFIRE® FILMARRAY®
Meningitis/Encephalitis (ME) Panel (BioMérieux) yielded positive
results for herpes simplex virus 1.
Therapy was continued only with acyclovir at 10
mg/kg three times. The CSF culture tested negative. His chest X-ray
was negative (Fig. 1). The blood
test results of the patient are presented in Table I.
 | Table ILaboratory findings at the time of
admission, at the time of VAP diagnosis and following
treatment. |
Table I
Laboratory findings at the time of
admission, at the time of VAP diagnosis and following
treatment.
| Laboratory
parameters (reference range) | At the time of
admission | At the time of VAP
diagnosis | Following treatment
for VAP |
|---|
| WBC, cells/mmc
(4,000-10,000) | 10,330 | 15,100 | 7,200 |
| Neutrophils, %
(40-75) | 73 | 85.6 | 57.3 |
| Lymphocytes, %
(25-50) | 18.3 | 7.4 | 30.4 |
| Monocytes, %
(2-10) | 8.8 | 6.3 | 9.8 |
| Platelets,
cells/mmc x103 (150-400) | 150 | 123 | 309 |
| Haemoglobin, g/dl
(12-16) | 13.1 | 12.6 | 12.4 |
| AST, UI/l
(15-35) | 19 | 17 | 23 |
| ALT, UI/l
(15-35) | 24 | 20 | 32 |
| Creatinine, mg/dl
(0.8-1.2) | 0.59 | 0.44 | 0.47 |
| Procalcitonin,
ng/ml (<0.5) | 0.04 | 0.04 | 0.01 |
| CRP, mg/dl
(0-0.5) | 0.5 | 10.5 | 0.07 |
Despite an initial improvement, 3 days following ICU
admission and endotracheal intubation, the patient's clinical
condition began to deteriorate, along with worsened respiratory
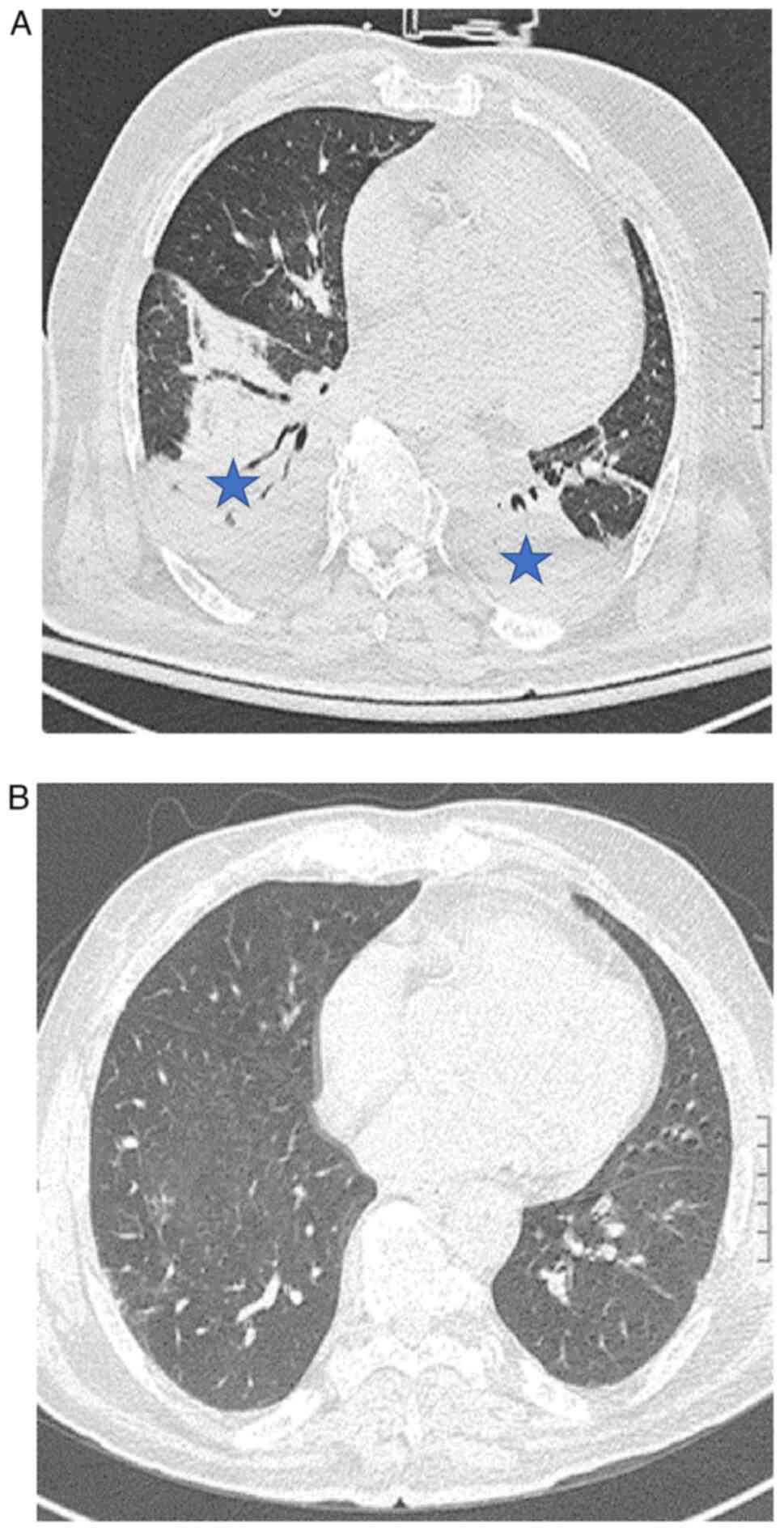
parameters and blood test results (Table I). A chest-CT scan (SOMATOM
Definition Flash, Siemens) revealed bilateral basal consolidative
opacities, along with bilateral pleural effusion (Fig. 2A).
Due to the diagnosis of VAP, antibiotic therapy was
empirically switched to intravenous meropenem at 1 g three times
daily plus intravenous linezolid at 600 mg twice daily.
Legionella and pneumococcal urinary antigens tested
negative, as well as a nasopharyngeal swab for severe acute
respiratory syndrome-coronavirus 2 (SARS-CoV-2). The analysis of
bronchoalveolar lavage (BAL) using the BIOFIRE®
FILMARRAY® Pneumonia Panel (PN) (BioFire Diagnostics)
tested positive for Pseudomonas aeruginosa (P.
aeruginosa).
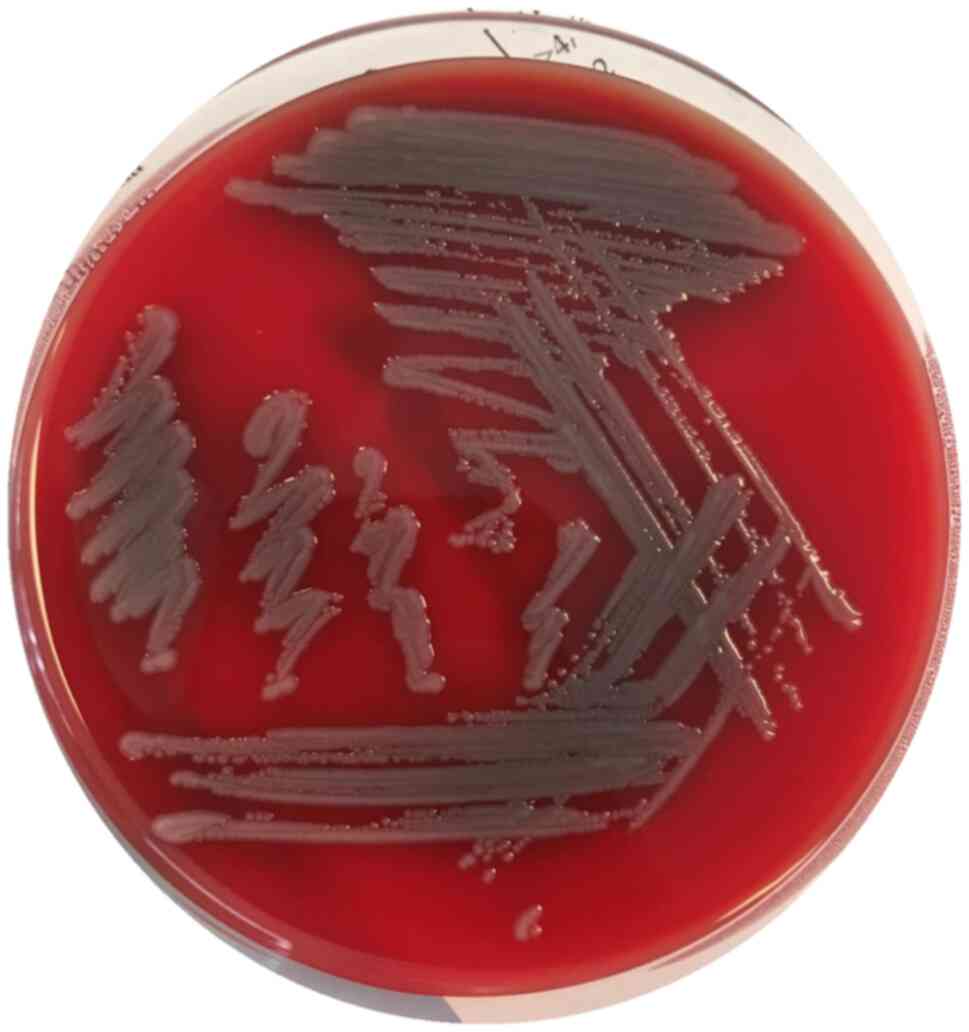
At 2 days after switching the therapy, the BAL
culture tested positive for S. maltophilia on a blood agar
plate (Vacutest Kima S.R.L.) (Fig.
3) with a total bacterial count of 105 CFU/ml
performed diluting the BAL sample at 1:100 in sterile saline and
plating 100 µl on Muheller Hintong agar (Vacutest Kima S.R.L.).
Species identification was assessed using the BD Phoenix system,
selecting a colony from the blood agar, and the antibiotic
susceptibility test performed using the MIC test strip (Liofilchem)
revealed susceptibility to TMP-SMX at an increased exposure [MIC 2
mg/l; according to the European Committee on Antimicrobial
Susceptibility Testing (EUCAST), breakpoints are expressed as the
trimethoprim concentration]; hence intravenous TMP-SMX at 12 mg/kg
(dosing was based on the TMP component) was commenced, while
linezolid treatment was terminated.
At 4 days after the addition of TMP-SMX to the
treatment schedule, the patient was extubated due to an
amelioration in respiratory function and improved blood tests
(Table I). The patient's mental
status also improved, with no evidence of neurological deficits.
The restored consciousness, along with a follow-up brain magnetic
resonance imaging (Magneton, Siemens) not revealing any
abnormalities, allowed for the discontinuation of acyclovir
administration after 17 days of therapy. Furthermore, due to the
resolution of consolidative opacities on a follow-up chest CT scan
(Fig. 2B) along with a good
general state, the antibiotic therapy with meropenem (14 days of
therapy) and TMP-SMX (11 days of therapy) was terminated. Finally,
the patient was discharged 7 days after the end of therapy, with no
evidence of respiratory or neurological sequelae (Fig. 4).
Discussion
Gram-negative drug-resistant bacteria pose a major
threat to physicians due to the increasing incidence of multi-drug
resistant (MDR) infections worldwide (12-14),
particularly among hospitalised patients (15,16).
VAP is a type of pneumonia that occurs >48 h following
intubation and is influenced by factors, such as immunosuppression,
a long duration of hospitalisation, and prolonged ventilation
(17,18).
The SARS-CoV-2 pandemic (19) may have contributed to an increase
the number of cases with VAP, due to the large number of affected
patients requiring ICU care and mechanical ventilation (20).
Gram-negative bacilli are the most commonly
identified causative agents of VAP, with P. aeruginosa,
Enterobacter spp. and Klebsiella spp. being the most
prevalent (21,22). MDR Gram-negative infections result
in poor outcomes of patients with hospital-acquired pneumonia/VAP
and their increasing prevalence makes it crucial for management to
consider the local ecology and patient risk factors (15,23).
The identification of causative agents of VAP also needs to be a
priority, due to the complexity of clinical and therapeutic
management (17).
In the case described herein, the patient developed
VAP due to S. maltophilia infection at 3 days after hospital
admission. The categorisation of early- and late-onset VAP, of
which late-onset VAP is more likely to be due to a MDR pathogen, is
being challenged, as the timing of VAP development needs to be
evaluated in the context of other risk factors (24,25).
Although often considered a colonizing pathogen,
S. maltophilia, an ubiquitous, motile, free-living, aerobic,
non-fermenting bacillus, multidrug-resistant Gram-negative
bacterium (1), represents the
causative agent of various infections, from skin manifestations to
bacteraemia (26), particularly in
patients with underlying illnesses (6). Recent research has reported that
S. maltophilia-associated pneumonia is increasingly being
isolated, describing this pathogen as one of the most common
infectious agents in patients in ICUs, with a high morbidity and
mortality rates. Risk factors for S. maltophilia-associated
pulmonary diseases include ICU admission and mechanical
ventilation, as well as prior broad-spectrum antibiotic therapy,
prolonged hospitalization, and immunosuppression (27).
As patients with respiratory tract infections
sustained by S. maltophilia are usually severely ill and
hospitalised, and due to the drug-resistant profile of this
bacteria, these conditions often lead to an increase in medical
expenses, prolonged durations of hospitalisation and higher
mortality rates. Therefore, identifying the right early targeted
treatment is the key to reducing the mortality rates associated
with S. maltophilia infection. A previously published
meta-analysis demonstrated that previous carbapenem treatment was
associated with lower respiratory tract infections by S.
maltophilia with the highest odd ratio (OR, 3.69), followed by
glycopeptide drugs (OR, 3.22), aminoglycoside drugs (OR, 2.57) and
β-lactamase inhibitors (OR, 1.76) (28). That study suggested that when the
clinical use of this type of drug is not effective, attention
should be paid to the possibility of S. maltophilia
infection; furthermore it was stated that more types of
antibiotics, longer treatment durations and a greater number
replacements can significantly increase the risk of respiratory
tract infections caused by this bacterium (28).
These data may be explained by S. maltophilia
features resulting in intrinsic or acquired resistance mechanisms
to several antibiotics. Resistance to β-lactams is primarily
mediated by L1 and L2, two
chromosomal-mediated inducible β-lactamases. L1 is a
molecular class B Zn2+-dependent metallo-β-lactamase,
whilst L2 is a molecular class A clavulanic
acid-sensitive cephalosporinase. The mrcA gene (encodes
penicillin-binding protein 1α) and regulatory proteins AmpR
(transcriptional regulator) and AmpN-AmpG (permease system)
influence basal β-lactamase activity. mrcA inactivation
causes L1/L2 β-lactamase hyperproduction
(29,30). S. maltophilia has
demonstrated resistance to aminoglycosides as well, through efflux
pumps and aminoglycoside modifying enzyme, such as
6'-N-aminoglycoside acetyltransferases. Fortunately, the new
generation cephalosporin, cefiderocol, appears to be able to evade
the chromosomally encoded L1 and L2
β-lactamases, as demonstrated by the 100% susceptibility of S.
maltophilia to this molecule (29,30),
as well as an in vivo model promising efficacy (31). The resistance of S.
maltophilia to quinolones is mainly caused by mutations in the
gyrA and parC genes at the target site (QRDRs) of the
DNA gyrase enzyme, which is also related to the outer membrane
barrier and high-efficiency efflux pump (32,33).
Additionally, S. maltophilia can accumulate multidrug efflux
pumps that reduce tetracycline and fluoroquinolone activity.
Increased fluoroquinolone MICs are also observed in isolates that
harbour the chromosomal Smqnr gene, the determinants of
which interfere through binding to gyrase and topoisomerase
(1). Furthermore, S.
maltophilia produces a wide variety of potential virulence
factors, such as biofilm and extracellular enzymes, thus rendering
treatment challenging (1,5,34).
Despite the lack of definitive evidence on the most
effective available treatment, the current IDSA guidelines suggest
TMP-SMX as the preferred treatment strategy for mild infections or
in combination with another antibacterial agent for moderate to
severe S. maltophilia infections. To date, combination
therapy fails to exhibit evident superiority, with similar rates of
clinical efficacy and resistance development compared with
monotherapy (7,35), as reported by Shah et al
(36) in a large retrospective
cohort study.
As with other MDR Gram-negative bacteria, such as
Acinetobacter spp., for which new antimicrobial agents are
intensively being studied due to limited therapeutic options
(37-39),
S. maltophilia poses a major threat, particularly when
resistant to TMP-SMX. When TMP-SMX is not a suitable treatment
option, due to resistance or patients' intolerance, minocycline,
tigecycline, other options include ticarcillin-clavulanate,
cefiderocol, or ceftazidime/avibactam plus aztreonam (8).
While interpretive criteria for seven antibiotics
have been established by the Clinical & Laboratory Standards
Institute (CLSI), EUCAST has only provided breakpoints for TMP-SMX
(40).
Of note, isolates resistant to this drug have
already been reported (41,42).
Previous studies have demonstrated that the sul1,
sul2 and drfA genes may represent important
determinants of TMP-SMX resistance in S. maltophilia
isolates (1,43,44).
In the case described in the present study, the
patient was diagnosed with VAP caused by a strain of S.
maltophilia with a MIC for TMP-SMX equal to 2 mg/l, the same
epidemiological cut-off value reported by the EUCAST. This value is
close to the resistance breakpoint (>4 mg/l) and its treatment
required an increased exposure to this antibiotic to assure the
success of the therapy. This led to the addition of a higher dose
of TMP-SMX. After only 11 days, the patient's condition improved,
and the pneumonia had resolved according to a follow-up chest CT
scan.
It is worth mentioning that while the IDSA
guidelines do not provide specific recommendations on therapy
duration, they suggest considering factors, such as the patient's
immune status, source control, and treatment response when deciding
when to terminate antibiotics (8).
However, the nationwide study by Guerci et al
(7) found no benefit in prolonged
antimicrobial therapy beyond 7 days for patients admitted to ICUs.
Nonetheless, the persistence of the infection caused a mistreatment
could lead to the development of resistance phenotypes,
particularly due to the commonly reported co-infection rate of
S. maltophilia with other species, and its capability to
obtain new resistance genes, such as sul1 and sul2,
through horizontal gene transfer (45).
Furthermore, according to previous studies, S.
maltophilia is often part of polymicrobial infections, with
other non-fermenting Gram-negative bacteria, such as P.
aeruginosa, Acinetobacter spp. and Burkholderia
cepacia complex (2,11). Interactions between these bacteria
may play a key role in clinical outcomes, with higher morbidity and
mortality rates (46). For
example, co-infection with P. aeruginosa and S.
maltophilia has been linked to a higher mortality rate in
patients with pneumonia. These two pathogens can form a
polymicrobial biofilm in the lungs, interact through quorum-sensing
signals and create a favourable environment for each other
(47). McDaniel et al
(48) found that the presence of
P. aeruginosa facilitated S. maltophilia persistence
in the lungs during polymicrobial infections.
In the case described herein, the
FILMARRAY® test of BAL was positive for P.
aeruginosa, even though it was not recovered on BAL culture.
Given the higher risk of mortality associated with VAP caused by
these two pathogens, it was decided to continue treatment with both
meropenem and TMP-SMX.
In conclusion, the present study aimed to emphasize
the importance of recognizing S. maltophilia as a potential
pathogen in hospital settings where MDR bacteria are prevalent. The
case described herein highlights the common co-occurrence of S.
maltophilia and P. aeruginosa infections, reinforcing
the need for considering this bacterium as a potential cause of
VAP.
However, inadequate initial antimicrobial treatment
is often the norm for S. maltophilia infections due to its
resistance to commonly used antibiotics such as β-lactams. Thus,
the consideration of alternative, effective treatment options for
patients with VAP who are not responding to broad-spectrum β-lactam
antibiotics is crucial, as well as in patients with S.
maltophilia identified through bronchoalveolar lavage
culture.
Notably however, more research is required in order
to better understand the risk factors of VAP caused by S.
maltophilia and to develop effective prevention strategies and
early antimicrobial treatments, ultimately improving the outcomes
of patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article as no
datasets were generated or analysed during the current study.
Authors' contributions
All authors (EC, AM, SS, RR, CM, GN, BC and MC)
contributed to the conception and design of the study. EC and AM
wrote the manuscript. SS, CM and MC revised the literature and
references. RR provided clinical assistance to the patient. BC was
responsible for the laboratory tests and pharmacological
treatments. GN and BC revised the manuscript. All authors have read
and approved the final manuscript. EC and AM confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient included in the present case report.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of his data in the present case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brooke JS: Advances in the microbiology of
Stenotrophomonas Maltophilia. Clin Microbiol Rev.
34(e0003019)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gajdács M and Urbán E: Epidemiological
trends and resistance associated with Stenotrophomonas
maltophilia Bacteremia: A 10-year retrospective cohort study in
a tertiary-care hospital in Hungary. Diseases. 7(41)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gröschel MI, Meehan CJ, Barilar I, Diricks
M, Gonzaga A, Steglich M, Conchillo-Solé O, Scherer IC, Mamat U,
Luz CF, et al: The phylogenetic landscape and nosocomial spread of
the multidrug-resistant opportunist Stenotrophomonas
maltophilia. Nat Commun. 11(2044)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hafiz TA, Aldawood E, Albloshi A, Alghamdi
SS, Mubaraki MA, Alyami AS and Aldriwesh MG: Stenotrophomonas
maltophilia epidemiology, resistance characteristics, and
clinical outcomes: Understanding of the recent three years's
trends. Microorganisms. 10(2506)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Saied WI, Merceron S, Schwebel C, Monnier
AL, Oziel J, Garrouste-Orgeas M, Marcotte G, Ruckly S, Souweine B,
Darmon M, et al: Ventilator-associated pneumonia due to
Stenotrophomonas maltophilia: Risk factors and outcome. J
Infect. 80:279–285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chang YT, Lin CY, Chen YH and Hsueh PR:
Update on infections caused by Stenotrophomonas maltophilia
with particular attention to resistance mechanisms and therapeutic
options. Front Microbiol. 6(893)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guerci P, Bellut H, Mokhtari M, Gaudefroy
J, Mongardon N, Charpentier C, Louis G, Tashk P, Dubost C,
Ledochowski S, et al: Outcomes of Stenotrophomonas
maltophilia hospital-acquired pneumonia in intensive care unit:
A nationwide retrospective study. Crit Care. 23(371)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tamma PD, Aitken SL, Bonomo RA, Mathers
AJ, van Duin D and Clancy CJ: Infectious diseases society of
America guidance on the treatment of AmpC β-Lactamase-producing
Enterobacterales, Carbapenem-Resistant Acinetobacter
baumannii, and Stenotrophomonas maltophilia Infections.
Clin Infect Dis. 74:2089–2114. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Baidya A, Kodan P, Fazal F, Tsering S,
Menon PR, Jorwal P and Chowdhury UK: Stenotrophomonas
maltophilia: More than just a colonizer! Indian J Crit Care
Med. 23:434–436. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Singhal L, Kaur P and Gautam V:
Stenotrophomonas maltophilia: From trivial to grievous.
Indian J Med Microbiol. 35:469–479. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mojica MF, Humphries R, Lipuma JJ, Mathers
AJ, Rao GG, Shelburne SA, Fouts DE, Van Duin D and Bonomo RA:
Clinical challenges treating Stenotrophomonas maltophilia
infections: An update. JAC Antimicrob Resist.
4(dlac040)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Morris S and Cerceo E: Trends,
epidemiology, and management of multi-drug resistant gram-negative
bacterial infections in the hospitalized setting. Antibiotics
(Basel). 9(196)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marino A, Campanella E, Stracquadanio S,
Ceccarelli M, Zagami A, Nunnari G and Cacopardo B:
Corynebacterium striatum bacteremia during SARS-CoV2
Infection: Case report, literature review, and clinical
considerations. Infect Dis Rep. 14:383–390. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Marino A, Munafò A, Zagami A, Ceccarelli
M, Campanella E, Cosentino F, Moscatt V, Cantarella G, Di Mauro R,
Bernardini R, et al: Ampicillin plus ceftriaxone therapy against
Enterococcus faecalis endocarditis: A case report,
guidelines considerations, and literature review. IDCases.
28(e01462)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cillóniz C, Dominedò C and Torres A: An
overview of guidelines for the management of hospital-acquired and
ventilator-associated pneumonia caused by multidrug-resistant
Gram-negative bacteria. Curr Opin Infect Dis. 32:656–662.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
El-Sokkary R, Uysal S, Erdem H, Kullar R,
Pekok AU, Amer F, Grgić S, Carevic B, El-Kholy A, Liskova A, et al:
Profiles of multidrug-resistant organisms among patients with
bacteremia in intensive care units: An international ID-IRI survey.
Eur J Clin Microbiol Infect Dis. 40:2323–2334. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kalil AC, Metersky ML, Klompas M,
Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP,
Bartlett JG, Carratalà J, et al: Management of adults with
hospital-acquired and ventilator-associated pneumonia: 2016
clinical practice guidelines by the infectious diseases society of
America and the American thoracic society. Clin Infect Dis.
63:e61–e111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Papazian L, Klompas M and Luyt CE:
Ventilator-associated pneumonia in adults: A narrative review.
Intensive Care Med. 46:888–906. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Marino A, Campanella E, Ceccarelli M,
Larocca L, Bonomo C, Micali C, Munafò A, Celesia BM, Nunnari G and
Cacopardo B: Sarilumab administration in patients with severe
COVID-19: A report of four cases and a literature review. World
Acad Sci J. 4(24)2022.
|
|
20
|
Ippolito M, Misseri G, Catalisano G,
Marino C, Ingoglia G, Alessi M, Consiglio E, Gregoretti C,
Giarratano A and Cortegiani A: Ventilator-associated pneumonia in
patients with covid-19: A systematic review and meta-analysis.
Antibiotics (Basel). 10(545)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rouzé A, Martin-Loeches I, Povoa P, Makris
D, Artigas A, Bouchereau M, Lambiotte F, Metzelard M, Cuchet P,
Geronimi CB, et al: Relationship between SARS-CoV-2 infection and
the incidence of ventilator-associated lower respiratory tract
infections: A European multicenter cohort study. Intensive Care
Med. 47:188–198. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Blonz G, Kouatchet A, Chudeau N, Pontis E,
Lorber J, Lemeur A, Planche L, Lascarrou JB and Colin G:
Epidemiology and microbiology of ventilator-associated pneumonia in
COVID-19 patients: A multicenter retrospective study in 188
patients in an un-inundated French region. Crit Care.
25(72)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Patro S, Sarangi G, Das P, Mahapatra A,
Mohapatra D, Paty B and Chayani N: Bacteriological profile of
ventilator-associated pneumonia in a tertiary care hospital. Indian
J Pathol Microbiol. 61:375–379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ferrer M, Liapikou A, Valencia M,
Esperatti M, Theessen A, Martinez JA, Mensa J and Torres A:
Validation of the American Thoracic Society-Infectious Diseases
Society of America guidelines for hospital-acquired pneumonia in
the intensive care unit. Clin Infect Dis. 50:945–952.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Restrepo MI, Peterson J, Fernandez JF, Qin
Z, Fisher AC and Nicholson SC: Comparison of the bacterial etiology
of early-onset and late-onset ventilator-associated pneumonia in
subjects enrolled in 2 large clinical studies. Respir Care.
58:1220–1225. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Looney WJ, Narita M and Mühlemann K:
Stenotrophomonas maltophilia: An emerging opportunist human
pathogen. Lancet Infect Dis. 9:312–323. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang N, Tang C and Wang L: Risk factors
for acquired Stenotrophomonas maltophilia pneumonia in
intensive care unit: A systematic review and meta-analysis. Front
Med (Lausanne). 8(808391)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Wang Y, Rong H, Guo Z, Xu J and
Huang X: Risk factors of lower respiratory tract infection caused
by Stenotrophomonas maltophilia: Systematic review and
meta-analysis. Front Public Health. 10(5410)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakamura R, Oota M, Matsumoto S, Sato T
and Yamano Y: In vitro activity and in vivo efficacy of cefiderocol
against Stenotrophomonas maltophilia. Antimicrob Agents
Chemother. 65:e01436–e01420. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Stracquadanio S, Torti E, Longshaw C,
Henriksen AS and Stefani S: In vitro activity of cefiderocol and
comparators against isolates of gram-negative pathogens from a
range of infection sources: SIDERO-WT-2014-2018 studies in Italy. J
Glob Antimicrob Resist. 25:390–398. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Petraitis V, Petraitiene R, Kavaliauskas
P, Naing E, Garcia A, Georgiades BN, Echols R, Bonomo RA, Yamano Y,
Satlin MJ and Walsh TJ: Efficacy of cefiderocol in experimental
Stenotrophomonas maltophilia pneumonia in persistently
neutropenic rabbits. Antimicrob Agents Chemother.
66(e0061822)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hotta G, Matsumura Y, Kato K, Nakano S,
Yunoki T, Yamamoto M, Nagao M, Ito Y, Takakura S and Ichiyama S:
Risk factors and outcomes of Stenotrophomonas maltophilia
bacteraemia: A comparison with bacteraemia caused by pseudomonas
aeruginosa and acinetobacter species. PLoS One.
9(e112208)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ba BB, Feghali H, Arpin C, Saux MC and
Quentin C: Activities of ciprofloxacin and moxifloxacin against
Stenotrophomonas maltophilia and emergence of resistant
mutants in an in vitro pharmacokinetic-pharmacodynamic model.
Antimicrob Agents Chemother. 48:946–953. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gil-Gil T, Martínez JL and Blanco P:
Mechanisms of antimicrobial resistance in Stenotrophomonas
maltophilia: A review of current knowledge. Expert Rev Anti
Infect Ther. 18:335–347. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Prawang A, Chanjamlong N, Rungwara W,
Santimaleeworagun W, Paiboonvong T, Manapattanasatein T,
Pitirattanaworranat P, Kitseree P and Kanchanasurakit S:
Combination therapy versus monotherapy in the treatment of
Stenotrophomonas maltophilia infections: A systematic review
and meta-analysis. Antibiotics (Basel). 11(1788)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shah MD, Coe KE, El Boghdadly Z, Wardlow
LC, Dela-Pena JC, Stevenson KB and Reed EE: Efficacy of combination
therapy versus monotherapy in the treatment of Stenotrophomonas
maltophilia pneumonia. J Antimicrob Chemother. 74:2055–2059.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bivona DA, Mirabile A, Bonomo C, Bonacci
PG, Stracquadanio S, Marino A, Campanile F, Bonaccorso C, Fortuna
CG, Stefani S, et al: Heteroaryl-ethylenes as new effective agents
for high priority gram-positive and gram-negative bacterial
clinical isolates. Antibiotics. 11(767)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Stracquadanio S, Bonomo C, Marino A,
Bongiorno D, Privitera GF, Bivona DA, Mirabile A, Bonacci PG and
Stefani S: Acinetobacter baumannii and Cefiderocol, between
Cidality and Adaptability. Microbiol Spectr.
10(e0234722)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Marino A, Stracquadanio S, Campanella E,
Munafò A, Gussio M, Ceccarelli M, Bernardini R, Nunnari G and
Cacopardo B: Intravenous fosfomycin: A potential good partner for
cefiderocol. Clinical experience and considerations. Antibiotics
(Basel). 12(49)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
The European Committee on Antimicrobial
Susceptibility Testing. Breakpoint tables for interpretation of
MICs and zone diameters. Version 13.0, 2023. http://www.eucast.org.
|
|
41
|
Biagi M, Tan X, Wu T, Jurkovic M,
Vialichka A, Meyer K, Mendes RE and Wenzler E: Activity of
potential alternative treatment agents for Stenotrophomonas
maltophilia isolates nonsusceptible to levofloxacin and/or
Trimethoprim-sulfamethoxazole. J Clin Microbiol. 58:e01603–e01619.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wu RX, Yu CM, Hsu ST and Wang CH:
Emergence of concurrent levofloxacin- and
trimethoprim/sulfamethoxazole-resistant Stenotrophomonas
maltophilia: Risk factors and antimicrobial sensitivity pattern
analysis from a single medical center in Taiwan. J Microbiol
Immunol Infection. 55:107–113. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chung HS, Kim K, Hong SS, Hong SG, Lee K
and Chong Y: The sul1 gene in Stenotrophomonas maltophilia
with high-level resistance to trimethoprim/sulfamethoxazole. Ann
Lab Med. 35:246–249. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hu LF, Chen GS, Kong QX, Gao LP, Chen X,
Ye Y and Li JB: Increase in the prevalence of resistance
determinants to trimethoprim/sulfamethoxazole in clinical
Stenotrophomonas maltophilia isolates in China. PLoS One.
11(157693)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Toleman MA, Bennett PM, Bennett DMC, Jones
RN and Walsh TR: Global emergence of trimethoprim/sulfamethoxazole
resistance in Stenotrophomonas maltophilia mediated by
acquisition of sul Genes. Emerg Infect Dis. 13:559–565.
2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yin C, Yang W, Meng J, Lv Y, Wang J and
Huang B: Co-infection of Pseudomonas aeruginosa and
Stenotrophomonas maltophilia in hospitalised pneumonia
patients has a synergic and significant impact on clinical
outcomes. Eur J Clin Microbiol Infect Dis. 36:2231–2235.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
McDaniel MS, Schoeb T and Swords WE:
Cooperativity between Stenotrophomonas maltophilia and
Pseudomonas aeruginosa during polymicrobial airway
infections. Infect Immun. 88:e00855–e00819. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mcdaniel MS, Lindgren NR, Billiot CE,
Valladares KN, Sumpter NA and Swords WE: Pseudomonas
aeruginosa promotes persistence of Stenotrophomonas
maltophilia via increased adherence to depolarized respiratory
epithelium. Microbiol Spectr. 11(e0384622)2022.PubMed/NCBI View Article : Google Scholar
|