Introduction
Coxiella burnetii (C. burnetii) is an
obligate intracellular bacterium and its common intermediate hosts
are cattle, sheep and goats. Pathogens transmitted by the
inhalation of biological product particles can travel a great
distance (several kilometers), in many cases, without the patient
being in direct contact with the pathogen (1). The most common infection caused by
C. burnetii is acute Q fever, characterized by pneumonia and
hepatitis. In some countries, outbreaks of >3,000 simultaneous Q
fever cases have been recorded, forming an outbreak with a
mortality rate of up to 2% (France in 2007 and The Netherlands in
2010) (2). El-Mahallawy et
al (3) evaluated the
prevalence of C. burnetii infection in a group of 180
healthy individuals in China and found the prevalence of C.
burnetii to be 25%. The results of their study revealed that
C. burnetii infection was a relatively common disease in
that country, in both urban and rural areas, similar to other
European countries (3). Chronic Q
fever due to C. burnetii infection usually accounts for 1-5%
of C. buretii infections (4). C. burnetii has a long
incubation period; the time recorded between first exposure and
clinical manifestations can vary from 1 year to >1 decade
(5). Common risk factors in
patients with C. burnetii endocarditis are the male sex
(75%), an age between 40 and 70 years, valvular disease (91%);
particularly the presence of prosthetic valves (30-55%) and
immunocompromised patients (32%) (5).
The manifestations of infective endocarditis due to
C. burnetii are non-specific and this is the cause of the
untimely diagnosis of this condition. Almost 50% of patients with
C. burnetii endocarditis have symptoms of acute heart
failure and the majority of patients have a fever (70%), and suffer
from weight loss, fatigue and anorexia (50%). Other manifestations
include a rash on the extremities and mucous membranes, changes in
the levels of hematological parameters, splenomegaly and renal
injury caused by immune disorders (2). It has been demonstrated that some
cases of endocarditis have negative blood cultures, as demonstrated
in the study by Houpikian and Raoult in 2005(6). Negative blood cultures can be caused
by a variety of factors, including the method used to obtain the
specimen, the culture medium used and previous antibiotic therapy
(7). The study performed in France
by Fournier et al (8)
developed a multimodal strategy for the diagnosis of negative
endocarditis when blood cultures were negative. The methods used in
their study included classical serology and PCR analysis of the
blood samples; PCR revealed an increase in diagnostic efficiency of
up to 24.3%, and the authors of that study thus suggested that
these tests should be used as a standard in studies on C.
Burnetii (8).
At the Cardiovascular Surgery Unit, Bach Mai
Hospital (Hanoi, Vietman), the authors also found that among the
patients undergoing cardiovascular surgery due to endocarditis,
there were some patients with post-operative infectious
complications, including some patients who had a negative blood
culture. Given the complications of endocarditis caused by C.
burnetii that have been previously reported, the present study
was performed in an aim to recommend further tests and treatment
regimens for patients with endocarditis (7).
Patients and methods
Patients
A total of 312 patients with endocarditis operated
at the Cardiovascular Surgery Unit of Bach Mai Hospital, from
January, 2022 to February, 2023, aged 17 to 74 years, male:female
ratio was 1.6:1 (193 male patients), were diagnosed with
endocarditis and required surgery. The patients were subjected to a
full range of examinations, such as hematological analysis,
coagulation analysis, microbiology tests, including hepatitis B
virus (HBV), hepatitis C virus (HCV) and HIV, as well as medical
history, if necessary. Following surgery, the heart valve tissue of
the patients was cultured. In the case that the results of the
culture are negative, the DNA was separated from the blood, and PCR
analysis for C. burnetii bacteria was performed using
specific primer pairs. All the aforementioned procedures were
approved by the Medical Ethics Committee of Bach Mai Hospital and
following the written consent of the patients or their parents (for
2 patients who were underage).
PCR technique and PCR cycle
Blood DNA was separated using the Qiagen kit
(Qiagen, Inc.). The primer sequences specific to C. burnetii
used were as follows: Forward, 5'-ACGGGTGAGTAATGCGTAGG-3' and
reverse, 5'-CAGTATCGGGTGCAATTCCCAG-3.
The PCR assays were performed using an Eppendorf
model 5382 Thermo Mixer C thermal cycler (BCE Vietnam) according to
the following procedure: An initial denaturation at 95˚C for 15
min; 45 cycles at 95˚C for 30 sec, 57˚C (for the first primer pair)
or 62˚C (for the second primer pair) for 30 sec and 72˚C for 30
sec; and a final elongation step at 72˚C for 7 min. The
amplification of 5 µl DNA was performed in a total volume of 25 µl
containing 10X PCR buffer (Qiagen, Inc.), 2.5 mM MgCl2,
0.25 mM deoxynucleotide triphosphate, 25 pmol of each primer, and 1
unit of Taq DNA polymerase (Qiagen, Inc.). Agarose gel
electrophoresis (2%) in the presence of ethidium bromide was used
to separate the PCR products.
Hematoxylin and eosin (H&E)
staining
For H&E staining, a bone marrow biopsy was
performed at the posterior superior iliac spine. The sample was
10-20 mm in length and was fixed with 5% formaldehyde, and
subjected to decalcification and decontamination with alcohol,
xylene and molded with paraffin melting at 61˚C. Staining was
performed using H&E (Diapath S.P.A.) at room temperature. The
thickness of sections was 0.2 mm. The sample was examined using a
light microscope (Olympus Corporation) with a 40X objective.
Patient treatment
Patients with positive results for C.
burnetii infection were treated with a regimen of doxycilin 600
mg/day for 7-10 days in combination with other antibiotics, such as
imipenem and cilastatin at a dose of 1-2 g/day and their clinical
progress was monitored, with periodical follow-up following
hospital discharge.
Results
Out of the total of 312 patients with infective
endocarditis who underwent surgery, 52 patients had negative blood
and cardiac tissue cultures following surgery. Using the PCR
technique with a 16s RNA primer pair of C. burnetii to
analyze the 52 negative bacterial culture samples, 13 samples
tested positive for C. burnetii at 460 bp; these patients
had both mitral and tricuspid valve lesions, abscesses and circuit
occlusion (Table I).
 | Table IResults of the analysis for
Coxiella burnetii using PCR. |
Table I
Results of the analysis for
Coxiella burnetii using PCR.
| Underlying
etiology | PCR-positive result
(no. of patients) | PCR-negative result
(no. of patients) |
|---|
| Mitral valve | 7 | 17 |
| Tricuspid valve | 6 | 12 |
| Circuit
occlusion | 3 | 5 |
| Abscess | 3 | 5 |
Patients with positive results for C.
burnetii were tested for other viruses, including HIV, HCV,
HBV, Epstein-Barr virus, vytomegalovirus and influenza, all of
which yielded negative results. Following surgery, the group of
patients positive for C. burnetii also had more severe
clinical manifestations than the group of patients with negative
results. The clinical lesions of the patients with C.
burnetii infection following surgery encountered included a
high fever >38˚C, pneumonia, weight loss, liver failure, kidney
failure, including 2 patients with severe multi-organ failure. All
patients with C. burnetii infection had a high fever
>38˚C, lasting for >14 days; the longest fever duration
observed was almost 40 days. Pneumonia and liver damage were
recorded at a high rate in this group of patients at a rate of 84.6
and 76.9%, respectively (Table
II).
 | Table IIClinical manifestations following
surgery in patients positive for Coxiella burnetii
infection. |
Table II
Clinical manifestations following
surgery in patients positive for Coxiella burnetii
infection.
| Clinical
manifestations | No. of patients | % |
|---|
| Fever (lasting for
>14 days) | 13 | 100 |
| Weight loss | 6 | 46.1 |
| Liver failure
(elevation in AST/ALT levels) | 11 | 84.6 |
| Impaired kidney
function | 8 | 61.5 |
| Pneumonia | 10 | 76.9 |
The mean duration of hospitalization in the C.
burnetii-positive group was 41.5 days, which was a markedly
longer post-operative hospitalization period than the patients with
negative C. burnetii results (Table III).
 | Table IIIAverage duration of
hospitalization. |
Table III
Average duration of
hospitalization.
| Coxiella
burnetii infection status | Average no. of
days |
|---|
| Endocarditis
Coxiella burnetii-negative (PCR) | 16 |
| Endocarditis
Coxiella burnetii-positive (PCR) | 41.5 |
All patients infected with C. burnetii in the
present study had anemia and thrombocytopenia; 3/13 patients had
leukopenia. The average hemoglobin level of the patients was 93.6
g/l (range, 74-110 g/l). 9 patients had mild anemia, and 1 patient
had moderate anemia. At its lowest, the level of hemoglobin was 74
g/l. A total of 3 patients had moderate or slightly elevated white
blood cell counts, with 3/13 cases having decreased white blood
cell counts, with a decreased neutrophil ratio (average, <35%).
The average platelet count was 85.9x109/l (Table IV).
 | Table IVChanges in the levels of
hematological parameters in patients positive for Coxiella
burnetii infection. |
Table IV
Changes in the levels of
hematological parameters in patients positive for Coxiella
burnetii infection.
| Patient no. | Hemoglobin
(g/l) | WBC
(x109/l) | PLT
(x109/l) |
|---|
| 1 | 92 | 6.71 | 121 |
| 2 | 94 | 4.32 | 102 |
| 3 | 101 | 8.93 | 68 |
| 4 | 102 | 2.78 | 93 |
| 5 | 83 | 3.90 | 67 |
| 6 | 74 | 1.62 | 45 |
| 7 | 87 | 16.45 | 101 |
| 8 | 95 | 10.81 | 82 |
| 9 | 96 | 12.18 | 38 |
| 10 | 103 | 9.87 | 66 |
| 11 | 110 | 13.62 | 83 |
| 12 | 89 | 1.96 | 76 |
| 13 | 91 | 4.09 | 92 |
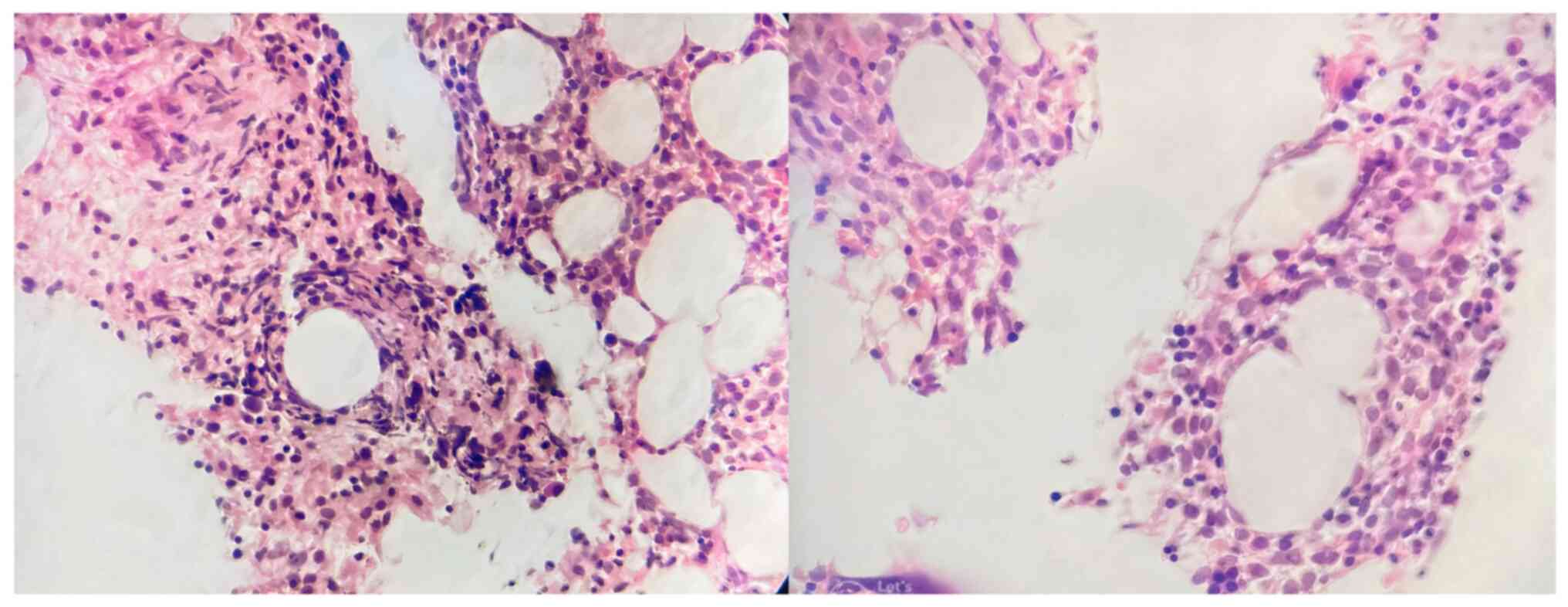
In particular, there was 1 case (patient no. 6;
Table IV) with pancytopenia and
neutrophils were reduced by 0.4x109/l. This patient
subsequently had a bone marrow biopsy and was found to have
multiple fibrin-ring granulomas (Fig.
1).
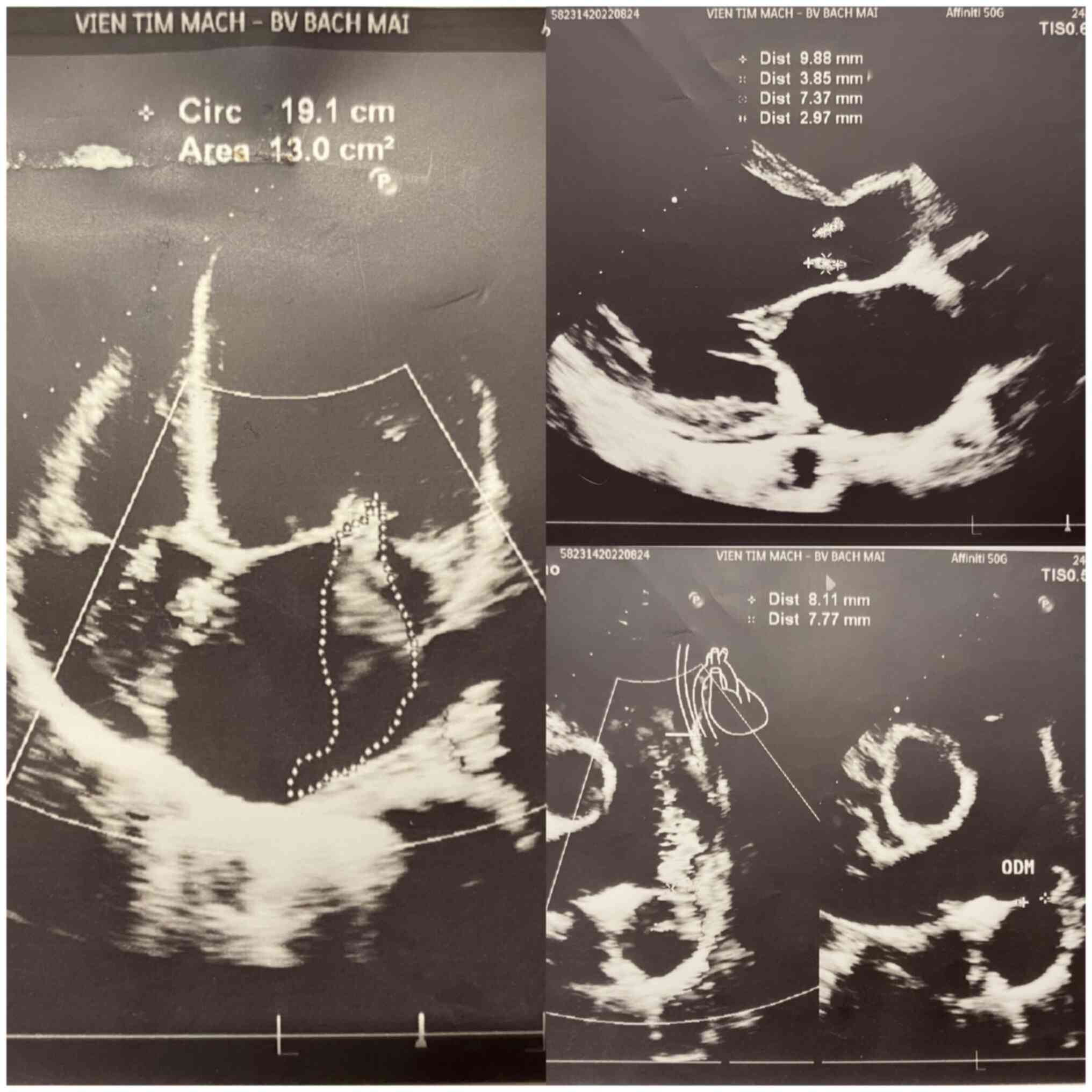
Among these cases, there were patients with both
mitral and tricuspid valve lesions, or both mitral valve lesions
and occlusion (Fig. 2). This
patient was admitted to the hospital with breathing difficulties,
which gradually increased for ~1 year. This patient was a male at
26 years of age. The tests to identify the cause of endocarditis
prior to surgery were all negative. This is also one of the 2 cases
of post-operative endocarditis with multi-organ failure.
Another patient succumbed 6 months following mitral
valve surgery due to continued damage to the tricuspid valve,
sepsis and multi-organ failure, who also tested positive for C.
burnetii.
Discussion
There were 52/312 patients with negative results for
bacterial culture following cardiovascular surgery, determined
using an automatic identification system. When analyzing these 52
samples, it was found that 13/52 cases had the presence of C.
burnetii bacteria in the analyzed blood samples (Table I). Blood cultures or tissue
fragments following surgery are often negative, which has been
explained by a number of factors that limit blood culture results,
including pre-operative antibiotic use, the specimen collection
method and culture medium used, as well as previous antibiotic
therapy (7). In the study by
Fournier et al (8), it was
found that the PCR method increased 24.3% sensitivity to detect the
presence of C. burnetii in the blood of patients. C.
burnetii is also the most commonly reported organism in cases
of culture-negative endocarditis. When studying 283 cases of
endocarditis with negative blood cultures, Fournier et al
(8) found that C. burnetii
was present in 27 cases (9.5%), exhibiting a higher proportion than
other cases. Other pathogens included Bartonella spp.,
Brucella spp., Tropheryma whipplei, Mycoplasma
spp. and Legionella spp., accounting for up to 5% of all
diagnoses of infective endocarditis (8). According to another study by
Houpikian and Raoult (6) in a
large study on culture-negative endocarditis from 1983-2001 in
France, it was found that C. burnetii accounted for 48% of
all cases diagnosed with infective endocarditis with negative blood
cultures. In the present study, 13/52 cases of endocarditis with
negative blood cultures were detected with C. burnetii
infection, accounting for 25%, similar to the results of the study
by Fournier et al (8), but
markedly lower than the research results of Houpikian and Raoult
(6). A few case reports of
post-operative complications due to C. burnetii have been
reported by cardiovascular surgeons, such as that of Deyell et
al in 2003(9). In the present
study, there was 1 patient who, after the first surgery to repair
the mitral valve lesions and remove the wart, had to have a second
surgery to correct the tricuspid valve; this patient then exhibited
signs of a continuous high fever, multi-organ failure and a severe
clinical course.
In a previous study, patients with valvular heart
disease and Q fever due to acute C. burnetii infection were
shown to have a 38.7% chance of developing endocarditis (10). Patient exposure to animals has been
reported in 70% of cases, with patients not even realizing they
have been infected (10).
Manifestations of C. burnetii causing infective endocarditis
are non-specific and this is the cause of the untimely diagnosis of
this condition. Almost 50% of patients have symptoms of acute heart
failure and the majority of patients suffer from fever (70%),
weight loss, fatigue and anorexia (50%). Manifestations include a
rash on the extremities and mucous membranes, changes in the levels
of hematological parameters, splenomegaly and kidney injury caused
by immune disorders (11), all of
which can lead the patients' conditions being confused with other
clinical conditions.
Molecular techniques for diagnosing endocarditis
from surgical tissues have been available for >20 years and have
become increasingly critical in the diagnosis of endocarditis
(12). These techniques detect the
causative organism in the majority of cases of blood
culture-negative endocarditis and may represent a major step
forward in the management of endocarditis cases in which
antibiotics are used before culture, in patients with inconclusive
serological results, in cases where culture and serology are
negative or, where serological testing is not available (13,14).
Furthermore, molecular sequencing improves the understanding of the
true etiology of endocarditis in different countries and represents
a major step forward in the diagnostic and management of this
disease (14,15).
Asian countries near Vietnam, such as China and
Korea have all recorded the presence of C. burnetii; the
study by Huang et al (16)
recorded an outbreak of a C. burnetii infection in a city in
China (16). The study by Bae
et al (17) conducted in
Korea, recorded 8/40 cases of C. burnetii negative blood
culture endocarditis using PCR analysis (17).
The aforementioned studies exhibit a common factor,
namely that the detection of C. burnetii endocarditis is
difficult using conventional bacterial culture alone, and the PCR
technique is considered a superior technique in determining the
presence of bacteria C. burnetii (13,14).
In the present study, the patients in the C.
burnetiii-positive group identified using the PCR method
exhibited worse clinical signs than the negative group, such as a
persistent high fever following surgery, pneumonia, elevated levels
of liver enzymes (aspartate aminotransferase/alanine
aminotransferase) and weight loss, leading to a longer
hospitalization period (Table
II). Deyell et al (9)
reported a case with complications requiring re-valve surgery due
to latent damage by C. burnetii infection. In the present
study, the majority of the patients had no/unrecorded cardiac
damage that warranted re-surgery. However, there was 1 case of
endocarditis with damage to both the mitral and tricuspid valves
(Fig. 2); following surgery, the
patient exhibited a severe clinical presentation, multi-organ
failure, and a 16 kg weight loss within 20 days; this patient was
found to be positive for C. burnetii using PCR, and the
patient was actively treated immediately after the infection was
detected and was discharged after 62 days of treatment. This was
the case with the longest hospitalization period. Multinucleated
giant cells without a fibrin ring have also been described in the
study by Jang et al (18)
on C. burnetii infection in patients with endocarditis who
had undergone surgery. Another patient in the present study died 6
months following mitral valve surgery due to continued damage to
the tricuspid valve, sepsis and multi-organ failure, who also
tested positive for C. burnetii infection. The delayed
detection of the presence of C. burnetii may reduce the
treatment efficacy. In the present study, patients positive for
C. burnetii infection had a longer hospitalization period
than the negative group (Table
III). This also becomes a burden for patients and their
families, doctors and as hospitals. To the best of our knowledge,
the present study is the first in Vietnam using molecular biology
to detect C. burnetii in the blood of patients with negative
culture results following surgery for endocarditis. Better better
research results can be achieved using 16S RNA primers analyzed on
surgically operated valvular tissue.
In conclusion, edocarditis caused by C.
burnetii infection is difficult to detect, and can cause a
number of cardiovascular complications, limiting the effectiveness
of cardiovascular surgery. The sources of C. burnetii
infection are diverse, and are derived from numerous hosts; in the
event that this type of infection is suspected, it is necessary to
send samples to reputable laboratories for identification, in order
to provide an effective intervention and treatment regimen for
affected patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HDD conceived the study and was the main surgeon for
the patients. ATVD and TMV obtained the patient samples and wrote
the manuscript. HTVB and ATVD performed the analysis of the samples
and PCR analysis. All authors have edited and agree to the reported
content of the manuscript and all authors have read and approved
the final manuscript. HDD and ATVD confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
The study received ethical approval from the Medical
Ethics Committee of Bach Mai Hospital (Hanoi, Vietnam) and informed
consent was obtained from all patients participating in the study.
For 2 patients who were underage, the parents provided the
consent.
Patient consent for publication
The patient whose Doppler echocardiography image is
presented in Fig. 2 provide
written informed consent for the publication of his data and the
related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maurin M and Raoult D: Q fever. Clin
Microbiol Rev. 12:518–553. 1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hanssen DAT, Morroy G, de Lange MMA,
Wielders CCH, van der Hoek W, Dijkstra F and Schneeberger PM:
Notification data and criteria during a large Q-fever epidemic
reassessed. Epidemiol Infect. 147(e191)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
El-Mahallawy HS, Kelly P, Zhang J, Yang Y,
Wei L, Tian L, Fan W, Zhang Z and Wang C: Serological and molecular
evidence of Coxiella burnetii in samples from humans and
animals in China. Ann Agric Environ Med. 23:87–91. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Habib G, Lancellotti P, Antunes MJ,
Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G,
Erba PA, Iung B, et al: 2015 ESC Guidelines for the management of
infective endocarditis: The Task Force for the Management of
Infective Endocarditis of the European Society of Cardiology (ESC).
Endorsed by: European Association for Cardio-Thoracic Surgery
(EACTS), the European Association of Nuclear Medicine (EANM). Eur
Heart J. 36:3075–3128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wilson HG, Neilson GH, Galea EG, Stafford
G and O'brien MF: Q fever endocarditis in Queensland. Circulation.
53:680–684. 1976.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Houpikian P and Raoult D: Blood
culture-negative endocarditis in a reference center: Etiologic
diagnosis of 348 cases. Medicine (Baltimore). 84:162–173.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brouqui P and Raoult D: New insight into
the diagnosis of fastidious bacterial endocarditis. FEMS Immunol
Med Microbiol. 47:1–13. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fournier PE, Gouriet F, Casalta JP, Lepidi
H, Chaudet H, Thuny F, Collart F, Habib G and Raoult D: Blood
culture-negative endocarditis: Improving the diagnostic yield using
new diagnostic tools. Medicine (Baltimore).
96(e8392)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Deyell MW, Chiu B, Ross DB and Alvarez N:
Q fever endocarditis: A case report and review of the literature.
Can J Cardiol. 22:781–785. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fenollar F, Fournier PE, Carrieri MP,
Habib G, Messana T and Raoult D: Risks factors and prevention of Q
fever endocarditis. Clin Infect Dis. 33:312–316. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Houpikian P, Habib G, Mesana T and Raoult
D: Changing clinical presentation of Q fever endocarditis. Clin
Infect Dis. 34:E28–E31. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Fournier PE, Thuny F, Richet H, Lepidi H,
Casalta JP, Arzouni JP, Maurin M, Célard M, Mainardi JL, Caus T, et
al: Comprehensive diagnostic strategy for blood culture-negative
endocarditis: A prospective study of 819 new cases. Clin Infect
Dis. 51:131–140. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Goldenberger D, Künzli A, Vogt P, Zbinden
R and Altwegg M: Molecular diagnosis of bacterial endocarditis by
broad-range PCR amplification and direct sequencing. J Clin
Microbiol. 35:2733–2739. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Moter A, Musci M and Schmiedel D:
Molecular methods for diagnosis of infective endocarditis. Curr
Infect Dis Rep. 12:244–252. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Calabrese F, Carturan E and Thiene G:
Cardiac infections: Focus on molecular diagnosis. Cardiovasc
Pathol. 19:171–182. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang M, Ma J, Jiao J, Li C, Chen L, Zhu
Z, Ruan F, Xing L, Zheng X, Fu M, et al: The epidemic of Q fever in
2018 to 2019 in Zhuhai city of China determined by metagenomic
next-generation sequencing. PLoS Negl Trop Dis.
15(e0009520)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bae M, Lee HJ, Park JH, Bae S, Jung J, Kim
MJ, Lee SO, Choi SH, Kim YS, Shin Y and Kim SH: Molecular diagnosis
of Coxiella burnetii in culture negative endocarditis and
vascular infection in South Korea. Ann Med. 53:2256–2265.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jang YR, Song JS, Jin CE, Ryu BH, Park SY,
Lee SO, Choi SH, Soo Kim Y, Woo JH, Song JK, et al: Molecular
detection of Coxiella burnetii in heart valve tissue from
patients with culture-negative infective endocarditis. Medicine
(Baltimore). 97(e11881)2018.PubMed/NCBI View Article : Google Scholar
|