Introduction
Platelet transfusions are currently performed
worldwide mainly for the treatment of hypoproliferative
thrombocytopenia. Since the early years of platelet transfusions,
it has been shown that platelet ABO and Rhesus D (RhD)
incompatibility does not preclude good clinical responses. However,
platelet transfusions non-identical to ABO and RhD have been found
to be associated with lower platelet counts increments following
transfusion, as well as with adverse reactions such as hemolytic
transfusion reactions and alloimmunization. Hence, despite the
long-term application of platelet transfusions, there is still a
lack of consensus guidelines, as well as a lack of specific
recommendations on ABO and RhD matching (1-4).
A key factor, which also affects platelet
transfusion practice regarding ABO and RhD matching, is their short
self-life which is up to 5 days, and the resulting limited stock of
this blood product, particularly in the absence of central
inventory management. The need to ensure adequate platelet supply,
along with the lack of strict transfusion guidelines as mentioned
above, explains the wide variability in practices associated with
the transfusion of ABO and RhD mismatched platelets by transfusion
centers worldwide (2,5).
The present study was conducted to assess, elucidate
and evaluate ABO and RhD matching in platelet transfusions in
Greece, given the upcoming centralization of blood services.
Materials and methods
Data collection
The present study was carried out from May to June,
2015 by the Working Committee of Transfusion Medicine and Apheresis
of the Hellenic Society of Hematology, as part of a national
survey. An electronic data collection form (Excel 2016,
Microsoft/Corp, WA, USA) was used, and all transfusion services in
Greece were invited to participate in the study. Data collection
was conducted using the aforementioned data forms that were filled
by the participating centers (6).
The study was approved by the Medical Ethics Committee of
Aretaieion Hospital, National and Kapodistrian University of Athens
(Athens, Greece). Informed consent was obtained from all patients
prior to ef8nrolment.
The data collected comprised the number of platelet
units produced, transfused and the platelet units and ABO/D blood
group of the patients. Platelet units consisted of random donor
platelets (RDPs), each of which was derived from a single whole
blood donation prepared from platelet-rich plasma and of
single-donor platelets (SDPs), that were prepared by apheresis.
Statistical analysis
Statistical analysis was performed using the Excel
electronic spreadsheet data forms and using SAS software version
9.3 for Windows (SAS Institute Inc.). For descriptive statistics
arithmetic data are presented as the mean value and standard
deviation (SD), while the categorical data are presented the
frequency of occurrence and the relevant percentage. The confidence
interval of the various calculated proportions was based on normal
approximation and the χ2 test statistical test was
applied for the comparisons of the percentages between groups. All
tests were two-sided. A P-value <0.05 was considered to indicate
a statistically significant difference.
Results
From the 97 transfusion services located all over
Greece that had been invited to join the study, 21 (21.6%)
participated in the study. The total number of platelet units
evaluated was 13,250; 12,061 RDPs and 1,189 SDPs.
Platelet transfusions by platelet product ABO blood
group and patient ABO group are shown in (Fig. 1) ABO identical platelet transfusion
was recorded in 58.99±0.88% of the cases for RDPs and in
45.75±2.87% for SDPs. Transfusion of platelets with major ABO
incompatibility was recorded in 15.92±0.66% of the cases for RDPs
and in 24.05±2.47% for SDPs. The transfusion of platelets with
minor ABO incompatibility was reported in 19.70±0.71% of the cases
for RDPs and in 22.96±2.43% for SDPs. However, a combination of
major and minor ABO incompatibility occurred in 5.38±0.41% and
7.23±1.51% of the cases for RDPs and SDPs, respectively (Fig. 1).
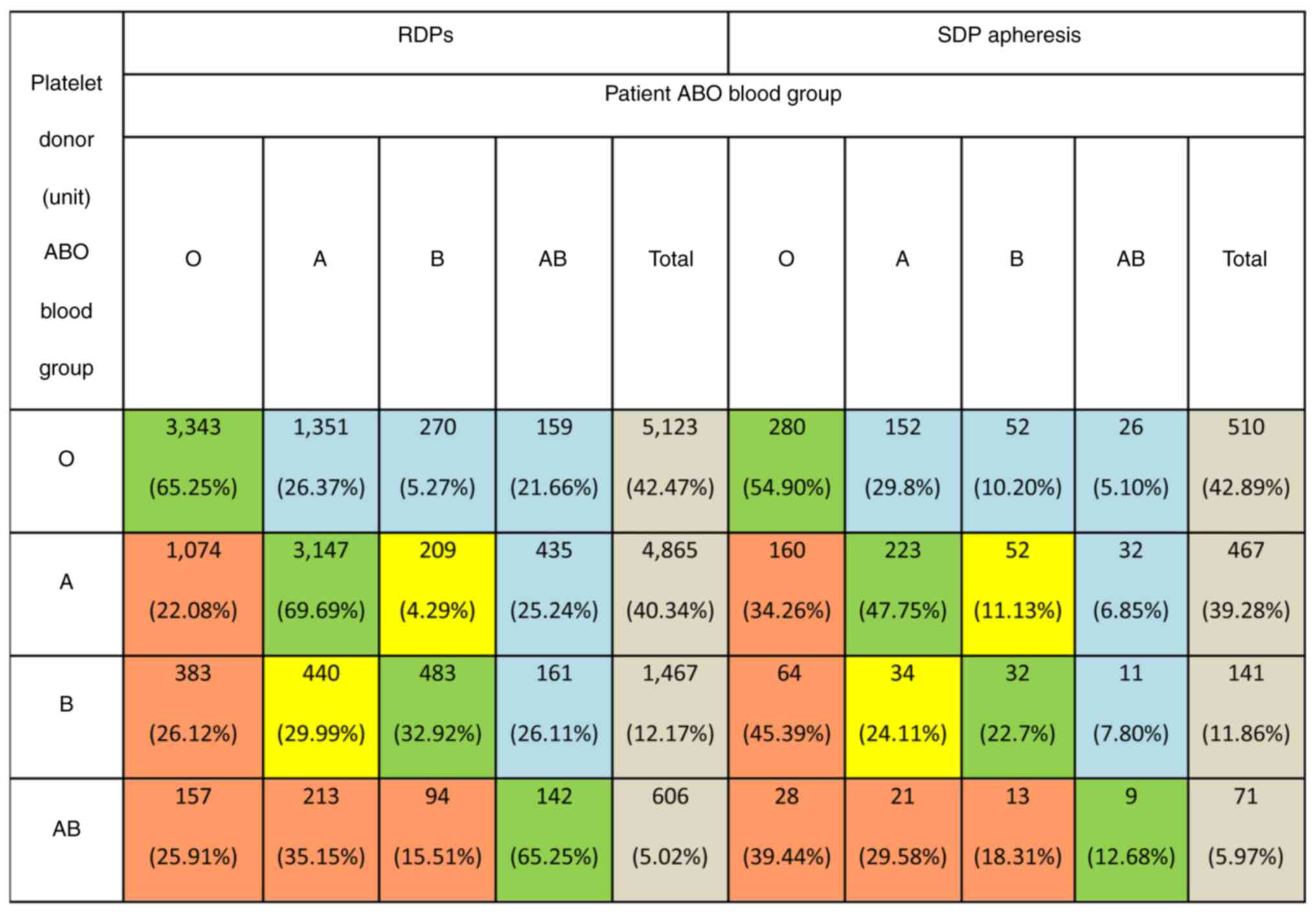 | Figure 1Transfusion of platelets according to
the ABO blood group may be ABO-identical (green boxes), major
ABO-incompatible-antigen incompatible (orange boxes), minor
ABO-incompatible-plasma incompatible (blue boxes), or both major
and minor incompatible (yellow boxes). In major
ABO-incompatibility, donor ABO antigens are incompatible with
recipient ABO antibodies (e.g., transfusion of group A platelets to
a group O recipient). In minor ABO-incompatibility, donor ABO
antibodies are incompatible with recipient ABO antigens. (e.g.,
transfusion of group O platelets to a group A recipient). In
combined major and minor ABO-incompatibility, both donor ABO
antigens are incompatible with recipient ABO antibodies and donor
ABO antibodies are incompatible with recipient ABO antigens (e.g.,
transfusion of group A platelets to a group B recipient). RDPs,
random donor platelets; SDPs, single donor platelets. |
As regards RhD, as shown in Table I, 729/10,892 (6.69%) RhD-positive
RDPs were transfused in RhD-negative patients and accordingly,
132/1,074 (12.29%) RhD-positive SDPs were transfused in
RhD-negative patients (both P<0.001). The percentage of
RhD-negative patients receiving at least one RhD-positive platelet
transfusion was 82.19% (729/887) for RDPs and 81.48% (132/162) for
SDPs.
 | Table IPlatelet transfusion according to
Rhesus D (RhD) type. |
Table I
Platelet transfusion according to
Rhesus D (RhD) type.
| | Patient's RhD
type |
|---|
| | RDPs | SDPs |
|---|
| Platelet donor (unit)
RhD type | RhD (-) (%) | RhD (+) (%) | Total (%) | RhD (-) (%) | RhD (+) (%) |
|---|
| RhD (-) | 158 (13.52) | 1,011 (86.48) | 1,169(100) | 30 (26.09) | 85 (73.91) |
| RhD (+) | 729 (6.69) | 10,163 (93.31) | 10,892(100) | 132 (12.29) | 942 (87.71) |
| Total | 887 (7.35) | 11,174 (92.65) | 12,061(100) | 162 (87.71) | 1,027 (86.38) |
Discussion
The transfusion of platelets with major ABO
incompatibility in the present study was 16% for RDPs and 24% for
SDPs, which is lower than the 32.4% and the 21% previously reported
for SDPs (5,7). The transfusion of platelets with
minor ABO incompatibility (incompatible plasma) was 20 and 23% for
RDPs and SDPs, respectively, in the present study, which is
consistent with the percentage estimated in the USA (10-40%), but
higher than the 12.6% reported by Dunbar et al (5) and the 15% reported by Adamidou et
al (7). No acute hemolytic
transfusion reactions related to platelet transfusion was recorded
by the Greek Hemovigilance Scheme in 2015(8). However, the fact that hemolytic
transfusion reactions due to plasma incompatible platelet
transfusions are under-reported, cannot be overlooked (9,10). A
s
The rather high rate of ABO non-identical platelet
transfusion recorded in the present study mas be attributed to the
lack of central inventory management. The geographical
particularities of Greece may hinder adequate platelet supply in
some cases. In addition, a number of transfusion services issue
platelets for transfusion mainly, according to the first in/first
out strategy to conserve resources and reduce wastage (1,11).
In the present study, ABO non-identical platelet
transfusion was more prominent in apheresis platelets and this may
also be attributed to the fact that SDPs are mainly provided from
non-remunerated replacement donors recruited from the family and
social environment of each patient. It is evident that it is not
always feasible to exchange or replace such donations according to
ABO in the absence of central inventory management (1,5,6).
The ABO and RhD distribution of platelets units
transfused depicted in Fig. 1,
reflects the ABO and RhD distribution in the Greek population
(12). Additionally, it reveals
the lack of an established policy to encourage A group apheresis
platelet donors, ideally A2, that have a weaker A antigen
expression on platelets and lower anti-B titers, instead of O group
donors, as in other developed countries (5,11,13).
As regards RhD in the present study, 86.48 and
73.91% of RhD-negative patients received at least one unit of
RhD-positive RDP or SDP, respectively, which is similar to the 83%
reported by Dunbar et al (5), but slightly higher than the 60.6%
recently reported by Gottschall et al (14). High immunogenic RhD antigen,
although it is not expressed on platelets, RhD group status is
labeled on platelet bags, as residual RBCs and microparticles can
cause alloimmunization in RhD-negative patients after being exposed
to as little as 0.5 ml of RhD-positive RBCs contaminating platelets
(15,16). Whole blood derived RDPs (a dose)
and pooled platelets have up to 0.3 ml of contaminating RBCs, while
SDPs apheresis platelets have <0.001 ml (17). Thus, the risk of alloimmunization
appears to be higher for whole blood derived RDPs than for SDPs
produced by apheresis (18). In
cancer patients however, older studies have indicated a rate of
anti-D alloimmunization greater than 7% (19-21),
although current studies suggest a much lower alloimmunization rate
of ~1% (10,15,22).
Nevertheless, anti-D alloimmunization is still particularly
important for RhD-negative girls or women of child-bearing
potential, due to the risk of hemolytic disease of the fetus and
newborn. Immunoprophylaxis with RhIG should be given in the case of
an inevitable RhD-positive platelet transfusion in this population.
A standard 300 µg dose provides prophylaxis for multiple
transfusions of RhD-positive platelets over a 2-4-week period in
RhD-negative individuals (23,24).
The present study represents a national survey
regarding ABO and RhD matching in platelet transfusion that
assessed 13,250 platelet units transfused. A limitation concerns
the lack of pre-transfusion and post-transfusion platelet count to
assess the impact of ABO major incompatibility, and the lack of the
rate of anti-D alloimmunization due to platelet transfusion of
RhD-positive platelet products to RhD-negative patients. A summary
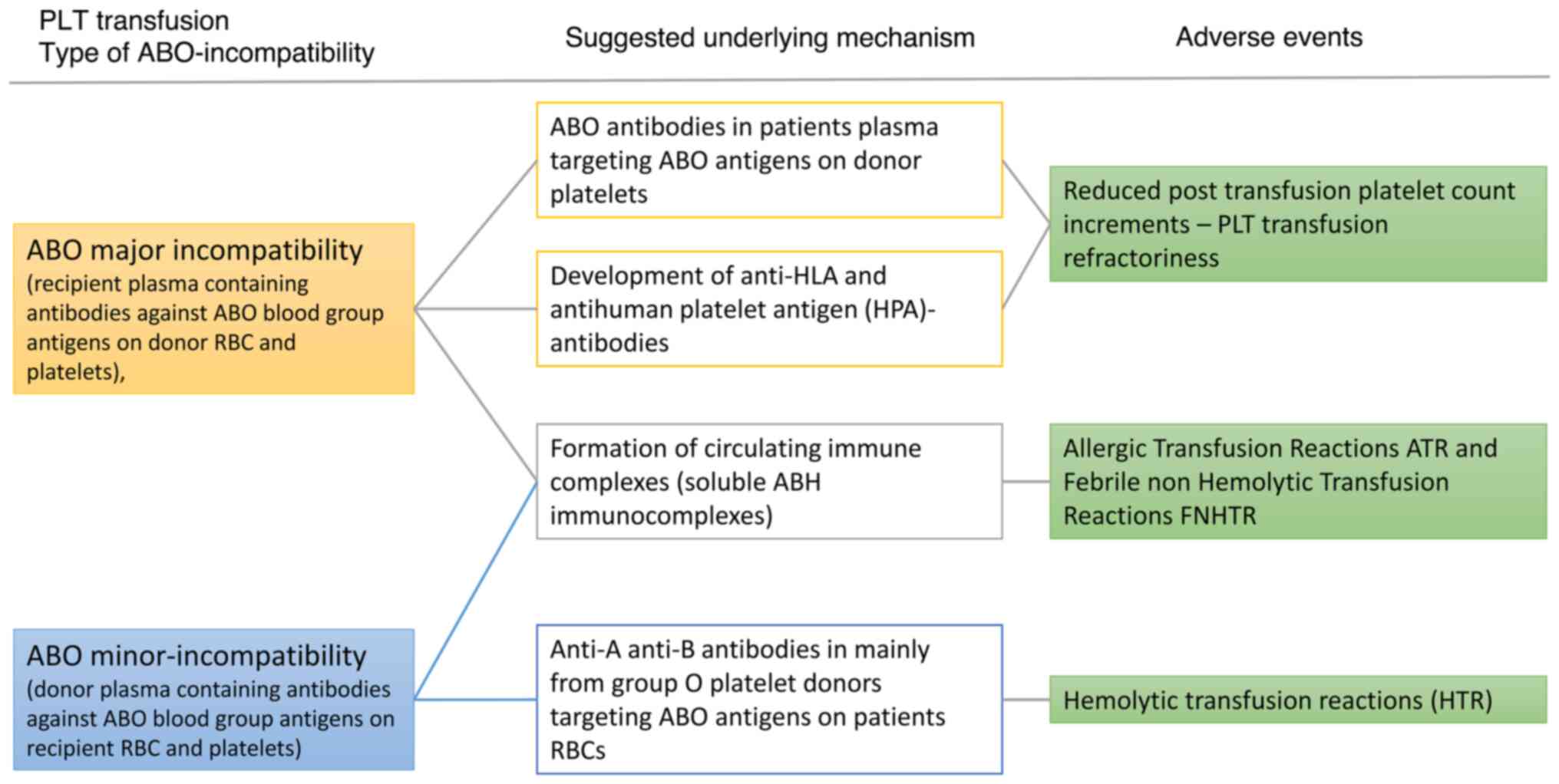
of the proposed mechanisms of ABO incompatibility, adverse events
and suggested underlying mechanisms is illustrated in Fig. 2.
In conclusion according to the real-world data
presented herein, ABO and RhD matching in platelet transfusion
practice varies in Greece, as also demonstrated by other
researchers (14,25), highlighting the necessity for
further studies to clarify the real impact of platelet ABO
compatibility in the management and outcomes of patients. Thus, the
implementation of specific strategies, such as screening group O
platelet donors for high titer ABO antibodies, and new initiatives
may further improve the platelet transfusion practice.
Acknowledgements
The authors would like to acknowledge Dr Evagelia
Triantafillou, Dr Evagelia Arvanitopoulou (both from the Blood
Transfusion Center, University Hospital of Patras, Patras, Greece),
Dr Fotios Girtovitis, Dr Virginia Voulgaridou (both from the Blood
Transfusion Department, University Hospital of Thessaloniki Ahepa,
Thessaloniki, Greece), Dr Aggeliki Megalou, Dr Paraskevi
Chronopoulou (both from the Blood Transfusion Department,
Evangelismos Hospital, Athens, Greece), Mr Andreas Papachronis, Mr
George Sakellarakis (both from the Blood Transfusion Department,
Laiko General Hospital, Athens, Greece), Dr Eleftheria Zervou10,
Mrs Christina Batsi (both from the Blood Transfusion Department,
University Hospital of Ioannina, Ioannina, Greece), Dr Kalliopi
Fountouli (Blood Transfusion Department, University Hospital of
Heraklion, Greece), Dr Aggelos Athanasopoulos (Blood Transfusion
Department, Metaxa Oncology Hospital, Athens, Greece), Dr Elias
Kyriakou (Laboratory of Hematology and Blood Bank Unit, ‘Attikon’
University Hospital, National and Kapodistrian Athens, Athens,
Greece), Dr Afrodite Cheropoulou (Blood Transfusion Department,
General Hospital Sismanoglio, Athens, Greece), Dr Anastasia Livada
(Department of Transfusion Service and Clinical Hemostasis, ‘Saint
Savvas’ Oncology Hospital of Athens, Athens, Greece), Dr
Konstantinos Lebessopoulos (Blood Transfusion Department, ‘Amalia
Fleming’ Hospital, Athens, Greece), Dr Maria Papakonstantinou
(Blood Transfusion Centre, General Hospital of Nikea, Athens,
Greece), Dr Anthi Gafou (Blood Transfusion Department, ‘Agioi
Anargyroi’ Hospital, Athens, Greece), Dr Despina Katopi (Blood
Transfusion Department, General Hospital Alexandra, Athens,
Greece), Dr George Martinis (Blood Transfusion Department,
University Hospital of Alexandroupolis, Alexandroupolis, Greece),
Dr Ioanna Dendrinou (Blood Transfusion Department, General Hospital
Nea Ionia ‘Agia Olga’, Athens, Greece), Dr Hrysanthi Katharopoulou
(Blood Transfusion Department, ‘Hatzikosta’ General Hospital of
Ioannina, Ioannina, Greece), Dr Marianna Politou (Hematology
Laboratory-Blood Bank, Aretaieion Hospital, National and
Kapodistrian University of Athens, Athens, Greece), Dr Margarita
Papadopoulou (Blood Transfusion Department, General Hospital of
Katerini, Katerini, Greece), Dr Paraskevi Papadopoulou (Blood
Transfusion Department, General Hospital of Kavala, Kavala,
Greece), Dr Ekaterini Manaka (Blood Transfusion Department, General
Hospital of Messologgi, Messologgi, Greece), Dr Konstantina Paneta
(Blood Transfusion Department, General Hospital of Pirgos, Pirgos,
Greece), Dr Chrissoula Alepi (Blood Transfusion Department, General
Hospital ‘Tzaneio’ of Piraeus, Athens, Greece), Dr Dimitra
Moshandreou (Department of Transfusion Service and Clinical
Hemostasis, ‘Saint Savvas’ Oncology Hospital of Athens, Athens,
Greece) and Dr Konstantinos Stamoulis (Hellenic National Blood
Transfusion Center, Athens, Greece) for contributing to the
acquisition of and for recording the data. The present study was
performed on behalf of the Working Committee of Transfusion
Medicine and Apheresis of the Hellenic Society of Hematology.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SV conceived, designed and supervised the study, and
wrote the manuscript. AA performed the data entry and evaluation,
and wrote the manuscript. GD analyzed the data, was involved in the
conception of the study and wrote a draft of the manuscript, MG and
EG contributed to study design and analyzed the data in the study.
AP performed the whole statistical analysis. SV and EG confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Aretaieion Hospital, National and Kapodistrian
University of Athens (Athens, Greece). All procedures were in
accordance with the ethical standards of the responsible committee
on human experimentation (institutional and national) and with
the1964 Declaration of Helsinki, and later versions. Informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dunbar NM: Does ABO and RhD matching
matter for platelet transfusion? Hematology Am Soc Hematol Educ
Program. 2020:512–517. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kaufman RM, Djulbegovic B, Gernsheimer T,
Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung
MK, Grossman BJ, et al: Platelet transfusion: A clinical practice
guideline from the AABB. Ann Intern Med. 162:205–213.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Kumar A, Mhaskar R, Grossman BJ, Kaufman
RM, Tobian AA, Kleinman S, Gernsheimer T, Tinmouth AT and
Djulbegovic B: AABB Platelet Transfusion Guidelines Panel. Platelet
transfusion: A systematic review of the clinical evidence.
Transfusion. 55:1116–1127, 1115. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee EJ and Schiffer CA: ABO compatibility
can influence the results of platelet transfusion. Results of a
randomized trial. Transfusion. 29:384–389. 1989.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dunbar NM, Katus MC, Freeman CM and
Szczepiorkowski ZM: Easier said than done: ABO compatibility and D
matching in apheresis platelet transfusions. Transfusion.
55:1882–1888. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Valsami S, Pouliakis A, Gavalaki M,
Argyrou A, Triantafillou E, Arvanitopoulou E, Girtovitis F,
Voulgaridou V, Megalou A, Chronopoulou P, et al: Platelets
transfusion in Greece: Where, when, why? A national survey. Asian J
Transfus Sci. 14:158–166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Adamidou D, Markantonatou A, Avramidou E,
Oikonomou M, Filippou A, Chroni A, Papadopoulou C, Abatzoglou E,
Sidi-Frankandrea V, Kourti M, et al: Efficacy of prophylactic
single donor platelets (SDP) transfusion is related to the time of
storage and not to the ABO compatibility in children with
malignancy. In: Proceedings of the 20th Congress of the European
Hematology Association. EHA, p407, Vienna, 2015.
|
|
8
|
Politis C, Richardson C, Asariotou M,
Grouzi EZ, E, Nomikou E, Katsarou O, Ganidou M, Martinis G,
Hatzitaki M and Halkia P: Transfusion adverse events (TAEs) and
errors/incorrect blood component transfused (IBCT) in Greece
2012-2017. Vox Sang. 114 (Suppl):S228–S229. 2019.
|
|
9
|
Quillen K: Hemolysis from platelet
transfusion: Call to action for an underreported reaction.
Transfusion. 52:2072–2074. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schiffer CA, Bohlke K, Delaney M, Hume H,
Magdalinski AJ, McCullough JJ, Omel JL, Rainey JM, Rebulla P,
Rowley SD, et al: Platelet transfusion for patients with cancer:
American society of clinical oncology clinical practice guideline
update. J Clin Oncol. 36:283–299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Valsami S, Dimitroulis D, Gialeraki A,
Chimonidou M and Politou M: Current trends in platelet transfusions
practice: The role of ABO-RhD and human leukocyte antigen
incompatibility. Asian J Transfus Sci. 9:117–123. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lialiaris T, Digkas E, Kareli D, Pouliliou
S, Asimakopoulos B, Pagonopoulou O and Simopoulou M: Distribution
of ABO and Rh blood groups in Greece: An update. Int J Immunogenet.
38:1–5. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Asllani A, Culler E and Ettkin L: A
simulation-based apheresis platelet inventory management model.
Transfusion. 54:2730–2735. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gottschall J, Wu Y, Triulzi D, Kleinman S,
Strauss R, Zimrin AB, McClure C, Tan S, Bialkowski W, Murphy E, et
al: The epidemiology of platelet transfusions: An analysis of
platelet use at 12 US hospitals. Transfusion. 60:46–53.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Curtis G, Scott M, Orengo L, Hendrickson
JE and Tormey CA: Very low rate of anti-D development in male,
primarily immunocompetent patients transfused with D-mismatched
platelets. Transfusion. 58:1568–1569. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gunson HH, Stratton F, Cooper DG and
Rawlinson VI: Primary immunization of Rh-negative volunteers. Br
Med J. 1:593–595. 1970.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cid J and Lozano M: Risk of Rh(D)
alloimmunization after transfusion of platelets from D+ donors to
D-recipients. Transfusion. 45:453–454. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Reckhaus J, Jutzi M, Fontana S, Bacher VU,
Vogt M, Daslakis M and Mansouri Taleghani B: Platelet transfusion
induces alloimmunization to D and non-D rhesus antigens. Transfus
Med Hemother. 45:167–172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baldwin ML, Ness PM, Scott D, Braine H and
Kickler TS: Alloimmunization to D antigen and HLA in D-negative
immunosuppressed oncology patients. Transfusion. 28:330–333.
1988.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Goldfinger D and McGinniss MH:
Rh-incompatible platelet transfusions-risks and consequences of
sensitizing immunosuppressed patients. N Engl J Med. 284:942–944.
1971.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McLeod BC, Piehl MR and Sassetti RJ:
Alloimmunization to RhD by platelet transfusions in autologous bone
marrow transplant recipients. Vox Sang. 59:185–189. 1990.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cid J, Lozano M, Ziman A, West KA, O'Brien
KL, Murphy MF, Wendel S, Vázquez A, Ortín X, Hervig TA, et al: Low
frequency of anti-D alloimmunization following D+ platelet
transfusion: the Anti-D alloimmunization after D-incompatible
platelet transfusions (ADAPT) study. Br J Haematol. 168:598–603.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ayache S and Herman JH: Prevention of D
sensitization after mismatched transfusion of blood components:
Toward optimal use of RhIG. Transfusion. 48:1990–1999.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Poston JN, Sugalski J, Gernsheimer TB,
Marc Stewart F and Pagano MB: Mitigation strategies for anti-D
alloimmunization by platelet transfusion in haematopoietic stem
cell transplant patients: A survey of NCCN® centres. Vox
Sang. 115:334–338. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Solves Alcaina P: Platelet transfusion:
And update on challenges and outcomes. J Blood Med. 11:19–26.
2020.PubMed/NCBI View Article : Google Scholar
|