1. Introduction
Dyslipidemia is a condition of elevated cholesterol
levels in the blood, which leads to premature atherosclerotic
cardiovascular disease. The common clinical features of this
condition are the development of xanthomas, early coronary
atherosclerosis, hemolytic anemia and liver dysfunction (1). The pathogenesis of dyslipidemia
involves lipid metabolism and several other signaling pathways.
Some of the known risk factors include obesity and diabetes. The
development and evolution of atherosclerotic lesions are influenced
by the pathological processes of lipid deposition in the artery
walls, localized inflammatory processes and endothelial dysfunction
(2). Previous research has
indicated that endoplasmic reticulum (ER) stress (ERS) signaling
pathways are crucial in the development of dyslipidemia and
associated cardiovascular diseases (CVDs). In eukaryotic cells, the
ER plays a crucial role in calcium homeostasis, protein synthesis,
folding and transport (3). ER
homeostasis may be disrupted by several pathogenic conditions,
including hyperlipidemia, oxidative stress and calcium imbalance.
ERS is then caused in the ER lumen by the presence of unfolded or
misfolded proteins (4). Through
various pathways, chronic ERS is connected to the development of
cardiovascular disorders; inflammatory response mechanisms and
apoptotic signaling pathways are also activated as a result of ERS
during this disease phase. This has an impact on lipid metabolism,
which results in dyslipidemia, cell dysfunction, and an alteration
in the creation and stability of atherosclerotic plaques, all of
which are vital factors in the emergence of CVD (5,6).
Recent therapeutic research has also revealed an important function
in a chaperone family known as heat shock protein (HSP)70, a
cytoprotective molecular chaperone involved in protein folding and
degradation. HSP70 is effective in treating and preventing type 2
diabetes mellitus (T2DM). As a result of ERS, mitochondrial
dysfunction and inflammation, HSP70 leads to the emergence of
insulin resistance (IR) as well (7,8). Due
to the crucial role of ERS signaling pathways and their modulation
of other pathogenic pathways, targeting ERS pathways may be the
most effective therapeutic strategy for dyslipidemia. Furthermore,
this may pave the way for a better understanding of the mechanisms
involved and may aid the development of novel therapeutics for this
condition. Thus, the present review discusses the role of the ERS
response and the chaperone system associated with dyslipidemia.
2. Biogenesis of dyslipidemia
It is fascinating to note that several aspects of
metabolic syndrome, including T2DM, hypertension, cerebrovascular
illness and IR, are associated with dyslipidemia (9). As obesity is driven by
pro-inflammatory adipokines and IR, vitamin D (VD) is associated
with maintaining the lipid status; thus, dyslipidemia also plays a
crucial role in obese individuals metabolically. Genes such as
proprotein convertase subtilisin/kexin type 9 (PCSK9) and
sphingosine-1-phosphate (S1P) are the key components that regulate
low-density lipoprotein (LDL) and high-density lipoprotein (HDL)
metabolism and function (10).
PCSK9 is also used as a novel pharmaceutical target for lowering
LDL cholesterol (LDL-C). It lowers the LDL-C intake from the
circulation by promoting the LDL receptor (LDLR) breakdown and
blocking LDLR recirculation to the cell surface (11). By contrast, S1P obtained by the
phosphorylation of sphingosine is catalyzed by sphingosine kinase
(SPHK)1 at the plasma membrane and SPHK2 in the ER, mitochondria
and nucleus. S1P contributes to dyslipidemia by its concentration,
i.e., a low S1P concentration leads to the vasodilation of isolated
arterioles, and a high S1P concentration promotes the circulating
lipids to accumulate inside the arterioles, leading to
vasoconstriction (12).
Nuclear receptors, such as the peroxisome
proliferator-activated receptor (PPAR) and the liver X receptor
(LXR) are considered to be the transcriptional regulators in lipid
metabolism, and they also play a crucial role in maintaining
glucose homeostasis and inflammation by making the receptor
signaling networks novel molecular targets for treating
lipid-related illnesses (13). The
nuclear hormone receptor superfamily's PPAR-α is a ligand-activated
transcription factor that is agonistic toward fibrates. Fibrate
medications are a significant class of medications used in the
treatment of dyslipidemia (14).
LXR, on the other hand, helps in maintaining cholesterol
homeostasis by preventing the uptake of cholesterol through the
intestinal tract, promoting the efflux of cholesterol from cells
into HDLs, facilitating the liver's conversion of HDL into bile
acids, and facilitating biliary excretion. Synthetic LXR agonists,
such as T0901317 and GW3965 mainly target dyslipidemia due to their
notable impact on reverse cholesterol transport, cholesterol
absorption and plasma HDL (15). A
diagrammatic representation of the mechanisms and functions of
PPAR, SP1, LXR and PCSK9 is presented in Fig. 1.
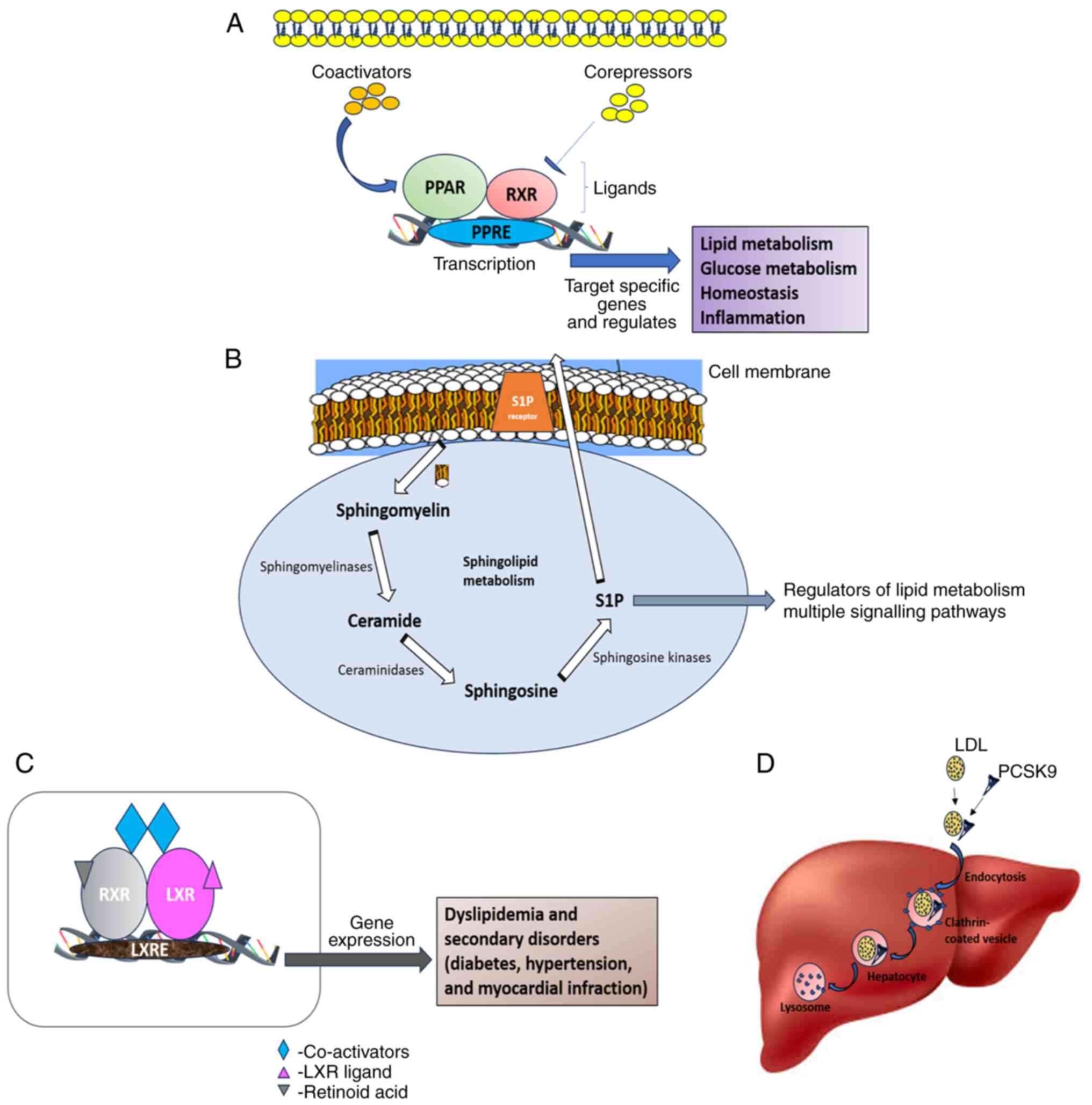 | Figure 1Schematic representation of the
mechanisms and role of (A) PPAR, (B) S1P, (C) LXR, and (D) PCSK9 in
dyslipidemia. PPAR, peroxisome proliferator-activated receptor;
S1P, sphingosine-1-phosphate; LXR, liver X receptor; PCSK9,
proprotein convertase subtilisin/kexin type 9; RXR, retinoid X
receptor; PPRE, PPAR response element; LXRE, LXR response
element. |
The most frequent cause of chronic liver disease,
non-alcoholic fatty liver disease (NAFLD), is considered to be the
hepatic manifestation of the metabolic disease and is intricately
linked to obesity, IR and dyslipidemia. Via hepatic and
extrahepatic routes, insulin affects the metabolism of glucose and
lipids in the liver, and IR serves as a critical factor in the
emergence of dyslipidemia associated with NAFLD, which ultimately
increases the risk of developing early cardiovascular illnesses,
the main cause of morbidity and mortality in patients with NAFLD
(16).
Several lipid molecules, such as phosphatidylcholine
(PC), lysophosphatidylcholine (LPC), sphingomyelin (SM),
diglyceride (DG), monoglyceride (MG) and sphingosine, are involved
in alterations in individuals with dyslipidemia; in these
individuals, it has been found that LPC, DG, MG and sphingosine
levels are upregulated, whereas PC and SM levels are downregulated.
By utilizing the phosphocholine from CDP-choline, DGs can be
transformed into PCs, and CDP-choline also contributes
phosphocholine for the biosynthesis of SMs. DGs interact with acyl
CoA to generate triglycerides (TGs) if they are not transformed
into PCs (17). A downregulation
in the levels of PCs and SMs may also be related to decreased
activities of triggering receptor expressed on myeloid cells 2
(TREM2), which are significant and potent ligands for activating
TREM2 signals that promote macrophage activation. Thus, it could be
concluded that the onset of elevated cholesterol levels in bodily
fluids provokes the immune response associated with various
signaling pathways involved in metabolism.
3. Proteins associated with
dyslipidemia
Dyslipidemia is a medical condition that affects the
levels of fat in the blood. It is characterized by increased levels
of LDL-C and TGs and/or decreased levels of HDL-cholesterol. This
dysfunction can lead to a high risk of developing metabolic
syndrome, obesity, NAFLD or combined hyperlipidemia (18). The levels of cholesterol and TGs in
the blood are closely linked to the number of LDL particles in the
circulation. LDL particles bind to receptors on the surface of
liver cells, including LDL receptor-related protein 1, fatty acid
translocase scavenger receptor (CD36) and LDLR. LDLR is the most
important of these receptors and plays a crucial role in the
removal of LDL particles from the blood (19). The activity of LDLR is modulated by
several factors, including hormones, growth factors and sterol
regulatory element-binding protein 2 (SREBP2). SREBP2 is a
transcription factor that controls the expression of the LDLR gene
(20). The degradation and
recirculation of LDLR to the plasma membrane surface are influenced
by proprotein convertase subtilisin/kexin type 9 serine protease
(PCSK9). PCSK9 binds to the EGF-A domain of LDLR through its
catalytic domain, and it directs LDLR to the endocytic pathway
towards the lysosomes (21).
The interaction between PCSK9 and LDLR is another
key perspective for the regulation of blood cholesterol levels.
Genetic studies have demonstrated that individuals with
loss-of-function mutations in PCSK9 have low cholesterol levels
(22). By contrast, individuals
with gain-of-function mutations in PCSK9 have high cholesterol
levels. Earlier studies on PCSK9 in patients treated with various
therapeutic strategies demonstrated that it may be a promising
therapeutic target for dyslipidemia. In addition to LDLR, PCSK9
also enhances the degradation of CD36(23). CD36 is a protein that has a number
of lipid-related functions in the liver, such as binding free
long-chain fatty acids, or LDL, facilitating their transport into
the cells. The level of CD36 has also been found to be increased in
hepatocytes under lipotoxic conditions or in NAFLD (24).
The PCSK9-mediated control of LDLR recycling is also
influenced by another protein known as Annexin A2 (ANXA2). When
ANXA2 binds to PCSK9, it causes a conformational change in the
protein and hampers its binding to LDLR. This reduces the
degradation of LDLR and increases its recycling to the plasma
membrane surface. As previously demonstrated, LDLR levels were
decreased by 20% in the liver of ANXA2-deficient mice. The
reduction was even more marked in tissues like the adrenal gland
and colon, which are known to be rich in ANXA2 and resistant to the
PCSK9 effect (25,26).
Ceramides are a type of lipid that can modulate
insulin sensitivity, cause metabolic derangement and stimulate cell
death. They are also involved in the oxidation of LDL particles.
The lipid composition of the diet can affect the levels of
ceramides in the body, and different diet patterns will have
differential effects on lipid profiles in hepatocytes. Some lipids
are more toxic, and they induce oxidative and inflammatory
processes. This can promote the progression and severity of
dyslipidemia-associated diseases, such as NAFLD (27). The difference between the effects
of ceramides and lipids is summarized in Table I.
 | Table IEffects of ceramides and lipids under
different metabolic conditions. |
Table I
Effects of ceramides and lipids under
different metabolic conditions.
| Metabolic
conditions | Effect of
ceramides | Effect of
lipids |
|---|
| Insulin
sensitivity | Decreased | Decreased |
| Metabolic
derangement | Increased | Increased |
| Cell death | Stimulated | Stimulated |
| Oxidation of LDL
particles | Increased | Increased |
| Oxidative
stress | Increased | Increased |
| Inflammation | Increased | Increased |
The most common cause of secondary hyperlipidemia in
children and adults is nephrotic syndrome, which causes the kidneys
to leak protein into the urine, which leads to various health
disorders, including high cholesterol levels. In patients with
nephrotic syndrome, the levels of lipoproteins, including
intermediate-density lipoprotein, very high LDL and LDL are
elevated. This is due to the fact that the liver produces more
lipoproteins in an attempt to compensate for the protein loss in
the urine. In addition, patients with nephrotic syndrome exhibit a
downregulation in hepatic lipase and lipoprotein lipase activities
that are responsible for breaking down lipoproteins. The
downregulation of such enzymatic events leads to an increase in the
levels of lipoproteins in the blood. Patients with nephrotic
syndrome also have high plasma PCSK9 levels (28). PCSK9 breaks down the LDL receptor
to remove LDL from the blood. Thus, the high levels of PCSK9 lead
to decreased LDL clearance and elevated plasma LDL-C levels. As a
result of these changes, patients with nephrotic syndrome are more
likely to develop atherosclerosis, which is a narrowing of the
arteries that can lead to heart attack, stroke, and other
cardiovascular complications. Furthermore, they are also more
likely to experience progressive kidney disease and premature death
(29).
4. Endoplasmic reticulum stress
In order to ensure that only proteins that have been
correctly folded may reach their destination, the main role of the
ER is to perform effective quality control (30). Specific chaperones of the reticular
compartment tightly control this maturation process, and three
groups may be formed from these: i) glucose regulated protein
(GRP)78 and GRP94, which promote the folding and assembly of
quasi-proteins (31); ii) lectins,
calreticulin and calnexin play a crucial role in the maturation
phase of glycoproteins (32),
whereas in collagens; iii) HSP47 plays a major role (33). Moreover, there is a diverse
functional family of enzymes, such as protein disulfide isomerases,
that catalyze the synthesis or destruction of disulfide bonds
[protein disulfide isomerases (PDIs)], which are essential for
protein complexity. The steps involved in protein folding are
illustrated in Fig. 2.
Endoplasmic reticulum-associated protein degradation
(ERAD) is the process by which broken or improperly folded proteins
are trapped and removed under physiological circumstances (34). Calcium is essential to this process
as reticular chaperones have a range of affinities for it, and
their activity is regulated by variations in its concentration
(35). Folding and refolding
constitute a net energy consumption in the form of ATP in any
circumstance that alters intracellular concentration levels, which
can inhibit their function. Reticular homeostasis is modified when
the capacity of the reticular antioxidant systems is saturated as
PDI activity involves a net generation of reactive oxygen species.
This stress response has the power to impair the ability of protein
complexes to ensure the proper folding of newly synthesized
proteins, leading to aggregation and protein misfolding, a state
more appropriately known as ‘reticular stress’ (36). Furthermore, it has been found that
the unfolded protein response (UPR) signal transduction system is
triggered in response to ERS. The UPR activation enables the cell
to manage the increased demand for protein folding in the ER.
Moreover, apart from the biological mechanisms, no
evidence has been found in clinical analyses (via biological
fluids) to prove that ERS is associated with chaperones. By
contrast, an important ERS moderator known as GRP78 has been
studied (37). Such circumstances
involve the ER-stress-dependent dysregulation of lipid metabolism
and its homeostasis.
5. Role of the ER in protein complexity
In ER, misfolded proteins trigger the production of
ER-resident chaperones, thus activating GRP78/binding
immunoglobulin protein (BiP) as a result, and temporarily reducing
protein synthesis. The UPR is regarded as an adaptive and
cytoprotective mechanism as it restores ER capacity by rebalancing
protein load and folding (38).
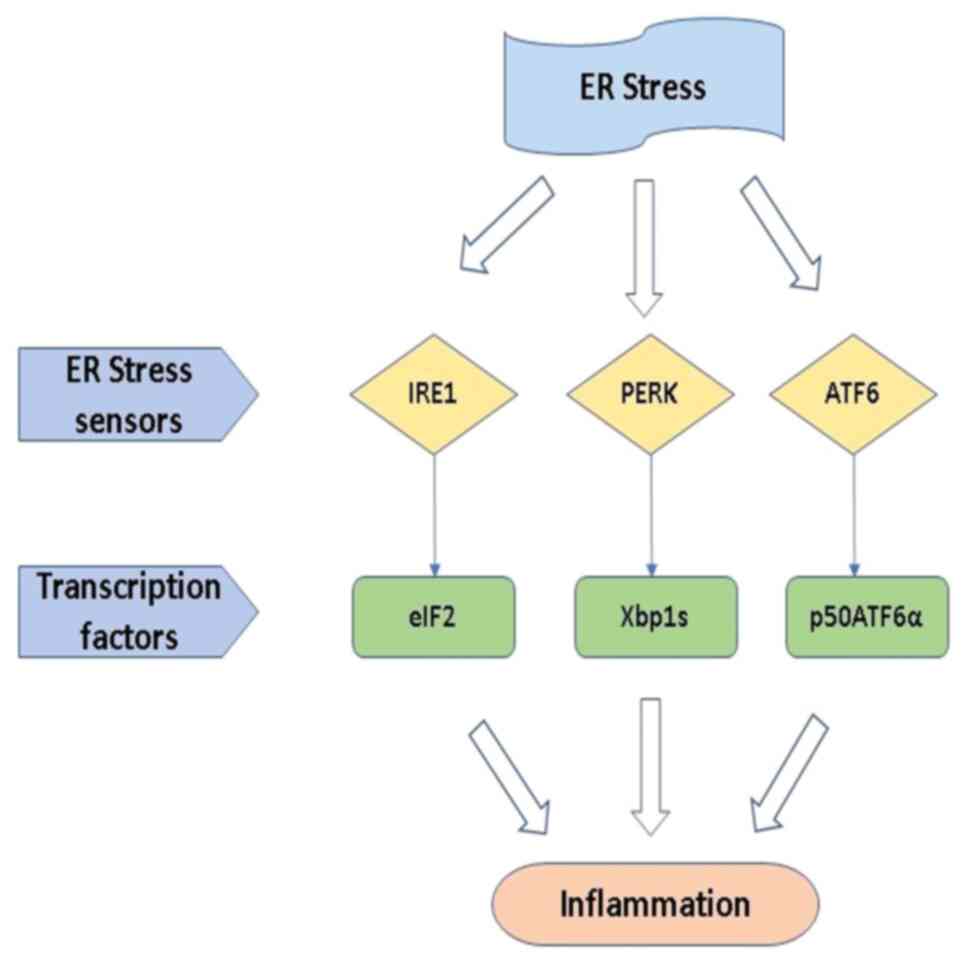
The inositol-requiring enzyme 1 (IRE1), activating transcription
factor 6 (ATF6) and protein kinase RNA-activated (PKR)-like ER
kinase (PERK) are the three main transmembrane protein/stress
sensors that regulate the UPR. These stress sensors are kept in an
inactive state by the ER chaperone GRP78/BiP, which is linked to
them. Unfolded or misfolded proteins bind to BiP when ERS causes it
to accumulate in the ER lumen, facilitating BiP dissociation and
activating downstream signaling (39,40).
Homodimerization and trans-autophosphorylation are used to activate
PERK when proteins are detached from Bip. The activated PERK
phosphorylates the α-subunit of eukaryotic translation initiation
factor 2 (eIF2) at Ser51, resulting in temporary inhibition of
protein synthesis. Activating transcription factor (ATF)4 is a
transcription factor whose translation is paradoxically made more
efficient by eIF2 phosphorylation in post-translational
modifications. To dephosphorylate eIF2 and restore overall mRNA
translation, ATF4 enhances the expression of its target genes,
including DNA damage-inducible protein 34 and growth arrest.
IRE1 is the same as PERK; activated IRE1 produces a
transcription factor (Xbp1s), and it regulates the gene expression
of ER protein folding, ERAD, and protein secretion from the cell.
ATF6 produces a cytosolic fragment (p50ATF6α), which acts as a
transcription factor to enhance the activity of ER capacity
(41). Hence, it is suggested that
further in vivo studies tuning the transcription factors of
Xbp1s and ATF6 with modified proteins or chemical components are
required to overcome the stress responses caused by liver disorders
and lipid abnormalities (42). The
overall possibilities of the ERS response in intracellular
signaling are illustrated in Fig.
3.
6. Role of chaperones in dyslipidemia
Chaperones are the molecules of the ER that
encounter proteins in folding mechanisms. Generally, chaperones can
be classified into three types based on their biological, chemical
and pharmacological properties. Unspecific molecular chaperones are
found in all living organisms and assist in protein folding.
Similarly, chemical chaperones are extracellular components that
aid in protein folding. By contrast, pharmacological chaperones,
which are G protein coupled receptor (GPCR) ligands, are
particularly beneficial for GPCR folding (43). Some of the key chaperone systems
linked to dyslipidemia include the following:
i) HSP70 and HSP72. HSPs are cytoprotective
as they protect proteins, lipids and nucleic acids from oxidative
stress, and additionally, they influence gene expression and cell
function, which contribute to maintaining protein homeostasis. Fat
deposition, the accumulation of oxidants, mitochondrial malfunction
and an overactive renin-angiotensin system are linked to nitric
oxide (NO), and nicotinamide adenine dinucleotide phosphate oxidase
activity is all associated with IR, leading to dyslipidemia. The
reduced NO release in the blood promotes HSP70, which protects
against oxidative stress damage, inflammation, and apoptosis in IR
and diabetes, whereas HSP72 is connected to vascular complications
brought on by a high-fat diet (44,45).
Thus, elucidating the functions of NO and HSPs is critical for use
in novel therapies, such as thermal therapy, nitrosylated
medications, chemical chaperones and exercise training.
VD and vitamin D receptor (VDR) play a crucial role
in the cardiovascular system and IR metabolism. Maintaining VD
concentrations is critical. Koroshi and Idrizi (46) proved that HSP70 is involved in
regulating the VDR concentrations of cells, and intracellular
VD-binding proteins associated with HSP70 control the metabolism of
VD. Thus, HSP70 in VD is concerned with the therapeutic advantage
of controlling the protein expression in the cell corresponding to
cardiovascular illness (47). It
is fascinating to note that T2DM, hypertension, cerebrovascular
illness and IR are several conditions of metabolic syndrome related
to Alzheimer's disease (AD), apart from dyslipidemia.
ii) Clusterin. Clusterin, otherwise known as
apolipoprotein J (ApoJ), functions as a molecular chaperone in
secreted protein folding, and it has been shown that altered
clusterin expression and protein levels have been linked to IR,
dyslipidemia and AD associated with CVDs (48). In our view, ApoJ is a heterodimeric
glycoprotein involved in muscle and nerve actions. Hence, it can be
utilized in the transportation of lipid molecules from one region
to another. In addition, it plays a major role in tissues and
membranes in maintaining protein folding stability. Recently, ApoJ
has been investigated in neurodegenerative disorders, such as
Parkinson's disease (49) and AD
(50).
iii) HSP47. HSP47 is a responsible chaperone
in collagen folding, otherwise known as collagen-specific stress
protein. In vivo studies on rats with hypercholesteremia
supplemented with a 2% cholesterol diet have demonstrated that
HSP47 induction serves as a treatment for hypercholesteremia by
contributing to glomerulosclerosis and collagen synthesis. Of note,
HSP47 is abundantly involved in liver fibrosis (a condition of
excess accumulation of collagen) (51) and further studies are required to
shed further light on its actions.
It is known that chaperones work based on ER signals
(intracellular signals), which play a significant role in
post-translational modifications. Thus, the further development of
chemically synthesized chaperones that have the capability to
reduce ERS may serve as novel therapeutics for diseases. In
addition, it has been shown that tauroursodeoxycholic acid (TUDCA;
a chemical chaperone) reduces ERS and rescues hypercholesteremia in
neutrophil extracellular traps (52).
A summary of the roles and differences between
chaperone systems in dyslipidemia is presented in Table II (53-60).
 | Table IIRole and differences between
chaperone systems. |
Table II
Role and differences between
chaperone systems.
| Chaperone
system | Role in
dyslipidemia | Differences |
Authors/(Refs.) |
|---|
| HSP70 | Aids in the
preservation of cholesterol homeostasis by encouraging the
breakdown of excess cholesterol and avoiding its cellular
build-up | All cells have the
ubiquitous chaperone system HSP70. It is engaged in many different
biological functions, such as the folding, transport, and
destruction of proteins | Gungor et al
(53), Wang et al (54) |
| HSP72 | In dyslipidemia, it
plays a similar role to HSP70, although it is more selectively
expressed in the liver and other organs involved in lipid
metabolism | Although HSP72 is a
chaperone belonging to the HSP70 family, it possesses several
distinct characteristics. For instance, it has a greater affinity
for certain proteins and is more resistant to denaturation than
HSP70 | Dong et al
(55), Johnson et al
(56) |
| Clusterin | A versatile protein
that chaperones lipids and lipoproteins among other types of
proteins. It is considered that clusterin contributes to the
prevention of cholesterol and other lipids from clumping together
in dyslipidemia | Clusterin is a
distinct chaperone system because it is not a member of the HSP
family of chaperones. It is also the only known chaperone mechanism
that is secreted from cells | Wittwer and Bradley
(57), Zhu et al (58) |
| HSP47 | A chaperone that
plays a specific role in collagen transport across the endoplasmic
reticulum membrane. HSP47 is hypothesized to have a unction in
limiting cholesterol buildup in the liver in dyslipidemia | HSP47 belongs to
the chaperone HSP40 family. HSP40 and HSP70 chaperones collaborate
to aid in protein folding and transport | Xu et al
(59), Sepulveda et al
(60) |
7. Summary and conclusions
The long-term consequences of ERS on protein
complexity were not discussed in the present review. To fully
comprehend the long-term effects of ERS on protein folding and
function, further investigations are required. The present study
did not account for individual differences in ERS responsiveness.
It is possible that some individuals are more vulnerable to the
effects of ERS than others. Although the ERS response is implicated
in other illnesses, including diabetes, obesity and cancer, the
present review concentrates on the involvement of the ERS response
in dyslipidemia and is based on a small amount of research.
Therefore, in order to create novel therapeutics that target the
ERS response, further studies are required to confirm the findings
discussed herein and clarify the function of protein complexity and
the ERS response in dyslipidemia. Furthermore, to comprehend the
significance of the ERS response in other disorders with the
potential to significantly influence the development of novel
therapeutics for dyslipidemia, the current review offers a fresh
and thorough assessment of the function of protein complexity and
the ERS response in dyslipidemia.
The present review provides an overview of the roles
played by protein complexity and the ERS response in dyslipidemia.
A significant risk factor for CVD is characterized by high blood
levels of TGs and/or cholesterol. Due to oxidative stress, calcium
imbalance and elevated cholesterol levels, dyslipidemia can result
in an overflow of unfolded proteins in the ER. This sets off the
ERS response, which can cause inflammation and mortality; however,
it can also have an adaptive effect by upregulating chaperone
expression and aiding in the restoration of ER function.
Aside from highlighting the significance of taking
oxidative stress, calcium imbalance and high cholesterol into
account when developing novel therapeutics for dyslipidemia, the
present review also provides new insight into the role of protein
complexity and the ERS response in the disease. It also discusses
the role of chaperones in the ERS response and how this may be used
to develop novel therapies for dyslipidemia.
8. Suggestions and future perspectives
The identification of multiple roles for ER in
physiology and disease was made possible by the progression in our
understanding of ER activities. Relevant opportunities to make use
of this knowledge were made possible by the ER, and several aspects
were identified, including homeostatic regulators and the UPR. The
UPR connects with a variety of physiological functions, including
protein folding, transcriptional regulation, translational control,
protein degradation, and the regulation of signaling pathways that
determine the destiny of a cell. Moreover, ERS regulators and
chaperones are considered to be novel molecular diagnostic
indicators and therapeutic targets. With this salient point, some
of the key techniques and molecules for the development of future
novel therapeutics are provide below, which include clustered
regularly interspaced short palindromic repeats (CRISPR)/CRISPR
associated protein 9 (Cas9), TUDCA, adenosine monophosphate
activated protein kinase (AMPK), C/EBP homologous protein (CHOP)
and PH domain and leucine rich repeat protein phosphatase 1
(PHLPP1).
i) FDA-approved compounds that can be utilized in
human medicine include 4-phenylbutyric acid and TUDCA, which are
chemically synthesized chaperones. It has been proven that TUDCA
can relieve ERS and prevent hypercholesterolemia in macrophages
(52). Additionally, sirtuin-1
chaperone activity is regulated to control raptor acetylation,
which in turn suppresses mammalian target of rapamycin complex
1-dependent protein synthesis and decreases ER overload (61).
ii) CHOP drives the main proapoptotic pathways
brought on by ERS. Hence, CHOP inhibitors can be designed
selectively to target the host to achieve a reduced ERS response.
Research on CHOP activation has determined that the upregulation of
microRNAs (miR-33) in atherosclerotic macrophages disrupted the ERS
response in lipid metabolism (62).
iii) PHLPP1, when disrupted using CRISPR/Cas9,
zebrafish larvae and C. elegans, exhibits less lipid
deposition in their intersegmental arteries, as well as lower
levels of total cholesterol and TGs in vivo analysis
(63). Thus, PHLPP1 can be
utilized in similar animal models to reduce the accumulation of
lipids in the arteries.
iv) The AMPK signaling pathway is considered to
regulate ERS (64). AMPK functions
as a physiological ERS inhibitor, and the effect of inhibition is
maintained by the sarco-endoplasmic reticulum calcium ATPase
activity and intracellular Ca2+. Among the widely used
statin drug types in dyslipidemia, it has been shown that
atorvastatin is an extensively researched pharmaceutical drug that
can decrease ERS through activating AMPK in in vivo studies
on atherosclerotic mice and cultured human umbilical vein-derived
endothelial cells (65).
v) Additionally, it is suggested that utilizing
herbal remedies may also serve as a preventative against
dyslipidemia by inhibiting the signaling pathways that are
upregulated during ERS. For instance, a study in the journal
‘Phytomedicine’, found that the herb ginger (Zingiber
officinale) was able to inhibit the expression of genes
involved in ERS and inflammation in mice with high cholesterol
levels. The study also found that ginger was able to reduce the
levels of cholesterol and TGs in the blood of mice (66). Another study was conducted on
turmeric (Curcuma longa) and explored similar results to
ginger (67). The mechanisms by
which ginger and turmeric exert these effects are not yet fully
understood, although it is considered that they may involve the
inhibition of enzymes called kinases, which are involved in the
signaling pathways that are upregulated during ERS. The detailed
perspectives and mechanisms involved are under research that may
conclude with an alternate remedy to decrease the levels of
cholesterol and TGs in the blood (66,68).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MSAH was involved in the conceptualization of the
study, in the submission of the final manuscript and in
correspondence. RK was involved in the reviewing and editing of the
manuscript. MSAH and RK were involved in the literature search for
studies to be included in the review. All the authors have read and
approved the manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
García-Giustiniani D and Stein R: Genetics
of Dyslipidemia. Arq Bras Cardiol. 106:434–438. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fuster JJ: Integrated stress response
inhibition in atherosclerosis. Preventing the Stressed-Out Plaque.
J Am Coll Cardiol. 73:1170–1172. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Engin F and Hotamisligil GS: Restoring
endoplasmic reticulum function by chemical chaperones: An emerging
therapeutic approach for metabolic diseases. Diabetes Obes Metab.
12 (Suppl 2):S108–S115. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dandekar A, Mendez R and Zhang K: Cross
talk between er stress, oxidative stress, and inflammation in
health and disease. Methods Mol Biol. 1292:205–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kruzliak P, Sabo J and Zulli A:
Endothelial endoplasmic reticulum and nitrative stress in
endothelial dysfunction in the atherogenic rabbit model. Acta
Histochemica. 117:762–766. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang C, Syed TW, Liu R and Yu J: Role of
endoplasmic reticulum stress, autophagy, and inflammation in
cardiovascular disease. Front Cardiovasc Med. 4(29)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Esser N, Legrand-Poels S, Piette J, Scheen
AJ and Paquot N: Inflammation as a link between obesity, metabolic
syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Krause M, Bock PM, Takahashi HK, Homem De
Bittencourt PI Jr and Newsholme P: The regulatory roles of NADPH
oxidase, intra- and extra-cellular HSP70 in pancreatic islet
function, dysfunction and diabetes. Clin Sci (Lond). 128:789–803.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gottesman RF, Albert MS, Alonso A, Coker
LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett
AR, et al: Associations Between Midlife Vascular Risk Factors and
25-Year Incident Dementia in the Atherosclerosis Risk in
Communities (ARIC) Cohort. JAMA Neurol. 74:1246–1254.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vekic J, Zeljkovic A, Stefanovic A,
Jelic-Ivanovic Z and Spasojevic-Kalimanovska V: Obesity and
dyslipidemia. Metabolism. 92:71–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lin XL, Xiao LL, Tang ZH, Jiang ZS and Liu
MH: Role of PCSK9 in lipid metabolism and atherosclerosis. Biomed
Pharmacother. 104:36–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen W, Lu H, Yang J, Xiang H and Peng H:
Sphingosine 1-phosphate in metabolic syndrome (Review). Int J Mol
Med. 38:1030–1038. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Beaven SW and Tontonoz P: Nuclear
receptors in lipid metabolism: Targeting the heart of dyslipidemia.
Annu Rev Med. 57:313–329. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Botta M, Audano M, Sahebkar A, Sirtori CR,
Mitro N and Ruscica M: PPAR Agonists and Metabolic Syndrome: An
Established Role? Int J Mol Sci. 19(1197)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang B and Tontonoz P: Liver X receptors
in lipid signalling and membrane homeostasis. Nat Rev Endocrinol.
14:452–463. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Akhtar DH, Iqbal U, Vazquez-Montesino LM,
Dennis BB and Ahmed A: Pathogenesis of insulin resistance and
atherogenic dyslipidemia in nonalcoholic fatty liver disease. J
Clin Transl Hepatol. 7:362–370. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ke C, Zhu X, Zhang Y and Shen Y:
Metabolomic characterization of hypertension and dyslipidemia.
Metabolomics. 14(117)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Csonka C, Baranyai T, Tiszlavicz L, Fébel
H, Szűcs G, Varga ZV, Sárközy M, Puskás LG, Antal O, Siska A, et
al: Isolated hypercholesterolemia leads to steatosis in the liver
without affecting the pancreas. Lipids Health Dis.
16(144)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Begg MJ, Sturrock ED and van der
Westhuyzen DR: Soluble LDL-R are formed by cell surface cleavage in
response to phorbol esters. Eur J Biochem. 271:524–533.
2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Streicher R, Kotzka J, Müller-Wieland D,
Siemeister G, Munck M, Avci H and Krone W: SREBP-1 Mediates
Activation of the Low Density Lipoprotein Receptor Promoter by
Insulin and Insulin-like Growth Factor-I. J Biol Chem.
271:7128–7133. 1996.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kwon HJ, Lagace TA, McNutt MC, Horton JD
and Deisenhofer J: Molecular basis for LDL receptor recognition by
PCSK9. Proc Natl Acad Sci USA. 105:1820–1825. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Demers A, Samami S, Lauzier B, Des Rosiers
C, Ngo Sock ET, Ong H and Mayer G: PCSK9 Induces CD36 Degradation
and Affects Long-Chain Fatty Acid Uptake and Triglyceride
Metabolism in Adipocytes and in Mouse Liver. Arterioscler Thromb
Vasc Biol. 35:2517–2525. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pepino MY, Kuda O, Samovski D and Abumrad
NA: Structure-Function of CD36 and Importance of Fatty Acid Signal
Transduction in Fat Metabolism. Annu Rev Nutr. 34:281–303.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Greco D, Kotronen A, Westerbacka J, Puig
O, Arkkila P, Kiviluoto T, Laitinen S, Kolak M, Fisher RM, Hamsten
A, et al: Gene expression in human NAFLD. Am J Physiol Gastrointest
Liver Physiol. 294:G1281–G1287. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zaid A, Roubtsova A, Essalmani R,
Marcinkiewicz J, Chamberland A, Hamelin J, Tremblay M, Jacques H,
Jin W, Davignon J, et al: Proprotein convertase subtilisin/kexin
type 9 (PCSK9): Hepatocyte-specific low-density lipoprotein
receptor degradation and critical role in mouse liver regeneration.
Hepatology. 48:646–654. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Seidah NG, Poirier S, Denis M, Parker R,
Miao B, Mapelli C, Prat A, Wassef H, Davignon J, Hajjar KA and
Mayer G: Annexin A2 is a natural extrahepatic inhibitor of the
PCSK9-Induced LDL receptor degradation. PLoS One.
7(e41865)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Németh K, Tóth B, Sarnyai F, Koncz A,
Lenzinger D, Kereszturi É, Visnovitz T, Kestecher BM, Osteikoetxea
X, Csala M, et al: High fat diet and PCSK9 knockout modulates lipid
profile of the liver and changes the expression of lipid
homeostasis related genes. Nutr Metab (Lond). 20(19)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Agrawal S, Zaritsky JJ, Fornoni A and
Smoyer WE: Dyslipidaemia in nephrotic syndrome: Mechanisms and
treatment. Nat Rev Nephrol. 14:57–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kongmalai T, Chuanchaiyakul N,
Srinoulprasert Y and Thongtang N: Injection of an improperly stored
proprotein convertase subtilisin/kexin type 9 monoclonal antibody
in a patient with secondary dyslipidemia from nephrotic syndrome: A
case report. J Med Case Rep. 17(89)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Almanza A, Carlesso A, Chintha C,
Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N,
Montibeller L, More S, et al: Endoplasmic reticulum stress
signalling – from basic mechanisms to clinical applications. FEBS
J. 286:241–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fu XL and Gao DS: Endoplasmic reticulum
proteins quality control and the unfolded protein response: The
regulative mechanism of organisms against stress injuries.
BioFactors. 40:569–585. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Williams DB: Beyond lectins: The
calnexin/calreticulin chaperone system of the endoplasmic
reticulum. J Cell Sci. 119(Pt 4):615–623. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ishida Y and Nagata K: Hsp47 as a
collagen-specific molecular chaperone. Methods Enzymol.
499:167–182. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hirsch C, Gauss R, Horn SC, Neuber O and
Sommer T: The ubiquitylation machinery of the endoplasmic
reticulum. Nature. 458:453–460. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Coe H and Michalak M: Calcium binding
chaperones of the endoplasmic reticulum. Gen Physiol Biophys 28
Spec No Focus: F96-F103, 2009.
|
|
36
|
Bhandary B, Marahatta A, Kim HR and Chae
HJ: An involvement of oxidative stress in endoplasmic reticulum
stress and its associated diseases. Int J Mol Sci. 14:434–456.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Al Zaidi M, Repges E, Sommer-Weisel S,
Jansen F, Zimmer S, Tiyerili V, Nickenig G and Aksoy A: Serum
levels of the endoplasmic-reticulum-stress chaperone GRP78 identify
patients with coronary artery disease and predict mortality. Eur
Heart J. 43 Suppl2(ehac544.1137)2022.
|
|
38
|
Sannino S and Brodsky JL: Targeting
protein quality control pathways in breast cancer. BMC Biol.
15(109)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Shen J, Chen X, Hendershot L and Prywes R:
ER Stress Regulation of ATF6 Localization by Dissociation of
BiP/GRP78 binding and unmasking of Golgi localization signals. Dev
Cell. 3:99–111. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Han J and Kaufman RJ: The role of ER
stress in lipid metabolism and lipotoxicity. J Lipid Res.
57:1329–1338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu X, Khalafalla M, Chung C, Gindin Y,
Hubchak S, LeCuyer B, Kriegermeier A, Zhang D, Qiu W, Ding X, et
al: Hepatic Deletion of X-Box Binding Protein 1 in FXR Null mice
leads to enhanced liver injury. J Lipid Res.
63(100289)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tao YX and Conn PM: Chaperoning G
Protein-Coupled Receptors: From cell biology to therapeutics.
Endocr Rev. 35:602–647. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Macario AJ and Conway de Macario E: Sick
chaperones, cellular stress, and disease. N Engl J Med.
353:1489–1501. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Molina MN, Ferder L and Manucha W:
Emerging role of nitric oxide and heat shock proteins in insulin
resistance. Curr Hypertens Rep. 18(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Koroshi A and Idrizi A: Renoprotective
effects of Vitamin D and renin-angiotensin system. Hippokratia.
15:308–311. 2011.PubMed/NCBI
|
|
47
|
Adams JS, Chen H, Chun RF, Nguyen L, Wu S,
Ren SY, Barsony J and Gacad MA: Novel regulators of vitamin D
action and metabolism: Lessons learned at the Los Angeles zoo. J
Cell Biochem. 88:308–314. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bradley D: Clusterin as a potential
biomarker of obesity-related Alzheimer's disease risk. Biomark
Insights. 15(1177271920964108)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Berdowska I, Matusiewicz M and
Krzystek-Korpacka M: HDL Accessory proteins in parkinson's
disease-focusing on clusterin (Apolipoprotein J) in regard to its
involvement in pathology and diagnostics-A review. Antioxidants
(Basel). 11(524)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fareed MM, Qasmi M, Aziz S, Völker E,
Förster CY and Shityakov S: The role of clusterin transporter in
the pathogenesis of Alzheimer's disease at the blood-brain barrier
interface: A systematic review. Biomolecules.
12(1452)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yuan W, Qiu T, Yao X, Wu C, Shi Y, Wang N,
Zhang J, Jiang L, Liu X, Yang G, et al: Hsp47 acts as a bridge
between NLRP3 inflammasome and hepatic stellate cells activation in
arsenic-induced liver fibrosis. Toxicol Lett. 370:7–14.
2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dhawan UK, Bhattacharya P, Narayanan S,
Manickam V, Aggarwal A and Subramanian M: Hypercholesterolemia
Impairs Clearance of Neutrophil Extracellular Traps and Promotes
Inflammation and Atherosclerotic Plaque Progression. Arterioscler
Thromb Vasc Biol. 41:2598–2615. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gungor B, Vanharanta L, Hölttä-Vuori M,
Pirhonen J, Petersen NHT, Gramolelli S, Ojala PM, Kirkegaard T and
Ikonen E: HSP70 induces liver X receptor pathway activation and
cholesterol reduction in vitro and in vivo. Mol Metab. 28:135–143.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang X, Chen M, Zhou J and Zhang X: HSP27,
70 and 90, anti-apoptotic proteins, in clinical cancer therapy. Int
J Oncol. 45:18–30. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Dong Y, Ma N, Fan L, Yuan L, Wu Q, Gong L,
Tao Z, Chen J and Ren J: GADD45β stabilized by direct interaction
with HSP72 ameliorates insulin resistance and lipid accumulation.
Pharmacol Res. 173(105879)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Johnson CN, McCoin CS, Kueck PJ, Hawley
AG, John CS, Thyfault JP, Swerdlow RH, Geiger PC and Morris JK:
Relationship of muscle apolipoprotein e expression with markers of
cellular stress, metabolism, and blood biomarkers in cognitively
healthy and impaired older adults. J Alzheimers Dis. 92:1027–1035.
2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wittwer J and Bradley D: Clusterin and its
role in insulin resistance and the cardiometabolic Syndrome. Front
Immunol. 12(612496)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhu H, Liu M, Zhai T, Pan H, Wang L, Yang
H, Yan K, Gong F and Zeng Y: High serum clusterin levels are
associated with premature coronary artery disease in a Chinese
population. Diabetes Metab Res Rev. 35(e3128)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xu H, Shen Y, Liang C, Wang H, Huang J,
Xue P and Luo M: Inhibition of the mevalonate pathway improves
myocardial fibrosis. Exp Ther Med. 21(224)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sepulveda D, Rojas-Rivera D, Rodríguez DA,
Groenendyk J, Köhler A, Lebeaupin C, Ito S, Urra H, Carreras-Sureda
A, Hazari Y, et al: Interactome screening identifies the ER Luminal
Chaperone Hsp47 as a regulator of the unfolded protein response
transducer IRE1α. Mol Cell. 69:238–252.e7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Fiorentino TV, Procopio T, Mancuso E,
Arcidiacono GP, Andreozzi F, Arturi F, Sciacqua A, Perticone F,
Hribal ML and Sesti G: SRT1720 counteracts glucosamine-induced
endoplasmic reticulum stress and endothelial dysfunction.
Cardiovasc Res. 107:295–306. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sun Y, Zhang D, Liu X, Li X, Liu F, Yu Y,
Jia S, Zhou Y and Zhao Y: Endoplasmic reticulum stress affects
lipid metabolism in atherosclerosis via CHOP activation and
over-expression of miR-33. Cell Physiol Biochem. 48:1995–2010.
2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Balamurugan K, Medishetti R, Kotha J,
Behera P, Chandra K, Mavuduru VA, Joshi MB, Samineni R, Katika MR,
Ball WB, et al: PHLPP1 promotes neutral lipid accumulation through
AMPK/ChREBP-dependent lipid uptake and fatty acid synthesis
pathways. iScience. 25(103766)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gao F, Chen J and Zhu H: A potential
strategy for treating atherosclerosis: Improving endothelial
function via AMP-activated protein kinase. Sci China Life Sci.
61:1024–1029. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhao WB, Fu H, Chang F, Liu J, Wang J, Li
F and Zhao J: Effects of various doses of atorvastatin on vascular
endothelial cell apoptosis and autophagy in vitro. Mol Med Rep.
19:1919–1925. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Carnuta MG, Deleanu M, Barbalata T, Toma
L, Raileanu M, Sima AV and Stancu CS: Zingiber officinale extract
administration diminishes steroyl-CoA desaturase gene expression
and activity in hyperlipidemic hamster liver by reducing the
oxidative and endoplasmic reticulum stress. Phytomedicine.
48:62–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zhu H, Fan Y, Sun H, Chen L and Man X:
Curcumin inhibits endoplasmic reticulum stress induced by cerebral
ischemia-reperfusion injury in rats. Exp Ther Med. 14:4047–4052.
2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Cai M, Wang H, Li JJ, Zhang YL, Xin L, Li
F and Lou SJ: The signaling mechanisms of hippocampal endoplasmic
reticulum stress affecting neuronal plasticity-related protein
levels in high fat diet-induced obese rats and the regulation of
aerobic exercise. Brain Behav Immun. 57:347–359. 2016.PubMed/NCBI View Article : Google Scholar
|