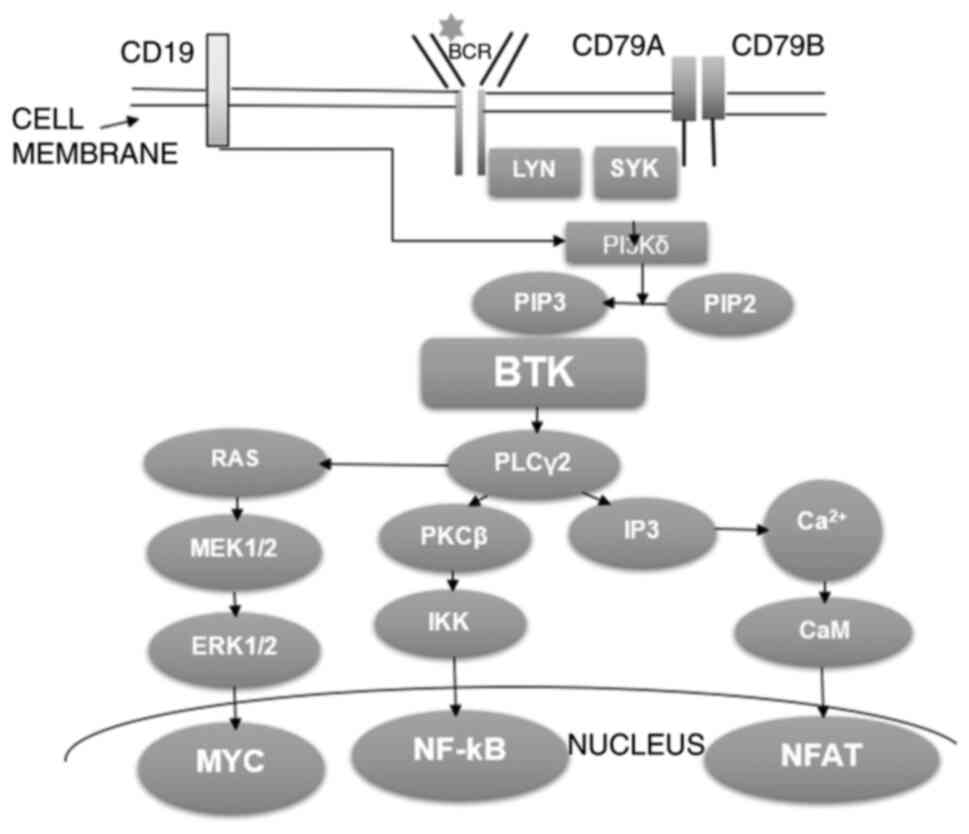
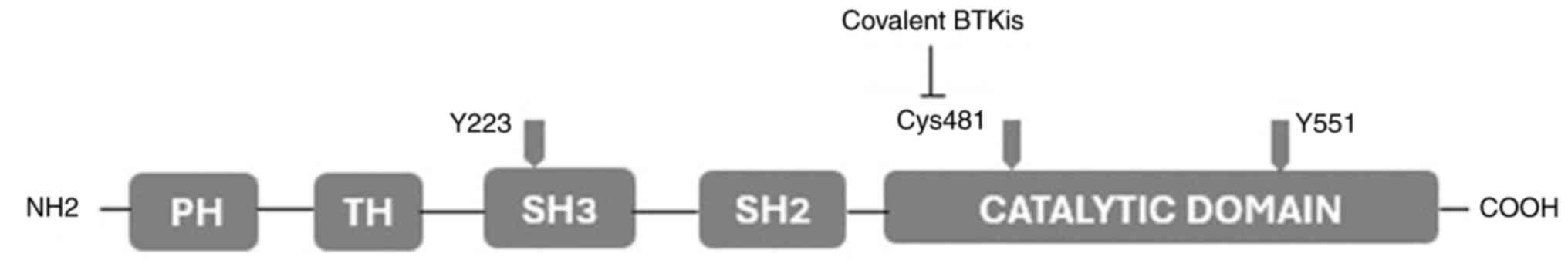
|
1
|
Shadman M: Diagnosis and treatment of
chronic lymphocytic leukemia: A review. JAMA. 329:918–932.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hampel PJ and Parikh SA: Correction:
Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer
J. 12(172)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen SS and Chiorazzi N: Functional
consequences of inhibition of Bruton's tyrosine kinase by ibrutinib
in chronic lymphocytic leukemia. Hematol Oncol. 41 (Suppl
1):S119–S128. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Wen T, Wang J, Shi Y, Qian H and Liu P:
Inhibitors targeting Bruton's tyrosine kinase in cancers: Drug
development advances. Leukemia. 35:312–332. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rey-Barroso J, Munaretto A, Rouquié N,
Mougel A, Chassan M, Gadat S, Dewingle O, Poincloux R, Cadot S,
Ysebaert L, et al: Lymphocyte migration and retention properties
affected by ibrutinib in chronic lymphocytic leukemia.
Haematologica. 109:809–823. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Song Y, Zhou K, Yang S, Hu J, Zou D, Gao
S, Pan L, Wang T, Yang H, Zhang H, et al: Indirect comparisons of
efficacy of zanubrutinib versus orelabrutinib in patients with
relapsed or refractory chronic lymphocytic leukemia/small
lymphocytic lymphoma or relapsed or refractory mantle cell
lymphoma. Invest New Drugs. 41:606–616. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rozkiewicz D, Hermanowicz JM, Kwiatkowska
I, Krupa A and Pawlak D: Bruton's tyrosine kinase inhibitors
(BTKIs): Review of preclinical studies and evaluation of clinical
trials. Molecules. 28(2400)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dhillon S: Tirabrutinib: First approval.
Drugs. 80:835–840. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Seymour C: FDA Approves Pirtobrutinib for
Previously Treated CLL/SLL. https://www.onclive.com/view/fda-approves-pirtobrutinib-for-previously-treated-cll-sll.
Available on February 21, 2024.
|
|
10
|
Bennett R, Anderson MA and Seymour JF:
Unresolved questions in selection of therapies for treatment-naïve
chronic lymphocytic leukemia. J Hematol Oncol.
16(72)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alu A, Lei H, Han X, Wei Y and Wei X: BTK
inhibitors in the treatment of hematological malignancies and
inflammatory diseases: Mechanisms and clinical studies. J Hematol
Oncol. 15(138)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nakhoda S, Vistarop A and Wang YL:
Resistance to Bruton tyrosine kinase inhibition in chronic
lymphocytic leukaemia and non-Hodgkin lymphoma. Br J Haematol.
200:137–149. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu W, Zhou K, Wang T, Yang S, Liu L, Hu Y,
Zhang W, Ding K, Zhou J, Gao S, et al: Orelabrutinib in relapsed or
refractory chronic lymphocytic leukemia/small lymphocytic lymphoma
patients: Multi-center, single-arm, open-label, phase 2 study. Am J
Hematol. 98:571–579. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cao XX, Jin J, Fu CC, Yi SH, Zhao WL, Sun
ZM, Yang W, Li DJ, Cui GH, Hu JD, et al: Evaluation of
orelabrutinib monotherapy in patients with relapsed or refractory
Waldenstrom's macroglobulinemia in a single-arm, multicenter,
open-label, phase 2 study. EClinicalMedicine.
52(101682)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kaptein A, de Bruin G, Emmelot-van Hoek M,
van de Kar B, de Jong A, Gulrajani M, Demont D, Covey T, Mittag D
and Barf T: Potency and selectivity of BTK inhibitors in clinical
development for B-cell malignancies. CLL: Therapy, excluding
transplantation: Poster I. Blood. 132 (Suppl 1)(S1871)2018.
|
|
16
|
Robak T, Witkowska M and Smolewski P: The
role of Bruton's kinase inhibitors in chronic lymphocytic leukemia:
Current status and future directions. Cancers (Basel).
14(771)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Berglöf A, Hamasy A, Meinke S, Palma M,
Krstic A, Månsson R, Kimby E, Österborg A and Smith CI: Targets for
ibrutinib beyond B cell malignancies. Scand J Immunol. 82:208–217.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dubovsky JA, Beckwith KA, Natarajan G,
Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu TM, Chang BY,
Larkin KM, et al: Ibrutinib is an irreversible molecular inhibitor
of ITK driving a Th1-selective pressure in T lymphocytes. Blood.
122:2539–2549. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Montoya S and Thompson MC: Non-covalent
bruton's tyrosine kinase inhibitors in the treatment of chronic
lymphocytic leukemia. Cancers (Basel). 15(3648)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li L, Su M, Lu W, Song H, Liu J, Wen X,
Suo Y, Qi J, Luo X, Zhou YB, et al: Triazine-based covalent
DNA-encoded libraries for discovery of covalent inhibitors of
target proteins. ACS Med Chem Lett. 13:1574–1581. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jensen JL, Mato AR, Pena C, Roeker LE and
Coombs CC: The potential of pirtobrutinib in multiple B-cell
malignancies. Ther Adv Hematol.
13(20406207221101697)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hillmen P, Pitchford A, Bloor A, Broom A,
Young M, Kennedy B, Walewska R, Furtado M, Preston G, Neilson JR,
et al: Ibrutinib and rituximab versus fludarabine,
cyclophosphamide, and rituximab for patients with previously
untreated chronic lymphocytic leukaemia (FLAIR): Interim analysis
of a multicentre, open-label, randomised, phase 3 trial. Lancet
Oncol. 24:535–552. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brown JR, Eichhorst B, Hillmen P, Jurczak
W, Kaźmierczak M, Lamanna N, O'Brien SM, Tam CS, Qiu L, Zhou K, et
al: Zanubrutinib or ibrutinib in relapsed or refractory chronic
lymphocytic leukemia. N Engl J Med. 388:319–332. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hillmen P, Eichhorst B, Brown JR, Lamanna
N, O'Brien SM, Tam CS, Qiu L, Kazmierczak M, Zhou K, Šimkovič M, et
al: Zanubrutinib versus ibrutinib in relapsed/refractory chronic
lymphocytic leukemia and small lymphocytic lymphoma: Interim
analysis of a randomized phase III trial. J Clin Oncol.
41:1035–1045. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mato AR, Woyach JA, Brown JR, Ghia P,
Patel K, Eyre TA, Munir T, Lech-Maranda E, Lamanna N, Tam CS, et
al: Pirtobrutinib after a covalent BTK inhibitor in chronic
lymphocytic leukemia. N Engl J Med. 389:33–44. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gill S, Vides V, Frey NV, Hexner EO,
Metzger S, O'Brien M, Hwang WT, Brogdon JL, Davis MM, Fraietta JA,
et al: Anti-CD19 CAR T cells in combination with ibrutinib for the
treatment of chronic lymphocytic leukemia. Blood Adv. 6:5774–5785.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ghia P, Pluta A, Wach M, Lysak D, Šimkovič
M, Kriachok I, Illés Á, de la Serna J, Dolan S, Campbell P, et al:
Acalabrutinib versus investigator's choice in relapsed/refractory
chronic lymphocytic leukemia: Final ASCEND trial results.
Hemasphere. 6(e801)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nguyen TT, Nhu NT, Tran VK, Nguyen TTH and
Lin CF: Efficacy and safety of Bruton tyrosine kinase inhibitor
monotherapy compared with combination therapy for chronic
lymphocytic leukemia and small lymphocytic lymphoma: A systematic
review and meta-analysis. Cancers (Basel). 15(1996)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee P, Kistler KD, Douyon L, Volodarsky R,
Young A, Karve S and Challagulla S: Systematic literature review of
real-world effectiveness results data for first-line ibrutinib in
chronic lymphocytic leukemia and small lymphocytic lymphoma. Drugs
Real World Outcomes. 10:11–22. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boriani G, Menna P, Morgagni R, Minotti G
and Vitolo M: Ibrutinib and Bruton's tyrosine kinase inhibitors in
chronic lymphocytic leukemia: focus on atrial fibrillation and
ventricular tachyarrhythmias/sudden cardiac death. Chemotherapy.
68:61–72. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Špaček M, Smolej L, Šimkovič M, Nekvindová
L, Křístková Z, Brychtová Y, Panovská A, Mašlejová S, Bezděková L,
Écsiová D, et al: Idelalisib plus rituximab versus ibrutinib in the
treatment of relapsed/refractory chronic lymphocytic leukaemia: A
real-world analysis from the chronic lymphocytic leukemia patients
registry (CLLEAR). Br J Haematol. 202:40–47. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Muhowski EM, Ravikrishnan J, Gordon B, Yu
L, Misra S, Walker B, Eathiraj S, Sampath D, Rogers KA, Byrd JC and
Woyach JA: Preclinical evaluation of combination nemtabrutinib and
venetoclax in chronic lymphocytic leukemia. J Hematol Oncol.
15(166)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Eyre TA and Riches JC: The evolution of
therapies targeting Bruton tyrosine kinase for the treatment of
chronic lymphocytic leukaemia: Future perspectives. Cancers
(Basel). 15(2596)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Perutelli F, Montalbano MC, Boccellato E,
Coscia M and Vitale C: Beyond ibrutinib: Novel BTK inhibitors for
the treatment of chronic lymphocytic leukemia. Curr Opin Oncol.
34:757–767. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Roeker LE, DerSarkissian M, Ryan K, Chen
Y, Duh MS, Wahlstrom SK, Hakre S, Yu L, Guo H and Mato AR:
Real-world comparative effectiveness of acalabrutinib and ibrutinib
in patients with chronic lymphocytic leukemia. Blood Adv.
7:4291–4301. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Munir T, Genovez V, Genestier V, Ryan K,
Liljas B and Gaitonde P: Cost-effectiveness of acalabrutinib
regimens in treatment-naïve chronic lymphocytic leukemia in the
United States. Expert Rev Pharmacoecon Outcomes Res. 23:579–589.
2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wallace DS, Zent CS, Baran AM, Reagan PM,
Casulo C, Rice G, Friedberg JW and Barr PM: Acalabrutinib and
high-frequency low-dose subcutaneous rituximab for initial therapy
of chronic lymphocytic leukemia. Blood Adv. 7:2496–2503.
2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Podoll T, Pearson PG, Kaptein A, Evarts J,
de Bruin G, Emmelot-van Hoek M, de Jong A, van Lith B, Sun H, Byard
S, et al: Identification and characterization of ACP-5862, the
major circulating active metabolite of acalabrutinib: Both are
potent and selective covalent Bruton tyrosine kinase inhibitors. J
Pharmacol Exp Ther. 384:173–186. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wolska-Washer A and Robak T: Zanubrutinib
for the treatment of lymphoid malignancies: Current status and
future directions. Front Oncol. 13(1130595)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Molica S, Tam C, Allsup D and Polliack A:
Advancements in the treatment of CLL: The rise of zanubrutinib as a
preferred therapeutic option. Cancers (Basel).
15(3737)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gu D, Li J and Miao Y: Evaluating
orelabrutinib as a novel treatment option for relapsed/refractory
chronic lymphocytic leukemia in China. Expert Opin Pharmacother.
23:1979–1986. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Robak P, Witkowska M, Wolska-Washer A and
Robak T: The preclinical discovery and development of orelabrutinib
as a novel treatment option for B-cell lymphoid malignancies.
Expert Opin Drug Discov. 18:1065–1076. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Munakata W, Ando K, Yokoyama M, Fukuhara
N, Yamamoto K, Fukuhara S, Ohmachi K, Mishima Y, Ichikawa S, Ogiya
D, et al: Long-term safety profile of tirabrutinib: final results
of a Japanese phase I study in patients with relapsed or refractory
B-cell malignancies. Int J Hematol. 117:553–562. 2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang H, Guo H, Yang J, Liu Y, Liu X, Zhang
Q and Zhou K: Bruton tyrosine kinase inhibitors in B-cell lymphoma:
Beyond the antitumour effect. Exp Hematol Oncol.
11(60)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yano M, Nunes J, Mo X, Rogers KA, Woyach
JA, Byrd JC and Muthusamy N: Differential regulation of CTLA4
expression through BTK-dependent and independent mechanisms in CLL.
Blood Adv. 6:5440–5448. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee HK, Hoechstetter MA, Buchner M, Pham
TT, Huh JW, Müller K, Zange S, von Buttlar H, Girl P, Wölfel R, et
al: Analysis of immune responses in patients with CLL after
heterologous COVID-19 vaccination. Blood Adv. 7:2214–2227.
2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Daniel A, Ghez D, Ravaiau C, Cavalieri D,
Tournilhac O, Herbaux C, Roriz M, Wemeau M, Guillet S, Bossard JB,
et al: Ibrutinib as a treatment of hematologic autoimmune disorders
in patients with indolent B-cell lymphoma. Eur J Haematol.
109:719–727. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yan Y, Lv R, Wang T, Yu Y, Huang Y, Xiong
W, Li Y, Sui W, Wang Q, Huang W, et al: Real-world treatment
patterns, discontinuation and clinical outcomes in patients with
B-cell lymphoproliferative diseases treated with BTK inhibitors in
China. Front Immunol. 14(1184395)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sharma S, Pepin X, Burri H, Zheng L,
Kuptsova-Clarkson N, de Jong A, Yu T, MacArthur HL, Majewski M,
Byrd JC, et al: Bioequivalence and relative bioavailability studies
to assess a new acalabrutinib formulation that enables
coadministration with proton-pump inhibitors. Clin Pharmacol Drug
Dev. 11:1294–1307. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Karadeniz M, Cinar OE, Erdogdu B, Malkan
UY, Goker H and Ozcebe OI: Hypophosphatemia related to the use of
ibrutinib. J Oncol Pharm Pract: 10781552231164504, 2023 (Epub ahead
of print).
|
|
51
|
Wan Q, Li Q, Lai X, Xu T, Hu J and Peng H:
Data mining and safety analysis of BTK inhibitors: A
pharmacovigilance investigation based on the FAERS database. Front
Pharmacol. 13(995522)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Awan FT, Addison D, Alfraih F, Baratta SJ,
Campos RN, Cugliari MS, Goh YT, Ionin VA, Mundnich S, Sverdlov AL,
et al: International consensus statement on the management of
cardiovascular risk of Bruton's tyrosine kinase inhibitors in CLL.
Blood Adv. 6:5516–5525. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gordon MJ, Jones JE, George B, Peterson C,
Burger JA, Jain N, Keating M, Wierda WG, Durand JB and Ferrajoli A:
Long-term outcomes in patients with chronic lymphocytic leukemia
treated with ibrutinib: Focus on hypertension and cardiovascular
toxicity. Cancer. 129:2192–2200. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Seymour JF, Byrd JC, Ghia P, Kater AP,
Chanan-Khan A, Furman RR, O'Brien S, Brown JR, Munir T, Mato A, et
al: Detailed safety profile of acalabrutinib vs ibrutinib in
previously treated chronic lymphocytic leukemia in the ELEVATE-RR
trial. Blood. 142:687–699. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Elaskalani O, Gilmore G, Hagger M, Baker
RI and Metharom P: Adenosine 2A receptor activation amplifies
ibrutinib antiplatelet effect; implications in chronic lymphocytic
leukemia. Cancers (Basel). 14(5750)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tham K, Prelewicz S, deHoll S, Stephens DM
and Gomez CA: Infectious complications among patients receiving
ibrutinib for the treatment of hematological malignancies. Am J
Health Syst Pharm. 81:112–119. 2024.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Diella L, Bavaro DF, Loseto G, Pasciolla
C, Minoia C, Di Gennaro D, Belati A, De Candia MS, Di Gennaro F,
Saracino A and Guarini A: Current therapies for chronic lymphocytic
leukemia: Risk and prophylaxis strategies for
secondary/opportunistic infections. Expert Rev Hematol. 16:267–276.
2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
No authors listed. Correction to: Managing
ibrutinib-intolerant patients with B-cell malignancies. Oncologist.
28(e487)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shadman M, Flinn IW, Levy MY, Porter RF,
Burke JM, Zafar SF, Misleh J, Kingsley EC, Yimer HA, Freeman B, et
al: Zanubrutinib in patients with previously treated B-cell
malignancies intolerant of previous Bruton tyrosine kinase
inhibitors in the USA: A phase 2, open-label, single-arm study.
Lancet Haematol. 10:e35–e45. 2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Salmerón-Navas FJ, Barreiro-Fernández EM
and Fénix-Caballero S: Adjusted indirect comparison of zanubrutinib
and ibrutinib in first-line treatment of chronic lymphocytic
leukemia. Farm Hosp. 48:9–15. 2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen S, Wei Y, Li S, Miao Y, Gu J, Cui Y,
Liu Z, Liang J, Wei L, Li X, et al: Zanubrutinib attenuates
bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β1
signaling pathway. Int Immunopharmacol. 113(109316)2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Blombery P, Thompson ER, Lew TE, Tiong IS,
Bennett R, Cheah CY, Lewis KL, Handunnetti SM, Tang CPS, Roberts A,
et al: Enrichment of BTK Leu528Trp mutations in patients with CLL
on zanubrutinib: Potential for pirtobrutinib cross-resistance.
Blood Adv. 6:5589–5592. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Naeem A, Utro F, Wang Q, Cha J, Vihinen M,
Martindale S, Zhou Y, Ren Y, Tyekucheva S, Kim AS, et al:
Pirtobrutinib targets BTK C481S in ibrutinib-resistant CLL but
second-site BTK mutations lead to resistance. Blood Adv.
7:1929–1943. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Takács F, Kotmayer L, Czeti Á, Szalóki G,
László T, Mikala G, Márk Á, Masszi A, Farkas P, Plander M, et al:
Revealing a phenotypical appearance of ibrutinib resistance in
patients with chronic lymphocytic leukaemia by flow cytometry.
Pathol Oncol Res. 28(1610659)2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Maher N, Mouhssine S, Matti BF, Alwan AF
and Gaidano G: Treatment refractoriness in chronic lymphocytic
leukemia: Old and new molecular biomarkers. Int J Mol Sci.
24(10374)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Woyach JA, Ghia P, Byrd JC, Ahn IE, Moreno
C, O'Brien SM, Jones D, Cheung LWK, Chong E, Kwei K, et al: B-cell
receptor pathway mutations are infrequent in patients with chronic
lymphocytic leukemia on continuous ibrutinib therapy. Clin Cancer
Res. 29:3065–3073. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Alsadhan A, Chen J, Gaglione EM,
Underbayev C, Tuma PL, Tian X, Freeman LA, Baskar S, Nierman P,
Soto S, et al: CD49d expression identifies a biologically distinct
subtype of chronic lymphocytic leukemia with inferior
progression-free survival on BTK inhibitor therapy. Clin Cancer
Res. 29:3612–3621. 2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Tissino E, Bomben R, Gattei V and
Zucchetto A: BCR/integrin interaction in CLL: A physiologic remnant
with clinical relevance. Clin Cancer Res. 29:3560–3562.
2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Thompson PA and Tam CS: Pirtobrutinib: A
new hope for patients with BTK inhibitor-refractory
lymphoproliferative disorders. Blood. 141:3137–3142.
2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chung C, Umoru G, Abboud K and Hobaugh E:
Sequencing and combination of current small-molecule inhibitors for
chronic lymphocytic leukemia: Where is the evidence? Eur J
Haematol. 111:15–28. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rizzuto A, Pirrera A, Gigliotta E, Mancuso
S, Vullo C, Camarda GM, Rotolo C, Roppolo A, Spoto C, Gentile M, et
al: Molecular-biology-driven frontline treatment for chronic
lymphocytic leukemia: A network meta-analysis of randomized
clinical trials. Int J Mol Sci. 24(9930)2023.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Nguyen TT, Thanh Nhu N, Tran VK, Van Cau N
and Lin CF: Efficacy and safety of Bruton tyrosine kinase inhibitor
plus anti-CD20 antibody therapy compared with chemoimmunotherapy as
front-line treatment for chronic lymphocytic leukemia: A systematic
review and meta-analysis of randomized controlled trials. J
Immunother. 46:299–309. 2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Nguyen TT, Nhu NT, Tran VK, Viet-Nhi NK,
Ho XD, Jhan MK, Chen YP and Lin CF: Efficacy and safety of add-on
anti-CD20 monoclonal antibody to Bruton tyrosine kinase inhibitor
treatment for chronic lymphocytic leukemia: A meta-analysis. Sci
Rep. 13(9775)2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Scarfò L, Heltai S, Albi E, Scarano E,
Schiattone L, Farina L, Moia R, Deodato M, Ferrario A, Motta M, et
al: Minimal residual disease-driven treatment intensification with
sequential addition of ibrutinib to venetoclax in R/R CLL. Blood.
140:2348–2357. 2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Cervantes-Gomez F, Lamothe B, Woyach JA,
Wierda WG, Keating MJ, Balakrishnan K and Gandhi V: Pharmacological
and protein profiling suggests venetoclax (ABT-199) as optimal
partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer
Res. 21:3705–3715. 2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Godwin CD, Bates OM, Garling EE, Beddoe
ME, Laszlo GS and Walter RB: The Bruton's tyrosine kinase inhibitor
ibrutinib abrogates bispecific antibody-mediated T-cell
cytotoxicity. Br J Haematol. 189:e9–e13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Mhibik M, Gaglione EM, Eik D, Kendall EK,
Blackburn A, Keyvanfar K, Baptista MJ, Ahn IE, Sun C, Qi J, et al:
BTK inhibitors, irrespective of ITK inhibition, increase efficacy
of a CD19/CD3-bispecific antibody in CLL. Blood. 138:1843–1854.
2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Nasnas P, Cerchione C, Musuraca G,
Martinelli G and Ferrajoli A: How I manage chronic lymphocytic
leukemia. Hematol Rep. 15:454–464. 2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Easaw S, Ezzati S and Coombs CC: SOHO
State of the art updates and next questions: Updates on BTK
inhibitors for the treatment of chronic lymphocytic leukemia. Clin
Lymphoma Myeloma Leuk. 23:697–704. 2023.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kim T, Kim K, Park I, Hong S and Park H:
Two-track virtual screening approach to identify the dual
inhibitors of wild type and C481S mutant of Bruton's tyrosine
kinase. J Chem Inf Model. 62:4500–4511. 2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Thus YJ, De Rooij MFM, Beijersbergen RL
and Spaargaren M: An Unbiased CRISPR-Cas9 screening method for the
identification of positive and negative regulatory proteins of cell
adhesion. Bio Protoc. 12(e4545)2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Mihăilă RG and Topîrcean D: The
high-performance technology CRISPR/Cas9 improves knowledge and
management of acute myeloid leukemia. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 165:249–257. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Sampietro M, Cassina V, Salerno D,
Barbaglio F, Buglione E, Marrano CA, Campanile R, Scarfò L,
Biedenweg D, Fregin B, et al: The nanomechanical properties of CLL
cells are linked to the actin cytoskeleton and are a potential
target of BTK inhibitors. Hemasphere. 7(e931)2023.PubMed/NCBI View Article : Google Scholar
|