1. Introduction
The median age of patients with chronic lymphocytic
leukemia (CLL) is 70 years. As many of these patients are
immunocompromised, they are thus prone to developing various
infections. Of note, 70-80% of patients with CLL are asymptomatic
at the time of diagnosis, and approximately one third of patients
will never require treatment for CLL (1). The CLL-International Prognostic Index
was validated as the optimal predictor of time to first therapy
among previously untreated patients (2).
The most commonly indicated drugs currently
available for first-line treatment are covalent Bruton's tyrosine
kinase (BTK) inhibitors (BTKis) and B-cell leukemia/lymphoma 2
(BCL2) inhibitors (1). Moreover,
they are currently the standard of care for use as frontline
therapy and for the treatment of refractory or relapsed (R/R)
CLL.
BTK is a member of the TEC-family non-receptor
protein-tyrosine kinases and is involved in the proliferation and
differentiation of B-lymphocytes. The activation of BTK is the
result of the activation of receptors, such as B-cell antigen
receptor, C-X-C chemokine receptor type 4 and various integrins,
including VLA-4. Once activated, BTK initiates trophic signals that
contribute to prevent cell death, and promote cell activation and
growth (3).
Ibrutinib was the first BTKi to be approved by the
FDA, in 2013. It constitutes a turning point in CLL therapy as it
allows for the avoidance of the toxicity associated with
chemotherapy. Its success was considerable, including from a
financial point of view (4). It is
a potent, covalent, irreversible, selective inhibitor of BTK, which
alters BTK-dependent adhesion and migration, which explains the
disruption of the retention of CLL cells in the supporting lymphoid
tissues. The relative expression of the receptors involved in lymph
node entry (CCR7) vs. exit (S1PR1) represent markers of the
clinically relevant treatment-produced lymphocytosis (5). Acalabrutinib and zanubrutinib are
covalent and irreversible second-generation BTKis, which aimed at
reducing off-target effects. They were approved in 2017 and 2019,
respectively (4). Orelabrutinib is
another novel next-generation, covalent and irreversible BTKi with
a high selectivity for BTK that was approved in China and Japan for
the treatment of R/R CLL in 2020 (6,7).
Tirabrutinib, another covalent and irreversible BTKi, was approved
in Japan, in 2020, for the treatment of recurrent or refractory
primary central nervous system lymphoma (8). The FDA has granted an accelerated
approval to pirtobrutinib, a non-covalent and reversible BTKi, as a
therapy for adult patients with CLL who have been treated with at
least two prior lines of therapy, including a BTK inhibitor and a
BCL2 inhibitor, in 2023(9).
Both BTKis, as well as venetoclax (a BCL-2
inhibitor) and next-generation anti-CD20 monoclonal antibodies,
have resulted in improved therapeutic results in patients with CLL,
even in those with del17p13 or TP53 mutation and unmutated
immunoglobulin heavy chain (IGHV) genes, which represent high-risk
features (2). Although the
venetoclax-obinutuzumab combination may be a limited treatment
solution, a BTKi is indicated if patients have the del17p, TP53
mutation, or unmutated IGHV, as it has greater efficacy; in these
cases, the combinations BTKi-venetoclax ± anti-CD20 monoclonal
antibody also appear to be useful (10).
For patients with multiple relapses of CLL, chimeric
antigen receptor T-cell (CAR-T) therapy with lisocabtagene
maraleucel is a solution that leads to a 45% complete response (CR)
rate. The allogeneic hematopoietic cell transplantation is the only
potentially curative solution, after use of targeted agents, in
selected patients (5).
The present review summarizes and discusses the
efficacy and safety of BTKis in patients with CLL. Articles
published in the PubMed and Web of Science databases between
October, 2022 and September, 2023 were searched, using the terms
‘chronic lymphocytic leukemia’ and ‘BTK inhibitors’.
2. Advantages associated with the use of
Bruton's tyrosine kinase inhibitors
BTK plays a role in B-cell receptor (BCR) signaling
(Fig. 1) (11): The binding of the antigen to the
BCR leads to the phosphorylation of its co-receptors, CD79A and
CD79B, by the recruited tyrosine kinases, LYN and SYK, thereby
recruiting SYK. SYK activates Pl3Kδ, which converts PIP2 to PIP3.
PIP3 constitutes a docking site for BTK. BTK phosphorylates and
activates phospholipase C γ2 (PLCγ2), which is involved in the
activation of protein kinase C (PKC)β. PKCβ phosphorylates IKK,
which activates NF-κB, a nuclear factor involved in the gene
expression necessary for B-lymphocyte survival and proliferation.
BTK stimulates PLCγ2 lipase activity, which produces
Ca2+ influx and nuclear factor of activated T-cell
activation via CaM; this nuclear factor also regulates gene
expression in lymphocytes. PLCγ2 is also involved in MYC (another
nuclear factor) activation through the RAS/MEK1/2 and ERK1/2
pathway; MYC activates the expression of a number of proliferative
genes.
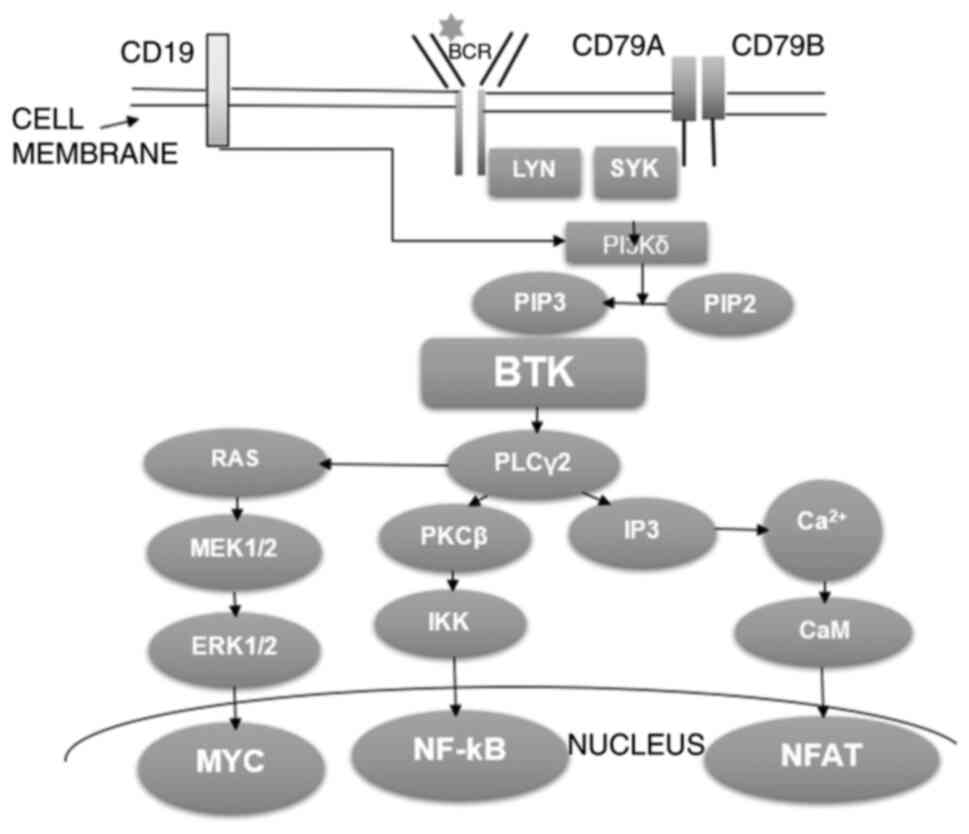 | Figure 1The mechanism of antigen-dependent
B-cell receptor signal transduction. The figure was adapted and
modified from the study by Alu et al (11) (https://creativecommons.org/publicdomain/zero/1.0/).
BCR, B-cell receptor; LYN, LYN tyrosine kinase; SYK, spleen
tyrosine kinase; Pl3Kδ, phosphoinositide 3-kinase δ; PIP2,
phosphatidylinositol 4,5-biphosphate; PIP3, phosphatidylinositol
3,4,5-triphosphate; BTK, Bruton's tyrosine kinase; PLCγ2,
phospholipase C γ2; RAS, signal transduction protein RAS; MEK,
mitogen-activated kinase MEK; ERK, extracellular signal-regulated
kinase; MYC, transcription factor MYC; PKCβ, protein kinase Cβ;
IKK, IκB kinase; NF-κB, nuclear factor κ-light-chain-enhancer of
activated B-cells; IP3, inositol 1,4,5-triphosphate; CAM,
calmodulin; NFAT, nuclear factor of activated T-cells. |
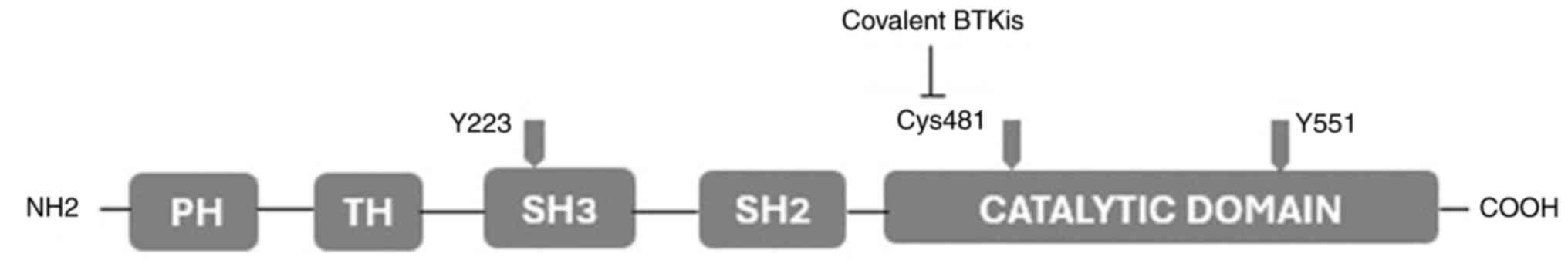
The structure of BTK is presented in Fig. 2. The activation of BTK requires the
phosphorylation of the Y551 and Y223 sites. The covalent BTKis have
as their main target Cys481 residues on the catalytic domain
(11).
The covalent BTKis are presented in Table I (4,12-18).
Ibrutinib binds irreversibly to the BTK, decreasing the
phosphorylation of this protein and thereby decreasing downstream
BCR signaling, a pathway that plays a crucial role in the survival
of CLL cells (19). The design of
a covalent DNA-encoded library and its selection method was
realized and published to facilitate the discovery of covalent
inhibitors for target proteins, including BTK (20). BTKis allow for the avoidance of the
adverse effects (AEs) of classical chemotherapy and their use has
led to deeper responses, including in high-risk patient categories.
Oral administration is another advantage of BTKis; however, the
therapy is continued until the disease progresses or until
discontinuation is required due to unacceptable toxicity (19).
 | Table IThe covalent BTKis. |
Table I
The covalent BTKis.
| BTKi | Biochemical
potencya | Selectivity | Inhibited
targets |
|---|
| Ibrutinib | +++ | + | TEC kinase, ITK,
BTK and the subsequent phosphorylation of BTK, phospholipase Cγ2,
AKT and ERK |
| Acalabrutinib | + | +++ | BTK, BMX kinase,
and human EGFR4; almost no inhibitory activity on EGFR, ITK, or TEC
kinase |
| Zanubrutinib | ++ | ++ | BTK, BMX kinase,
and human EGFR4; almost no inhibitory activity on EGFR; less
activity on TEC and ITK |
| Orelabrutinib | + | +++ | Significant
inhibition only of BTK |
| Tirabrutinib | + | +++ | BTK |
The non-covalent BTKis (ncBTKis) are the following:
Pirtobrutinib-a third-generation BTKi with a selectivity for BTK of
>300-fold higher than >98% of other kinases, and a nanomolar
potency against both wild-type and C481-mutant BTK (21). The following drugs that are at
various stages of clinical testing: Nemtabrutinib (less selective
than other ncBTKis) (11),
vecabrutinib-a selective, reversible inhibitor of BTK, B-lymphocyte
kinase, insulin-like growth factor 1 receptor,
interleukin-2-inducible T-cell kinase (ITK), LCK and TEC, but not
EGFR, with a potency against BTK with a half-maximal inhibitory
concentration] (19) and
fenebrutinib (a selective BTKi). The latter two drugs have been
discontinued in B-cell malignancies (19), due to adverse effects. The results
of clinical trials with BTKis are presented in Table II (13,22-27).
 | Table IIClinical trials of BTKis. |
Table II
Clinical trials of BTKis.
| The investigated
drugs | Studied
population | Results | Adverse
effects | (Refs.) |
|---|
| Ibrutinib +
rituximab/FCR | 771 Previously
untreated patients with CLL | PFS at 53
months=non-reached with ibrutinib and rituximab, and 67 months in
FCR group; OS was similar between groups | Grade 3 and 4
leukopenia-more frequent in the group treated with FCR; a small
number of sudden unexplained or cardiac deaths was in the ibrutinib
+ rituximab group | (22) |
|
Ibrutinib/zanubrutinib | 652 Patients with
relapsed or refractory CLL or SLL | PFS at 24
months=78.4% in the zanubrutinib group and 65.9% in the ibrutinib
group; longer PFS in those with 17p deletion, a TP53 mutation, or
both in the zanubrutinib group | Fewer cardiac
events and adverse events that required treatment discontinuation
in the zanubrutinib group | (23) |
|
Zanubrutinib/ibrutinib | 415 Patients with
relapsed or refractory CLL | ORR at 15
months=78.3% in the zanubrutinib group and 62.5% in the ibrutinib
group; PFS at 12 months= 94.9% with zanubrutinib and 84.0% with
ibrutinib; ORR was higher with zanubrutinib vs. ibrutinib in
patients with del(17p)/TP53 mutations and del(11q) | Fewer patients had
cardiac events (including the development of atrial fibrillation),
major hemorrhages, and adverse events that required treatment
discontinuation or leading to death in the zanubrutinib group | (24) |
| Pirtobrutinib | CLL or SLL
including 247 patients previously treated with a BTKi | ORR=73.3%; median
PFS= 19.6 months | The most frequent
were infections, bleeding, and neutropenia; less frequently
appeared: hypertension, atrial fibrillation or flutter, and major
hemorrhage | (25) |
| Ibrutinib +
autologous huCART-19 | 19 Patients with
CLL without CR after ≥6 months of ibrutinib treatment | CR rate at 3
months=44%; the estimated OS and PFS at 48 months=84% and 70%,
respectively; undetectable MRD at 12 months= 72% of tested
patients | Cytokine release
syndrome- in 15 of 18 subjects; neuro- toxicity in 5 patients | (26) |
|
Acalabrutinib/investigator's choice | 310 Patients with
R/R CLL | PFS at 42
months=62% (acalabrutinib) vs. 19%; 42-month OS at 42 months= 78%
(acalabrutinib) vs. 65% | During
acalabrutinib treatment. Atrial fibrillation/flutter, 8%;
hypertension, 8%; major hemorrhage, 3%' grade ≥3 infections, 29%;
and second primary malignancies without non-melanoma skin cancer,
7% | (27) |
| Orelabrutinib | 80 Patients with
R/R CLL or SLL | ORR, 92.5%;
CR=21.3%; PR=60.0%; PR with lymphocytosis 11.3%; high response rate
also in the subgroup of patients with unfavorable prognostic
risk | Approximately 86.8%
of AEs were grade 1 or 2 | (13) |
A recent meta-analysis included 1,510 patients
treated for CLL/small lymphocytic lymphoma with BTKis or
combination therapy. The progression-free survival (PFS) and
overall response rate (ORR) were significantly longer for patients
who received BTKis compared to the combination therapy, although
the overall survival (OS) and CR rate did not differ between the
two study arms (28). It has been
shown that the survival rates of patients are ~88% at 4 years under
acalabrutinib treatment, 94% at 2 years under zanubrutinib
treatment and 78% at 7 years under ibrutinib treatment (1).
Covalent BTKis. Ibrutinib
The effectiveness of ibrutinib administered to naïve
patients with CLL in real-world clinical settings was previously
examined in a systematic literature review. The PFS rates were
between 89 and 93%, and the ORR was between 71 and 90% after a
1-year follow-up period. Following an 18-month follow-up period,
the OS rate was 91%. These data support the high efficacy of
ibrutinib in real-life (29).
Other results of ibrutinib therapy are presented in Table II.
Following an 8-year follow-up, it was found that
ibrutinib reduced all-cause mortality, and produced few cases of
ventricular arrhythmias and sudden cardiac death, independently of
QT lengthening (28). In the case
that AEs occur, both the reduction of the dose of ibrutinib and the
careful management of arrhythmia can allow for long-term treatment
and reduce all-cause mortality with a prolonged PFS and a reduced
all-cause mortality (30).
In a real-world retrospective analysis, it was found
that ibrutinib had better efficacy and tolerability than the
rituximab-idelalisib combination in patients with R/R CLL (31). It has been found that ibrutinib and
venetoclax work synergistically and they have been proven to be
effective in clinical trials (32).
In order to reduce the number of AEs associated with
the use of ibrutinib, more specific inhibitors of BTK were
produced; thus, acalabrutinib and zanubrutinib have
equivalent/enhanced efficacy and improved tolerability (33).
Acalabrutinib. Acalabrutinib has been
approved for CLL therapy and has an efficacy comparable to that of
ibrutinib, although with fewer AEs (34). As a result, is was previously
demonstrated that patients who received acalabrutinib had a 41%
lower risk of discontinuation and a longer time to discontinuation
compared to those treated with ibrutinib (35). Acalabrutinib monotherapy given in
treatment-naïve patients with CLL was found to be cost-effective
compared to chlorambucil + obinutuzumab (36). Acalabrutinib, unlike ibrutinib,
does not inhibit anti-CD20 monoclonal antibody-dependent cellular
phagocytosis; thus, its association with rituximab is suitable
(37). As with acalabrutinib, its
major metabolite, ACP-5862, is more selective towards BTK compared
to ibrutinib and zanubrutinib. ACP-5862 is involved in the clinical
efficacy of acalabrutinib treatment (38).
Zanubrutinib. Zanubrutinib, a BTKi with a
greater specificity than ibrutinib, has been proven to be superior
to it in terms of ORR in patients with R/R CLL (23). Zanubrutinib has a similar action to
acalabrutinib, but is less active against ITK and TEC tyrosine
kinase (39). It has been shown
that zanubrutinib has excellent response rates and its approval is
awaited. It produces fewer AEs, apart from high rates of
neutropenia (34). Zanubrutinib
has a lower risk of atrial fibrillation/flutter and major bleeding
events (39). Zanubrutinib has
been shown to significantly increase PFS vs. bendamustine-rituximab
in first-line therapy (39,40).
Other covalent BTKis. Orelabrutinib is a
highly selective covalent BTKi that targets a single kinase, BTK
(41). Preclinical studies claim
that orelabrutinib has a high selectivity, good efficacy and very
good safety profile in B-cell lymphoproliferation (42). It has been proven to be effective
and safe, including in Chinese patients with CLL (41).
Tirabrutinib is an irreversible and covalent BTKi.
Administered in a Japanese study in patients with B-cell
lymphoproliferations, it was shown to result in an ORR and a median
duration of response of 76.5% and 2.59 years, respectively
(43).
ncBTKis
ncBTKis have been produced to overcome resistance to
BTKis; among the tested products, pirtobrutinib has been proven to
be promising, with manageable toxicities (33).
Nemtabrutinib is a potent reversible BTKi of the new
generation, effective in treatment-naïve and ibrutinib-refractory
CLL cells ex vivo. In a previous study in a mouse model of
CLL, the combination of nemtabrutinib and venetoclax led to longer
survival rates vs. treatment with ibrutinib and venetoclax
(32).
3. Immunomodulatory effects
BTKis inhibit various specific immune receptors,
such as T-cell receptor and Toll-like receptors. As BTKis also
inhibit other kinases, such as ITK, TEC and SRC family kinases,
EGFR, they also affect the function of other cells, such as
T-cells, natural killer cells, cardiomyocytes and platelets. These
pathways explain the marked clinical efficacy of BTKis, but also
their AEs, among which are infections, atrial fibrillation and
bleeding (44).
Apart from the BTKi effect, ibrutinib is involved in
suppressing the expression and trafficking of cytotoxic
T-lymphocyte antigen 4, a key immune checkpoint and target for
cancer immunotherapy. This is another immune benefit of ibrutinib
(45).
T-lymphocyte responses following anti-SARS CoV-2
vaccination in patients with CLL occurred independently of the
treatment status, although higher humoral response rates were
observed in those under BTKi treatment and following B-lymphocyte
reconstitution. Boosting was more effective in patients with
improved immunity by leukemia treatment (46).
In a previous study, ibrutinib was shown to be
useful for the treatment of 25 patients with R/R B-cell lymphoma or
leukemia and hematological immune manifestation; ~67% of the immune
manifestations responded to ibrutinib; the CR rate was 44%
(47).
4. Limitations of Bruton's tyrosine kinase
inhibitors
The main limitations of BTIs are the following: The
emergence of drug resistance, low complete remission rates, the
need for an indefinite treatment duration (39) and the possible occurrence of AEs,
which vary depending on the product, and can be responsible for
intolerance.
The permanent discontinuation of BTKis within the
first 6 months of their administration, most of the time due to
progressive disease, has led to a median post-discontinuation
survival of only 6.9 months (48).
The bioavailability of acalabrutinib from capsules
decreases if proton-pump inhibitors are co-administered. However,
tablets of acalabrutinib maleate with pH-independent release were
produced, to avoid this limitation (49).
AEs
Some of the AEs of ibrutinib are due to the
inhibition of kinases other than BTK (32). A recent meta-analysis found that
the risk of developing grade ≥3 AEs did not differ significantly
between patients treated with BTKis compared to those treated with
combination therapy. Moreover, the risk of developing grade ≥3 AEs
was significantly lower in the group of patients treated with
second-generation BTKis compared to the combination therapy
(28).
Hypophosphatemia can appear during treatment with
tyrosine kinase inhibitors (TKis); it has the same mechanism that
is involved in the production of secondary hyperparathyroidism and
renal tubulopathy and can be managed with alternating doses of TKis
(50).
Males are more prone to the occurrence of AEs during
ibrutinib and acalabrutinib treatment (51). Ventricular arrhythmias and sudden
cardiac death are a class effect of BTKis (29), as well as hypertension, atrial
fibrillation, heart failure (52),
bleeding (10) and
gastrointestinal symptoms (50).
Hypertension that occurs during treatment with ibrutinib has been
proven to be reversible following the discontinuation of treatment.
The factors associated with hypertension in these patients were the
following: An older age, the male sex, tobacco use and chronic
kidney disease. Baseline hypertension did not lead to major
cardiovascular complications (53).
In accordance with the international consensus
statement on the management of the cardiovascular risk of patients
with CLL who are to be treated with BTKis, it is indicated to
establish their cardiovascular diseases, risk factors and level,
and perform the necessary investigations, including an
electrocardiogram (52).
In the case that the patients have a high
cardiovascular risk, it is indicated that a multidisciplinary team
determines whether treatment with a BTKi is indicated: If the
answer is positive, a selective BTKi (acalabrutinib or
zanubrutinib) will be preferred. It is recommended to avoid the use
of ibrutinib in patients with ventricular arrhythmias, and any BTKi
in those with a history of heart failure. The multidisciplinary
team must contribute to the management of AEs that occur during
BTKi therapy; thus, the control of hypertension, the therapy of
arrhythmias and heart failure will help to maintain the therapy
with BTKi (52).
The following AEs have been found to occur more
frequently with ibrutinib than with acalabrutinib: Arthralgia, back
pain, diarrhea, urinary tract infection, dyspepsia and muscle
spasms, atrial fibrillation/flutter, hypertension, and bleeding.
Instead, cough and headaches have been found to be more frequent
with acalabrutinib (54).
Approximately a third of the B-lymphocytes of
patients with CLL express CD73, the nucleotidase that produces
adenosine. Adenosine 2A receptor activation leads to the
amplification of the anti-platelet aggregation effect of ibrutinib
(55).
In patients treated with ibrutinib, the first
infection has been shown to occur after a median period of 125 days
from the initiation of therapy. Risk factors for a severe infection
are the following: Previous allogeneic hematopoietic stem cell
transplantation and corticotherapy (56). The anti-infective prophylaxis of
patients treated with BTKi should target opportunistic infections
and it is indicated that it should be performed in collaboration
with an infectious disease specialist (57).
The side-effects associated with the use of
ibrutinib can be treated with supportive care or dose reduction,
continuation with another covalent BTKi, or a ncBTKi, or another
medication (58). Compared to
ibrutinib, zanubrutinib has a better safety profile and an enhanced
clinical efficacy, effects due to its higher selectivity for the
kinase binding site (39).
Acalabrutinib and zanubrutinib produce less episodes of atrial
fibrillation, compared to ibrutinib (12).
As previously demonstrated, ~70% of the AEs that
occurred during treatment with ibrutinib were not present with
zanubrutinib treatment and ~80% of those that occurred during
therapy with acalabrutinib did not recur with zanubrutinib
(59).
Cardiovascular events have been found to occur less
frequently in patients treated with zanubrutinib compared to those
who received ibrutinib, although zanubrutinib produced a higher
incidence of secondary cancers (60).
Among the main causes of mortality in patients
treated with ibrutinib and acalabrutinib are infections, pneumonia,
pleural effusion, diarrhea and fall. Cardiac disorders, such as
atrial fibrillation and cardiac failure, are an important cause of
mortality among those treated with ibrutinib (49).
Zanubrutinib has been shown to attenuate
bleomycin-induced lung fibrosis in an experimental model in mice,
by inhibiting the TGF-β1 signaling mechanism (61).
Resistance to BTKis
Resistance to BTKis can be primary or acquired, and
can occur due to various mechanisms, such as gene mutations, the
activation of bypass signaling mechanisms and the influence of the
tumor microenvironment (12).
It is considered that the most common mechanism
involved in the emergence of resistance to covalent BTKis
(including ibrutinib), under which the disease progresses, is a
mutation in the BTK 481 cysteine, a residue to which the inhibitors
bind covalently (62,63). A high CD27 and CD86 expression
associated with BTKC481S mutation has been found in patients with
CLL resistant to ibrutinib. A higher expression of CD27, CD69 and
CD86 has been found 3 months prior to the appearance of clinical
resistance. Monitoring these phenotypic markers using flow
cytometry could be useful to detect ibrutinib resistance (64).
Point mutations of the phospholipase C-γ2
gene, such as R665W are other causes of resistance to BTKis
(65) and progressive disease.
Mutations in BTK, PLCG2 or both genes are rare before
any treatment for CLL (3, 2 and 1% of patients, respectively).
Under treatment with ibrutinib, following a median follow-up of 35
months, in patients who did not have progressive disease at last
sample, it was found that there were mutations in BTK (30%),
PLCG2 (7%), or both genes (5%), particularly in patients
with R/R CLL (66).
BTK Leu528Trp mutation was observed in some patients
treated with zanubrutinib and proved cross-resistance to
pirtobrutinib, a non-covalent inhibitor (62). Homogeneous and bimodal
CD49d-positive CLL cells have been shown to have a shorter time to
progression (6.6 years) compared to homogeneously CD49d-CLL cells.
During acalabrutinib therapy, signaling through NF-κB and JAK/STAT
increases, as well as the adhesion, survival and migratory capacity
of CD49d+ CLL cells (67). CD49d remains activated despite
therapy with ibrutinib or acalabrutinib, and can be attenuated by
PI3K inhibitors (68). CD49d/VLA-4
expression is a contributing factor to BTKi resistance (67).
5. Advantages associated with the use of
non-covalent Bruton's tyrosine kinase inhibitors
ncBTKis have a different mechanism of binding to BTK
(32). They reversibly bind the
BTK target, a fact that explains the rare occurrence of toxicity
and acquired resistance (19).
Their use is associated with fewer AEs than the covalent BTKis, and
they have shown promising efficacy and safety profiles in clinical
trials (44). Furthermore, they
have the potential to overcome resistance of CLL cells due to
mutations (32).
Pirtobrutinib is a ncBTKi that ensures high response
rates in CLL cases that are refractory to covalent BTKis,
regardless of the mechanism of this resistance (69). Pirtobrutinib potently inhibits cell
viability, BCR signaling, and CCL3/CCL4 chemokine production, not
only in BTK wild-type, but also in C481S-mutant CLL cells (63).
Pirtobrutinib has been shown to result in an ORR
>70% following the failure of covalent BTKis and venetoclax
(1,19). Early studies with pirtobrutinib
found that it has a safety profile that can recommend it for use in
therapeutic combinations (69).
The acquired resistance to pirtobrutinib has been
recently observed; the mechanisms can include a novel acquired
mutations in BTK outside of the C481 position (19,63).
6. Therapeutic combinations
The longer the treatment duration, the lower the
response rate of CLL cells to the treatment, particularly if it is
represented by a BTKi. Therefore, therapeutic combinations have
been tested. In addition, they often have a synergistic effect,
contribute to the reduction of the proliferation of resistant
clones, and sometimes allow for the treatment duration to be
shortened, with the reduction of AEs and costs (70).
A recent meta-analysis established the superiority
of the combination of anti-CD20 monoclonal antibodies + BTKi or
BCL2i compared to chemotherapy in the first-line treatment of CLL
(71).
Another systematic review and meta-analysis
evaluated four clinical trials with patients with treatment-naïve
or R/R CLL and concluded that BTKis administered in combination
with anti-CD20 antibodies led to the prolongation of PFS and ORR,
but not OS and CR compared with chemoimmunotherapy. The risk of
severe AEs induced by the two types of treatment was comparable
(72). The addition of anti-CD20
monoclonal antibodies to BTKi therapy was compared to BTKi
monotherapy in another systematic review and meta-analysis; PFS was
significantly improved in the first group, as well as the CR and
undetectable minimal residual disease rate, but not the OS; the
risk of severe AEs was comparable in the two groups (73).
As previously demonstrated, the combination
obinutuzumab-acalabrutinib was able to produce longer PFS compared
to acalabrutinib, but this fact was not observed with the
combination rituximab-ibrutinib; in addition, the AEs may be more
important (10).
It has been shown that ibrutinib is able to produce
deep responses in combination with venetoclax (74). The combined treatment with
ibrutinib-venetoclax is more cytotoxic against CLL cells than any
of the drugs used alone (75). The
combination of BTKis with BCL-2 antagonists can be tested with the
aim of increasing the anti-leukemic efficacy and reducing the risk
of acquired resistance (16). In a
previous study, patients with R/R CLL, who did not obtain
undetectable measurable residual disease (uMRD) with venetoclax
monotherapy at cycle 12 day 1, were treated with ibrutinib and both
drugs were continued. Following a median of 7 months of combined
treatment, 84% of patients achieved uMRD (<10-4) and
treatment was terminated; 2 patients with minimal residual disease
continued ibrutinib until progression or toxicity (74).
In another study, the triple combination of
BTKi-venetoclax-anti-CD20 monoclonal antibody led to similar rates
of CR compared to the venetoclax-obinutuzumab combination, although
with more potential AEs (3).
The broad involvement of BTK in immunological
mechanisms, and particularly the influence of ibrutinib on
T-lymphocytes, is a reason to combine BTKis with specific
immunotherapies, such as immune checkpoint inhibitors, including
the programmed cell death-ligand 1 (PD-L1) inhibitors, CAR-T
therapy, or bispecific antibodies (BiAbs), particularly as a
therapeutic solution for R/R diseases (44). However, it is known that the
inhibition of BTK with a BTKi produces changes in immune cell
numbers. It appears that the decrease in the number of T-cells
occurs in parallel with the receding tumor burden. It is
explainable why patients with R/R CLL have higher T-lymphocyte
numbers than untreated and non-progressive patients. Combining
ibrutinib with PD-L1 inhibitors has a synergistic effect compared
to PD-L1 inhibition alone in experimental models of lymphoma.
Clinical trials have found that the activity of the combination of
nivolumab or pembrolizumab with ibrutinib is limited in CLL cells,
but it is promising in patients with Richter transformation. BTK
appears to be expressed in effector/memory T-cells and plays a key
role in T-cell activation; thus, BTKis can target this mechanism
(44). It has been established
that ibrutinib is a clinically relevant and physiologically potent
inhibitor of ITK (18,44). By inhibiting ITK, it decreases Th2
and Th17 cell numbers and potentiates Th1-based immune responses.
The specific advantage given to Th1 lymphocytes may allow for the
effective generation of antitumor immunity (18). Acalabrutinib and zanubrutinib have
a weak effect on ITK and, as a result, do not alter the Th1/Th2
cell numbers (44).
Pre-treatment with ibrutinib before leukapheresis
can reverse T-cell dysfunction and improve CAR-T cell production,
which may be used as a bridging therapy before CAR-T cell therapy.
Furthermore, ibrutinib or acalabrutinib, together with CAR-T cell
therapy, can increase the number and function of T-lymphocytes,
contributing to the increase in the engraftment and expansion of
CAR-T cells, improving the anti-leukemic efficacy of CAR-T cells,
and decreasing cytokine release syndrome in patients with CLL
(44).
BiAbs, such as CD19/CD3-BsAb, recruit autologous
T-cell cytotoxicity against CLL cells in vitro.
Broad-spectrum BTKis can acutely abrogate the cytotoxicity of
T-cell-directed BiAbs and CAR T-cells in vitro (76). Acute exposure to BTKis impairs
T-cell activation and the lysis of target cells upon treatment with
CD3-directed BiAbs, through an effect independent of BTK
inhibition. This acute effect may be compensated in CLL, due to the
direct toxicity of BTKis to tumor cells. T-lymphocytes from
ibrutinib-treated patients have a greater in vitro antitumor
efficacy than T-lymphocytes from ibrutinib-naïve patients when
combined with BiAbs (76). It was
demonstrated that T-lymphocytes from these patients expanded more
rapidly and had superior cytotoxic activity in response to the
BiAbs. BTKis enhance BiAb-induced cytotoxicity by relieving
T-lymphocytes of immunosuppressive restraints imposed by CLL cells
(77).
7. Conclusions and future perspectives
BTKis have contributed to improving the therapeutic
results of patients with newly diagnosed or R/R CLL, for which they
have become the standard of care. PFS and ORR are significantly
longer with BTKis compared to the classical chemotherapy. BTKis are
also indicated for patients with an unfavorable prognosis.
Ibrutinib has immunomodulatory properties, and
selective BTKis have advantages compared to ibrutinib: An improved
PFS or ORR, and produce fewer AEs (particularly cardiac events and
adverse events that require treatment discontinuation).
The use of ibrutinib is not recommended for patients
with ventricular arrhythmias, and the use of any BTKi is not
recommended in those with a history of heart failure. A
multidisciplinary team must contribute to the management of AEs
that occur during BTKi therapy.
The combination of BTKis with an anti-CD20
monoclonal antibody and/or a BCL2 inhibitor aims at reducing the
proliferation of resistant clones, and sometimes allows for the
shortening of treatment duration. Studies using the combination of
zanubrutinib with venetoclax, obinutuzumab and other drugs are
underway (39). The combinations
of BTKi and venetoclax have been proven to be well-tolerated and
able to induce deep remissions (78).
The use of a ncBTKi or BCL2 inhibitor is a solution
for patients who develop resistance to covalent BTKis. Patients
with acquired resistance to BTKis could be treated with novel
agents, such as BTK degraders [which act by ubiquitination and
proteasomal degradation (32)],
BiAb therapy, CAR T-cell therapy, PKCβ inhibitors, or various
combinations (e.g., pirtobrutinib and venetoclax) that may
contribute to overcoming this acquired resistance (19). BTK degraders function by removing
BTK and could remain efficacious independent of BTK resistance
mutations (79).
Due to the numerous immunological pathways in which
BTKis are involved, their combination with other immunotherapeutic
agents, such as immune checkpoint inhibitors or CAR-T-cell therapy,
for the treatment of patients with relapsed or refractory CLL is
under discussion (44).
A synthetic chemical product,
6,7-dimethoxy-N-(pyridin-3-yl)quinazolin-4-amine, was found through
the screening of a large chemical library; it can serve as an
effective molecular core from which various druggable dual
inhibitors of the wild-type BTK and the C481S mutant would be
produced (80).
Integrin-mediated homing and the retention of the
malignant B-lymphocytes in the lymphoid organs can be achieved with
the use of ibrutinib. However, there are patients with CLL
intrinsically resistant to ibrutinib or who develop resistance to
this drug (81). The clustered
regularly interspaced short palindromic repeats
(CRISPR)-CRISPR-associated protein 9 (Cas9) system has been used in
recent years for gene insertions or deletions into the genome of
eukaryotic cells (82). An
unbiased screening method uses functional genomic CRISPR-Cas9 to
identify novel proteins involved in B-lymphocyte
receptor-controlled integrin-mediated adhesion; these proteins can
represent novel therapeutic targets to overcome ibrutinib
resistance (81).
Actomyosin complex organization and altered
mechanical properties of CLL cells may be involved in a novel
mechanism of drug resistance. BTKis are able to restore the
mechanical properties of the CLL cells to a healthy phenotype and
are involved in actomyosin complex activation. Actin cytoskeleton
organization could be a novel potential therapeutic target in CLL
(83).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RGM contributed to the preparation and design of the
manuscript, drafting and editing the manuscript, and in the design
of the tables. The author has read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
Shadman M: Diagnosis and treatment of
chronic lymphocytic leukemia: A review. JAMA. 329:918–932.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hampel PJ and Parikh SA: Correction:
Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer
J. 12(172)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen SS and Chiorazzi N: Functional
consequences of inhibition of Bruton's tyrosine kinase by ibrutinib
in chronic lymphocytic leukemia. Hematol Oncol. 41 (Suppl
1):S119–S128. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Wen T, Wang J, Shi Y, Qian H and Liu P:
Inhibitors targeting Bruton's tyrosine kinase in cancers: Drug
development advances. Leukemia. 35:312–332. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rey-Barroso J, Munaretto A, Rouquié N,
Mougel A, Chassan M, Gadat S, Dewingle O, Poincloux R, Cadot S,
Ysebaert L, et al: Lymphocyte migration and retention properties
affected by ibrutinib in chronic lymphocytic leukemia.
Haematologica. 109:809–823. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Song Y, Zhou K, Yang S, Hu J, Zou D, Gao
S, Pan L, Wang T, Yang H, Zhang H, et al: Indirect comparisons of
efficacy of zanubrutinib versus orelabrutinib in patients with
relapsed or refractory chronic lymphocytic leukemia/small
lymphocytic lymphoma or relapsed or refractory mantle cell
lymphoma. Invest New Drugs. 41:606–616. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rozkiewicz D, Hermanowicz JM, Kwiatkowska
I, Krupa A and Pawlak D: Bruton's tyrosine kinase inhibitors
(BTKIs): Review of preclinical studies and evaluation of clinical
trials. Molecules. 28(2400)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dhillon S: Tirabrutinib: First approval.
Drugs. 80:835–840. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Seymour C: FDA Approves Pirtobrutinib for
Previously Treated CLL/SLL. https://www.onclive.com/view/fda-approves-pirtobrutinib-for-previously-treated-cll-sll.
Available on February 21, 2024.
|
|
10
|
Bennett R, Anderson MA and Seymour JF:
Unresolved questions in selection of therapies for treatment-naïve
chronic lymphocytic leukemia. J Hematol Oncol.
16(72)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alu A, Lei H, Han X, Wei Y and Wei X: BTK
inhibitors in the treatment of hematological malignancies and
inflammatory diseases: Mechanisms and clinical studies. J Hematol
Oncol. 15(138)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nakhoda S, Vistarop A and Wang YL:
Resistance to Bruton tyrosine kinase inhibition in chronic
lymphocytic leukaemia and non-Hodgkin lymphoma. Br J Haematol.
200:137–149. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu W, Zhou K, Wang T, Yang S, Liu L, Hu Y,
Zhang W, Ding K, Zhou J, Gao S, et al: Orelabrutinib in relapsed or
refractory chronic lymphocytic leukemia/small lymphocytic lymphoma
patients: Multi-center, single-arm, open-label, phase 2 study. Am J
Hematol. 98:571–579. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cao XX, Jin J, Fu CC, Yi SH, Zhao WL, Sun
ZM, Yang W, Li DJ, Cui GH, Hu JD, et al: Evaluation of
orelabrutinib monotherapy in patients with relapsed or refractory
Waldenstrom's macroglobulinemia in a single-arm, multicenter,
open-label, phase 2 study. EClinicalMedicine.
52(101682)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kaptein A, de Bruin G, Emmelot-van Hoek M,
van de Kar B, de Jong A, Gulrajani M, Demont D, Covey T, Mittag D
and Barf T: Potency and selectivity of BTK inhibitors in clinical
development for B-cell malignancies. CLL: Therapy, excluding
transplantation: Poster I. Blood. 132 (Suppl 1)(S1871)2018.
|
|
16
|
Robak T, Witkowska M and Smolewski P: The
role of Bruton's kinase inhibitors in chronic lymphocytic leukemia:
Current status and future directions. Cancers (Basel).
14(771)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Berglöf A, Hamasy A, Meinke S, Palma M,
Krstic A, Månsson R, Kimby E, Österborg A and Smith CI: Targets for
ibrutinib beyond B cell malignancies. Scand J Immunol. 82:208–217.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dubovsky JA, Beckwith KA, Natarajan G,
Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu TM, Chang BY,
Larkin KM, et al: Ibrutinib is an irreversible molecular inhibitor
of ITK driving a Th1-selective pressure in T lymphocytes. Blood.
122:2539–2549. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Montoya S and Thompson MC: Non-covalent
bruton's tyrosine kinase inhibitors in the treatment of chronic
lymphocytic leukemia. Cancers (Basel). 15(3648)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li L, Su M, Lu W, Song H, Liu J, Wen X,
Suo Y, Qi J, Luo X, Zhou YB, et al: Triazine-based covalent
DNA-encoded libraries for discovery of covalent inhibitors of
target proteins. ACS Med Chem Lett. 13:1574–1581. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jensen JL, Mato AR, Pena C, Roeker LE and
Coombs CC: The potential of pirtobrutinib in multiple B-cell
malignancies. Ther Adv Hematol.
13(20406207221101697)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hillmen P, Pitchford A, Bloor A, Broom A,
Young M, Kennedy B, Walewska R, Furtado M, Preston G, Neilson JR,
et al: Ibrutinib and rituximab versus fludarabine,
cyclophosphamide, and rituximab for patients with previously
untreated chronic lymphocytic leukaemia (FLAIR): Interim analysis
of a multicentre, open-label, randomised, phase 3 trial. Lancet
Oncol. 24:535–552. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brown JR, Eichhorst B, Hillmen P, Jurczak
W, Kaźmierczak M, Lamanna N, O'Brien SM, Tam CS, Qiu L, Zhou K, et
al: Zanubrutinib or ibrutinib in relapsed or refractory chronic
lymphocytic leukemia. N Engl J Med. 388:319–332. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hillmen P, Eichhorst B, Brown JR, Lamanna
N, O'Brien SM, Tam CS, Qiu L, Kazmierczak M, Zhou K, Šimkovič M, et
al: Zanubrutinib versus ibrutinib in relapsed/refractory chronic
lymphocytic leukemia and small lymphocytic lymphoma: Interim
analysis of a randomized phase III trial. J Clin Oncol.
41:1035–1045. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mato AR, Woyach JA, Brown JR, Ghia P,
Patel K, Eyre TA, Munir T, Lech-Maranda E, Lamanna N, Tam CS, et
al: Pirtobrutinib after a covalent BTK inhibitor in chronic
lymphocytic leukemia. N Engl J Med. 389:33–44. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gill S, Vides V, Frey NV, Hexner EO,
Metzger S, O'Brien M, Hwang WT, Brogdon JL, Davis MM, Fraietta JA,
et al: Anti-CD19 CAR T cells in combination with ibrutinib for the
treatment of chronic lymphocytic leukemia. Blood Adv. 6:5774–5785.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ghia P, Pluta A, Wach M, Lysak D, Šimkovič
M, Kriachok I, Illés Á, de la Serna J, Dolan S, Campbell P, et al:
Acalabrutinib versus investigator's choice in relapsed/refractory
chronic lymphocytic leukemia: Final ASCEND trial results.
Hemasphere. 6(e801)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nguyen TT, Nhu NT, Tran VK, Nguyen TTH and
Lin CF: Efficacy and safety of Bruton tyrosine kinase inhibitor
monotherapy compared with combination therapy for chronic
lymphocytic leukemia and small lymphocytic lymphoma: A systematic
review and meta-analysis. Cancers (Basel). 15(1996)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee P, Kistler KD, Douyon L, Volodarsky R,
Young A, Karve S and Challagulla S: Systematic literature review of
real-world effectiveness results data for first-line ibrutinib in
chronic lymphocytic leukemia and small lymphocytic lymphoma. Drugs
Real World Outcomes. 10:11–22. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boriani G, Menna P, Morgagni R, Minotti G
and Vitolo M: Ibrutinib and Bruton's tyrosine kinase inhibitors in
chronic lymphocytic leukemia: focus on atrial fibrillation and
ventricular tachyarrhythmias/sudden cardiac death. Chemotherapy.
68:61–72. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Špaček M, Smolej L, Šimkovič M, Nekvindová
L, Křístková Z, Brychtová Y, Panovská A, Mašlejová S, Bezděková L,
Écsiová D, et al: Idelalisib plus rituximab versus ibrutinib in the
treatment of relapsed/refractory chronic lymphocytic leukaemia: A
real-world analysis from the chronic lymphocytic leukemia patients
registry (CLLEAR). Br J Haematol. 202:40–47. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Muhowski EM, Ravikrishnan J, Gordon B, Yu
L, Misra S, Walker B, Eathiraj S, Sampath D, Rogers KA, Byrd JC and
Woyach JA: Preclinical evaluation of combination nemtabrutinib and
venetoclax in chronic lymphocytic leukemia. J Hematol Oncol.
15(166)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Eyre TA and Riches JC: The evolution of
therapies targeting Bruton tyrosine kinase for the treatment of
chronic lymphocytic leukaemia: Future perspectives. Cancers
(Basel). 15(2596)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Perutelli F, Montalbano MC, Boccellato E,
Coscia M and Vitale C: Beyond ibrutinib: Novel BTK inhibitors for
the treatment of chronic lymphocytic leukemia. Curr Opin Oncol.
34:757–767. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Roeker LE, DerSarkissian M, Ryan K, Chen
Y, Duh MS, Wahlstrom SK, Hakre S, Yu L, Guo H and Mato AR:
Real-world comparative effectiveness of acalabrutinib and ibrutinib
in patients with chronic lymphocytic leukemia. Blood Adv.
7:4291–4301. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Munir T, Genovez V, Genestier V, Ryan K,
Liljas B and Gaitonde P: Cost-effectiveness of acalabrutinib
regimens in treatment-naïve chronic lymphocytic leukemia in the
United States. Expert Rev Pharmacoecon Outcomes Res. 23:579–589.
2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wallace DS, Zent CS, Baran AM, Reagan PM,
Casulo C, Rice G, Friedberg JW and Barr PM: Acalabrutinib and
high-frequency low-dose subcutaneous rituximab for initial therapy
of chronic lymphocytic leukemia. Blood Adv. 7:2496–2503.
2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Podoll T, Pearson PG, Kaptein A, Evarts J,
de Bruin G, Emmelot-van Hoek M, de Jong A, van Lith B, Sun H, Byard
S, et al: Identification and characterization of ACP-5862, the
major circulating active metabolite of acalabrutinib: Both are
potent and selective covalent Bruton tyrosine kinase inhibitors. J
Pharmacol Exp Ther. 384:173–186. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wolska-Washer A and Robak T: Zanubrutinib
for the treatment of lymphoid malignancies: Current status and
future directions. Front Oncol. 13(1130595)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Molica S, Tam C, Allsup D and Polliack A:
Advancements in the treatment of CLL: The rise of zanubrutinib as a
preferred therapeutic option. Cancers (Basel).
15(3737)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gu D, Li J and Miao Y: Evaluating
orelabrutinib as a novel treatment option for relapsed/refractory
chronic lymphocytic leukemia in China. Expert Opin Pharmacother.
23:1979–1986. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Robak P, Witkowska M, Wolska-Washer A and
Robak T: The preclinical discovery and development of orelabrutinib
as a novel treatment option for B-cell lymphoid malignancies.
Expert Opin Drug Discov. 18:1065–1076. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Munakata W, Ando K, Yokoyama M, Fukuhara
N, Yamamoto K, Fukuhara S, Ohmachi K, Mishima Y, Ichikawa S, Ogiya
D, et al: Long-term safety profile of tirabrutinib: final results
of a Japanese phase I study in patients with relapsed or refractory
B-cell malignancies. Int J Hematol. 117:553–562. 2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang H, Guo H, Yang J, Liu Y, Liu X, Zhang
Q and Zhou K: Bruton tyrosine kinase inhibitors in B-cell lymphoma:
Beyond the antitumour effect. Exp Hematol Oncol.
11(60)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yano M, Nunes J, Mo X, Rogers KA, Woyach
JA, Byrd JC and Muthusamy N: Differential regulation of CTLA4
expression through BTK-dependent and independent mechanisms in CLL.
Blood Adv. 6:5440–5448. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee HK, Hoechstetter MA, Buchner M, Pham
TT, Huh JW, Müller K, Zange S, von Buttlar H, Girl P, Wölfel R, et
al: Analysis of immune responses in patients with CLL after
heterologous COVID-19 vaccination. Blood Adv. 7:2214–2227.
2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Daniel A, Ghez D, Ravaiau C, Cavalieri D,
Tournilhac O, Herbaux C, Roriz M, Wemeau M, Guillet S, Bossard JB,
et al: Ibrutinib as a treatment of hematologic autoimmune disorders
in patients with indolent B-cell lymphoma. Eur J Haematol.
109:719–727. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yan Y, Lv R, Wang T, Yu Y, Huang Y, Xiong
W, Li Y, Sui W, Wang Q, Huang W, et al: Real-world treatment
patterns, discontinuation and clinical outcomes in patients with
B-cell lymphoproliferative diseases treated with BTK inhibitors in
China. Front Immunol. 14(1184395)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sharma S, Pepin X, Burri H, Zheng L,
Kuptsova-Clarkson N, de Jong A, Yu T, MacArthur HL, Majewski M,
Byrd JC, et al: Bioequivalence and relative bioavailability studies
to assess a new acalabrutinib formulation that enables
coadministration with proton-pump inhibitors. Clin Pharmacol Drug
Dev. 11:1294–1307. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Karadeniz M, Cinar OE, Erdogdu B, Malkan
UY, Goker H and Ozcebe OI: Hypophosphatemia related to the use of
ibrutinib. J Oncol Pharm Pract: 10781552231164504, 2023 (Epub ahead
of print).
|
|
51
|
Wan Q, Li Q, Lai X, Xu T, Hu J and Peng H:
Data mining and safety analysis of BTK inhibitors: A
pharmacovigilance investigation based on the FAERS database. Front
Pharmacol. 13(995522)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Awan FT, Addison D, Alfraih F, Baratta SJ,
Campos RN, Cugliari MS, Goh YT, Ionin VA, Mundnich S, Sverdlov AL,
et al: International consensus statement on the management of
cardiovascular risk of Bruton's tyrosine kinase inhibitors in CLL.
Blood Adv. 6:5516–5525. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gordon MJ, Jones JE, George B, Peterson C,
Burger JA, Jain N, Keating M, Wierda WG, Durand JB and Ferrajoli A:
Long-term outcomes in patients with chronic lymphocytic leukemia
treated with ibrutinib: Focus on hypertension and cardiovascular
toxicity. Cancer. 129:2192–2200. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Seymour JF, Byrd JC, Ghia P, Kater AP,
Chanan-Khan A, Furman RR, O'Brien S, Brown JR, Munir T, Mato A, et
al: Detailed safety profile of acalabrutinib vs ibrutinib in
previously treated chronic lymphocytic leukemia in the ELEVATE-RR
trial. Blood. 142:687–699. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Elaskalani O, Gilmore G, Hagger M, Baker
RI and Metharom P: Adenosine 2A receptor activation amplifies
ibrutinib antiplatelet effect; implications in chronic lymphocytic
leukemia. Cancers (Basel). 14(5750)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tham K, Prelewicz S, deHoll S, Stephens DM
and Gomez CA: Infectious complications among patients receiving
ibrutinib for the treatment of hematological malignancies. Am J
Health Syst Pharm. 81:112–119. 2024.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Diella L, Bavaro DF, Loseto G, Pasciolla
C, Minoia C, Di Gennaro D, Belati A, De Candia MS, Di Gennaro F,
Saracino A and Guarini A: Current therapies for chronic lymphocytic
leukemia: Risk and prophylaxis strategies for
secondary/opportunistic infections. Expert Rev Hematol. 16:267–276.
2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
No authors listed. Correction to: Managing
ibrutinib-intolerant patients with B-cell malignancies. Oncologist.
28(e487)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shadman M, Flinn IW, Levy MY, Porter RF,
Burke JM, Zafar SF, Misleh J, Kingsley EC, Yimer HA, Freeman B, et
al: Zanubrutinib in patients with previously treated B-cell
malignancies intolerant of previous Bruton tyrosine kinase
inhibitors in the USA: A phase 2, open-label, single-arm study.
Lancet Haematol. 10:e35–e45. 2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Salmerón-Navas FJ, Barreiro-Fernández EM
and Fénix-Caballero S: Adjusted indirect comparison of zanubrutinib
and ibrutinib in first-line treatment of chronic lymphocytic
leukemia. Farm Hosp. 48:9–15. 2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen S, Wei Y, Li S, Miao Y, Gu J, Cui Y,
Liu Z, Liang J, Wei L, Li X, et al: Zanubrutinib attenuates
bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β1
signaling pathway. Int Immunopharmacol. 113(109316)2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Blombery P, Thompson ER, Lew TE, Tiong IS,
Bennett R, Cheah CY, Lewis KL, Handunnetti SM, Tang CPS, Roberts A,
et al: Enrichment of BTK Leu528Trp mutations in patients with CLL
on zanubrutinib: Potential for pirtobrutinib cross-resistance.
Blood Adv. 6:5589–5592. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Naeem A, Utro F, Wang Q, Cha J, Vihinen M,
Martindale S, Zhou Y, Ren Y, Tyekucheva S, Kim AS, et al:
Pirtobrutinib targets BTK C481S in ibrutinib-resistant CLL but
second-site BTK mutations lead to resistance. Blood Adv.
7:1929–1943. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Takács F, Kotmayer L, Czeti Á, Szalóki G,
László T, Mikala G, Márk Á, Masszi A, Farkas P, Plander M, et al:
Revealing a phenotypical appearance of ibrutinib resistance in
patients with chronic lymphocytic leukaemia by flow cytometry.
Pathol Oncol Res. 28(1610659)2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Maher N, Mouhssine S, Matti BF, Alwan AF
and Gaidano G: Treatment refractoriness in chronic lymphocytic
leukemia: Old and new molecular biomarkers. Int J Mol Sci.
24(10374)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Woyach JA, Ghia P, Byrd JC, Ahn IE, Moreno
C, O'Brien SM, Jones D, Cheung LWK, Chong E, Kwei K, et al: B-cell
receptor pathway mutations are infrequent in patients with chronic
lymphocytic leukemia on continuous ibrutinib therapy. Clin Cancer
Res. 29:3065–3073. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Alsadhan A, Chen J, Gaglione EM,
Underbayev C, Tuma PL, Tian X, Freeman LA, Baskar S, Nierman P,
Soto S, et al: CD49d expression identifies a biologically distinct
subtype of chronic lymphocytic leukemia with inferior
progression-free survival on BTK inhibitor therapy. Clin Cancer
Res. 29:3612–3621. 2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Tissino E, Bomben R, Gattei V and
Zucchetto A: BCR/integrin interaction in CLL: A physiologic remnant
with clinical relevance. Clin Cancer Res. 29:3560–3562.
2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Thompson PA and Tam CS: Pirtobrutinib: A
new hope for patients with BTK inhibitor-refractory
lymphoproliferative disorders. Blood. 141:3137–3142.
2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chung C, Umoru G, Abboud K and Hobaugh E:
Sequencing and combination of current small-molecule inhibitors for
chronic lymphocytic leukemia: Where is the evidence? Eur J
Haematol. 111:15–28. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rizzuto A, Pirrera A, Gigliotta E, Mancuso
S, Vullo C, Camarda GM, Rotolo C, Roppolo A, Spoto C, Gentile M, et
al: Molecular-biology-driven frontline treatment for chronic
lymphocytic leukemia: A network meta-analysis of randomized
clinical trials. Int J Mol Sci. 24(9930)2023.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Nguyen TT, Thanh Nhu N, Tran VK, Van Cau N
and Lin CF: Efficacy and safety of Bruton tyrosine kinase inhibitor
plus anti-CD20 antibody therapy compared with chemoimmunotherapy as
front-line treatment for chronic lymphocytic leukemia: A systematic
review and meta-analysis of randomized controlled trials. J
Immunother. 46:299–309. 2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Nguyen TT, Nhu NT, Tran VK, Viet-Nhi NK,
Ho XD, Jhan MK, Chen YP and Lin CF: Efficacy and safety of add-on
anti-CD20 monoclonal antibody to Bruton tyrosine kinase inhibitor
treatment for chronic lymphocytic leukemia: A meta-analysis. Sci
Rep. 13(9775)2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Scarfò L, Heltai S, Albi E, Scarano E,
Schiattone L, Farina L, Moia R, Deodato M, Ferrario A, Motta M, et
al: Minimal residual disease-driven treatment intensification with
sequential addition of ibrutinib to venetoclax in R/R CLL. Blood.
140:2348–2357. 2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Cervantes-Gomez F, Lamothe B, Woyach JA,
Wierda WG, Keating MJ, Balakrishnan K and Gandhi V: Pharmacological
and protein profiling suggests venetoclax (ABT-199) as optimal
partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer
Res. 21:3705–3715. 2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Godwin CD, Bates OM, Garling EE, Beddoe
ME, Laszlo GS and Walter RB: The Bruton's tyrosine kinase inhibitor
ibrutinib abrogates bispecific antibody-mediated T-cell
cytotoxicity. Br J Haematol. 189:e9–e13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Mhibik M, Gaglione EM, Eik D, Kendall EK,
Blackburn A, Keyvanfar K, Baptista MJ, Ahn IE, Sun C, Qi J, et al:
BTK inhibitors, irrespective of ITK inhibition, increase efficacy
of a CD19/CD3-bispecific antibody in CLL. Blood. 138:1843–1854.
2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Nasnas P, Cerchione C, Musuraca G,
Martinelli G and Ferrajoli A: How I manage chronic lymphocytic
leukemia. Hematol Rep. 15:454–464. 2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Easaw S, Ezzati S and Coombs CC: SOHO
State of the art updates and next questions: Updates on BTK
inhibitors for the treatment of chronic lymphocytic leukemia. Clin
Lymphoma Myeloma Leuk. 23:697–704. 2023.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kim T, Kim K, Park I, Hong S and Park H:
Two-track virtual screening approach to identify the dual
inhibitors of wild type and C481S mutant of Bruton's tyrosine
kinase. J Chem Inf Model. 62:4500–4511. 2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Thus YJ, De Rooij MFM, Beijersbergen RL
and Spaargaren M: An Unbiased CRISPR-Cas9 screening method for the
identification of positive and negative regulatory proteins of cell
adhesion. Bio Protoc. 12(e4545)2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Mihăilă RG and Topîrcean D: The
high-performance technology CRISPR/Cas9 improves knowledge and
management of acute myeloid leukemia. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 165:249–257. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Sampietro M, Cassina V, Salerno D,
Barbaglio F, Buglione E, Marrano CA, Campanile R, Scarfò L,
Biedenweg D, Fregin B, et al: The nanomechanical properties of CLL
cells are linked to the actin cytoskeleton and are a potential
target of BTK inhibitors. Hemasphere. 7(e931)2023.PubMed/NCBI View Article : Google Scholar
|