Introduction
Anemia is a global health issue; in 2019, the World
Health Organization (WHO) estimated that 29.9% of all women of
reproductive age, 39.9% of all children and 60.2% of all African
children are anemic (1). Anemia
can lead to several adverse health effects that have a negative
impact on cognitive functioning in children and on work capacity in
adults (1,2). Iron deficiency anemia (IDA) comprises
66.2% of all types of anemia, rendering it a crucial health issue,
particularly for women of reproductive age and children (3,4).
Several factors for anemia have been reported, including dietary
causes, hemoglobinopathies, malaria and other infectious diseases
(3).
Glycated hemoglobin (HbA1c) is a hemoglobin fraction
that is used as a diagnostic tool for diabetes mellitus and its
related complications (5).
Previous studies have shown inconsistent results regarding the
association between HbA1c, iron depletion and IDA (6-11).
While some studies have reported a low level of HbA1c in IDA
(12,13), others have found that anemia/IDA is
associated with high HbA1c levels (6-11).
In Sudan, there is a high prevalence of anemia among
various age groups, including children (49.4%) (14), adults (25.2%) (15) and pregnant women (37.0%) (16). There is also a high prevalence of
diabetes mellitus in Sudan; 20% of adults have diabetes mellitus
and 80.0% of these adults have uncontrolled diabetes mellitus
(17). The high prevalence of
anemia may underestimate the diagnosis of diabetes mellitus. It is
crucial to assess whether HbA1c levels are influenced by IDA and
whether this can lead to the revaluation of the cut-off for HbA1c
in diagnosing diabetes mellitus, as well as the level of control of
diabetes mellitus. Such results would prove helpful for clinicians
who provide patient care, health planners and academicians. The
present study was conducted in an aim to compare the HbA1c levels
in adolescents with IDA to those in their peers without IDA.
Patients and methods
Study population
A school-based case-control study was conducted in
Northern Sudan from September to December, 2022. The number of
adolescents from each school was determined by the total number of
adolescents in that school, following a probability proportional to
size. Thus, schools with more students greatly contributed to the
study sample. The assigned sample size was selected from the list
of students in each school through the use of a simple random
technique (lottery method). The present study was carried out in
accordance with the Declaration of Helsinki and good clinical
practices. The study obtained ethics approval from the Ethical
Board of the Faculty of Medicine, University of Khartoum, Khartoum,
Sudan, under reference no. 9, 2021. All included students and their
guardians agreed to participate and signed a written informed
consent form. The author followed all measures to ensure the
participants' privacy, safety and confidentiality, such as
excluding personal identifiers during data collection.
Inclusion and exclusion criteria
The study population comprised of adolescents (aged
10-19 years) with IDA and no other diseases; the controls were
adolescents with no such anemia (and otherwise healthy, as
described below). On the other hand, students whose guardians or
parents did not provide their consent to participate in the study
and those who were ill, pregnant, or lactating were excluded from
the study.
Study variables and measures
The questionnaire included data on various
sociodemographic characteristics, such as age in years, sex (male
or female), parental educational levels (less than secondary or
greater than or equal to secondary), the occupational status of the
mother (housewife or employed), the occupational status of the
father (laborer or skilled worker), anthropometric measurements,
including weight and height [expressed as body mass index (BMI)
z-score] and hematological parameters, including serum ferritin.
The investigator trained five medical research assistants to
collect the data; all trainings were complete prior to the
commencement of the study. The training emphasized proper
measurement techniques for weight and height, effective utilization
of the study questionnaire, and adherence to the study
protocol.
The medical research assistants (three males and two
females) approached the selected adolescents after the participants
and their guardians had agreed to participate and signed an
informed consent form. The selected adolescents were informed about
the aims of the study and provided with all necessary information,
including their voluntary participation in the study and their
right to withdraw at any time without needing to give a reason. The
study also took preventive measures to ensure the privacy and
confidentiality of the participants, such as excluding personal
identifiers during data collection, further reinforcing the study's
ethical integrity.
Weight, height, anemia and serum ferritin levels
were measured using standard procedures, as detailed below
(sociodemographic data, BMI and hematological parameters).
Weight and height measurements
The weights of the adolescents were measured in kg
using standard procedures (i.e., well-calibrated scales adjusted to
zero prior to each measurement). Weight was measured to the nearest
100 g. Shoes and excess clothing were removed before the
adolescents stood with minimal movement, with their hands by their
sides. Height was measured to the nearest 0.1 cm, with the
adolescents standing straight with their backs against the wall and
their feet together. The BMI for the age z-score was determined
based on the WHO standards (18).
Blood sample processing
To analyze hemoglobin and serum ferritin levels, 5
ml blood were obtained from each student and transferred into an
EDTA tube under aseptic conditions. These samples were used as part
of a complete blood count. An automated hematology analyzer (Sysmex
KX-21, Sysmex Corporation) was used to measure the hemoglobin
levels (19). Based on the
recommendation of the WHO for adolescents, a hemoglobin
concentration <12 g/dl in females and 13 g/dl in males was
considered a sign of anemia (20).
Furthermore, the hemoglobin values were categorized as mild (>10
g/dl), moderate (7-9.9 g/dl) and severe (<7 g/dl) (21) anemia.
The remaining blood samples (3 ml) were centrifuged
(at 3,000 x g, for 10 min at room temperature) and the serum was
stored at -20˚C in the laboratory. Serum ferritin levels were
subsequently measured using a radioimmunoassay gamma counter
(Riostad, Berthold Technologies GmbH & Co. KG) and kits
provided by Beijing Isotope Nuclear Electronic Co. (22). HbA1c levels were measured in all
participants, including those who were later diagnosed with mild,
moderate, or severe anemia, using enzymatic methods on the
ARCHITECT clinical chemistry analyzer (Abbott Pharmaceutical Co.
Ltd.).
In accordance with previous studies (23,24),
a serum ferritin level ≥15 µg/ was considered normal, while a level
<15 µg/l was considered low (iron deficiency). In the present
study, IDA was defined as the presence of anemia as per the
definition of the WHO (a hemoglobin level <12 g/dl and serum
ferritin level <15 µg/l). To ensure accurate and consistent
results, both the automated hematology analyzer and the
radioimmunoassay gamma counter were calibrated on a monthly basis.
Additionally, quality control measures were taken prior to daily
operation of these instruments.
Sample size calculation
OpenEpi Menu software was used to compute the
desired sample size (25). The
present study required a sample of 47 cases and 94 controls (a
ratio of 1:2). The mean (standard deviation) of HbA1c was set at
5.5% (0.3) for the cases and 5.7% (0.4) for the controls. This
sample size would have a confidence interval (CI) of 95% (1.96) and
a margin of error of 5% (0.05).
Statistical analysis
The data were entered into IBM Statistical Product
and Service Solutions (SPSS) for Windows (Version 22.0; IBM Corp.)
for analysis. Continuous data, such as age, BMI, and hemoglobin and
serum ferritin levels, were evaluated for normality using the
Kolmogorov-Smirnov test. They were found to be non-normally
distributed; therefore, they were expressed as a median
[interquartile range (IQR)]. Additionally, the Mann-Whitney U test
was used to compare the continuous variables between adolescents
with IDA and adolescents without IDA. The Chi-squared test was sued
to compare proportions between two groups). Univariate analysis was
conducted initially, and all variables were then shifted for
multivariate logistic regression to adjust for covariates. Adjusted
multivariate analysis was performed on anemia and iron deficiency
as categorical variables, as dependent variables, and
independently, as well as on sociodemographic characteristics (age,
sex, BMI z-score as independent variables). Adjusted odds ratios
(AORs) and 95% CIs were calculated as they were applied. A
two-sided P-value <0.05 was considered to indicate a
statistically significant difference.
Results
General characteristics of the study
subjects
A total of 141 adolescents were enrolled in the
present study. Of these, 75 (53.2%) were females, and 66 (46.8%)
were males. The median (IQR) of the adolescents' age was 14.1
(12.3-16.1) years (Table I).
 | Table IComparison of the age, body mass
index, HbA1c levels, parents' education and occupation of
adolescents with and without iron deficiency anemia in Sudan in
2022. |
Table I
Comparison of the age, body mass
index, HbA1c levels, parents' education and occupation of
adolescents with and without iron deficiency anemia in Sudan in
2022.
| Variables | Total adolescents
(n=141) | Adolescents with IDA
(n=47) | Adolescents without
IDA (n=94) | P-value |
|---|
| Age, years; median
(interquartile range)a | 14.1 (12.3-16.1) | 14.1 (12.4-16.2) | 14.3 (12.2-16.1) | 0.832 |
| Body mass index,
kg/m2; median (interquartile range)a | 17.0 (15.3-19.8) | 16.9 (15.0-19.8) | 17.1 (15.4-19.9) | 0.875 |
| Blood glucose,
mg/dl2; median (interquartile range)a | 80.1 (71.5-90.0) | 83.0 (75.0-95.0 | 80.0 (70.0-88.0) | 0.174 |
| HbA1c, %; median
(interquartile range)a | 5.5 (5.2-5.7) | 5.4 (5.3-5.7) | 5.5 (5.1-5.8) | 0.890 |
| Serum ferritin, µg/l;
median (interquartile range)a | 18.3
(10.2-37.4)) | 10.0 (10.0-11.6) | 29.1
(18.1-53.7)) | <0.001 |
| Sex, n
(%)b | | | | |
|
Male | 66 (46.8) | 20 (42.6) | 46 (48.9) | 0.591 |
|
Female | 75 (53.1) | 27 (57.4) | 48 (51.1) | |
| Mother's education, n
(%)b | | | | |
|
>
Secondary level | 97 (68.8) | 32 (68.1) | 65 (69.1) | 0.522 |
|
≤ Secondary
level | 44 (31.2) | 15 (31.9) | 29 (30.9) | |
| Mother's occupation,
n (%)b | | | | |
|
Housewife | 119 (84.8) | 39 (83.0 | 80 (85.1) | 0.460 |
|
Employed | 22 (15.6) | 8 (17.0) | 14 (14.9) | |
| Father's education, n
(%)b | | | | |
|
>
Secondary level | 102 (72.3) | 34 (72.3) | 68 (72.3) | 0.999 |
|
≤ Secondary
level | 39 (27.7) | 13 (27.7) | 26 (27.7 | |
| Father's occupation,
n (%)b | | | | |
|
Skilled | 49 (34.8) | 16 (34.0) | 33 (35.1) | 0.999 |
|
Labourer | 92 (65.2) | 31 (66.0) | 61 (64.9) | |
In total, 47 cases (adolescents with IDA) and 94
controls (adolescents without IDA) were enrolled in the present
study. The two groups exhibited no significant differences in age,
BMI, or blood glucose levels, using Mann-Whitney U test (Table I). Of note, 27 of the cases (57.4%)
and 48 of the controls (51.1%) were females (P=0.591).
Factors associated with HbA1c
levels
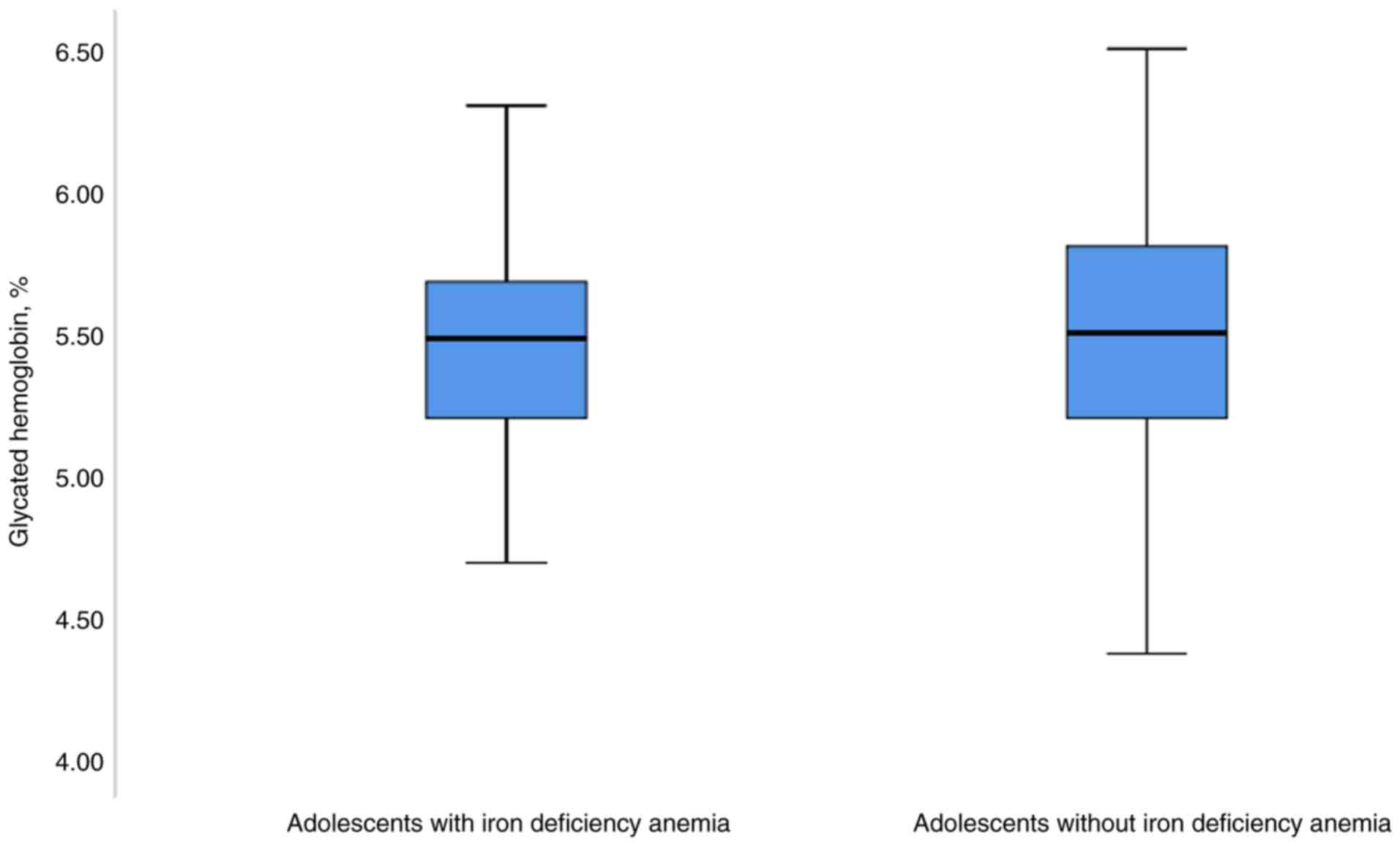
The median (IQR) of HbA1c was 5.4% (5.3-5.7%) in the
cases and 5.5% (5.1-5.8%) in the controls; the Mann-Whitney U test
did not reveal any significant differences between the two groups
(P=0.890; Fig. 1 and Table I). In the univariate linear
analysis, age, sex, BMI and iron deficiency (IDA: coefficient,
0.008; 95% CI, -0.1570-0.174; P=0.921) were not found to be
associated with HbA1c (Table II).
Moreover, in the multivariate linear analysis, age, sex, BMI and
iron deficiency (IDA: coefficient, 0.008; 95% CI, -0.16-0.17;
P=0.929) were also not found to be associated with HbA1c (Table II).
 | Table IIUnivariate and multivariate linear
regression analysis of the factors associated with HbA1c in
adolescents in Sudan, 2022. |
Table II
Univariate and multivariate linear
regression analysis of the factors associated with HbA1c in
adolescents in Sudan, 2022.
| | Univariate linear
analysis | Multivariate linear
analysis |
|---|
| Variables | Coefficient | 95% confidence
interval | P-value | Coefficient | 95% confidence
interval | P-value |
|---|
| Age, years | -0.007 | -0.038-0.024 | 0.658 | -0.007 | -0.04-0.03 | 0.717 |
| Body mass index,
kg/m2 | -0.002 | -0.022-0.017 | 0.826 | 1.11 | -0.023-0.023 | 0.999 |
| Sex | | | | | | |
|
Male | Reference | | | | | |
|
Female | -0.014 | -0.170-0.143 | 0.865 | -0.004 | -0.17-0.16 | 0.958 |
| Iron deficiency
anemia | | | | | | |
|
No | Reference | | | | | |
|
Yes | 0.008 | -0.1570-0.174 | 0.921 | 0.008 | -0.16-0.17 | 0.929 |
Discussion
The main findings of the present study were that no
difference was found in HbA1c between adolescents with and without
IDA, and HbA1c was not associated with IDA. This is consistent with
the findings of a recent meta-analysis that included six studies
and demonstrated that IDA had no effect on HbA1c in patients with
diabetes mellitus (26). However,
the results of the meta-analysis revealed that IDA had an effect on
HbA1c in non-diabetic individuals (26). By contrast, it has been reported
that in 263 patients without diabetes mellitus, HbA1c levels were
significantly lower in patients with IDA than in their peers who
had no such anemia (6). Moreover,
a moderate correlation has been reported between HbA1c and serum
iron levels in children with type 1 diabetes (13).
As aforementioned, the results of previous studies
on HbA1c levels in iron deficiency have been inconsistent. Building
upon earlier results that demonstrated that IDA is associated with
high HbA1c (in diabetic patients), a previous study found that a
high level of glycated hemoglobin was markedly corrected
(decreased) following treatment with iron supplements (6). By contrast, other research has
suggested that a low level of HbA1c is associated with IDA
(12). Furthermore, a recent
systematic review concluded that iron replacement therapy reduced
HbA1c in patients with uncontrolled diabetic mellitus who had an
iron deficiency (27). However, in
another study, the mean HbA1c level was higher in anemic than
non-anemic Indian euglycemic patients (7). In addition, HbA1c was significantly
increased in iron-deficient patients (non-diabetic) compared to the
control group (8).
In a large-scale retrospective study, patients with
IDA had higher HbA1c levels than those without anemia (10). A later study confirmed these
results and found that patients with nutritional anemia (such as
IDA) exhibited a higher mean level of HbA1c, which was then reduced
in response to the treatment of anemia (9). Intra et al (10) demonstrated that the mean HbA1c was
higher in anemic patients than in non-anemic patients.
In Dutch children with diabetes, the mean HbA1c did
not differ in patients with an iron deficiency (28). However, an inverse finding was
reported among Egyptian children with type 1 diabetes, who had
significantly higher levels of HbA1c than non-anemic diabetic
children (11). In addition, serum
iron has been negatively correlated with glycated hemoglobin in
children and adolescents with type 1 diabetes mellitus (13). The diverse sociodemographic
characteristics of the enrolled participants and the varied
prevalence of anemia and severe anemia in different areas may
explain these variable results. Moreover, some of these studies
assessed HbA1c and iron deficiency in diabetic patients, while
others investigated these variables among healthy cohorts.
Although the exact mechanism by which HbA1c
increases in association with IDA remains unclear; it is considered
that the reduction in iron and ferritin levels affects the rate of
red blood cell turnover. This leads to a prolonged half-life of the
red blood cells eligible for destruction, thereby increasing the
rate of hemoglobin glycation (29). Additionally, hemoglobin with a
lower iron content is more vulnerable to oxidative stress,
resulting in elevated malondialdehyde levels, which further
accelerate hemoglobin glycation (30).
From a clinical perspective, in cases of IDA, it is
essential to correct hemoglobin and iron levels before interpreting
HbA1c, as falsely elevated results are likely. Furthermore, it is
advisable to measure hemoglobin levels whenever elevated HbA1c is
detected to rule out potential false-positive results.
The present study has certain limitations that
warrant consideration. Potential confounding factors that could
influence HbA1c levels, such as recent dietary habits, physical
activity and a family history of diabetes, were not examined. As a
result, further research is needed to address these variables and
provide a more comprehensive understanding of the relationship
between IDA and HbA1c levels. In conclusion, the present study did
not find any notable differences in HbA1c levels between
adolescents with IDA and those without IDA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
The author AM was involved in the conceptualization
of the study, in the design of the study methodology, in data
curation and formal analysis, as well as in the investigation and
procedural aspects of the study. The author AM was also drafted and
reviewed the manuscript. AM confirms the authenticity of all the
raw data. The author has read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was carried out in accordance with
the Declaration of Helsinki and good clinical practices. The study
obtained ethics approval from the ethical board of the Faculty of
Medicine, University of Khartoum, Khartoum, Sudan, under reference
no. 9, 2021. All included students and their guardians agreed to
participate and signed a written informed consent form. The author
followed all measures to ensure the participants' privacy, safety,
and confidentiality, such as excluding personal identifiers during
data collection.
Patient consent for publication
Not applicable.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
World Health Organization (WHO): WHO
Global Anaemia estimates, 2021 edition. WHO, Geneva, 2022.
|
|
2
|
Gardner WM, Razo C, McHugh TA, Hagins H,
Vilchis-Tella VM, Hennessy C, Taylor H, Jean P, Nandita F, Kia C,
et al: Prevalence, years lived with disability, and trends in
anaemia burden by severity and cause, 1990-2021: Findings from the
global burden of disease study 2021. Lancet Haematol. 10:e713–e734.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
The Institute for Health Metrics and
Evaluation (IHME): The Lancet: New study reveals global anemia
cases remain persistently high among women and children. Anemia
rates decline for men. IHME, Seattle, WA, 2023.
|
|
4
|
Hamdan SZ, Hamdan HZ, Nimieri M and Adam
I: The association between Helicobacter Pylori infection and iron
deficiency anemia in children: A systematic review and
meta-analysis. J Pediatr Infect Dis. 17:59–70. 2022.
|
|
5
|
International Expert Committee.
International Expert Committee report on the role of the A1C assay
in the diagnosis of diabetes. Diabetes Care. 32:1327–1334.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aydın B, Özçelik S, Kilit TP, Eraslan S,
Çelik M and Onbaşı K: Relationship between glycosylated hemoglobin
and iron deficiency anemia: A common but overlooked problem. Prim
Care Diabetes. 16:312–317. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharda M, Gandhi N, Bansal D and Gadhwal
M: Correlation of anemia with glycated hemoglobin among euglycemic
type 2 diabetic patients. J Assoc Physicians India. 71:11–12.
2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alzahrani BA, Salamatullah HK, Alsharm FS,
Baljoon JM, Abukhodair AO, Ahmed ME, Malaikah H and Radi S: The
effect of different types of anemia on HbA1c levels in
non-diabetics. BMC Endocr Disord. 23(24)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pilla R, Palleti SK, Rayala R, SKSS SR,
Abdul Razzack A and Kalla S: Glycated haemoglobin (HbA1c)
variations in nondiabetics with nutritional anemia. Cureus.
12(e11479)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Intra J, Limonta G, Cappellini F, Bertona
M and Brambilla P: Glycosylated hemoglobin in subjects affected by
iron-deficiency anemia. Diabetes Metab J. 43:539–544.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mahgoob MH and Moussa MM: Glycated albumin
versus HbA1c as indicators of glycemic control in type I diabetic
children with iron deficiency anemia. Clin Pediatr Endocrinol.
29:151–157. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Solomon A, Hussein M, Negash M, Ahmed A,
Bekele F and Kahase D: Effect of iron deficiency anemia on HbA1c in
diabetic patients at Tikur Anbessa specialized teaching hospital,
Addis Ababa Ethiopia. BMC Hematol. 19(2)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Satriawibawa IWE, Arimbawa IM, Ariawati K,
Suparyatha IBG, Putra IGNS and Hartawan INB: Serum iron is
negatively correlated with the HbA1c level in children and
adolescents with type 1 diabetes mellitus. Clin Pediatr Endocrinol.
31:242–249. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elmardi KA, Adam I, Malik EM, Ibrahim AA,
Elhassan AH, Kafy HT, Nawai LM, Abdin MS and Kremers S: Anaemia
prevalence and determinants in under 5 years children: Findings of
a cross-sectional population-based study in Sudan. BMC Pediatr.
20(538)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Musa IR, Hassan AA and Adam I:
Multimorbidity and its associated risk factors among adults in
northern Sudan: A community-based cross-sectional study. J Health
Popul Nutr. 43(13)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abbas W, Elmugabil A, Hamdan HZ, Rayis DA
and Adam I: Iron deficiency and thyroid dysfunction among sudanese
women in first trimester of pregnancy: A cross-sectional study. BMC
Endocr Disord. 23(223)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Omar SM, Musa IR, ElSouli A and Adam I:
Prevalence, risk factors, and glycaemic control of type 2 diabetes
mellitus in eastern Sudan: A community-based study. Ther Adv
Endocrinol Metab. 27(2042018819860071)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
World Health Organization (WHO):
BMI-for-age (5-19 years). https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age.
|
|
19
|
Ali SA, Hassan AA and Adam I: History of
pica, obesity, and their associations with anemia in pregnancy: A
community-based cross-sectional study. Life (Basel).
13(2220)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
World Health Organization (WHO):
Haemoglobin concentrations for the diagnosis of anaemia and
assessment of severity. WHO, Geneva, 2011.
|
|
21
|
Thomas D, Chandra J, Sharma S, Jain A and
Pemde HK: Determinants of nutritional anemia in adolescents. Indian
Pediatr. 52:867–869. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Abbas W, Adam I, Rayis DA, Hassan NG and
Lutfi MF: Higher rate of iron deficiency in obese pregnant sudanese
women. Open Access Maced J Med Sci. 5:285–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shaban L, Al-Taiar A, Rahman A, Al-Sabah R
and Mojiminiyi O: Anemia and its associated factors among
Adolescents in Kuwait. Sci Rep. 10(5857)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Scott S, Lahiri A, Sethi V, de Wagt A,
Menon P, Yadav K, Varghese M, Joe W, Vir SC and Nguyen PH: Anaemia
in Indians aged 10-19 years: Prevalence, burden and associated
factors at national and regional levels. Matern Child Nutr.
18(e13391)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dean AG, Sullivan KM and Soe MM: OpenEpi:
Open Source Epidemiologic Statistics for Public Health, version
2.3.1. http://www.OpenEpi.com.
|
|
26
|
Kuang L, Li W, Xu G, You M, You M, Wu W
and Li C: Systematic review and meta-analysis: Influence of iron
deficiency anemia on blood glycosylated hemoglobin in diabetic
patients. Ann Palliat Med. 10:11705–11713. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
AlQarni AM, Alghamdi AA, Aljubran HJ,
Bamalan OA, Abuzaid AH and AlYahya MA: The effect of iron
replacement therapy on HbA1c levels in diabetic and nondiabetic
patients: A systematic review and meta-analysis. J Clin Med.
12(7287)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Akkermans MD, Mieke Houdijk ECA, Bakker B,
Boers AC, van der Kaay DCM, de Vries MC, Claire Woltering M, Mul D,
van Goudoever JB and Brus F: Iron status and its association with
HbA1c levels in Dutch children with diabetes mellitus type 1. Eur J
Pediatr. 177:603–610. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guo W, Zhou Q, Jia Y and Xu J: Increased
levels of glycated hemoglobin A1c and iron deficiency anemia: A
review. Med Sci Monit. 25:8371–8378. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hellman R: When are HBA1C values
misleading? AACE Clin Case Rep. 2:e377–e379. 2016.
|