Introduction
Diabetes mellitus (DM) is one of the global burden
diseases and a major cause of mortality, with a reduced life
expectancy for affected individuals (1). In 2021, there were approximately 537
million individuals worldwide affected by diabetes, according to
the International Diabetes Federation; without proper medical
implementation and effective prevention methods, however, these
numbers are predicted to increase to as high as 783 million
individuals by 2045 (1,2). In Indonesia, ~19.5 million adults
were diagnosed with diabetes in 2021, of which 6.7 million
succumbed due to the complications of this disease (2). Moreover, high management costs lead
to the further worsening of the socio-economic conditions affecting
this disease. Diabetes itself is a chronic, non-communicable
disease that is characterized by hyperglycemia and impaired glucose
tolerance. Specifically, type 2 diabetes (T2DM) is characterized by
insulin resistance and impaired β-cell function, and this is mainly
associated with β-cell death (3).
However, recent studies have shown that this dysfunctionality
occurs due to a more complex network of interactions among the
environment and several molecular pathways in cell biology
(4-6).
Generally, T2DM develops as a consequence of unhealthy lifestyles,
including poor diet and a lack of physical activity. This is also
associated with an increase in the levels of markers of chronic
systemic inflammation (low grade), including interleukin 6 and
tumor necrosis factor- α (TNF-α), and this phenomenon induces
metabolic inflammation (4,7-9).
Although therapies for T2DM comprising anti-diabetic
medication and insulin therapy are well established, both the
number of complications and the mortality rate remain relatively
high. Hence, alternative management therapies for T2DM are urgently
required. Over the past few years, regenerative medicine has
yielded promising results, and the use of mesenchymal stem cells
(MSCs) has garnered attention as a potential therapeutic approach.
Human umbilical cord-derived MSCs (hUC-MSCs) have been demonstrated
to have the ability to differentiate into various cell types, to
modulate immune responses, and to secrete bioactive molecules that
exert a role in tissue repair and regeneration (10,11).
These secreted factors are found in the medium in which cells are
cultured under certain conditions. Therefore, this medium is termed
conditioned medium (CM) (12,13).
CM provides an attractive alternative strategy for the management
of T2DM due to its regenerative capability via its paracrine
activities. Robust studies have previously explored the potential
of CM for the treatment of type 1 diabetes (14); however, only a few of these studies
have investigated the CM from hUC-MSCs as an alternative treatment
for T2DM (15-17).
Therefore, the present study aimed to evaluate the therapeutic
potential of CM from hUC-MSCs as an alternative treatment for
hyperglycemia management in T2DM-induced rats, and the findings
demonstrated herein will highlight the regenerative effects of CM
from hUC-MSCs in T2DM therapy.
Materials and methods
Ethical approval
All animal procedures performed in the present study
were approved by the Institutional Animal Care and Use Committee
(IACUC) of the Faculty of Medicine, Tarumanagara University,
Jakarta, Indonesia, with the ethical approval no.
019.KEPH/UPPM/FK/VI/2024. The duration of the animal experiments
was 4 months, upon animal arrival until the study endpoint.
CM production
The CM used in the present study was derived from
hUC-MSCs and produced in Tarumanagara Human Cell Technology
Laboratory, Jakarta, according to a previously published protocol
(18). In brief, MSCs were
isolated from fresh umbilical cords obtained from caesarian
delivery with parental consent (ethical approval no. PPZ20192062,
obtained from the Universitas Tarumanagara Human Research Ethics
Committee, Tarumanagara University). MSCs were cultured until they
had reached 80% confluency at passages 5-6, and subsequently the
medium was replaced with serum-free media [Gibco®
Minimum Essential Medium Alpha (MEMα); Thermo Fisher Scientific,
Inc.] and cultured under hypoxic conditions (in the presence of 5%
O2) for 3 days. Finally, the cultured medium was
collected and filtered as a CM.
Animal experiments
Male Sprague-Dawley rats, aged 8-10 weeks (n=33),
were obtained from the Indonesian Food and Drug Authority, Jakarta,
Indonesia. The number of rats was accounted for using resource
equation ANOVA (19,20), also taking into consideration the
3Rs principle (Replacement, Refinement and Reduction). The rats
were group-housed and acclimatized to the laboratory conditions
(12-h light:dark cycle, with access to water and feed provided
ad libitum) for 1 week, and fed a standard feed (PT Surya
Sains Indonesia) during this period, until their body weight (BW)
had reached 180-200 g. The animals were maintained in a controlled
environment and monitored daily. At the start of the experiment,
individual housing was implemented to enable the accurate
measurement of individual feed intake. Structural environmental
enrichment was provided throughout the study period and animal
behavior was monitored daily. Humane endpoints were employed to
minimize animal suffering throughout the study. These included
severe pain or distress indicated by the inability to eat or drink
for >24 h, body weight loss >20% within 1 week or 10% within
24 h, persistent hunched posture for >8 h, or any other clinical
signs indicating a poor prognosis, as determined by the attending
veterinarian.
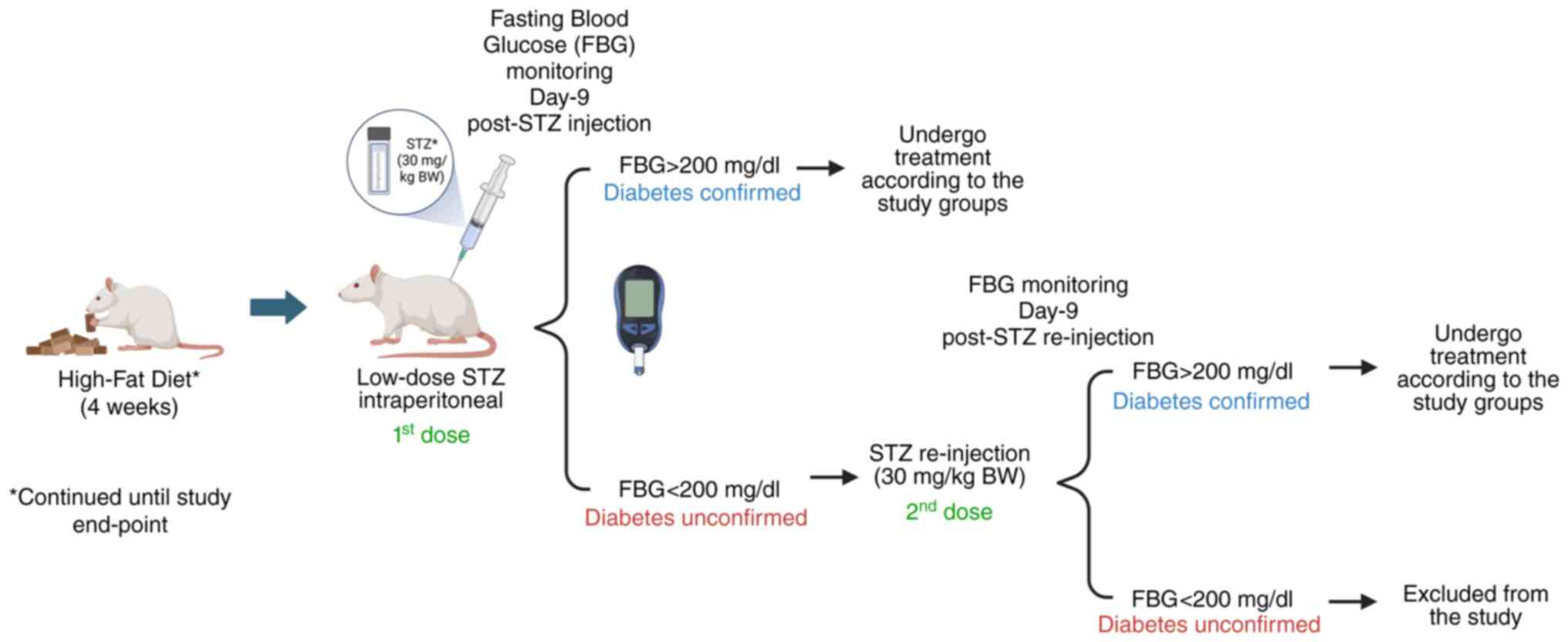
Development of the animal model of
T2DM
The T2DM model was rendered in 28 rats, constructed
as previously described (21,22).
Essentially, T2DM induction was performed by feeding the rats a
high-fat diet (HFD) comprising 33% fat, 37% carbohydrate and 13%
protein content feed (PT Surya Sains Indonesia) combined with
injections of streptozotocin (STZ) (MilliporeSigma). Rats receiving
HFD feed were adapted for 3 days (details provided below) before
being entirely administered HFD feed. During the adaptation
process, feeds were administered in decreasing percentage ratios
between standard feed and HFD for each consecutive day, namely
75:25, 50:50 and 25:75% (Fig. 1).
Subsequently, the group fed the HFD was administered 100% HFD feed
for 4 weeks. Both the condition of the animals and the feed intake
were monitored on a daily basis. Fasting blood glucose (FBG) levels
were examined in all animals using a glucometer
(Accu-chek®; Roche Diagnostics), with a minimum of 6 h
fasting prior to every blood glucose check. At the end of 4 weeks
of providing the rats a HFD, the FBG level was examined as the
baseline prior to a low dosage injection of STZ (30 mg/kg BW via
the intraperitoneal route). The dosage of STZ administered was
based on previous studies, although with modifications (21,22).
STZ was freshly prepared by dissolving the compound in 0.09 M
sodium citrate buffer (MilliporeSigma) under sterile and dark
conditions. The FBG levels of the T2DM-modeled rats were monitored
on day 3 and 9 post-STZ administration, in order to confirm the
establishment of T2DM. Rats were confirmed to be diabetic if the
FBG level was >200 mg/dl; re-injection of STZ (30 mg/kg BW, via
the intraperitoneal route) was performed if the FBG level was
<200 mg/dl. The level of FBG was subsequently monitored again on
days 3 and 9 post re-injection. Rats with FBG levels >200 mg/dl
were then included in the present study, whereas those with a FBG
level that persisted <200 mg/dl were earmarked to be excluded
from the study. Of the 28 rats induced with STZ, 8 were found dead
3 days after the first STZ injection possibly due to an acute STZ
toxicity (23,24), leaving 20 rats eligible for
treatment. During the study, appropriate medical interventions were
applied when necessary, including insulin administration for FBG
levels >400 mg/dl, subcutaneous fluid (Ringer's lactate
solution, B. Braun) infusion for signs of dehydration, and
paracetamol for indications of pain. Following this time point,
rats were continuously fed a HFD throughout the remainder of the
study period (Fig. 2).
Intervention of CM from hUC-MSCs,
insulin and serum-free media
Rats were randomly divided into five experimental
groups (n=5 rats per group), and the investigators were blinded to
the group allocation. The groups were designated as follows: i) The
normal group, wherein the rats were fed without any diabetic
interventions; ii) the control DM group, wherein diabetic rats were
treated with serum-free medium (MEMα), administered 0.5 ml
intraperitoneally; iii) the DM + insulin group, wherein diabetic
rats were treated with insulin (NovoRapid®; Novo
Nordisk), administered 1 unit subcutaneously; iv) the DM + CM
group, wherein diabetic rats were treated with CM, administered 0.5
ml intraperitoneally (25,26); and v) the DM + insulin + CM group,
wherein the diabetic rats were injected with insulin (1 unit
subcutaneously) and CM (0.5 ml intraperitoneally). The treatments
were started immediately following T2DM confirmation, and the rats
received the treatments two times per week for 4 weeks (Fig. 3). The FBG level was examined prior
to each treatment, and subsequently monitored for up to 2 weeks
after the final treatment.
Performances of the oral glucose
tolerance test (OGTT) and intraperitoneal insulin tolerance test
(IPITT)
At 2 weeks following the final treatment, an OGTT
and IPITT were performed. For the OGTT procedure, the rats were
fasted for at least 4 h, and the observed FBG level was regarded as
the baseline. Subsequently, glucose solution (2 g/kg BW) was
administered via oral gavage (17). Blood glucose levels were examined
at 30, 60, 90 and 120 min post-glucose administration. IPITT was
performed similarly to the OGTT procedure, with the exception that
insulin (1 unit) was administered intraperitoneally instead of
glucose solution. Blood glucose levels were then examined at 30,
60, 90 and 120 min post-insulin administration to observe the
corresponding insulin sensitivity of the rats. To more effectively
assess the overall glucose intolerance and insulin sensitivity, the
area under the curve (AUC) (mg/dl∙min) was calculated using the
trapezoidal rule (27).
Euthanasia and blood analyses
On the following day, the rats were euthanized via
an exsanguination process by cardiac puncture under deep
anesthesia. Anesthesia was induced by intraperitoneal injection of
10% ketamine (80 mg/kg BW) and 2% xylazine (10 mg/kg BW) prior to
blood collection (8-10 ml). Death was confirmed by the attending
veterinarian or veterinary paramedic through noting the absence of
a heartbeat or respiration. Whole blood was used to measure the
glycated hemoglobin (HbA1c) level, and the serum was subjected to
enzyme-linked immunosorbent assay (ELISA) to measure the levels of
insulin (Elabscience® cat no. #E-EL-R3034; Elabscience
Bionovation, Inc.) and insulin-like growth factor-1 (IGF-1)
(Invitrogen® cat no. #ERIGF1; Thermo Fisher Scientific,
Inc.). Homeostasis model assessment for insulin resistance
(HOMA-IR) was also calculated using the FBG level (mg/dl) x the
fasting insulin serum level (mU/l)/405 (22,28).
Hepatic inflammation analyses
At the endpoint of the experiment, liver tissue was
obtained, weighed within an accuracy of ±0.1 g, and immediately
stored in a deep freezer (-80˚C) prior to protein isolation.
Protein isolation was performed according to the manufacturer's
instructions. Cold radioimmunoprecipitation assay (RIPA) lysis
buffer (Elabscience® cat no. #E-BC-R327; Elabscience
Bionovation, Inc.) containing 1% protease inhibitor cocktail (cat
no. #HY-K0010; MedChemExpress) was used to isolate the whole liver
protein. The total protein concentration was subsequently measured
using a Bradford kit assay (Elabscience® cat no.
#E-BC-K168-M; Elabscience Bionovation, Inc.). Standardized protein
samples were then subjected to ELISA to determine the levels of the
pro-inflammatory marker, TNF-α (cat. no. #DY510-05; R&D
Systems, Inc.).
Histopathological analyses
Pancreatic tissue was collected at the endpoint of
the experiment, and fixed in 4% paraformaldehyde solution (4˚C, 72
h). The fixed pancreas tissues were stained with hematoxylin for 10
min at room temperature (ScyTek Laboratories Inc.) and eosin for 1
min at room temperature (Thermo Fisher Scientific, Inc.) (H&E)
to observe the morphology of islets of Langerhans. Apart from
H&E staining, the fixed pancreatic tissue was also stained with
Gomori Aldehyde Fuchsin stains for 1 h at room temperature
(Fuchsin: Gurr Certistain, BDH Laboratory; paraldehyde:
MilliporeSigma) to identify the numbers of β-cells. The tissue was
also subjected to immunohistochemical staining to measure the
expression of insulin in a process that involved the use of
specific antibodies to detect the presence of insulin in the
tissue. This procedure was performed at the Pathology Laboratory,
Primate Research Center, Bogor Agricultural University, Bogor,
Indonesia.
Statistical analysis
All results are expressed as the mean ± SD. The
normality of the data was assessed using the Shapiro-Wilk test. For
multiple comparisons, one-way ANOVA or repeated measures ANOVA was
used, and followed by Tukey's post-hoc test for normally
distributed data. Otherwise, Kruskal-Wallis with pairwise
comparisons followed by Dunn's post-hoc test was used. For
comparison within groups, the paired Student's t-test was used for
normally distributed data, otherwise the Wilcoxon signed rank test
was used. P<0.05 was considered to indicate a statistically
significant difference, whereas P<0.01 was considered to
indicate a highly statistically significant difference.
Results
Successful induction of diabetes
Diabetes was successfully induced in the rats that
were assigned to the diabetic group, with an 85% success rate
obtained after the first dose of STZ, followed by the remaining 15%
of the rats achieving the successful induction of T2DM after the
second dose. Therefore, no animals were excluded in the present
study. During the study period, BW, food and calorific intake were
monitored daily. Although all diabetic groups exhibited a slight
increase in BW between the post-STZ (namely, prior to the
administration of the specific treatments) and post-treatment
periods, these differences were found not to be statistically
significant. Similarly, no significant changes were observed in
either food or calorific intake comparing between the post-STZ and
post-treatment periods, although an increase in calorie intake was
observed in the DM + insulin and DM + CM experimental groups
(Table I).
 | Table IBody weight, food and calorie intake
of the rats (n=5/group). |
Table I
Body weight, food and calorie intake
of the rats (n=5/group).
| | Body weight
(g) | | Food intake
(g) | Calorie intake
(kcal) | |
|---|
| Groups |
Post-STZa |
Post-treatmenta |
P-valueb | Post-STZ | Post-treatment | Post-STZ | Post-treatment |
P-valuec |
|---|
| Normal | 271.40±7.44 | 314.40±9.18 | 0.042d | 20.00±0.00 | 19.40±1.34 | 60.59±0.00 | 58.77±4.06 | 0.317 |
| Control DM | 252.00±20.38 | 264.20±31.34 | 0.678 | 13.60±2.30 | 13.80±2.02 | 67.13±11.36 | 68.12±9.96 | 0.686 |
| DM + insulin | 263.80±33.98 | 279.40±39.22 | 0.532 | 11.00±3.94 | 13.70±0.57 | 54.30±19.43 | 67.63±2.81 | 0.216 |
| DM + CM | 243.80±19.60 | 245.20±19.03 | 0.957 | 10.60±3.05 | 12.70±2.36 | 52.32±15.05 | 62.69±11.66 | 0.225 |
| DM + insulin +
CM | 259.60±20.91 | 275.60±15.93 | 0.329 | 12.80±3.70 | 12.80±1.48 | 63.18±18.27 | 63.18±7.32 | 0.892 |
CM from hUC-MSCs reduces blood glucose
levels in rats with T2DM
To determine whether the CM derived from hUC-MSCs
could exert any therapeutic effects in the management of
hyperglycemia in rats with T2DM, CM from hUC-MSCs was administered
intraperitoneally according to the design of the various
experimental groups. Repeated blood glucose monitoring revealed
that CM-treated groups (namely, the DM + CM and DM + insulin + CM
groups) exhibited lower blood glucose levels compared with the
other groups (namely, the control DM and DM + insulin groups)
(Fig. 4A). This decrease was found
to be significant during the injection period. However, at 1 and 2
weeks following the final injection, no significant differences
were observed among the groups. Rats in the DM + insulin + CM group
exhibited the lowest blood glucose levels compared with those in
the other groups, suggesting the positive effect of CM on blood
glucose regulation in T2DM. In accordance with this finding, the DM
+ insulin + CM group also displayed the lowest HbA1c levels
(6.5±1.6%) compared with the control group (7.6±0.6%) (P=0.358)
(Fig. 4B). This highlights the
additive effect of combining CM and insulin therapy in terms of
reducing HbA1c levels in T2DM. On the other hand, fasting insulin
serum levels did not exhibit any significant differences across all
the treatment groups, and these were also similar to the normal
group (Fig. 4C). The HOMA-IR
score, which estimates insulin resistance, was shown to be
significantly higher in all diabetic groups compared with the
normal group (Fig. 4D). Finally,
although not statistically significant, the DM + insulin + CM group
achieved the lowest insulin resistance index (4.17±1.28) among the
diabetic groups, suggesting a potential improvement of insulin
sensitivity through the use of combined therapy.
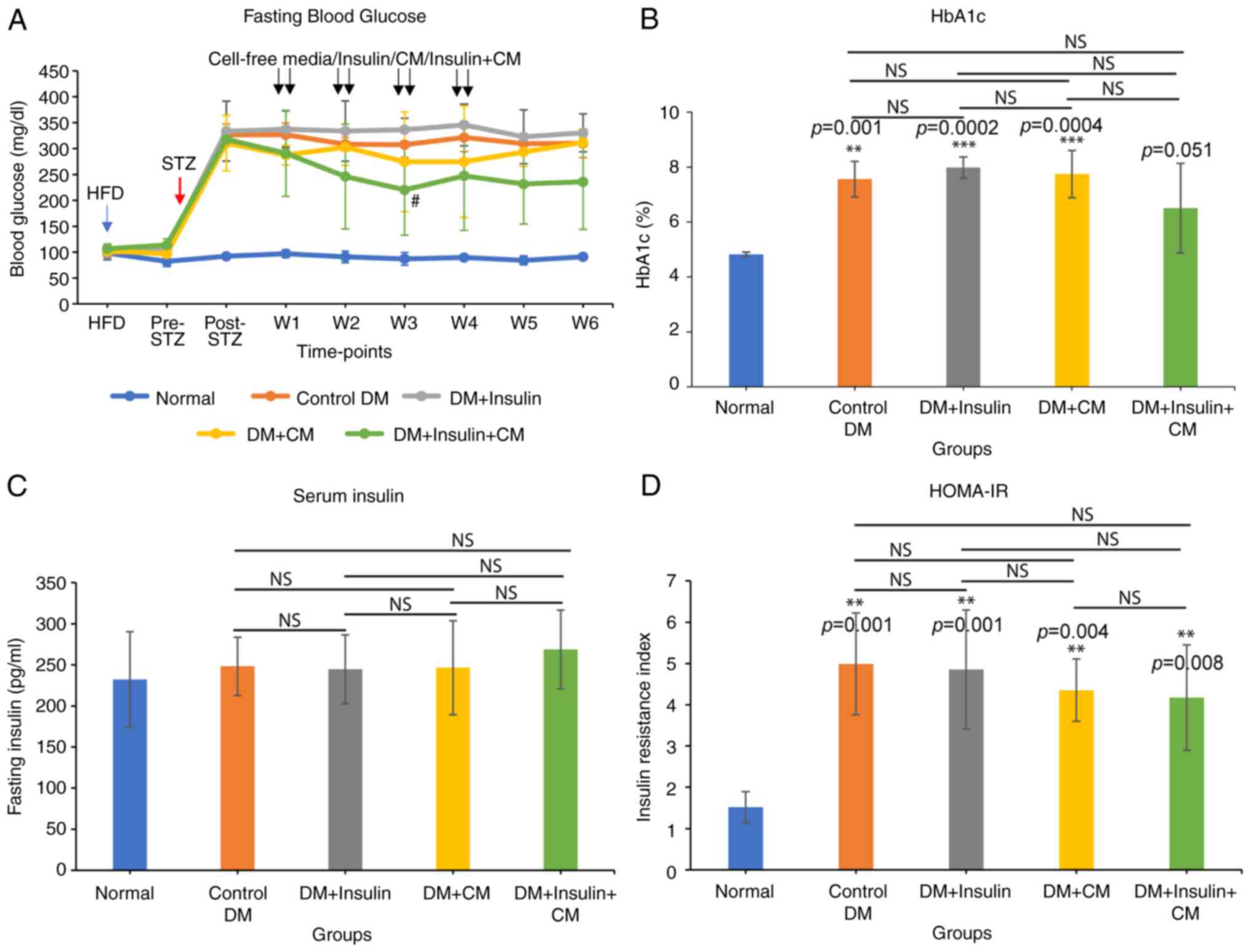 | Figure 4CM positively regulated blood glucose
levels in rats with diabetes. Comparison of (A) fasting blood
glucose levels, (B) HbA1c, (C) fasting serum insulin level, and (D)
insulin resistance index between groups. HFD indicates the starting
point of HFD feed; Pre-STZ represents 4 weeks post-HFD and before
the STZ injection; Post-STZ represents diabetes confirmation; W1-W4
represents weeks 1-4 of treatment; W5-W6 represents weeks 5-6 (2
weeks after the final treatment). Data are expressed as the mean ±
SD (n=5/group). #P<0.05, significant difference
compared to the DM + insulin group, **P<0.01 and
***P<0.001, significant difference compared to the
normal group (P-values are indicated above the bars); NS, not
significant. Statistical analysis was determined using (A) repeated
measures ANOVA and (B-D) one-way ANOVA. DM, diabetes mellitus; CM,
conditioned medium; HFD, high-fat diet; HbA1c, glycated
hemoglobin. |
CM from hUC-MSCs promotes glucose
tolerance and insulin sensitivity in rats with T2DM
The glucose tolerance level and insulin sensitivity
were subsequently assessed using an OGTT and IPITT, respectively.
Blood glucose levels were measured at 0, 30, 60, 90 and 120 min
following glucose administration (OGTT) or insulin injection
(IPITT). In the OGTT, blood glucose levels in all diabetic groups
were found to increase sharply during the first 60 min post-glucose
administration. Furthermore, glucose levels in the normal group
were relatively stable throughout the time point (Fig. 5A). Further analysis, performed by
measuring the AUC, which provides an indication of glucose
intolerance, revealed significantly higher AUC values (P<0.001)
in all the diabetic groups compared with the normal group,
confirming the existence of glucose intolerance under diabetic
conditions (Fig. 5B). Among all
the diabetic groups, the DM + insulin + CM group exhibited an
improved glucose tolerance, although this improvement was found not
to be statistically significant.
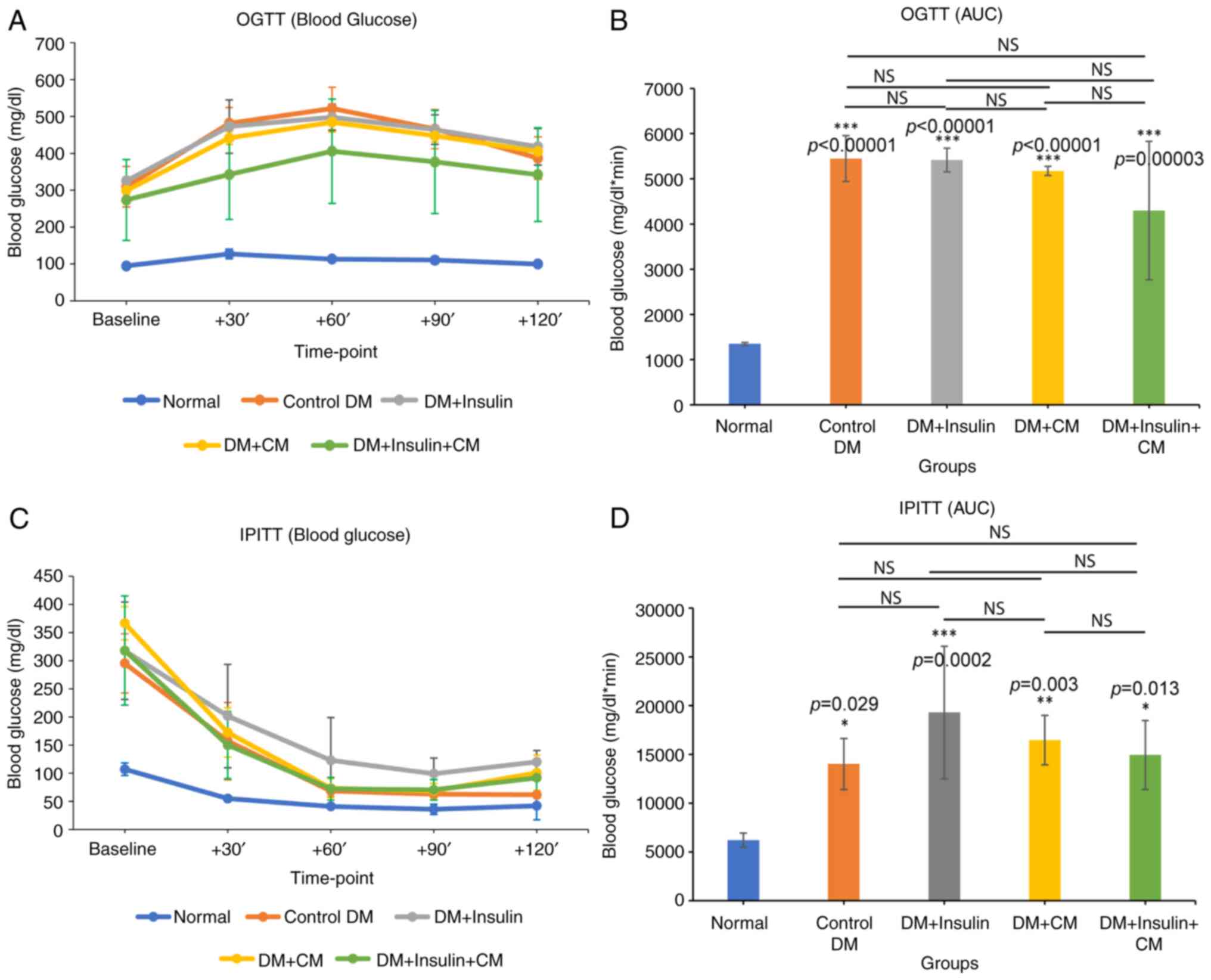 | Figure 5The glucose tolerance test and
insulin sensitivity test. (A) OGTT of each group was determined at
0, 30, 60, 90 and 120 min post-glucose administration. (B) Area
under the curve of OGTT. (C) IPITT of each group was determined at
0, 30, 60, 90 and 120 min post-insulin administration. (D) Area
under the curve of IPITT. Data are expressed as the mean ± SD
(n=5/group). *P<0.05, **P<0.01 and
***P<0.001, significant difference compared to the
normal group (P-values are indicated above the bars); NS, not
significant. Statistical analysis was determined using (A and C)
repeated measures ANOVA and (B and D) one-way ANOVA. OGTT, oral
glucose tolerance test; IPITT, intraperitoneal insulin tolerance
test; DM, diabetes mellitus; CM, conditioned medium. |
As regards the IPITT, blood glucose levels exhibited
a decrease in all groups up to 90 min following insulin
administration. By the 120 min time point, all groups exhibited a
slight increase in blood glucose levels, with the exception of the
control diabetic group, which maintained the same level as at 90
min (Fig. 5C). Although not
statistically significant, the decreases in the level of blood
glucose in the rats with T2DM treated with exogenous insulin alone
(namely, the DM + insulin group) were less pronounced compared with
those of the other groups (Fig.
5C). Accordingly, the AUC analysis demonstrated that all
diabetic groups had significantly higher AUC values compared with
the normal group, with the DM + insulin group displaying the
largest AUC value, indicating its poor insulin sensitivity compared
with the other diabetic groups (Fig.
5D). Although no significant differences were identified among
the diabetic groups, both of the CM-treated groups (namely, the DM
+ CM and the DM + insulin + CM groups) demonstrated an improved
insulin sensitivity compared with the DM + insulin group.
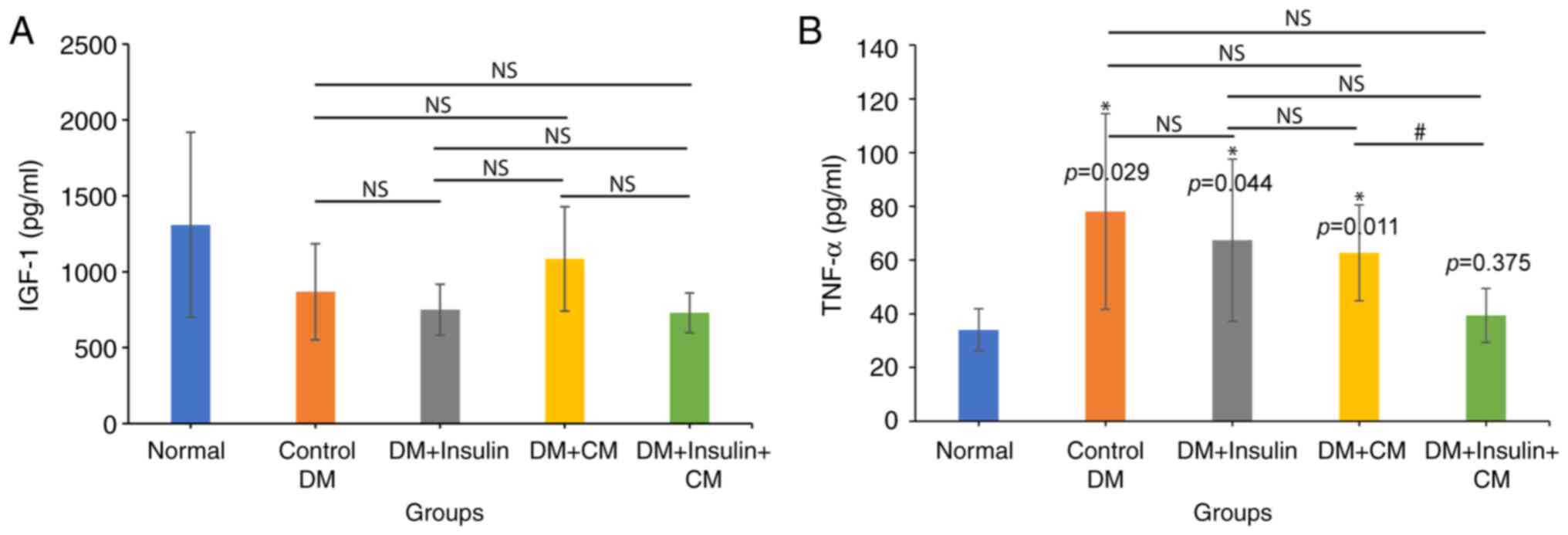
CM from hUC-MSCs exhibits an elevated
level of IGF-1 secretion in rats with T2DM
IGF-1 is a key cytokine that regulates glucose
uptake and insulin sensitivity. It is primarily produced by the
liver, and released into the bloodstream. In T2DM, however,
circulating IGF-1 levels are altered due to insulin resistance,
which has been shown to impair the signaling pathways involved in
IGF-1 production (29). In the
present study, the analysis of serum IGF-1 levels revealed that the
DM + CM group had the highest level of IGF-1 compared with the
other diabetic groups, with a value of 1,085±343 pg/ml, almost
comparable with that of the normal group (1,308±610 pg/ml)
(Fig. 6A). By contrast, the groups
treated with exogenous insulin exhibited no improvement in serum
IGF-1 levels, where these remained the lowest.
CM from hUC-MSCs reverses hepatic
inflammation in rats with T2DM
Improvements in blood markers would be
representative of the therapeutic effects of insulin + CM therapy
in a T2DM setting. As the primary site for gluconeogenesis,
glyconeogenesis and glycogen storage, the liver has a central part
in maintaining glucose homeostasis (30). Hence, from a mechanistic
perspective, the present study examined whether CM from hUC-MSCs
could protect the liver against inflammatory damage in rats with
T2DM. In diabetic rats, the hepatic TNF-α level was found to be
higher compared with that in normal rats (78.08±36.43 pg/ml in the
DM group compared with 33.99±7.88 pg/ml in the normal group;
P=0.029). Although the administration of insulin or CM alone was
shown to reduce hepatic TNF-α levels (67.38±30.22 pg/ml in the DM +
insulin group, and 62.69±17.85 pg/ml in the DM + CM group),
administering the combination therapy of insulin + CM resulted in a
further decrease in the TNF-α concentration to 39.36±10.07 pg/ml
(Fig. 6B), which approached the
level observed in the normal group. These results demonstrated the
positive effect of CM in attenuating hepatic inflammation.
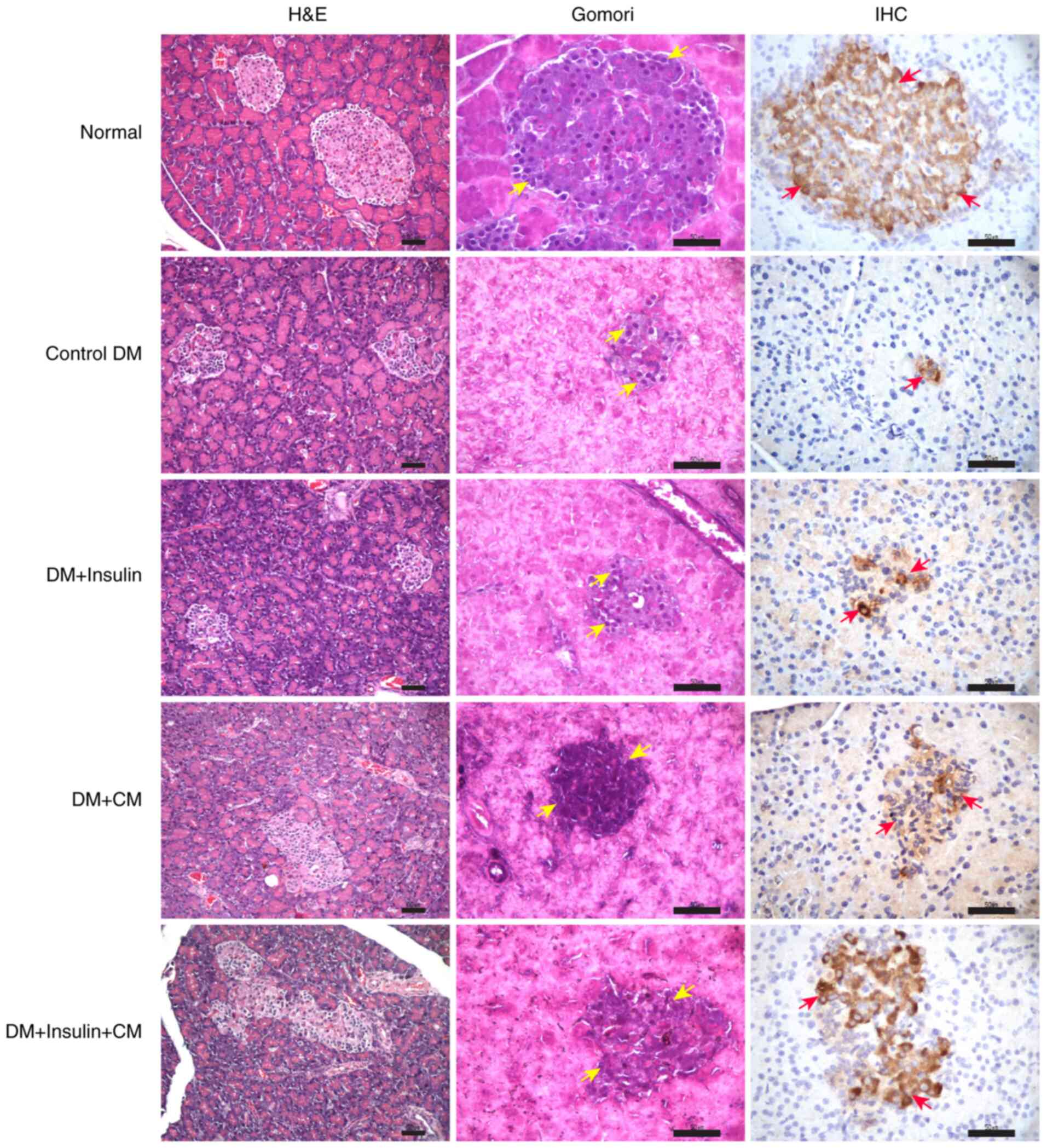
CM from hUC-MSCs results in increased
numbers of pancreatic β-cells, with an improved ability to produce
insulin
To assess the effects of CM from hUC-MSCs on
pancreatic function under T2DM conditions, a microscopic evaluation
was performed via the methods of H&E, Gomori and insulin
staining to examine, in turn, the morphology of islets of
Langerhans, the numbers of β-cells and insulin production,
respectively. H&E staining of pancreatic tissue sections
revealed a reduction in both the size and numbers of the islet of
Langerhans in all diabetic rats compared with the normal group
(Fig. 7). Infiltration by a small
number of lymphocytes was observed in the DM + insulin group, but
not in the other experimental groups. The mean number of β-cells,
as determined using Gomori staining, was found to be significantly
higher in the DM + insulin + CM group compared with the control DM
group (Table II). Similarly, the
mean number of insulin-positive cells was also significantly higher
in both the CM-treated groups compared with the control DM and DM+
insulin groups (Table II). Taken
together, these findings suggested that the intraperitoneal
administration of insulin + CM led to beneficial effects in terms
of enhancing pancreatic β-cell viability and function.
 | Table IIAverage number of β-cells per field
of view and insulin-positive cells per field of view on the
pancreatic tissue (n=5/group). |
Table II
Average number of β-cells per field
of view and insulin-positive cells per field of view on the
pancreatic tissue (n=5/group).
| | Gomori
staining |
Immunohistochemistry |
|---|
| Groups | No. of β-cells per
field of view | P-value | Νο. of
insulin-positive cells per field of view | P-value |
|---|
| Normal | 57±27 | | 131±36 | |
| Control DM | 10±2 | | 13±4 | |
| DM + insulin | 13±8 | | 15±4 | |
| DM + CM | 20±11 | | 27±10 | 0.009b, 0.047c |
| DM + insulin +
CM | 27±15 | 0.037a | 33±7 | 0.009b, 0.009d |
Discussion
Poor diet and a sedentary lifestyle are major
contributors to the development of T2DM. As the underlying cause,
insulin resistance over time results in the exhaustion of
pancreatic β-cells, eventually leading to inadequate insulin
production and elevated blood glucose levels (26-28).
This condition is also associated with impaired glucose tolerance
and low insulin sensitivity (34).
At the same time, persistent hyperglycemia may lead to other
complications, as for example, cardiovascular disease, nephropathy
and neuropathy (3,35). The current standard therapy relies
upon the use of exogenous insulin to achieve glycemic control;
however, insulin resistance is associated with multiple organ
dysfunctions, and merely lowering the level of blood glucose, as a
therapeutic strategy, is insufficient to address the metabolic and
inflammatory dysregulations that are observed in T2DM. Moreover,
hyperinsulinemia induced by exogenous insulin can also exacerbate
oxidative stress and alter lipid metabolism, further worsening
insulin resistance. As a result, insulin therapy fails to reduce
the levels of inflammatory markers, with the consequence that
neither cardiovascular burden nor mortality outcomes are
effectively lowered in T2DM (36).
Stem cell therapy has emerged as a promising
alternative for the treatment of various degenerative diseases
through a more comprehensive approach. However, several challenges
with stem cell therapy still remain, including immunogenicity
risks, invasive procedures and low cell survival
post-transplantation (37,38). To overcome these challenges, CM has
garnered attention as a cell-free product with comparable
regenerative properties. CM derived from MSCs is known to contain
various bioactive molecules, including growth factors,
extracellular vesicles (EVs), cytokines and exosomes (12,39).
Recently, several studies have highlighted the regenerative
capabilities of EVs or exosomes alone in a variety of disease
models, including osteoarthritis, cardiovascular disease,
neurodegenerative diseases and liver fibrosis, among others
(35-41).
Additionally, MSC-derived exosomes have been explored as
therapeutic agents for the treatment of diabetes-related
complications. The study by Li et al (47) demonstrated that hUC-MSC-derived
exosomes shuffle the miRNA miR-17-3p, which targets the STAT1
signaling pathway, thereby alleviating inflammatory reactions and
oxidative injury in diabetic retinopathy mice. Similarly, Yang
et al (48) reported that
exosomes incorporated into pluronic F-127 hydrogel could enhance
diabetic wound healing through promoting wound closure, stimulating
tissue regeneration, and upregulating the expression of vascular
endothelial growth factor (VEGF) and transforming growth factor
β-1. Despite its potential, however, the effects of CM have not yet
been extensively investigated, particularly as regards the
treatment of T2DM.
The present preliminary study explored the potential
of whole CM as an adjuvant therapy to exogenous insulin for the
management of hyperglycemia in rats with T2DM. In the present
study, it was found that the intraperitoneal administration of
insulin + CM alleviated the pancreatic dysfunction that arises
under T2DM conditions, particularly by enhancing the proliferation
of β-cells and their insulin-producing capability. These findings
are consistent with those of previous studies, which demonstrated
the positive effects of MSCs and their CM in terms of promoting
β-cell proliferation and function (49,50).
MSCs have the ability to regenerate pancreatic islet β-cells, to
protect them from apoptosis, and even to improve insulin resistance
in peripheral tissues, a process that is mainly driven by the
activities of their paracrine factors (51,52).
Moreover, insulin + CM therapy has previously been shown to exert
protective effects against hepatic inflammation, as revealed by
lower levels of the pro-inflammatory protein, TNF-α. In the context
of T2DM, elevated hepatic TNF-α levels disrupt insulin receptor
signaling, leading to impaired glucose regulation and enhanced
insulin resistance (53,54).
The improvements in pancreatic function, and the
reduction in hepatic inflammation, that resulted from insulin + CM
treatment were consistent with an improved control of the blood
glucose level in this experimental group (namely, the insulin + CM
group) compared with the other diabetic groups. This was in spite
of the fact that, during the 2 weeks following the final
intervention, blood glucose levels in the CM-treated groups were
found to fluctuate, suggesting a need for prolonged or continuous
therapy. Furthermore, the HbA1c level was comparatively lower in
the DM+ insulin + CM group; in fact, it exhibited the lowest
insulin resistance index among all treatment groups. CM treatment,
however, was found not to affect the fasting insulin serum levels.
Taken together, these findings align with those of previous
studies, where CM was demonstrated to reduce both the blood glucose
and HbA1c levels in patients with type 1 diabetes (55), whereas it did not affect the
fasting insulin serum concentration (17).
Herein, CM was also found to improve glucose
tolerance and insulin sensitivity, as demonstrated by performing
OGTT and IPITT, respectively. Typically, blood glucose levels
peaked at 30 min following glucose administration, before gradually
returning to the baseline level, as observed in the normal group.
However, in a previously published study (56), in cases of impaired glucose
tolerance (namely, the control DM group), blood glucose levels
remained elevated for up to 60 min. The intervention of insulin +
CM resulted in the lowest blood glucose level, thereby reflecting
improved glucose tolerance compared with the other diabetic groups.
All these observations demonstrated that combination therapy with
CM and insulin may improve the glucose tolerance of patients with
T2DM (27,57). Insulin sensitivity, another key
issue in T2DM, was also shown to be elevated in the CM-treated
groups. In line with these findings, Sun et al (17) demonstrated that exosomes from
hUCM-MSCs, but not exogenous insulin, led to an increase in insulin
sensitivity by activating the insulin-signaling pathway and
enhancing glycogen synthesis.
As a key factor in determining insulin resistance,
IGF-1 regulates insulin sensitivity and glucose uptake in
peripheral tissues via pathways similar to the insulin-signaling
pathway (53-55).
Reductions in the IGF-1 level were found to lead to a deterioration
in insulin resistance, especially in cases of T2DM (60). IGF-1 is mainly produced by the
liver; hence, in conjunction with the suppression of hepatic
inflammation, the results of the present study demonstrated that CM
enhanced IGF-1 secretion. By contrast, treatment with exogenous
insulin did not lead to any increases in IGF-1 secretion, and this
finding corroborates those of previous studies, which identified
that subcutaneous insulin treatment over an extended period may
even reduce IGF-1 levels (61,62).
Notably, in the present study, rats treated with
insulin alone had the poorest insulin sensitivity, accompanied by
persistently elevated blood glucose levels throughout the study
compared with other treatment groups. These findings were similar
to those of a previous study, which reported that insulin-treated
diabetic rats displayed glucose tolerance and insulin-sensitivity
profiles that were comparable with those of the control DM group
(17). This result may be
attributed to the use of short-acting insulin, which was
administered at a frequency equivalent to that of the CM regimen.
The selection of insulin type and dosage were selected based on
preliminary findings and previous studies (63,64),
in which insulin administration exceeding 1 unit was shown to often
lead to severe hypoglycemia and subsequent mortality. Notably, only
the co-administration of insulin with CM led to disease
alleviation, partly through repair of the functions of the pancreas
and liver.
Previously, the authors have demonstrated that the
CM from hUC-MSCs contains several growth factors, including VEGF,
basic fibroblast growth factor (bFGF) and hepatocyte growth factor
(HGF) (18). Although the specific
mechanism through which hUC-MSC-derived paracrine activity
alleviates the T2DM condition has not been clearly elucidated,
these growth factors may play a major role in alleviating disease
progression. Proangiogenic factors, such as VEGF, bFGF and HGF, are
known to promote the proliferation of pancreatic β-cells,
stimulating nutrient and blood flow delivery into these cells
(60-62).
Other in vitro studies (14,52)
have demonstrated that CM from hUC-MSCs enhances glucose uptake
through upregulating the expression of membranous glucose
transporter 4 (GLUT4) and activating the insulin signaling pathway,
which eventually leads to an improvement in insulin resistance.
Similarly, Sun et al (17)
found that exosomes from hUC-MSCs were responsible for an increase
in the expression and the membrane translocation of GLUT4 in
muscle, thereby enhancing glycogen storage in the liver to maintain
glucose homeostasis. Moreover, it can reverse peripheral insulin
resistance and relieve β-cell destruction. The administration of
hUC-MSC exosomes has been shown to lower blood glucose levels and
to enhance glucose uptake in skeletal muscle and liver cells
(17,68,69).
In the presence of insulin, these exosomes stimulate the expression
of key insulin signaling proteins, including protein kinase B/AKT
and insulin receptor substrate 1, which exert crucial roles in
glucose transport and metabolism. Consequently, this process
attenuates the development of insulin resistance (47,66-68).
hUC-MSC-derived exosomes have also been shown to restore islet
architecture and to inhibit the STZ-induced apoptosis of β-cells
(68,69).
However, there were several limitations associated
with the present study. Although the CM from hUC-MSCs demonstrated
potential in alleviating T2DM, some of the results lacked
statistical significance, probably due to the relatively small
sample size of the study. Additionally, variability in CM
composition between production batches may have represented a
potential confounding factor. Factors such as the umbilical cord
source and manufacturing process have previously been shown to
markedly influence the quantity and types of factors secreted into
the medium (73). Therefore,
future studies with larger sample sizes are required to investigate
the appropriate dosing and mechanisms of action. Furthermore, it is
essential to analyze the components of CM that may contribute to
immunogenic responses in order to assess its potential
immunogenicity. In addition, standardizing the production process
is also critical to ensure the efficacy and reliability of CM from
hUC-MSCs for T2DM clinical applications. The half-lives of secreted
factors in the CM are crucial for determining both the effective
duration of CM use and the optimal dosing frequency in vivo
(14). Taken together, however,
these results have shown that CM from hUC-MSCs, when administered
as an adjuvant therapy to insulin, may positively influence T2DM by
lowering blood glucose and HbA1c levels, as well as improving
insulin sensitivity and the secretion of IGF-1.
In conclusion, finding suitable therapies against
T2DM is crucial, as T2DM is a debilitating disease, and effective
blood glucose management is essential. CM derived from hUC-MSCs has
the potential to reduce the levels of both blood glucose and HbA1c,
particularly if administered as an adjuvant therapy to insulin.
Additionally, CM may also improve glucose tolerance and insulin
sensitivity, particularly when combined with insulin. It also has
the potential to enhance IGF-1 secretion, which may partly decrease
insulin resistance. Finally, CM may also increase both the numbers
of pancreatic β-cells and their functionality, which deteriorate
under diabetic conditions. The present study has provided a
knowledge base for future research in terms of the application of
CM as an adjuvant therapeutic approach in the management of
T2DM.
Acknowledgements
The authors would like to thank Baermed, Zürich,
Switzerland, and the Institute for Research and Community Service
of Tarumanagara University, Jakarta, Indonesia, for their valuable
technical support in this research. The authors also would like to
thank Mrs. Permanawati, Doctor of Veterinary Medicine (DVM)
(Primate Research Center, Bogor, Indonesia) for her contributions
towards and the support of this research.
Funding
Funding: This research was funded by a grant from the
Directorate General of Higher Education, Research, and Technology,
Ministry of Education, Culture, Research, and Technology, Indonesia
(grant no.
105/E5/PG.02.00.PL/2024-829/LL3/AL.04/2024-0614-Int-KLPPM/UNTAR/VI/2024).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
All authors (SH, JL, OM, LT, HUB and SG) were
involved in the conception and design of the study. JL and OM were
responsible for data collection and administration. SH, JL and OM
were responsible for data calculation and analysis. SH and JL were
responsible for the writing of the manuscript (the original draft
preparation). SH, LT, HUB and SG were responsible for data
interpretation. OM, LT, HUB and SG were responsible for the writing
of the manuscript (reviewing and editing). and SH and HUB
supervised the project. SH, SG confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal study protocol was approved by the
Institutional Animal Care and Use Committee of Faculty of Medicine,
Tarumanagara University, Jakarta, Indonesia, (grant no.
019.KEPH/UPPM/FK/VI/2024; date of approval: 11 June 2024). MSCs
were isolated from fresh umbilical cords obtained from caesarian
delivery with parental consent (ethical approval no. PPZ20192062,
obtained from the Universitas Tarumanagara Human Research Ethics
Committee, Tarumanagara University).
Patient consent for publication
Not applicable.
Competing interests
HUB is the Director of Baermed, Center of Abdominal
Surgery, Zürich, Switzerland. The other authors declare that they
have no competing interests.
References
|
1
|
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X,
Song X, Ren Y and Shan PF: Global, regional, and national burden
and trend of diabetes in 195 countries and territories: An analysis
from 1990 to 2025. Sci Rep. 10:1–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Magliano DJ and Boyko EJ: IDF diabetes
atlas. 10th ed. International Diabetes Federation, Brussels,
2021.
|
|
3
|
Ohiagu FO, Chikezie PC and Chikezie CM:
Pathophysiology of diabetes mellitus complications: Metabolic
events and control. Biomed Res Ther. 8:4243–4257. 2021.
|
|
4
|
Galicia-Garcia U, Benito-Vicente A, Jebari
S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H and Martín C:
Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci.
21:1–34. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Halban PA, Polonsky KS, Bowden DW, Hawkins
MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L and Weir GC:
β-Cell failure in type 2 diabetes: Postulated mechanisms and
prospects for prevention and treatment. J Clin Endocrinol Metab.
99:1983–1992. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Christensen AA and Gannon M: The beta cell
in type 2 diabetes. Curr Diab Rep. 19(81)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pradhan AD, Manson JE, Rifai N, Buring JE
and Ridker PM: C-reactive protein, interleukin 6, and risk of
developing type 2 diabetes mellitus. J Am Med Assoc. 286:327–334.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Esser N, Legrand-Poels S, Piette J, Scheen
AJ and Paquot N: Inflammation as a link between obesity, metabolic
syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bunney PE, Zink AN, Holm AA, Billington CJ
and Kotz CM: Orexin activation counteracts decreases in nonexercise
activity thermogenesis (NEAT) caused by high-fat diet. Physiol
Behav. 176:139–148. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang B, Cheng X, Wang H, Huang W, la Ga
hu Z, Wang D, Zhang K, Zhang H, Xue Z, Da Y, et al: Mesenchymal
stem cells and their secreted molecules predominantly ameliorate
fulminant hepatic failure and chronic liver fibrosis in mice
respectively. J Transl Med. 14(45)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fan CG, Zhang QJ and Zhou JR: Therapeutic
potentials of mesenchymal stem cells derived from human umbilical
cord. Stem Cell Rev Rep. 7:195–207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pawitan JA: Prospect of stem cell
conditioned medium in regenerative medicine. Biomed Res Int.
2014:7–9. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim HO, Choi SM and Kim HS: Mesenchymal
stem cell-derived secretome and microvesicles as a cell-free
therapeutics for neurodegenerative disorders. Tissue Eng Regen Med.
10:93–101. 2013.
|
|
14
|
Isildar B, Ozkan S and Koyuturk M:
Therapeutic potential of mesenchymal stem cell-derived conditioned
medium for diabetes mellitus and related complications. Adv Ther.
6(2300216)2023.
|
|
15
|
Nagaishi K, Ataka K, Echizen E, Arimura Y
and Fujimiya M: Mesenchymal stem cell therapy ameliorates diabetic
hepatocyte damage in mice by inhibiting infiltration of bone
marrow-derived cells. Hepatology. 59:1816–1829. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ormazabal V, Nova-Lampeti E, Rojas D,
Zúñiga FA, Escudero C, Lagos P, Moreno A, Pavez Y, Reyes C, Yáñez
M, et al: Secretome from human mesenchymal stem cells-derived
endothelial cells promotes wound healing in a type-2 diabetes mouse
model. Int J Mol Sci. 23(941)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang
B, Wu P, Shi Y, Mao F, Yan Y, et al: Human mesenchymal stem cell
derived exosomes alleviate type 2 diabetes mellitus by reversing
peripheral insulin resistance and relieving β-cell destruction. ACS
Nano. 12:7613–7628. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hendrawan S, Marcelina O, Tan ST and Baer
HU: Immobilization of hUC-MSCs conditioned medium on 3D PLLA
collagen-coated matrix enhances diabetic wound healing progression.
Eng Regen. 5:421–431. 2024.
|
|
19
|
Charan J and Kantharia N: How to calculate
sample size in animal studies? J Pharmacol Pharmacother. 4:303–306.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Arifin WN and Zahiruddin WM: Sample size
calculation in animal studies using resource equation approach.
Malaysian J Med Sci. 24:101–105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gunawan S, Aulia A and Soetikno V:
Development of rat metabolic syndrome models: A review. Vet World.
14:1774–1783. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gunawan S, Munika E, Wulandari ET,
Ferdinal F, Purwaningsih EH, Wuyung PE, Louisa M and Soetikno V:
6-gingerol ameliorates weight gain and insulin resistance in
metabolic syndrome rats by regulating adipocytokines. Saudi Pharm
J. 31:351–358. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
GBD 2017 Disease and Injury Incidence and
Prevalence Collaborators: Global, regional, and national incidence,
prevalence, and years lived with disability for 354 diseases and
injuries for 195 countries and territories, 1990-2017: A systematic
analysis for the global burden of disease study 2017. Lancet.
392:1789–1858. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kume E, Fujimura H, Matsuki N, Ito M,
Aruga C, Toriumi W, Kitamura K and Doi K: Hepatic changes in the
acute phase of streptozotocin (SZ)-induced diabetes in mice. Exp
Toxicol Pathol. 55:467–480. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Karimi Z, Daryabor G and Masjedi F:
Effects of conditioned media derived from human Wharton's jelly
mesenchymal stem cells on diabetic nephropathy and hepatopathy via
modulating TGF-β and apelin signaling pathways in male rats. BMC
Endocr Disord. 24:1–15. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Utami A, Putra A, Wibowo JW, Amalina ND
and Irawan RCS: Hypoxic secretome mesenchymal stem cells inhibiting
interleukin-6 expression prevent oxidative stress in type 1
diabetes mellitus. Med Glas. 20:148–155. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Leng YP, Qiu N, Fang WJ, Zhang M, He ZM
and Xiong Y: Involvement of increased endogenous asymmetric
dimethylarginine in the hepatic endoplasmic reticulum stress of
type 2 diabetic rats. PLoS One. 9(e97125)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wallace TM, Levy JC and Matthews DR: Use
and abuse of HOMA modeling. Diabetes Care. 27:1487–1495.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Biadgo B, Tamir W and Ambachew S:
Insulin-like growth factor and its therapeutic potential for
diabetes complications mechanisms and metabolic links: A review.
Rev Diabet Stud. 16:24–34. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ,
Ding L, Chen Q, Li YH, Wang JJ, Kang YM and Zhu GQ: Irisin inhibits
hepatic gluconeogenesis and increases glycogen synthesis via the
PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci
(Lond). 129:839–850. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee SH, Park SY and Choi CS: Insulin
resistance: From mechanisms to therapeutic strategies. Diabetes
Metab J. 46:15–37. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Campbell JE and Newgard CB: Mechanisms
controlling pancreatic islet cell function in insulin secretion.
Nat Rev Mol Cell Biol. 22:142–158. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
De la Cruz Concepción B, Flores-Cortez YA,
Barragán-Bonilla MI, Mendoza-Bello JM and Espinoza-Rojo M: Insulin:
A connection between pancreatic β cells and the hypothalamus. World
J Diabetes. 14:76–91. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tangvarasittichai S: Oxidative stress,
insulin resistance, dyslipidemia and type 2 diabetes mellitus.
World J Diabetes. 6:456–480. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mauricio D, Alonso N and Gratacòs M:
Chronic diabetes complications: The need to move beyond classical
concepts. Trends Endocrinol Metab. 31:287–295. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mendez CE, Walker RJ, Eiler CR, Mishriky
BM and Egede LE: Insulin therapy in patients with type 2 diabetes
and high insulin resistance is associated with increased risk of
complications and mortality. Postgrad Med. 131:376–382.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mitrousis N, Fokina A and Shoichet MS:
Biomaterials for cell transplantation. Nat Rev Mater. 3:441–456.
2018.
|
|
38
|
Sortwell CE, Pitzer MR and Collier TJ:
Time course of apoptotic cell death within mesencephalic cell
suspension grafts: Implications for improving grafted dopamine
neuron survival. Exp Neurol. 165:268–277. 2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hsiao STF, Asgari A, Lokmic Z, Sinclair R,
Dusting GJ, Lim SY and Dilley RJ: Comparative analysis of paracrine
factor expression in human adult mesenchymal stem cells derived
from bone marrow, adipose, and dermal tissue. Stem Cells Dev.
21:2189–2203. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li S, Liu J, Liu S, Jiao W and Wang X:
Chitosan oligosaccharides packaged into rat adipose mesenchymal
stem cells-derived extracellular vesicles facilitating cartilage
injury repair and alleviating osteoarthritis. J Nanobiotechnology.
19(343)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li S, Liu J, Liu S, Jiao W and Wang X:
Mesenchymal stem cell-derived extracellular vesicles prevent the
development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9
axis. J Nanobiotechnology. 19(194)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cheng H, Chang S, Xu R, Chen L, Song X, Wu
J, Qian J, Zou Y and Ma J: Hypoxia-challenged MSC-derived exosomes
deliver miR-210 to attenuate post-infarction cardiac apoptosis.
Stem Cell Res Ther. 11(224)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhu D, Liu S, Huang K, Wang Z, Hu S, Li J,
Li Z and Cheng K: Intrapericardial exosome therapy dampens cardiac
injury via activating Foxo3. Circ Res. 131:e135–e150.
2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kandeel M, Morsy MA, Alkhodair KM and
Alhojaily S: Mesenchymal stem cell-derived extracellular vesicles:
An emerging diagnostic and therapeutic biomolecules for
neurodegenerative disabilities. Biomolecules.
13(1250)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu B, Feng J, Guo J, Wang J, Xiu G and Xu
J, Ning K, Ling B, Fu Q and Xu J: ADSCs-derived exosomes ameliorate
hepatic fibrosis by suppressing stellate cell activation and
remodeling hepatocellular glutamine synthetase-mediated glutamine
and ammonia homeostasis. Stem Cell Res Ther. 13(494)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Allan D, Tieu A, Lalu M and Burger D:
Mesenchymal stromal cell-derived extracellular vesicles for
regenerative therapy and immune modulation: Progress and challenges
toward clinical application. Stem Cells Transl Med. 9:39–46.
2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li W, Jin L, Cui Y and Xie N: Human
umbilical cord mesenchymal stem cells-derived exosomal
microRNA-17-3p ameliorates inflammatory reaction and antioxidant
injury of mice with diabetic retinopathy via targeting STAT1. Int
Immunopharmacol. 90(107010)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang J, Chen Z, Pan D, Li H and Shen J:
umbilical cord-derived mesenchymal stem cell-derived exosomes
combined pluronic F127 hydrogel promote chronic diabetic wound
healing and complete skin regeneration. Int J Nanomedicine.
15:5911–5926. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Masithoh DBH, Fibrianto YH, Anggita M,
Nugroho WS and Budipitojo T: Mesenchymal stem cell-conditioned
medium improve the recovery of pancreatic α and β cells in type 1
diabetes mellitus. In: Proceedings of the 20th FAVA Congress and
the 15th KIVNAS PDHI. Bali, Nov 1-3, pp 172–174, 2018. https://journal.ipb.ac.id/hemera/article/view/23814/15670.
|
|
50
|
Liu X, Zheng P, Wang X, Dai G, Cheng H,
Zhang Z, Hua R, Niu X, Shi J and An Y: A preliminary evaluation of
efficacy and safety of Wharton's jelly mesenchymal stem cell
transplantation in patients with type 2 diabetes mellitus. Stem
Cell Res Ther. 5(57)2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zang L, Hao H, Liu J, Li Y, Han W and Mu
Y: Mesenchymal stem cell therapy in type 2 diabetes mellitus.
Diabetol Metab Syndr. 9:1–11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kim KS, Choi YK, Kim MJ, Hwang JW, Min K,
Jung SY, Kim SK, Choi YS and Cho YW: Umbilical cord-mesenchymal
stem cell-conditioned medium improves insulin resistance in c2c12
cell. Diabetes Metab J. 44:260–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang X, Li Z, Liu D, Xu X, Shen W and Mei
Z: Effects of probucol on hepatic tumor necrosis factor-α,
interleukin-6 and adiponectin receptor-2 expression in diabetic
rats. J Gastroenterol Hepatol. 24:1058–1063. 2009.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Klover PJ, Zimmers TA, Koniaris LG and
Mooney RA: Chronic exposure to interleukin-6 causes hepatic insulin
resistance in mice. Diabetes. 52:2784–2789. 2003.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shalaby MS, Abdel-Reheim ES, Almanaa TN,
Alhaber LA, Nabil A, Ahmed OM, Elwan M and Abdel-Moneim A:
Therapeutic effects of mesenchymal stem cell conditioned media on
streptozotocin-induced diabetes in Wistar rats. Regen Ther.
28:1–11. 2025.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kuo FY, Cheng KC, Li Y and Cheng JT: Oral
glucose tolerance test in diabetes, the old method revisited. World
J Diabetes. 12:786–793. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu KF, Niu CS, Tsai JC, Yang CL, Peng WH
and Niu HS: Comparison of area under the curve in various models of
diabetic rats receiving chronic medication. Arch Med Sci.
18:1078–1087. 2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kasprzak A: Insulin-like growth factor 1
(IGF-1) signaling in glucose metabolism in colorectal cancer. Int J
Mol Sci. 22(6434)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kim SH and Park MJ: Effects of growth
hormone on glucose metabolism and insulin resistance in human. Ann
Pediatr Endocrinol Metab. 22:145–152. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Clemmons DR: Role of insulin-like growth
factor iin maintaining normal glucose homeostasis. Horm Res
Paediatr. 62:77–82. 2004.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Van Dijk PR, Logtenberg SJJ, Chisalita SI,
Hedman CA, Groenier KH, Gans ROB, Kleefstra N, Arnqvist HJ and Bilo
HJG: Different effects of intraperitoneal and subcutaneous insulin
administration on the GH-IGF-1 axis in type 1 diabetes. J Clin
Endocrinol Metab. 101:2493–2501. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Nijenhuis-Noort EC, Berk KA, Neggers SJCMM
and van der Lely AJ: The fascinating interplay between growth
hormone, insulin-like growth factor-1, and insulin. Endocrinol
Metab (Seoul). 39:83–89. 2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li N, Guenancia C, Rigal E, Hachet O,
Chollet P, Desmoulins L, Leloup C, Rochette L and Vergely C:
Short-term moderate diet restriction in adulthood can reverse
oxidative, cardiovascular and metabolic alterations induced by
postnatal overfeeding in mice. Sci Rep. 6(30817)2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Su W, Yu S, Yin Y, Li B, Xue J, Wang J, Gu
Y, Zhang H, Lyu Z, Mu Y and Cheng Y: Diabetic microenvironment
preconditioning of adipose tissue-derived mesenchymal stem cells
enhances their anti-diabetic, anti-long-term complications, and
anti-inflammatory effects in type 2 diabetic rats. Stem Cell Res
Ther. 13:1–14. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zeinhom A, Fadallah SA and Mahmoud M:
Human mesenchymal stem/stromal cell based-therapy in diabetes
mellitus: Experimental and clinical perspectives. Stem Cell Res
Ther. 15(384)2024.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kono TM, Sims EK, Moss DR, Yamamoto W, Ahn
G, Diamond J, Tong X, Day KH, Territo PR, Hanenberg H, et al: Human
adipose-derived stromal/stem cells protect against STZ-induced
hyperglycemia: Analysis of hASC-derived paracrine effectors. Stem
Cells. 32:1831–1842. 2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
El-Kersh AOFO, El-Akabawy G and Al-Serwi
RH: Transplantation of human dental pulp stem cells in
streptozotocin-induced diabetic rats. Anat Sci Int. 95:523–539.
2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yang M, Chen J and Chen L: The roles of
mesenchymal stem cell-derived exosomes in diabetes mellitus and its
related complications. Front Endocrinol (Lausanne). 13:1–16.
2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yap SK, Tan KL, Rahaman NY, Hamid NF, Ooi
DJ, Tor YS, Looi QH, Tan LK, How CW and Foo JB: Human umbilical
cord mesenchymal stem cell-derived small extracellular vesicles
ameliorated insulin resistance in type 2 diabetes mellitus rats.
Pharmaceutics. 14(649)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kim JE, Lee JW, Cha GD and Yoon JK: The
potential of mesenchymal stem cell-derived exosomes to treat
diabetes mellitus. Biomimetics (Basel). 10(49)2025.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Keshtkar S, Azarpira N and Ghahremani MH:
Mesenchymal stem cell-derived extracellular vesicles: Novel
frontiers in regenerative medicine. Stem Cell Res Ther.
9(63)2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Abu-Rmeileh NME, Husseini A, Capewell S
and O'Flaherty M: MEDCHAMPS project. Preventing type 2 diabetes
among Palestinians: Comparing five future policy scenarios. BMJ
Open. 3(e003558)2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Laggner M, Gugerell A, Bachmann C,
Hofbauer H, Vorstandlechner V, Seibold M, Lechner GG, Peterbauer A,
Madlener S, Demyanets S, et al: Reproducibility of GMP-compliant
production of therapeutic stressed peripheral blood mononuclear
cell-derived secretomes, a novel class of biological medicinal
products. Stem Cell Res Ther. 11:1–16. 2020.PubMed/NCBI View Article : Google Scholar
|