1. Introduction
Rheumatoid arthritis (RA) and periodontitis are two
prevalent chronic inflammatory conditions with profound systemic
and local consequences. RA, a systemic autoimmune disease,
predominantly affects synovial joints, leading to progressive joint
destruction and systemic comorbidities, including cardiovascular
disease and osteoporosis. Periodontitis, on the other hand, is a
destructive inflammatory disease of periodontal tissues
characterized by bone loss and potential tooth loss if left
untreated (1). While they affect
different anatomical regions, pathophysiological mechanisms
underlying both diseases show considerable overlap, particularly in
the context of immune-mediated inflammation.
Recent systematic reviews and large population
cohorts confirm a bidirectional association between periodontitis
and RA, with periodontitis linked to a higher risk of developing RA
and patients with RA exhibiting a greater periodontal burden
(2-4).
However, current evidence still supports an association rather than
a definitive causal association (5). The present review summarizes the
interconnectedness of oral health conditions, examining
inflammatory mechanisms, the role of oral pathogens and clinical
implications for the management of these conditions.
2. Historical background
RA and periodontitis, although distinct in their
anatomical manifestations, share a rich and evolving medical
history marked by significant advancements in understanding their
pathogenesis and clinical management. RA has been a subject of
medical interest since the 19th century, with Alfred B. Garrod
formally distinguishing it from other forms of arthritis in
1859(6). Early conceptions of RA
were grounded in the notion of chronic synovial inflammation;
however, the discovery of rheumatoid factor (RF) in the early 20th
century and the subsequent identification of autoantibodies, such
as the anti-perinuclear factor in the 1960s, later recognized as a
precursor to the modern anti-citrullinated protein antibody (ACPA)
tests, marked a pivotal shift toward recognizing RA as an
autoimmune condition. These immunologic insights lay the groundwork
for the development of biological and targeted therapies, most
notably tumor necrosis factor-α (TNF-α) inhibitors, which markedly
alter disease outcomes by reducing inflammation, attenuating joint
destruction and improving the quality of life of patients. Despite
these advancements, RA continues to be a progressive systemic
disorder with multifaceted manifestations beyond joint involvement
(7).
In parallel, periodontitis has been recognized as a
significant oral health concern for the millennia, with
archeological evidence of periodontal destruction found in ancient
human remains. However, it was not until the 20th century that its
microbial etiology was firmly established. The identification of
specific periodontal pathogens, such as Porphyromonas
gingivalis, Tannerella forsythia and Treponema
denticola, revolutionized the understanding of periodontitis as
a biofilm-mediated disease (8).
This paradigm shift was further reinforced in the 1970s with the
emergence of the microbial biofilm concept, which emphasized the
structured and cooperative nature of bacterial communities in
dental plaque. In addition to microbial research, increasing
attention has been paid to the host immune response, particularly
the role of inflammatory cytokines in mediating periodontal tissue
breakdown (9). Together, these
discoveries have shaped the modern view of periodontitis as a
chronic inflammatory disease driven by complex interactions between
dysbiotic microbial communities and a dysregulated host immune
response.
The historical development of knowledge surrounding
RA and periodontitis highlights a shared trajectory wherein
advances in immunology and microbiology have informed both
diagnostic and therapeutic approaches. This convergence has sparked
growing interest in their potential bidirectional association,
particularly in the context of systemic inflammation and immune
modulation (10).
3. Epidemiological data
RA and periodontitis share common epidemiological
features in that both conditions are prevalent worldwide and
exhibit sex-based differences. RA affects ~0.5-1% of the global
population, with women being more frequently affected than men.
Onset typically occurs between the ages of 30 and 50 years. Global
epidemiological studies suggest that periodontitis affects >50%
of adults, with estimates ranging from 45-55% for any form of
disease. Notably, ~10-15% of adults are affected by severe
periodontitis, while the remainder exhibit mild-to-moderate
disease. The prevalence varies substantially depending on
population, socioeconomic status, and risk factors (11).
Numerous studies have highlighted the connection
between RA and periodontitis, with several population-based studies
revealing a greater prevalence of periodontitis in patients with RA
than in healthy controls (12).
The meta-analysis by de Pablo et al demonstrated that
patients with RA are at a 2-fold greater risk of developing
moderate-to-severe periodontitis. Additionally, patients with
periodontitis show a greater prevalence of RA, particularly when
the levels of serological markers, such as anti-cyclic
citrullinated peptide antibodies are elevated. Current evidence
supports an association, but does not confirm causality (13).
One particularly notable finding was derived from
the study of Porphyromonas gingivalis, a key periodontal
pathogen. This bacterium is capable of generating citrullinated
peptides, which are major targets of autoantibodies in RA,
suggesting a microbial trigger for the autoimmune response observed
in RA (14).
4. Pathophysiology
RA and periodontitis are both chronic inflammatory
diseases characterized by dysregulated immune responses and
progressive tissue destruction, albeit occurring in different
anatomical sites. RA primarily affects the synovial joints and is
classified as an autoimmune disease. It arises from a complex
interplay of genetic predispositions, environmental exposures and
immune dysregulation (15). A key
genetic factor linked to RA susceptibility is the presence of
specific HLA-DRB1 alleles, particularly those encoding the ‘shared
epitope’, a conserved amino acid sequence associated with an
increased risk. Environmental triggers, such as cigarette smoking
and exposure to certain microbes, can initiate an aberrant immune
response in genetically susceptible individuals, leading to chronic
synovial inflammation (2,15).
Immunologically, RA is characterized by the
infiltration of immune cells, including CD4+ T-cells,
B-cells, macrophages and neutrophils into the synovial membrane.
These cells release pro-inflammatory cytokines, such as TNF-α,
interleukin (IL)-1β, IL-6 and IL-17, which sustain the inflammatory
environment and contribute to synovial hyperplasia and pannus
formation (16). The receptor
activator of nuclear factor-kappa B ligand (RANKL)/osteoprotegerin
(OPG) axis is critically involved in bone remodeling in RA; an
imbalance favoring RANKL promotes osteoclastogenesis and bone
resorption, leading to characteristic joint destruction (15).
Similarly, periodontitis is driven by a chronic
immune-inflammatory response initiated by the accumulation of
pathogenic subgingival bacteria, such as Porphyromonas
gingivalis, Tannerella forsythia and Treponema
denticola. These organisms form structured biofilms on tooth
surfaces and subvert the host immune response, leading to a
persistent inflammatory state. Immune cell infiltration in gingival
tissues involves neutrophils, macrophages, dendritic cells, and T
helper 17 (Th17) cells, with IL-17 playing a pivotal role in
sustaining inflammation. IL-17 enhances neutrophil chemotaxis and
stimulates the release of matrix metalloproteinases (MMPs), which
degrade extracellular matrix components and contribute to
connective tissue breakdown (17).
Moreover, the RANKL/OPG axis is also dysregulated in
periodontitis. The increased expression of RANKL by activated
T-cells and osteoblasts enhances osteoclast differentiation and
activity, resulting in alveolar bone resorption and periodontal
attachment loss. The overlapping pathogenic mechanisms,
particularly the roles of proinflammatory cytokines, Th17 cells and
the RANKL/OPG pathway, highlight the shared molecular pathways
between RA and periodontitis, supporting the growing body of
evidence linking these two chronic inflammatory diseases (18).
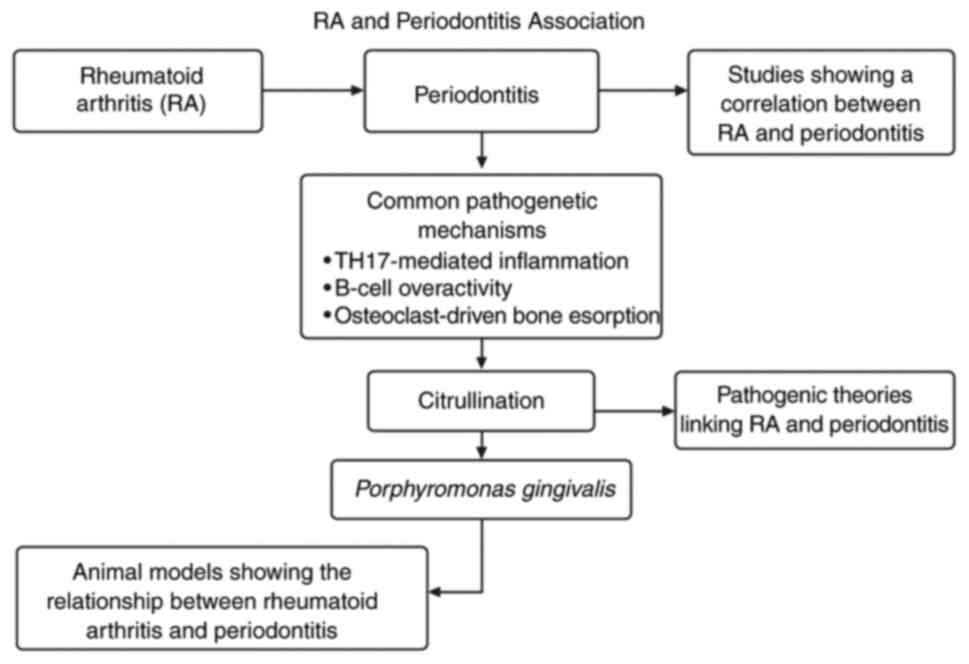
5. Common pathogenetic mechanisms
The overlapping immunopathological mechanisms
between RA and periodontitis suggest a bidirectional association
(Fig. 1). Both diseases are
characterized by excessive inflammation mediated by Th17 cells and
increased levels of IL-17. This cytokine promotes the activation of
osteoclasts via the RANKL/OPG axis, leading to bone resorption in
both the joints in RA and the alveolar bone in periodontitis
(19).
Another key intersection is the presence of
citrullinated proteins. Among periodontal pathogens,
Porphyromonas gingivalis produces peptidyl arginine
deiminase, which catalyzes the citrullination of host proteins,
thereby providing a possible link to ACPA formation. In addition,
Aggregatibacter actinomycetemcomitans can induce neutrophil
hypercitrullination, representing another microbial mechanism
potentially associated with RA. These findings highlight that
multiple periodontal pathogens may contribute to autoimmune
responses, although a definitive causal role has not yet been
established. These citrullinated peptides can initiate an
autoimmune response in genetically susceptible individuals,
contributing to the development of RA (16,20).
Systemic inflammation is another common feature of
both diseases, with elevated levels of pro-inflammatory cytokines,
such as TNF-α, IL-6 and C-reactive protein (CRP). These markers not
only reflect disease activity, but may also contribute to the
pathogenesis of both RA and periodontitis (1).
6. Correlative studies and animal
models
Several observational studies have confirmed the
association between RA and periodontitis. The Epidemiological
Investigation of Rheumatoid Arthritis (EIRA) study, for example,
revealed that individuals with severe periodontitis are at an
increased risk of developing RA (21). Other studies have shown that
patients with RA with periodontitis exhibit increased levels of
systemic inflammation and worse disease outcomes (5,7).
Clinical intervention studies have also demonstrated
the benefits of periodontal therapy in the management of RA. For
example, Kaushal et al (22) reported that non-surgical
periodontal treatment improved the disease activity score in 28
joints (DAS-28) in patients with RA, suggesting that managing
periodontal health can have a positive impact on systemic
inflammation in patients with RA.
Animal models have also been instrumental in
elucidating the bidirectional association between RA and
periodontitis. In a previous study, in a mouse model of
collagen-induced arthritis, it was shown that when combined with
Porphyromonas gingivalis infection, animals developed more
severe joint damage (23).
Similarly, the ligature-induced periodontitis model in rats has
been used to determine how periodontal disease exacerbates systemic
inflammation and joint pathology (24).
Double-hit models, in which both RA and
periodontitis are induced simultaneously, provide further evidence
for the synergistic association between these two diseases. These
models reveal elevated levels of IL-17, RANKL and MMPs, mimicking
the clinical scenario observed in human patients (25,26).
7. Pathogenic theories
There are two primary theories that explain the
connection between RA and periodontitis, as follows:
i) Autoimmune hypothesis
Periodontal inflammation leads to the systemic
dissemination of citrullinated peptides, which prime the immune
system to produce ACPAs. These autoantibodies target citrullinated
proteins, leading to the development of RA (27).
ii) Microbial hypothesis
Periodontal pathogens, including Porphyromonas
gingivalis and Aggregatibacter actinomycetemcomitans,
may translocate to joints or initiate immune responses that
cross-react with joint tissues. DNA from oral bacteria has been
detected in synovial fluid, supporting their potential association
with the pathogenesis of RA, although direct causality remains
unproven (28).
8. Clinical manifestations
The clinical manifestations of RA and periodontitis
involve inflammation and progressive tissue destruction, with
overlapping inflammatory pathways that complicate diagnosis and
management when both conditions are present.
RA typically presents with symmetrical
polyarthritis, affecting both large and small joints, often
beginning in the hands and feet. Symptoms include joint pain,
stiffness, swelling and warmth of the affected joints (15,20).
Morning stiffness that persists for at least 1 h tends to improve
with movement. Over time, joint damage may cause deformities, such
as ulnar deviation and swan-neck deformities. Systemic symptoms
include fatigue, low-grade fever, weight loss and anemia.
Extra-articular manifestations, such as rheumatoid nodules,
cardiovascular complications, such as atherosclerosis, pulmonary
fibrosis and severe rheumatoid vasculitis can also occur, affecting
small blood vessels and causing skin lesions and ulcers (1,29).
Periodontitis, an inflammatory disease affecting the
supporting structures of the teeth, is characterized by gingival
inflammation, pocket formation, alveolar bone loss and tooth
mobility. Clinical signs include bleeding gums, swelling and
redness, which worsen with brushing or probing. As the disease
progresses, deep periodontal pockets form, leading to tooth
mobility and visible bone loss on radiographs. Gingival recession
exposes tooth roots, causing sensitivity and aesthetic concerns.
Severe periodontitis may result in abscesses, swelling, pain and
pus formation. Halitosis often accompanies the disease due to
bacterial activity in the pockets (30,31).
The bidirectional association between RA and
periodontitis complicates clinical management. Patients with RA are
often more susceptible to developing periodontal disease due to
factors, such as immunosuppressive therapy, reduced manual
dexterity and medication side-effects such as xerostomia. In turn,
periodontitis in patients with RA can exacerbate systemic
inflammation, worsen joint symptoms, and create a vicious cycle
that accelerates joint damage and decreases treatment
effectiveness. Chronic periodontal inflammation can significantly
affect RA disease activity, rendering both conditions more
difficult to manage (32).
9. Diagnostic considerations
The diagnosis of both RA and periodontitis involves
a combination of clinical evaluation, laboratory testing and
imaging studies. For patients with overlapping RA and
periodontitis, early and accurate diagnosis is crucial for
implementing effective treatment strategies.
Diagnosis of RA
The diagnosis of RA begins with a thorough clinical
history and examination. Symmetrical joint involvement and morning
stiffness lasting for >1 h are key symptoms of RA. Specific
serological markers, including RF, which is positive in 60-80% of
patients with RA, and ACPAs, which have high specificity for RA,
help confirm the diagnosis. Additionally, elevated erythrocyte
sedimentation rates and CRP levels are indicative of systemic
inflammation and provide evidence of active disease (1).
Imaging analyses play a crucial role in assessing
joint damage. Radiographs reveal joint space narrowing, erosions
and periarticular osteopenia, which are characteristic of RA.
Ultrasound and MRI are valuable tools for detecting early
inflammatory changes and assessing synovial thickening and fluid
accumulation, often before radiographic damage is evident (33,34).
Diagnosis of periodontitis
The diagnosis of periodontitis is based on clinical
and radiographic criteria. A clinical evaluation includes measuring
the probing depth and clinical attachment level, which assess the
extent of pocket formation and tissue destruction. Indicators, such
as bleeding on probing and tooth mobility are suggestive of active
disease. Radiographs provide essential information regarding the
extent of alveolar bone loss and the severity of periodontal
involvement (30).
Periodontitis is classified on the basis of its
severity and extent, ranging from mild gingivitis to severe
periodontitis, which is characterized by significant bone loss and
deep periodontal pockets (5). This
classification is vital for determining the appropriate treatment
approach.
10. Screening for overlap and prognosis
Given the potential for increased disease severity
when both RA and periodontitis co-occur, screening for periodontal
disease in patients with RA and vice versa is strongly recommended.
Routine oral assessments for patients with RA can aid in the early
detection and management of periodontal disease, which may, in
turn, aid in controlling the disease activity of RA. Conversely,
screening patients with RA for periodontal involvement can improve
the management of both conditions through a coordinated care
approach (29,31).
Prognosis
The prognosis of patients with RA and periodontitis
is complex due to the interaction between systemic inflammation and
oral health. Both diseases contribute to the other's progression,
with periodontal inflammation exacerbating RA symptoms and vice
versa. In patients with RA, periodontitis increases systemic
inflammation, leading to elevated CRP and pro-inflammatory cytokine
levels, which may exacerbate joint symptoms. Some research suggests
that this could reduce responsiveness to disease-modifying
anti-rheumatic drugs and biologics, although evidence remains
limited and further studies are required to confirm this
association (11). Evidence
suggests that periodontal treatment can improve RA symptoms by
reducing systemic inflammation and potentially slowing joint
destruction (12). In patients
with periodontitis, RA can contribute to the rapid progression of
periodontal disease, and immunosuppressive therapies can impair the
ability of the body to manage periodontal pathogens, leading to
more severe disease. Integrated care for improved prognosis is
crucial, and collaboration between rheumatologists and
periodontists can help manage both conditions more effectively
(6,35).
11. Management and treatment
The management of RA and periodontitis requires a
comprehensive, interdisciplinary approach that addresses both local
and systemic factors. Treatment for both conditions aims to control
inflammation, prevent further damage and improve the overall
quality of life of affected individuals.
Management of RA
The primary goal of RA treatment is to control joint
inflammation and prevent further damage (Table I) (36,37).
 | Table IManagement of rheumatoid
arthritis. |
Table I
Management of rheumatoid
arthritis.
| Treatment
Modality | Description | Considerations in
periodontal context |
|---|
| NSAIDs | Alleviate pain and
control inflammation; provide symptomatic relief. | Useful for managing
inflammation but do not alter disease progression. |
| DMARDs (e.g.,
methotrexate) | Slow disease
progression and prevent joint destruction. | May improve
systemic inflammation; immune modulation can affect periodontal
healing. |
| Biological agents
(e.g., TNF inhibitors) | Target
proinflammatory cytokines for severe RA treatment. | Enhanced control of
inflammation may benefit periodontal outcomes. |
|
Corticosteroids | Used during
flare-ups to control inflammation; long-term use limited due to
side-effects. | Long-term use may
cause osteoporosis, complicating periodontal therapy and bone
health. |
| Nonpharmacological
interventions | Physical therapy,
exercise, occupational therapy to maintain joint function and.
mobility | Improved physical
health and reduced disability can enhance oral hygiene
practices. |
| Surgical
interventions | Repair or
replacement of severely damaged joints. | Restoration of
function may indirectly improve oral hygiene ability and overall
health. |
Management of periodontitis
The management of periodontitis primarily focuses on
eliminating the source of infection, reducing inflammation and
regenerating lost tissue (Table
II) (38-40).
 | Table IIManagement of periodontitis. |
Table II
Management of periodontitis.
| Treatment
Modality | Description | Purpose/impact |
|---|
| Scaling and root
planing (SRP) | Mechanical removal
of plaque and calculus from root surfaces; smoothing of roots. | Reduces bacterial
load, promotes healing, and establishes a healthier oral
environment. |
| Antibiotic
therapy | Use of topical or
systemic antibiotics targeting specific pathogens (e.g.,
Porphyromonas gingivalis). | Enhances bacterial
control, particularly in aggressive or refractory cases. |
| Surgical
therapy | Procedures to
regenerate lost periodontal tissue or reduce deep pockets (e.g.,
flap surgery, bone grafts). | Improves access for
debridement, restores periodontal architecture, and reduces pocket
depth. |
| Host modulation
therapy | Use of agents such
as low-dose doxycycline to modify the inflammatory response of the
host. | Reduces tissue
destruction, preserves periodontal support, and enhances treatment
outcomes. |
Although some clinical research has reported
improvements in periodontal parameters with anti-TNF-α and
anti-IL-6 therapy, findings are not consistent across populations
(7). Thus, any potential
periodontal benefit of RA biologics should be interpreted with
caution, as current evidence does not establish a definitive
therapeutic effect.
Impact of periodontal treatment on
RA
Effective periodontal treatment has been shown to
have a significant positive effect on RA by reducing systemic
inflammation, a key contributor to disease pathogenesis. It has
been demonstrated that periodontal therapy can lead to lower levels
of CRP, a marker of systemic infection, and improve the DAS-28,
reflecting better control over RA symptoms (41). The bidirectional association
between RA and periodontitis suggests that managing one condition
can help improve the outcomes of the other, reinforcing the need
for a holistic and interdisciplinary approach to patient care
(20,42). Recent randomized trials and
systematic reviews have reported that non-surgical periodontal
therapy (scaling and root planning ± local/systemic adjuncts) can
reduce systemic inflammatory markers and modestly reduce RA disease
activity (mean DAS-28 reductions reported in meta-analyses ~0.3-0.5
points), although the results are heterogeneous and
patient-selection dependent. These data support routine periodontal
screening in RA clinics and collaborative management pathways where
periodontitis is present (43).
These findings underscore the importance of an integrated approach
to treatment, where addressing periodontal disease can directly
enhance the management of RA.
12. Conclusions and future directions
Research gaps and future
directions
Despite progress being made in the understanding of
the association between RA and periodontitis, there gaps remain in
research. Longitudinal studies are required to determine whether
treating one disease can prevent the onset or progression of the
other, with a focus on biomarkers of disease progression and the
effect of periodontal treatment on RA progression. Specific
biomarker candidates include: Citrullinated vimentin (anti-MCV),
implicated in osteoclast activation and bone resorption;
citrullinated α-enolase/CEP-1 and related ACPA specificities that
cross-react with bacterial enolases; neutrophil-derived proteases
(e.g., elastase, proteinase-3) detectable in saliva and gingival
crevicular fluid reflecting neutrophil hyperactivation; and oral
microbiome signatures (16S/shotgun panels) that may distinguish
early RA or predict periodontal disease progression. Evidence of
the role of citrullinated vimentin in bone resorption has been
demonstrated in animal models, and clinical studies have revealed
increased levels of antibodies against citrullinated bacterial
epitopes in early-stage RA (32,44,45).
Incorporating these markers into prospective, standardized cohort
studies will clarify temporal relations and may enable precision
screening in high-risk patients. Potential candidates under current
investigation include citrullinated vimentin and α-enolase as
autoantigens, salivary protease markers reflective of periodontal
tissue destruction, and microbiome signatures specific to
periodontitis. Incorporating these markers into longitudinal
studies may clarify the temporal association between RA and
periodontitis and guide precision medicine approaches.
Understanding the role of specific microbes and their interactions
with the host immune system could provide new insights into the
pathogenesis of both diseases and the genetic basis for the overlap
in susceptibility to RA and periodontitis. Future clinical trials
are required to evaluate the efficacy of co-management strategies
for RA and periodontitis, assessing whether integrated care
improves patient outcomes in both oral and systemic health.
Limitations of current evidence
While numerous studies support an association
between RA and periodontitis, the current body of evidence has
several limitations. A number of investigations are cross-sectional
in nature, rendering it difficult to establish temporal
associations or causality. Potential confounding factors, such as
smoking, socioeconomic status, diabetes and other systemic
comorbidities may influence both diseases, and complicate the
interpretation of the associations. Furthermore, heterogeneity in
diagnostic criteria for both RA and periodontitis across studies
reduces comparability. Sample sizes in interventional trials are
often small, limiting generalizability. Longitudinal and
multicenter studies with standardized definitions are thus
warranted to strengthen the evidence base.
In conclusion, the association between RA and
periodontitis is complex, with inflammation being a common factor
accelerating their progression. This highlights the need for
integrated care approaches that address both systemic and oral
health. Early diagnosis and effective management of periodontitis
in patients with RA can improve patient outcomes, reduce joint
damage and enhance treatment response. Collaborative efforts
between rheumatologists and periodontists are essential to unravel
the underlying mechanisms linking these diseases. Future research
into biomarkers, genetic predispositions and microbial interactions
will lead to personalized therapies and to an improved quality of
life for affected patients. Bridging the gap between oral and
systemic health care is now necessary for holistic,
patient-centered treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
The current review represents an original synthesis
by the authors, integrating immunopathological, clinical and
therapeutic perspectives on the RA-periodontitis relationship. AS
was responsible for the conceptualization of the study, conducted
the literature search, curation of data from the literature and
drafted the manuscript. DGK and NS supervised the study, critically
reviewed the content, and provided expert input on periodontology
and systemic disease interactions. All authors contributed to
refining the manuscript, ensuring accuracy, and reading and
approving the final version. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Batool H, Afzal N, Shahzad F and Kashif M:
Relationship between rheumatoid arthritis and chronic
periodontitis. J Med Radiol Pathol Surg. 2:11–14. 2016.
|
|
2
|
Dolcezza S, Flores-Fraile J, Lobo-Galindo
AB, Montiel-Company JM and Zubizarreta-Macho Á: Relationship
between rheumatoid arthritis and periodontal disease-systematic
review and meta-analysis. J Clin Med. 14(10)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chang Y, Chung MK, Park JH and Song TJ:
Association of oral health with risk of rheumatoid arthritis: A
nationwide cohort study. J Pers Med. 13(340)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bolstad AI, Fevang BTS and Lie SA:
Increased risk of periodontitis in patients with rheumatoid
arthritis: A nationwide register study in Norway. J Clin
Periodontol. 50:1022–1032. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zielińska A and Tabarkiewicz J:
Correlation between rheumatoid arthritis and periodontitis. Eur J
Clin Exp Med. 20:451–458. 2022.
|
|
6
|
Bartold PM and Lopez-Oliva I:
Periodontitis and rheumatoid arthritis: An update 2012-2017.
Periodontol 2000. 83:189–212. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hamed MN and Ali BG: Serum level of TNF-α
and IL-17 in patient have chronic periodontitis associated
rheumatoid arthritis. J Bagh Coll Dent. 29:104–110. 2017.
|
|
8
|
Hussain SB, Botelho J, Machado V, Zehra
SA, Mendes JJ, Ciurtin C, Orlandi M and D'Aiuto F: Is there a
bidirectional association between rheumatoid arthritis and
periodontitis? A systematic review and meta-analysis. Semin
Arthritis Rheum. 50:414–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Agnihotri R and Gaur S: Rheumatoid
arthritis in the elderly and its relationship with periodontitis: A
review. Geriatr Gerontol Int. 14:8–22. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
de Pablo P, Chapple ILC, Buckley CD and
Dietrich T: Periodontitis in systemic rheumatic diseases. Nat Rev
Rheumatol. 5:218–224. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mercado FB, Marshall RI, Klestov AC and
Bartold PM: Relationship between rheumatoid arthritis and
periodontitis. J Periodontol. 72:779–787. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Leech MT and Bartold PM: The association
between rheumatoid arthritis and periodontitis. Best Pract Res Clin
Rheumatol. 29:189–201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Pablo P, Dietrich T and Karlson EW:
Antioxidants and other novel cardiovascular risk factors in
subjects with rheumatoid arthritis in a large population sample.
Arthritis Rheum. 57:953–962. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ceccarelli F, Orrù G, Pilloni A,
Bartosiewicz I, Perricone C, Martino E, Lucchetti R, Fais S, Vomero
M, Olivieri M, et al: Porphyromonas gingivalis in the tongue
biofilm is associated with clinical outcome in rheumatoid arthritis
patients. Clin Exp Immunol. 194:244–252. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chatterjee A, Jayaprakasan M, Chakrabarty
AK, Lakkaniga NR, Bhatt BN, Banerjee D, Narwaria A, Katiyar CK and
Dubey SK: Comprehensive insights into rheumatoid arthritis:
Pathophysiology, current therapies and herbal alternatives for
effective disease management. Phytother Res. 39:2764–2799.
2024.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Poryadin GV, Zakhvatov AN and Parshina AY:
Pathogenetic relationship of immunological disorders in chronic
generalized periodontitis and rheumatoid arthritis. Russ Arch
Intern Med. 12:203–211. 2022.
|
|
17
|
Syniachenko OV, Yermolaieva MV, Havilei
DO, Liventsova KV, Verzilov SM and Pylypenko VV: Clinical and
pathogenetic features of the joint syndrome in rheumatoid arthritis
with comorbid periodontitis. Travma. 21:10–15. 2020.
|
|
18
|
Na HS, Kim SY, Han H, Kim HJ, Lee JY, Lee
JH and Chung J: Identification of potential oral microbial
biomarkers for the diagnosis of periodontitis. J Clin Med.
9(1549)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Arkema EV, Karlson EW and Costenbader KH:
A prospective study of periodontal disease and risk of rheumatoid
arthritis. J Rheumatol. 37:1800–1804. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Acharya A, Li S, Liu X, Pelekos G, Ziebolz
D and Mattheos N: Biological links in periodontitis and rheumatoid
arthritis: Discovery via text-mining PubMed abstracts. J
Periodontal Res. 54:318–328. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eriksson K, Nise L, Kats A, Luttropp E,
Catrina AI, Askling J, Jansson L, Alfredsson L, Klareskog L,
Lundberg K and Yucel-Lindberg T: Prevalence of periodontitis in
patients with established rheumatoid arthritis: A Swedish
population based case-control study. PLoS One.
11(e0155956)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaushal S, Singh AK, Lal N, Das SK and
Mahdi AA: Effect of periodontal therapy on disease activity in
patients of rheumatoid arthritis with chronic periodontitis. J Oral
Biol Craniofac Res. 9:128–132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shimizu M, Kondo Y, Tanimura R, Furuyama
K, Yokosawa M, Asashima H, Tsuboi H, Matsumoto I and Sumida T:
T-bet represses collagen-induced arthritis by suppressing Th17
lineage commitment through inhibition of RORγt expression and
function. Sci Rep. 11(17357)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Srivastava M, Neupane YR, Kumar P and
Kohli K: Nanoemulgel (NEG) of Ketoprofen with eugenol as oil phase
for the treatment of ligature-induced experimental periodontitis in
Wistar rats. Drug Deliv. 23:2228–2234. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shimizu S, Hayashi K, Takeuchi Y, Tanabe
G, Churei H, Kobayashi H, Ueno T and Fueki K: Effect of
Porphyromonas gingivalis infection on healing of skeletal
muscle injury: An in vivo study. Dent J (Basel).
12(346)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sakuraba K, Oyamada A, Fujimura K, Spolski
R, Iwamoto Y, Leonard WJ, Yoshikai Y and Yamada H: Interleukin-21
signaling in B cells, but not in T cells, is indispensable for the
development of collagen-induced arthritis in mice. Arthritis Res
Ther. 18(188)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kharlamova N, Jiang X, Sherina N, Potempa
B, Israelsson L, Quirke AM, Eriksson K, Yucel-Lindberg T, Venables
PJ, Potempa J, et al: Antibodies to Porphyromonas gingivalis
indicate interaction between oral infection, smoking, and risk
genes in rheumatoid arthritis etiology. Arthritis Rheumatol.
68:604–613. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yilmaz D, Niskanen K, Gonullu E,
Tervahartiala T, Gürsoy UK and Sorsa T: Salivary and serum levels
of neutrophil proteases in periodontitis and rheumatoid arthritis.
Oral Dis. 30:1660–1668. 2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kaczyński T, Wroński J, Głuszko P and
Górska R: Link between rheumatoid arthritis and chronic
periodontitis. Postepy Hig Med Dosw (Online). 72:69–80. 2018.
|
|
30
|
Research, Science and Therapy Committee.
Position paper: Diagnosis of periodontal diseases. J Periodontol.
74:1237–1247. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ranade SB and Doiphode S: Is there a
relationship between periodontitis and rheumatoid arthritis? J
Indian Soc Periodontol. 16:22–27. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rajkarnikar J, Thomas BS and Rao SK:
Inter-relationship between rheumatoid arthritis and periodontitis.
Kathmandu Univ Med J (KUMJ). 11:22–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tan YK and Conaghan PG: Imaging in
rheumatoid arthritis. Best Pract Res Clin Rheumatol. 25:569–584.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Østergaard M and Boesen M: Imaging in
rheumatoid arthritis: The role of magnetic resonance imaging and
computed tomography. Radiol Med. 124:1128–1141. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Varshney S, Sharma M, Kapoor S and
Siddharth M: Association between rheumatoid arthritis and
periodontitis in an adult population-a cross sectional study. J
Clin Exp Dent. 13:e980–e986. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Krutyhołowa A, Strzelec K, Dziedzic A,
Bereta GP, Łazarz-Bartyzel K, Potempa J and Gawron K: Host and
bacterial factors linking periodontitis and rheumatoid arthritis.
Front Immunol. 13(980805)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Monsarrat P, Fernandez de Grado G,
Constantin A, Willmann C, Nabet C, Sixou M, Cantagrel A, Barnetche
T, Mehsen-Cetre N, Schaeverbeke T, et al: The effect of periodontal
treatment on patients with rheumatoid arthritis: The ESPERA
randomised controlled trial. Joint Bone Spine. 86:600–609.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jajoo NS, Shelke AU and Bajaj RS:
Periodontitis and rheumatoid arthritis: The common thread. Clin Rev
Bone Miner Metab. 18:18–30. 2020.
|
|
39
|
Bingham CO III and Moni M: Periodontal
disease and rheumatoid arthritis: The evidence accumulates for
complex pathobiologic interactions. Curr Opin Rheumatol.
25:345–353. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Koziel J and Potempa J: Pros and cons of
causative association between periodontitis and rheumatoid
arthritis. Periodontol 2000. 89:83–98. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Amos RS, Constable TJ, Crockson RA,
Crockson AP and McConkey B: Rheumatoid arthritis: Relation of serum
C-reactive protein and erythrocyte sedimentation rates to
radiographic changes. Br Med J. 1:195–197. 1997.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wolf DL and Lamster IB: Contemporary
concepts in the diagnosis of periodontal disease. Dent Clin North
Am. 55:47–61. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Inchingolo F, Inchingolo AM, Avantario P,
Settanni V, Fatone MC, Piras F, Di Venere D, Inchingolo AD, Palermo
A and Dipalma G: The effects of periodontal treatment on rheumatoid
arthritis and of anti-rheumatic drugs on periodontitis: A
systematic review. Int J Mol Sci. 24(17228)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shindo S, Pierrelus R, Ikeda A, Nakamura
S, Heidari A, Pastore MR, Leon E, Ruiz S, Chheda H, Khatiwala R, et
al: Extracellular release of citrullinated vimentin directly acts
on osteoclasts to promote bone resorption in a mouse model of
periodontitis. Cells. 12(1109)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sherina N, de Vries C, Kharlamova N, Sippl
N, Jiang X, Brynedal B, Kindstedt E, Hansson M, Mathsson-Alm L,
Israelsson L, et al: Antibodies to a citrullinated Porphyromonas
gingivalis epitope are increased in early rheumatoid arthritis,
and can be produced by gingival tissue B cells: Implications for a
bacterial origin in RA etiology. Front Immunol.
13(804822)2022.PubMed/NCBI View Article : Google Scholar
|