Introduction
Globally, thyroid pathologies constitute a major
public health challenge, with prevalence rates reaching ~5% across
populations. Predominant manifestations include autoimmune thyroid
disease (AITD), particularly Hashimoto's thyroiditis (HT) and
Graves' disease (GD). These conditions develop through
immune-mediated inflammatory processes involving lymphocyte
invasion and elevated concentrations of thyroid-specific antibodies
(1). The thyroid is exceptionally
susceptible to autoimmune dysfunction (2). Within populations maintaining an
adequate iodine consumption, the aetiology of thyroid malfunction
predominantly involves autoimmune pathways rather than dietary
insufficiencies (3).
Building on this autoimmune framework, HT is a
chronic autoimmune condition typically causing hypothyroidism. It
is characterised by the destruction of thyroid follicles, leading
to low T3/T4 and elevated thyroid-stimulating hormone (TSH) levels,
and is often accompanied by anti-thyroid peroxidase (anti-TPO)
antibodies (4). Conversely, GD
causes hyperthyroidism with thyrotoxicosis, goitre and orbitopathy
driven by stimulating thyrotropin receptor antibodies (1). Both conditions are markedly more
common among women and can progress through hyper-, eu-, and
hypothyroid phases as thyroid damage evolves (5).
Concurrent with these autoimmune thyroid disorders,
vitamin B12 deficiency represents a clinically significant
nutritional concern, as this water-soluble vitamin serves as a
vital cofactor for DNA biosynthesis and cellular metabolism. Due to
a low dietary intake or malabsorption, B12 deficiency is relatively
common and may produce non-specific, yet severe haematological and
neurological effects (6). The
clinical evaluation of B12 status relies on total serum B12 as the
primary biomarker, supplemented by holotranscobalamin for enhanced
diagnostic precision (7,8).
Moreover, the association between B12 deficiency and
autoimmune conditions warrants particular attention. While
affecting only 3 to 4% of the general population, vitamin B12
deficiency demonstrates a significantly higher prevalence among
patients with autoimmune diseases, including autoimmune thyroiditis
(AIT) (9). Given these patterns,
the B12-thyroid association is particularly relevant in the Iraqi
population, among whom both AITDs and nutritional deficiencies,
such as vitamin B12 deficiency, are prevalent health concerns
(10,11). This association is strengthened by
the frequent coexistence of AITDs with other autoimmune disorders,
including type 1 diabetes, vitiligo and particularly pernicious
anaemia, which directly impairs B12 absorption through autoimmune
gastritis (1). Furthermore, recent
research investigating the roles of B12, folate, vitamin D and
anaemia in HT has suggested that the metabolic status can influence
thyroid autoimmunity, underlining the importance of nutritional
factors in thyroid immunity (12).
Considering such convergences, B12 deficiency
symptoms mimic thyroid dysfunction, requiring diligent evaluation.
Thus, the early identification of B12 deficiency is essential in
patients with thyroid disorders. Symptoms such as fatigue,
cognitive impairment and neuropathy often overlap with thyroid
disorders, potentially delaying diagnosis and leading to suboptimal
treatment outcomes (13). A direct
comparison of the vitamin B12 status between patients with HT and
GD could therefore reveal disease-specific patterns of deficiency,
inform targeted screening strategies, and lead to an improvement in
patient management protocols in clinical practice.
While serum vitamin B12 levels have been examined in
the context of thyroid dysfunction, prior research reveals
contradictory evidence (14).
Direct comparisons of the vitamin B12 status between patients with
HT and GD, particularly in Middle Eastern populations, such as in
Iraq, remain limited, despite their clinical importance. Therefore,
in order to strengthen the evidence-based research in this
understudied area, the present case-control study was designed to
assess and compare vitamin B12 levels across patients with HT, GD
and healthy controls, while investigating correlations with thyroid
function parameters [TSH and free thyroxine (FT4)] and autoimmune
markers [thyroid peroxidase antibodies (anti-TPO) and thyrotropin
receptor antibodies (TRAbs)].
Subjects and methods
Research design and sample size
determination
Designed with a case-control method, the present
study was conducted between December, 2024 and March, 2025 at the
Thyroid Centre, Smart Health Tower, Sulaymaniyah, Iraq. Patient
recruitment involved the random selection of cases among
individuals with confirmed HT or GD. All diagnoses were established
prior to study inclusion by a team of specialised physicians
following standard clinical protocols. Healthy controls were
recruited from among hospital staff and individuals presenting for
routine check-ups, after ensuring they had no history of thyroid or
autoimmune diseases.
In order to establish the required sample size, a
power analysis was performed using G*Power statistical software for
Windows (Universität Düsseldorf, Düsseldorf, Germany), setting
included α at 0.05, power at 0.95 and effect size at 0.4 (14,15).
The minimal sample size required was determined as 102
participants, which was increased to 120 to compensate for
potential procedural errors. The three experimental arms consisted
of the HT, GD and control groups, preserving a balanced 1:1:1
proportion across the enrolment process. Each group was composed of
an equal number of 40 participants.
Participant selection criteria
The inclusion criteria comprised of adults aged
25-60 years with a confirmed diagnosis of HT or GD for the case
groups and the absence of autoimmune or major health conditions for
the control group. The exclusion criteria included recent vitamin
B12 supplementation over the past 6 months, the presence of
non-thyroidal autoimmune diseases, a history of thyroidectomy,
gastrointestinal surgery affecting nutrient absorption, a
vegetarian diet and pregnancy or lactation. In order to minimise
the potential for confounding variables, the ages and sex of the
participants were balanced across the case and control groups
(16).
Data collection and laboratory
assessments
In order to capture a comprehensive patient profile
for research, a structured questionnaire was designed to obtain
demographic data, such as age and sex, medical backgrounds
including surgical history, thyroid treatment use, a family history
of autoimmune conditions and disease duration, alongside lifestyle
factors, such as the use of supplements, dietary habits, smoking
status and alcohol consumption, and any relevant health symptoms.
The body mass index (BMI), measured in kg/m2, was
determined by dividing the recorded weight by the height squared.
Based on WHO standards, participants were classified as
underweight, healthy weight, overweight, or obese according to
their BMI thresholds of <18.5, 18.5 to 24.9, 25.0 to 29.9, and
≥30.0 in kg/m2, respectively (17).
All study participants provided a blood sample
without prior fasting. For patients with thyroid disorders,
sampling coincided with routine clinical visits for thyroid
monitoring, whereas control participants provided samples within
the same time frame. To minimise temporal variability, samples were
collected at comparable times for all participants. A volume of 3
ml venous blood was drawn into VACUETTE® serum separator
tubes (Greiner Bio-One) containing a clot activator and separation
gel. The sample was allowed to clot at room temperature for ~20
min, and then centrifuged at 2,500 x g for 15 min at 20-25˚C using
a Hettich RotoFix 32A centrifuge. The separated serum was
immediately analysed for the quantitative determination of vitamin
B12, TSH, FT4, anti-TPO and TRAb concentrations. Laboratory
analyses were conducted using the cobas® pro e 801
analysers (Roche Diagnostics GmbH), which employ
electrochemiluminescence technology for immunoassay detection with
daily internal quality controls and standard calibration. Reference
ranges were established according to manufacturer specifications as
follows: Serum vitamin B12: Reference range, 197-771 pg/ml;
categorised as deficiency: <197 pg/ml; borderline, 197-300
pg/ml; and normal, 300-771 pg/ml; TSH: Reference range, 0.4-4.2
µIU/ml; FT4: Reference range, 12-22 pmol/l; anti-TPO: Reference
range, <35 IU/ml; and TRAbs: Reference range, <1.75 IU/l
(18,19). While the guidelines exhibit some
inconsistency in terms of the exact threshold values for vitamin
B12 deficiency and no single universal cut-off is recognised, this
scheme is concordant with commonly adopted international
thresholds, which typically define deficiency at <200 pg/ml and
a borderline range of 200-300 pg/ml (20,21).
Anti-TPO was measured specifically in the HT cases, and TRAbs in
the GD cases, while both antibodies were evaluated in the control
group.
Ethical considerations
The present study was executed in strict adherence
to the ethical principles of the Declaration of Helsinki,
safeguarding the rights and dignity of the participants. Formal
approval was granted by the Ethics Board of the College of Health
and Medical Technology at Sulaimani Polytechnic University
(reference no. 30/245, dated December 1, 2024). After receiving a
thorough explanation of the purpose of the study, processes,
possible risks and advantages, participants provided written
informed consent. Additionally, they were made aware of their
freedom to discontinue participation at any point during the study
without consequence. All data were collected and stored in
accordance with institutional privacy and confidentiality
guidelines (22).
Statistical analysis
The evaluation of data in the present study was
carried out using IBM SPSS statistical software, version 26.0 (IBM
Corp.), to execute a range of statistical computations necessary
for the analysis. The normality of continuous variables was tested
using the Shapiro-Wilk test to ascertain their distribution
patterns. It was observed that the majority of continuous
variables, including TSH, FT4, anti-TPO, TRAbs and vitamin B12
concentrations, did not conform to a normal distribution, leading
to the decision to report the results as the median and quartile
range (QR) for a precise depiction of central tendencies and
variability. For comparisons between two distinct groups, the
Mann-Whitney U test was used for non-parametric data, and the
independent samples t-test was applied for parametric data to
detect significant differences, while the Kruskal-Wallis test was
applied for analyses involving three or more groups to assess
variations across categories. When the Kruskal-Wallis test
indicated statistical significance, post hoc pairwise comparisons
were performed using Dunn's test with Bonferroni correction to
adjust for multiple testing. Categorical data, encompassing
variables such as sex, smoking status, residential location,
familial history, vitamin B12 status, and BMI classifications-were
summarised as frequencies and corresponding percentages, with
differences being evaluated using either the Chi-squared test or
Fisher's exact test, selected based on the expected frequencies in
contingency tables. To explore the potential correlations between
serum vitamin B12 levels and thyroid-related markers, such as TSH,
FT4, anti-TPO, and TRAbs, Spearman's rank correlations were
reported with 95% confidence intervals (CIs) to indicate the
precision of association estimates. Independent associations
between vitamin B12 status and thyroid autoimmunity were initially
evaluated using univariate logistic regression and subsequently
assessed with multivariate logistic regression adjusted for BMI,
residence, and smoking. All statistical tests were conducted as
two-tailed to ensure a thorough evaluation. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
The baseline demographic and clinical
characteristics of the 120 study participants are presented in
Table I. The median age of all the
participants was 42.5 years, with no significant difference
observed between the patient group (median, 42.0 years) and the
healthy group (median, 43.0 years) (P=0.640). Similarly, there was
no significant difference in sex distribution between the patients
(65.8% males and 67.1% females) and the healthy controls (34.2%
males and 32.9% females) (P=0.890). The mean BMI was comparable
between the patient group (29.50±5.4) and the healthy group
(28.83±4.58), with no statistically significant difference
(P=0.515). Significant differences were found in terms of the
family history of autoimmune thyroiditis (AIT), smoking status and
residence of the participants. A significantly higher proportion of
patients (78.9%) reported a family history of AIT compared to the
healthy individuals (21.1%) (P=0.007). As regards smoking status, a
significantly greater percentage of patients (93.3%) were smokers
compared to the healthy group (6.7%) (P=0.019). Furthermore, a
significantly higher proportion of patients resided in rural areas
(93.2%) compared to the healthy participants (6.8%), while a larger
percentage of healthy individuals resided in urban areas (48.7%)
compared to the patients (51.3%) (P<0.001) (Table I).
 | Table IFoundational demographic and clinical
attributes of the study participants. |
Table I
Foundational demographic and clinical
attributes of the study participants.
| Variables | Total | Patients | Healthy | P-value |
|---|
| Age (years), median
(QR) | 42.5
(35.0-47.0) | 42.0
(34.5-47.0) | 43.0
(35.5-46.5) | 0.640 |
| Sex, n (%) | | | | |
|
Male | 38 (100.0) | 25 (65.8) | 13 (34.2) | 0.890 |
|
Female | 82 (100.0) | 55 (67.1) | 27 (32.9) | |
| BMI (mean ±
SD) | 29.3±5.1 | 29.50±5.4 | 28.83±4.58 | 0.515 |
| Family history of
AIT, n (%) | | | | |
|
Yes | 57 (100.0) | 45 (78.9) | 12 (21.1) | 0.007 |
|
No | 63 (100.0) | 35 (55.6) | 28 (44.4) | |
| Smoking status, n
(%) | | | | |
|
Yes | 15 (100.0) | 14 (93.3) | 1 (6.7) | 0.019 |
|
No | 105 (100.0) | 66 (62.9) | 39 (37.1) | |
| Place of residence,
n (%) | | | | |
|
Urban | 76 (100.0) | 39 (51.3) | 37 (48.7) | <0.001 |
|
Rural | 44 (100.0) | 41 (93.2) | 3 (6.8) | |
Among the 120 study participants, vitamin B12
deficiency was identified in 13 individuals (10.8%), with the
highest prevalence observed in the healthy control group (n=6,
46.2%), followed by the participants with GD (n=5, 38.5%) and HT
(n=2, 15.4%). Borderline vitamin B12 levels were documented in 38
participants (31.7%), with the majority concentrated in the HT
group (n=18, 47.4%), while the GD and healthy control groups each
contributed 10 participants (26.3% each). A normal vitamin B12
status was found in 69 participants (57.5%), distributed relatively
evenly across the three groups: GD (n=25, 36.2%), healthy controls
(n=24, 34.8%) and HT (n=20, 29.0%). Statistical analysis
demonstrated no significant association between the vitamin B12
status categories and study group classification (P=0.215). In
alignment with the categorical distribution of the vitamin B12
status, the analysis of serum vitamin B12 concentrations revealed
no statistically significant differences among the study groups
(P=0.556). The median vitamin B12 level for the total study
population was 318.0 pg/ml (QR, 255-427.5). When assessed by group,
the HT group demonstrated a median value of 301.5 pg/ml (QR,
250.5-370.5), the GD group had a median of 321.0 pg/ml (QR,
269.0-445.0), and the healthy control group exhibited a median of
347.5 pg/ml (QR, 247.0-438.0) (Table
II).
 | Table IIComparison of vitamin B12 status,
thyroid function parameters and thyroid autoantibodies among
participants with Hashimoto's thyroiditis, Graves' disease and
healthy controls. |
Table II
Comparison of vitamin B12 status,
thyroid function parameters and thyroid autoantibodies among
participants with Hashimoto's thyroiditis, Graves' disease and
healthy controls.
| Variables | Total | Hashimoto's
thyroiditis | Graves'
disease | Healthy
controls | HT vs.
GDa | HT vs. healthy
a | GD vs
healthya | P-value |
|---|
| Vitamin B12
status | | | | | | | | |
|
Deficient | 13 (100.0) | 2 (15.4) | 5 (38.5) | 6 (46.2) | - | - | - | 0.215 |
|
Borderline | 38 (100.0) | 18 (47.4) | 10 (26.3) | 10 (26.3) | | | | |
|
Normal | 69 (100.0) | 20 (29.0) | 25 (36.2) | 24 (34.8) | | | | |
| Vitamin B12, median
(QR) | 318.0
(255-427.5) | 301.5
(250.5-370.5) | 321.0
(269.0-445.0) | 347.5
(247.0-438.0) | - | - | - | 0.556 |
| | | | | | - | - | - | |
| TSH level, median
(QR) | 1.875
(0.925-3.990) | 5.045
(2.020-8.505) | 0.626
(0.005-2.935) | 1.565
(1.330-2.075) | <0.001 | <0.001 | <0.001 | <0.001 |
| FT4, median
(QR) | 15.85
(13.45-18.50) | 16.25
(12.47-18.75) | 16.0
(12.5-32.20) | 15.50
(14.35-17.55) | - | - | - | 0.876 |
| Anti-TPO, median
(QR) | 26.55
(11.90-280.50) | 280.50
(146.0-511.5) | - | 11.90
(9.72-15.55) | - | - | - | <0.001 |
| TRAb antibody,
median (QR) | 1.37
(0.87-3.55) | - | 3.55
(2.11-9.28) | 0.88
(0.80-1.05) | - | - | - | <0.001 |
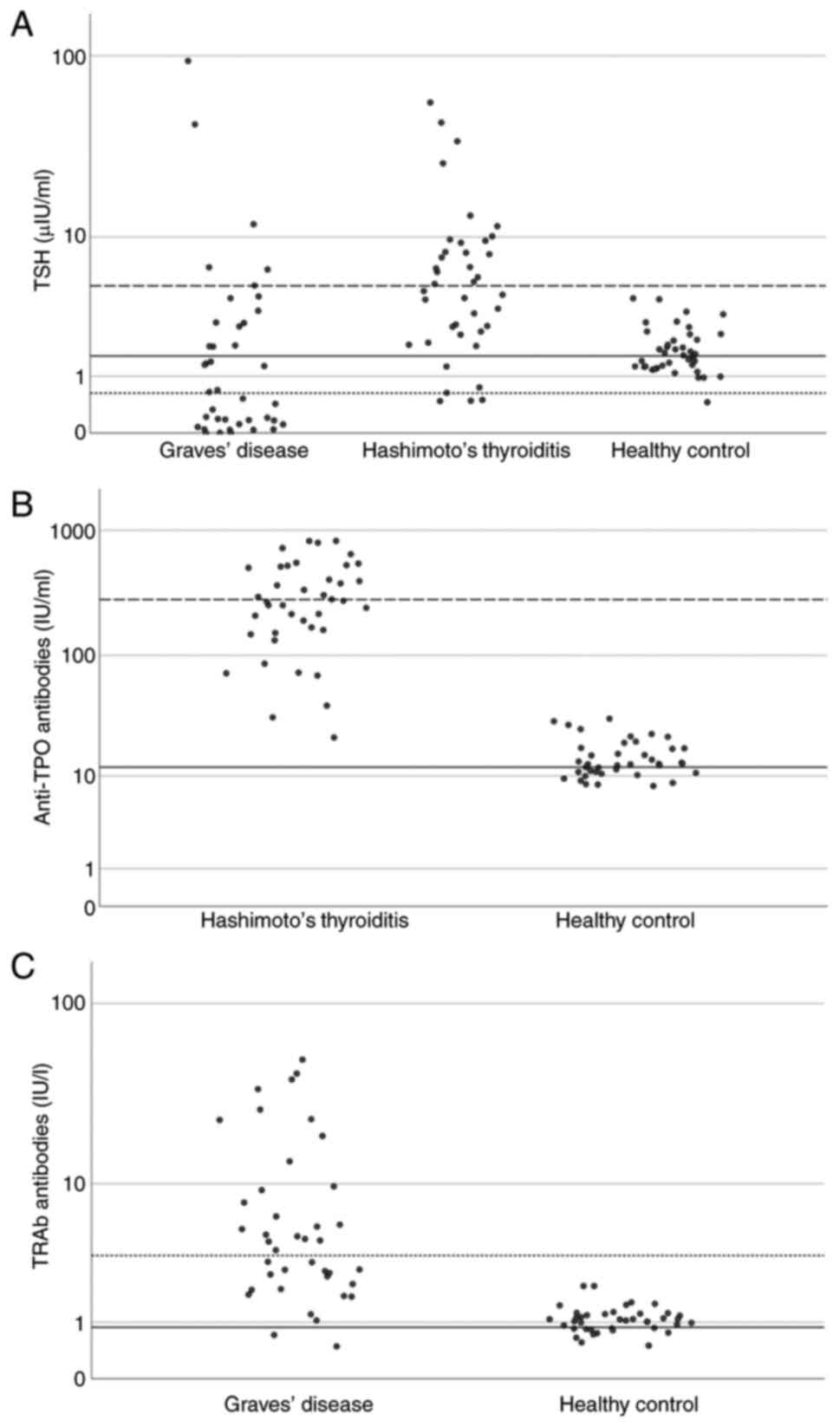
Thyroid function parameters demonstrated distinct
patterns across the study groups. The TSH levels varied
significantly among the participants, with the HT group exhibiting
the highest median concentration at 5.045 µIU/ml (QR, 2.020-8.505),
while the GD group exhibited the lowest at 0.626 µIU/ml (QR,
0.005-2.935) and the healthy controls had intermediate values at
1.565 µIU/ml (QR, 1.330-2.075). Statistical analysis confirmed a
significant difference in the TSH concentrations between the three
groups (P<0.001) (Table II and
Fig. 1A). Conversely, the FT4
levels remained comparable across all groups, with values of 16.25
pmol/l (QR, 12.47-18.75), 16.0 pmol/l (QR, 12.5-32.20) and 15.50
pmol/l (QR, 14.35-17.55) for HT, GD and healthy controls,
respectively (P=0.876) (Table
II).
Thyroid autoantibody measurements revealed
characteristic patterns specific to each disease group. The
anti-TPO antibody concentrations were substantially higher in the
HT group, with a median of 280.50 IU/ml (QR, 146.0-511.5). This
contrasted sharply with the healthy controls, who had a median of
11.90 IU/ml (QR, 9.72-15.55). The overall median anti-TPO antibody
level across all participants was 26.55 IU/ml (QR,11.90-280.50),
with statistical analysis confirming a highly significant
difference between groups (P<0.001). TRAb concentrations
exhibited a similar pattern of group-specific elevation, with
participants in the GD group exhibiting markedly increased levels
at 3.55 IU/l (QR, 2.11-9.28) compared to healthy controls at 0.88
IU/l (QR, 0.80-1.05). The overall median TRAb concentration was
1.37 IU/l (QR, 0.87-3.55), with statistical analysis confirming
significant differences between the groups (P<0.001) (Table II, and Fig. 1B and C).
These distinct biochemical and immunological
profiles are clearly illustrated in Fig. 1. TSH values cluster at elevated
levels for HT and are suppressed in GD. The anti-TPO concentrations
exhibited a pronounced increase in the HT group compared to the
controls, and TRAb values were distinctly elevated in the GD group,
but remained low in the controls. This graphical representation
reinforces the specific diagnostic and pathophysiological features
distinguishing each group (Table
II and Fig. 1).
The analysis of the vitamin B12 status concerning
demographic and clinical characteristics revealed that obesity was
the predominant BMI category among the participants with vitamin
B12 deficiency (61.5%), followed by the normal BMI (23.1%) and
overweight (15.4%) categories. A similar pattern was observed in
the borderline group, where obesity also constituted the largest
subgroup (44.7%), with the overweight (39.5%) and normal BMI
(15.8%) categories also represented. Among the individuals with
normal vitamin B12 levels, the overweight category was the most
prevalent (47.8%), while the obesity (31.9%) and normal BMI (20.3%)
categories were also noted (Table
III).
 | Table IIIDistribution of demographic and
clinical characteristics by vitamin B12 status. |
Table III
Distribution of demographic and
clinical characteristics by vitamin B12 status.
| | Vitamin B12
status | |
|---|
| Parameters, n
(%) | Deficient
(n=13) | Borderline
(n=38) | Normal (n=69) | P-value |
|---|
| BMI group | | | | |
|
Underweight | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.168 |
|
Healthy
weight | 3 (23.1) | 6 (15.8) | 14 (20.3) | |
|
Overweight | 2 (15.4) | 15 (39.5) | 33 (47.8) | |
|
Obese | 8 (61.5) | 17 (44.7) | 22 (31.9) | |
| Sex | | | | |
|
Male | 2 (15.4) | 11 (28.9) | 25 (36.2) | 0.326 |
|
Female | 11 (84.6) | 27 (71.1) | 44 (63.8) | |
| Age group,
years | | | | 0.533 |
|
25-30 | 0 (0.0) | 4 (10.5) | 9 (13.0) | |
|
31-35 | 2 (15.4) | 5 (13.2) | 12 (17.4) | |
|
36-40 | 1 (7.7) | 8 (21.1) | 10 (14.5) | |
|
41-45 | 8 (61.5) | 8 (21.1) | 13 (18.8) | |
|
46-50 | 2 (15.4) | 8 (21.1) | 16 (23.2) | |
|
51-55 | 0 (0.0) | 4 (10.5) | 6 (8.7) | |
|
56-60 | 0 (0.0) | 1 (2.6) | 3 (4.3) | |
| Disease
durationa | | | | 0.649 |
|
<1
year | 2 (28.6) | 7 (25.0) | 15 (33.3) | |
|
From 1 to 3
years | 3 (42.9) | 6 (21.4) | 10 (22.2) | |
|
From 3 to 5
years | 2 (28.6) | 7 (25.0) | 8 (17.8) | |
|
>5
years | 0 (0.0) | 8 (28.6) | 12 (26.7) | |
As regards sex distribution, females constituted the
majority across all vitamin B12 classifications, notably among
those exhibiting deficiency (84.6%) and borderline levels (71.1%).
As for age, vitamin B12 deficiency was notably clustered within the
41-45-year cohort (61.5%). By contrast, the participants with a
borderline status demonstrated a more uniform age distribution,
primarily spanning the 36-50-year range. Those with normal vitamin
B12 levels exhibited a wider age spread, with the 46-50-year
category containing the largest proportion (23.2%) (Table III).
The assessment of disease duration within the
patients with AIT indicated that vitamin B12 deficiency was most
prevalent among individuals diagnosed for 1 to 3 years (42.9%); the
remaining deficient cases were equally divided between disease
durations of <1 year and 3 to 5 years (28.6% each). Participants
with borderline vitamin B12 levels displayed a varied distribution
of disease duration: 28.6% had a duration >5 years, 25.0% each
were in the <1 year and 3 to 5 year categories, and 21.4% had a
duration of 1 to 3 years. Among the individuals with normal vitamin
B12 levels, the largest subgroup (33.3%) had a disease duration of
<1 year, followed by those with a duration of >5 years
(26.7%) (Table III).
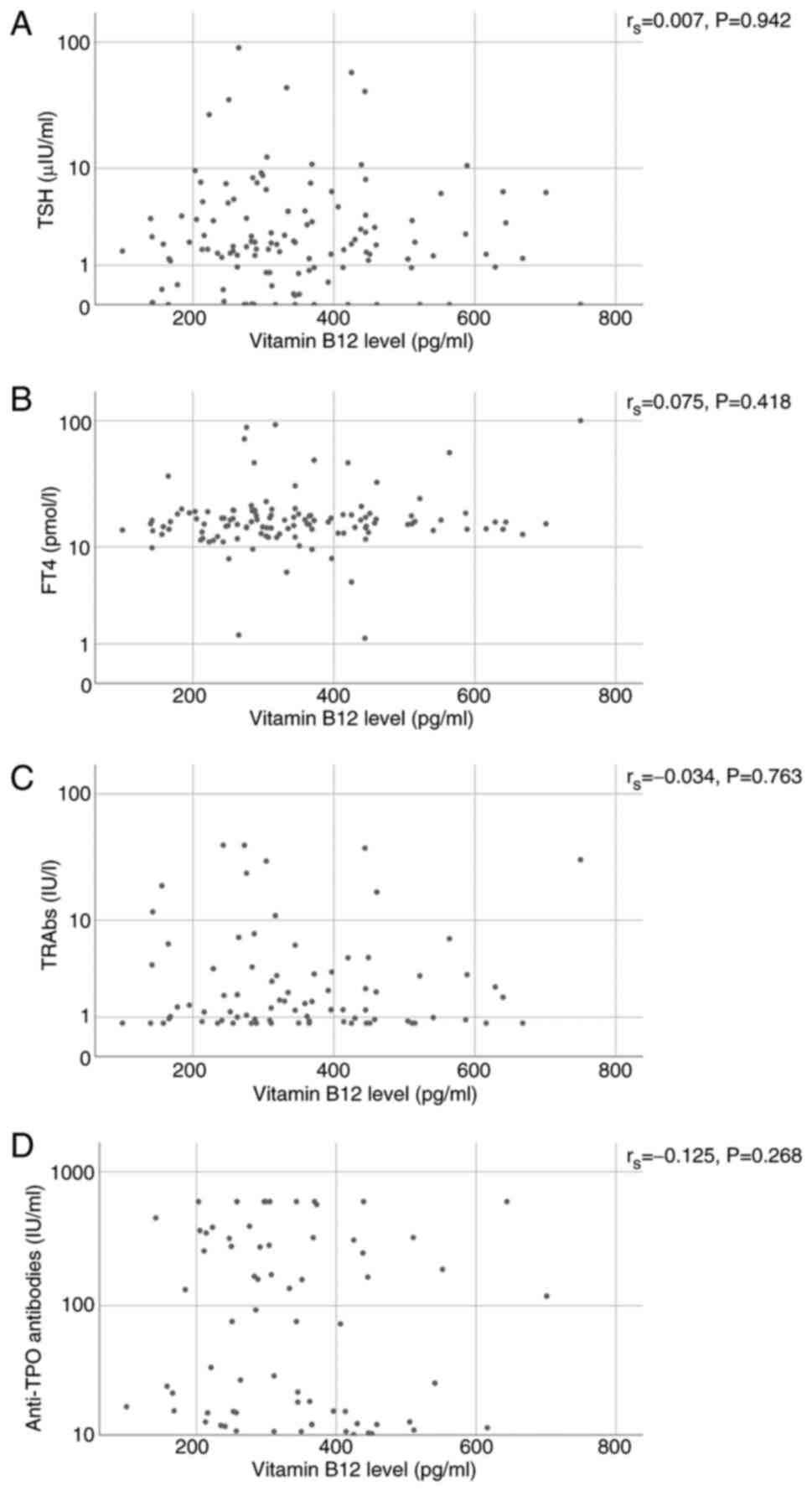
Spearman's correlation analysis was performed to
assess the correlation between serum vitamin B12 concentrations and
various thyroid-related parameters, including TSH, FT4, TRAbs and
anti-TPO antibodies. The choice of Spearman's correlation analysis
was based on the non-normal distribution of the data. The analysis
revealed that the correlation coefficient (rs) between vitamin B12
and TSH was 0.007, with a P-value of 0.942, indicating no
observable correlation between these variables (95% CI, -0.158 to
0.183). Similarly, the correlation between vitamin B12 and FT4 was
weakly positive (rs=0.075; P=0.418), suggesting a negligible
association (95% CI, -0.098 to 0.234). For the thyroid
autoantibodies, TRAbs displayed a slight negative correlation with
vitamin B12 (rs=-0.034; P=0.763; 95% CI, -0.275 to 0.214), while
anti-TPO antibodies exhibited a modest negative correlation
(rs=-0.125, P=0.268; 95% CI, -0.339 to 0.088). However, none of
these associations reached statistical significance, as all
P-values exceeded the conventional threshold (Table IV and Fig. 2).
 | Table IVSpearman's rank correlation analysis
between serum vitamin b12 levels and thyroid-related parameters in
study participants. |
Table IV
Spearman's rank correlation analysis
between serum vitamin b12 levels and thyroid-related parameters in
study participants.
| Variables | No. of
participants | rs | P-value | 95% CI lower | 95% CI upper |
|---|
| Vitamin B12 level
and TSH | 120 | .007 | .942 | -0.158 | 0.183 |
| Vitamin B12 level
and FT4 | 120 | .075 | .418 | -0.098 | 0.234 |
| Vitamin B12 level
and TRAba | 80 | -.034 | .763 | -0.275 | 0.214 |
| Vitamin B12 l level
and anti-TPOb | 80 | -.125 | .268 | -0.339 | 0.088 |
To further explore the determinants of the vitamin
B12 status among the study participants, a univariate logistic
regression analysis was initially conducted (Table V). BMI exhibited an odds ratio (OR)
of 0.946 (95% CI, 0.846-1.058; P=0.329), rural residence had an OR
of 0.483 (95% CI, 0.125-1.859; P=0.290), and smoking status had an
OR of 1.806 (95% CI, 0.218-14.989; P=0.584); none of these factors
reached statistical significance. A subsequent multivariate
logistic regression model including the same variables confirmed
these finding. Similarly, none of the examined factors emerged as
statistically significant predictors of the vitamin B12 status.
Specifically, BMI [odds ratio (OR), 1.046; 95% CI, 0.933-1.174;
P=0.441], the place of residence (urban vs. rural: OR, 0.507; 95%
CI, 0.131-1.969; P=0.327) and smoking status (OR, 1.506; 95% CI,
0.172-13.157; P=0.711) did not demonstrate independent associations
with vitamin B12 deficiency following adjustment for confounding
factors.
 | Table VUnivariate and multivariate logistic
regression of factors associated with vitamin B12 status. |
Table V
Univariate and multivariate logistic
regression of factors associated with vitamin B12 status.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | B | SE | Exp(B) (OR) | 95% CI for OR | P-value | B | SE | Exp(B) (OR) | 95% CI for OR | P-value |
|---|
| BMI | -0.056 | 0.057 | 0.946 | 0.846-1.058 | 0.329 | -0.045 | 0.059 | 1.046 | 0.933-1.174 | 0.441 |
| District
(residence) | | | | | | | | | | |
|
Urbana | | | | | | | | | | |
|
Rural | -0.728 | 0.688 | 10.483 | 0.125-1.859 | 0.290 | -0.679 | 0.692 | 10.507 | 0.131-1.969 | 0.327 |
| Smoking status | | | | | | | | | | |
|
Yesa | | | | | | | | | | |
|
No | -0.591 | 1.080 | 11.806 | 0.218-14.989 | 0.584 | -0.409 | 1.106 | 11.506 | 0.172-13.157 | 0.711 |
Discussion
The analysis of the demographic data in the present
study revealed patterns that are largely consistent with both
regional and international literature. The pronounced female
predominance in the HT and GD groups is a well-established
epidemiological feature of AITD, consistent with the findings from
the studies by Zoori and Mousa (23) in Nasiriya, Iraq, and by Aon et
al (24) in Kuwait, which
similarly identified a notably higher occurrence of AITD among
females in their respective regions. The median age of the
participants in the present study was 42.5 years, which was also
comparable to the mean age of 42.48 years reported in the Indian
study by Kaur et al (25)
and that of 41.21 years in the Iraqi study by Abed et al
(26) for patients with an
autoimmune hypothyroid and GD, positioning the present study cohort
within a typical age range for AITD diagnosis. The significantly
higher rates of a positive family history of AITD in the patient
groups herein reinforce the strong genetic component of these
diseases. The observed associations with smoking and rural
residence are novel findings that warrant further investigation, as
they may point to specific environmental triggers or lifestyle
factors relevant to our regional population.
The present study validated participant grouping
with distinct thyroid function and autoantibody profiles across HT,
GD and the controls, demonstrating significant differences in TSH,
anti-TPO and TRAb levels (all P<0.001), while FT4 remained
comparable (P=0.876). The hormonal and immunological patterns align
with the synthesis in the study by Vargas-Uricoechea (27), who confirmed TSH and FT4 as the
foundational laboratory tests in the initial assessment of thyroid
dysfunction and noted the defining roles of TRAb and anti-TPO
antibodies in differentiating autoimmune thyroid disease entities.
The comparable FT4 levels across groups suggest that a number of
participants were in subclinical stages or under effective therapy.
This biochemical distinction confirms the precision of the disease
classification and underpins the reliability of the analyses in the
present study.
The principal finding of the present study was the
lack of a statistically significant association between the serum
B12 level and the presence of either HT or GD in the Iraqi cohort
(P=0.215). The analysis demonstrated no significant variations in
themedian B12 concentrations or the prevalence of B12 deficiency
among the HT, GD and healthy control groups (P=0.556). Moreover,
vitamin B12 levels did not significantly correlate with any of the
measured thyroid function or autoimmune markers. This outcome
contributes a crucial, albeit null, finding to a field
characterised by markedly conflicting evidence and highlights the
complexity of the relationship between vitamin B12 and autoimmune
thyroid disorder.
The absence of significant group differences may be
explained by several physiological and genetic factors. For
example, pernicious anaemia, a manifestation of autoimmune
gastritis, leads to vitamin B12 malabsorption. Notably, intrinsic
factor antibodies and anti-parietal cell antibodies, which play a
decisive role in B12 absorption (28), were not screened in the present
study cohort. Their absence may partially explain the lack of B12
deficiency detected, although they are recognised mediators in the
link between gastric and thyroid autoimmunity. Moreover, pernicious
anaemia is observed only in a subset of patients with thyroid
disorders. According to Vaqar and Shackelford (28), up to one-quarter of individuals
with autoimmune gastritis develop pernicious anaemia, and these
patients often present with coexisting autoimmune conditions,
including thyroiditis. However, such an overlap may vary across
populations, potentially reflecting a low prevalence of gastric
autoantibody positivity in some regions or differing genetic
backgrounds compared with populations in which thyroid-gastric
associations are more common. A recent genome-wide association
study identified susceptibility loci for pernicious anaemia, such
as PTPN22, HLA-DQB1, IL2RA and AIRE, that overlap with loci
implicated in autoimmune thyroid disease (29). In other words, only patients with
thyroid disorders carrying these susceptibility variants are prone
to B12 malabsorption, while those without such predispositions are
likely to maintain normal B12 levels. Consequently, the combined
effects of the incomplete expression of gastric autoimmunity and
genetic heterogeneity may explain the null association observed in
our study.
The results of the present study are strongly
corroborated by a notable subset of studies that also failed to
find a clear association. Notably, the study by Al-Mousawi et
al (9) performed in Duhok,
Iraq, aligns with the findings of the present study, as it reported
no significant difference in total vitamin B12 levels between
patients with subclinical hypothyroidism and healthy controls. This
regional alignment suggests that in some Iraqi populations, a
strong link may not be present. Similarly, the large meta-analysis
performed by Benites-Zapata et al (14), which included >28,000
participants, provides nuanced support for the findings of the
present study. Whereas it did find lower B12 levels in patients
with overt hypothyroidism, it notably found no significant
difference in B12 levels for patients with hyperthyroidism, AITD or
subclinical hypothyroidism when compared to healthy controls
(14), which perfectly mirrors the
results from our well-defined AITD cohorts. Further support comes
from the study by Aon et al (24), who found no statistically
significant variation in the occurrence of vitamin B12 deficiency
among the hypothyroid groups compared to the control group.
Additionally, the literature reviews performed by Collins and
Pawlak (13), and Kacharava et
al (30) also emphasise the
highly variable and inconsistent findings across the field,
highlighting that the link is far from established and validating
the importance of null findings such as those of the present study.
Conversely, the findings of the present study are in contrast to
certain other scientific studies that have reported a significant
association between thyroid dysfunction and B12 deficiency. For
instance, both Chatterjee et al (31) and Kaur et al (25) reported a high prevalence of B12
deficiency (68 and 70%, respectively) in their hypothyroid cohorts
within their studies conducted in India.
Furthermore, another notable result of the present
study was the non-existence of a statistically significant
correlation with the 95% CI values for these correlations all
including zero, reinforcing the absence of any meaningful
association between vitamin B12 levels and the main thyroid
function parameters (TSH) or autoimmunity (anti-TPO and TRAb).
Similarly, this result is supported by the findings reported in the
studies by Bhuta et al (32), Sinha et al (33) and Chatterjee et al (31), who all reported no significant
correlations between TSH levels and vitamin B12 in their
hypothyroid cohorts (31-33).
In addition to this, the observation in the present study of a
non-significant association with thyroid autoantibodies is
consistent with several key investigations, including the studies
by Aon et al (24) and
Kumari et al (34), who
also failed to establish a significant associative link between B12
status and anti-TPO levels. Similarly, the regional Iraqi study by
Al-Mousawi et al (9) failed
to establish a significant link between B12 status and hypothyroid
autoantibodies However, this body of evidence stands in stark
contrast to other published research. For instance, Chatterjee
et al (31) demonstrated a
strong negative correlation between vitamin B12 levels and the
level of anti-TPO, a finding echoed in the studies by Aktaş
(35) and Kacharava et al
(36), who also reported weak, yet
significant negative correlations between B12 and anti-TPO levels.
This clear dichotomy in the literature, in which the present study
aligns firmly with one perspective, underlines the ongoing
scientific debate. This suggests that the association between
vitamin B12 and thyroid autoimmunity is not necessarily direct or
universal, and may instead be influenced by other unmeasured
confounding factors, such as co-occurring autoimmune conditions or
specific population genetics, which vary between study cohorts.
A granular examination of the present study cohort
revealed several descriptive trends that, while not reaching
statistical significance, provide valuable context. The apparent
preponderance of females in the B12-deficient group, which
comprised 84.6% of diagnosed cases, is most plausibly interpreted
as a reflection of the underlying demographics of autoimmune
thyroid disorder, a pattern well-documented by studies, such as
those by Zoori and Mousa (23),
and Aon et al (24), rather
than an independent sex-based risk for deficiency. Furthermore, the
descriptive clustering of deficiency within the 41-45-year age
bracket and its prevalence among patients with a 1-3-year disease
duration suggests a potential temporal window of vulnerability
post-diagnosis. However, the lack of statistical significance in
the present study is in agreement with the previous study by Kumari
et al (34), who also
reported no significant correlation between B12 levels and either
patient age or disease duration. This convergence suggests that
while these patterns may be observable, they do not represent
robust, independent predictors of B12 status in AITD
populations.
Univariate and multivariate analyses revealed no
independent predictive role for BMI, residential location, or
smoking status in determining vitamin B12 deficiency. This finding
contributes to a complex and inconsistent body of literature on the
B12-obesity association. For example, while the study on adults by
Sun et al (37) in the USA
reported a significant inverse association, other research such as
a large randomised trial in a European cohort by de Araghi et
al (38) found no significant
link. The results of the present study, however, align more closely
with evidence from the Middle East, such as the study by Abu-Shanab
et al (39) on Jordanian
adults, in which similar demographic variables showed poor
predictive value. Furthermore, although a national Iraqi survey
identified rural-urban disparities in B12 level (10), the adjusted model used herein did
not detect a significant residence effect. Collectively, these
results suggest that BMI, residential location and smoking status
are not robust predictors of vitamin B12 deficiency in patients
with AIT.
The discordant findings within the literature and
the contrast between the results of the present study and studies
reporting a positive association may likely be attributed to marked
methodological heterogeneity. Firstly, a number of studies
aggregate all hypothyroid patients, regardless of aetiology,
whereas the present study differentiated between HT and GD.
Secondly, the lack of a standardised diagnostic threshold for
vitamin B12 deficiency, with cut-offs varying across different
studies, greatly affects reported prevalence rates. As demonstrated
by Kumari et al (34),
raising the cut-off value from <145 to <200 pg/ml increased
the observed prevalence from 45.5 to 55%. Of note, beyond such
methodological heterogeneity, deeper population-based factors play
a critical role in generating divergent results across studies.
Genetic predisposition, such as varying frequencies of HLA types
and immunoregulatory gene variants, differs substantially among
ethnicities and may influence both thyroid disease risk and
comorbid gastrointestinal autoimmunity such as pernicious anaemia
(29). Nutritional habits,
particularly with respect to animal product intake, impact baseline
B12 status; this was demonstrated in a study from northern India,
in which 86% of patients with vitamin B12 deficiency were pure
vegetarians (40). It was found
that, among those with both B12 deficiency and hypothyroidism, 17
of 19 were strict vegetarians (40). Collectively, these considerations
underline that results from one study population may not be
directly translatable to another, thereby highlighting the
necessity of region-specific research and interpretation.
The present study possesses several methodological
strengths that enhance the validity of its findings. A key strength
is the inclusion of two distinct, well-defined AITD cohorts (HT and
GD) alongside a healthy control group. This design allows for a
more granular analysis than studies that aggregate all hypothyroid
patients or focus on only one AITD type. The groups were
well-matched for age and sex, and we utilised robust statistical
power analysis to ensure an adequate sample size. The carefully
crafted inclusion and exclusion criteria minimised the potential
for confounding variables to influence the results. Additionally,
the present study addressed a key research gap, as there is
insufficient evidence to establish a direct correlation between
vitamin B12 levels and TRAb titres in GD. Finally, the use of a
single, high-precision ECLIA platform for all hormonal and vitamin
assays reduced inter-assay variability and increased the
reliability and consistency of our measurements.
Notwithstanding the merits of the present
case-control study, certain constraints need to be recognised.
Initially, although age and sex were matched between cases and
controls, the omission of matching for additional demographic and
lifestyle variables may have affected the findings. Moreover, the
significant overrepresentation of female participants, in line with
the epidemiology of AITD, limits the ability to thoroughly examine
sex-specific associations and impedes direct comparisons between
males and females. Additionally, despite drawing participants from
varied regions within the province, the single-centre framework may
constrain the applicability of the findings to other populations
with distinct healthcare environments or demographic
characteristics. Finally, the focus of the present study precludes
any determination of causality concerning B12 deficiency. Future
longitudinal or multi-centre studies, which should include thorough
matching for these demographic and dietary factors, as well as
relevant biomarkers and factors, such as gastric autoantibodies,
are essential for advancing comprehension of this intricate
association.
The findings of the present study suggest that
routine screening for vitamin B12 deficiency in all patients with
autoimmune thyroid disease may not be justified in low-prevalence
populations. Instead, testing should be considered for those with
clinical symptoms, risk factors, such as vegetarian diets, or
concomitant gastric autoimmunity. This targeted approach can
improve resource allocation and reduce unnecessary interventions.
When B12 deficiency is detected, timely supplementation remains
critical to prevent neurological and haematological complications.
Ultimately, the present study supports adopting individualised
screening strategies in clinical practice rather than blanket
testing for all thyroid autoimmunity cases.
In conclusion, the present study demonstrates no
significant association between serum vitamin B12 levels and
autoimmune thyroid diseases, including HT and GD, with similar
findings for thyroid function markers and disease-specific
autoantibodies. These null results contribute to the existing
literature by highlighting the variability in B12-AITD associations
across populations, potentially influenced by genetic, nutritional,
or methodological factors. While routine B12 screening may not be
warranted for all patients with AITDs in similar settings, targeted
assessment is advisable for those with relevant risk factors. In
order to better elucidate potential influences and long-term
patterns, future multicentre longitudinal studies are
recommended.
Acknowledgements
The authors extend their sincere gratitude to the
medical team of Dr Abdul Wahid at the Thyroid Centre, Smart Health
Tower, Sulaymaniyah, Iraq, for their collaboration during the data
collection process.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Both authors (BHA and KRK) contributed equally to
the writing, revising and finalisation of the manuscript, as well
as to data analysis and table creation. BHA and KRK confirm the
authenticity of all the raw data. Both authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study received official approval from
the Ethics Board of the College of Health and Medical Technology at
Sulaimani Polytechnic University (reference no. 30/245, dated
December 1, 2024). All participants gave written informed consent
after receiving a comprehensive explanation of the study's
objectives, procedures, potential risks, and benefits. They were
also informed of their right to withdraw from the study at any
time.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ben Salah R, Hadj Kacem F, Soomauro S,
Chouaib S, Frikha F, Charfi N, Abid M and Bahloul Z: Autoimmune
thyroiditis associated with autoimmune diseases. Electron J Gen
Med. 19(em409)2022.
|
|
2
|
Lichtiger A, Fadaei G and Tagoe CE:
Autoimmune thyroid disease and rheumatoid arthritis: Where the
twain meet. Clin Rheumatol. 43:895–905. 2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Soetedjo NN, Agustini D and Permana H: The
impact of thyroid disorder on cardiovascular disease: Unraveling
the connection and implications for patient care. Int J Cardiol
Heart Vasc. 55(101536)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Verma MK, Tripathi P, Saxena N and Singh
AN: Association of vitamin B12, folate and ferritin with thyroid
hormones in hypothyroidism. Ann Int Med Dent Res. 5:2395–2814.
2019.
|
|
5
|
Dyrka K, Obara-Moszyńska M and Niedziela
M: Autoimmune thyroiditis: An update on treatment possibilities.
Endokrynol Pol. 75:461–472. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mucha P, Kus F, Cysewski D, Smolenski RT
and Tomczyk M: Vitamin B12 metabolism: A network of multi-protein
mediated processes. Int J Mol Sci. 25(8021)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Harrington DJ, Stevenson E and
Sobczyńska-Malefora A: The application and interpretation of
laboratory biomarkers for the evaluation of vitamin B12 status. Ann
Clin Biochem. 62:22–33. 2025.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nexo E and Parkner T: Vitamin B12-related
biomarkers. Food Nutr Bull. 45 (Suppl 1):S28–S33. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al-Mousawi M, Salih S, Ahmed A and
Abdullah B: Serum vitamin B12 and holotranscobalamin levels in
subclinical hypothyroid patients in relation to thyroid-stimulating
hormone (TSH) levels and the positivity of anti-thyroid peroxidase
antibodies: A case-control study. Cureus. 16(e61513)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sabeeh HK, Ali SH and Al-Jawaldeh A: Iraq
is moving forward to achieve global targets in nutrition. Children.
9(215)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zaman BA, Rasool SO, Sabri SM, Raouf GAM,
Balatay AA, Abdulhamid MA, Hussein DS, Odisho SK, George ST, Hassan
SM, et al: Prevalence of thyroid dysfunctions in a large,
unselected population in Duhok city, Iraqi Kurdistan: A
cross-sectional study. J Biol Res (Italy). 94(10067)2021.
|
|
12
|
Aslan ES and Gür S: Evaluation and
epigenetic impact of B12, vitamin D, folic acid and anemia in
Hashimoto's thyroiditis: A clinical and molecular docking study. J
Health Sci Med. 6:705–712. 2023.
|
|
13
|
Collins AB and Pawlak R: Prevalence of
vitamin B-12 deficiency among patients with thyroid dysfunction.
Asia Pac J Clin Nutr. 25:221–226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Benites-Zapata VA, Ignacio-Cconchoy FL,
Ulloque-Badaracco JR, Hernandez-Bustamante EA, Alarcón-Braga EA,
Al-kassab-Córdova A and Herrera-Añazco P: Vitamin B12 levels in
thyroid disorders: A systematic review and Meta-analysis. Front
Endocrinol (Lausanne). 14(1070592)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kang H: Sample size determination and
power analysis using the G*Power software. J Educ Eval Health Prof.
18(17)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lutsey PL: Case-control studies:
Increasing scientific rigor in control selection. Res Pract Thromb
Haemost. 7(100090)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Purnell JQ: Definitions, classification,
and epidemiology of obesity. Endotext. 2023. https://www.ncbi.nlm.nih.gov/books/NBK279167/.
|
|
18
|
Roche Diagnostics: Elecsys Vitamin B12 II.
Version 3.0. Roche Diagnostics GmbH, Mannheim, 2024. https://elabdoc-prod.roche.com/eLD/api/downloads/21d33a84-4470-e911-0b9a-00215a9b3428?countryIsoCode=us.
|
|
19
|
Roche Diagnostics: Reference Intervals for
Children and Adults-Elecsys® Thyroid Tests. Roche
Diagnostics GmbH, Mannheim, 2020. https://www.labgids.be/files/bijlages/12960/Elecsys_Thyroid_Test_Reference_Brochure_2020.pdf.
|
|
20
|
Obeid R, Andrès E, Češka R, Hooshmand B,
Guéant-Rodriguez RM, Prada GI, Sławek J, Traykov L, Ta Van B,
Várkonyi T, et al: Diagnosis, treatment and Long-term management of
vitamin B12 deficiency in adults: A Delphi expert consensus. J Clin
Med. 13(2176)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aroda VR, Edelstein SL, Goldberg RB,
Knowler WC, Marcovina SM, Orchard TJ, Bray GA, Schade DS, Temprosa
MG, White NH, et al: Long-term metformin use and vitamin B12
deficiency in the diabetes prevention program outcomes study. J
Clin Endocrinol Metab. 101:1754–1761. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
The Innovation Editorial Team: Advancing
human dignity: The latest updates to the Declaration of Helsinki.
Innovation, 2025.
|
|
23
|
Zoori A and Mousa H: Prevalence of thyroid
disorders in Nasiriya City, Iraq. Univ Thi Qar J Sci. 10:122–127.
2023.
|
|
24
|
Aon M, Taha S, Mahfouz K, Ibrahim MM and
Aoun AH: Vitamin B12 (cobalamin) deficiency in overt and
subclinical primary hypothyroidism. Clin Med Insights Endocrinol
Diabetes. 15(11795514221086634)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kaur R, Singh H and Yadav RS: Prevalence
of vitamin B deficiency in patients with thyroid disorders: A study
from Himalayan region. Int J Clin Biochem Res. 9:123–126. 2022.
|
|
26
|
Abed RM, Abdulmalek HW, Yaaqoob LA, Altaee
MF and Kamona ZK: Serum level and genetic polymorphism of IL-38 and
IL-40 in autoimmune thyroid disease. Iraqi J Sci. 64:2786–2797.
2023.
|
|
27
|
Vargas-Uricoechea H, Nogueira JP,
Pinzón-Fernández MV and Schwarzstein D: The usefulness of thyroid
antibodies in the diagnostic approach to autoimmune thyroid
disease. Antibodies (Basel). 12(48)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vaqar S and Shackelford KB: Pernicious
anemia. In: StatPearls [Internet]. StatPearls Publishing, Treasure
Island, FL, 2023.
|
|
29
|
Laisk T, Lepamets M, Koel M and Abner E:
Estonian Biobank Research Team. Mägi R: Genome-wide association
study identifies five risk loci for pernicious anemia. Nat Commun.
12(3761)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kacharava T, Giorgadze E, Janjgava S and
Lomtadze N: Correlation between vitamin B12 deficiency and
autoimmune thyroid diseases (literature review). Exp Clin Med
Georgia: doi: 10.52340/jecm.2022.06.009.
|
|
31
|
Chatterjee T, Gupta R and Choudhary S: A
study on vitamin B12 levels in hypothyroid patients presenting to a
tertiary care teaching hospital. J Assoc Physicians India.
71(1)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bhuta P, Shah A and Muley A: A study of
anemia in hypothyroidism with reference to vitamin B12 deficiency.
Int J Adv Med. 6:1667–1671. 2019.
|
|
33
|
Sinha MK, Sinha M and Usmani F: Study of
the correlation between vitamin B12, folic acid and ferritin with
thyroid hormones in hypothyroidism. Int J Health Sci (Qassim).
16:6877–6884. 2022.
|
|
34
|
Kumari SJ, Bantwal G, Devanath A, Aiyyar V
and Patil M: Evaluation of serum vitamin B12 levels and its
correlation with anti-thyroperoxidase antibody in patients with
autoimmune thyroid disorders. Indian J Clin Biochem. 30:217–220.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Aktaş HŞ: Vitamin B12 and vitamin D levels
in patients with autoimmune hypothyroidism and their correlation
with Anti-thyroid peroxidase antibodies. Med Princ Pract.
29:364–370. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kacharava T, Giorgadze E, Janjgava S,
Lomtadze N and Taboridze I: Correlation between vitamin B12
deficiency and autoimmune thyroid diseases. Endocr Metab Immune
Disord Drug Targets. 23:86–94. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun Y, Sun M, Liu B, Du Y, Rong S, Xu G,
Snetselaar LG and Bao W: Inverse association between serum vitamin
B12 concentration and obesity among adults in the United States.
Front Endocrinol (Lausanne). 10(414)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Araghi SO, Braun KVE, van der Velde N, van
Dijk SC, van Schoor NM, Zillikens MC, de Groot LCPGM, Uitterlinden
AG, Stricker BH, Voortman T, et al: B-vitamins and body
composition: Integrating observational and experimental evidence
from the B-PROOF study. Eur J Nutr. 59:1253–1262. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Abu-Shanab A, Zihlif M, Rbeihat MN,
Shkoukani ZW, Khamis A, Isleem U and Dardas LA: Vitamin B12
deficiency among the healthy Jordanian adult population: Diagnostic
levels, symptomology and risk factors. Endocr Metab Immune Disord
Drug Targets. 21:1107–1114. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Singh J, Dinkar A, Gupta P and Atam V:
Vitamin B12 deficiency in northern India tertiary care: Prevalence,
risk factors and clinical characteristics. J Family Med Prim Care.
11:2381–2388. 2022.PubMed/NCBI View Article : Google Scholar
|