Introduction
According to the World Health Organization (WHO),
body mass index (BMI) is the most effective marker for measuring
overweight and obesity in the population; BMI is determined in the
same manner in both sexes and adults of all ages (1). A BMI of 25-29.9 kg/m2 is
classified as overweight, while and a BMI >30 kg/m2
is classified as obese in adults, regardless of sex or age to
predict body fat percentage in the population (2).
Obesity is defined as the abnormal or excessive
accumulation of fat that can affect the health of an individual
(3). It is caused by a disruption
in the homeostatic control of food intake, which results in
increased energy intake in relation to the metabolic demands of the
body and consequently, increased energy intake in relation to
energy expenditure, and hence weight gain (4). Previous epidemiological studies have
demonstrated that body weight is related to a risk of developing
infections and disease (5-7).
However, in children and adolescents, being underweight increases
the chances of developing a variety of infections. It has been
shown that being both obese or underweight is associated with an
increased U-shaped risk of acquiring infections in adults,
suggesting that a normal weight is associated with the lowest risk
of acquiring infections in the majority of participants (7).
In previous research, there are variations in
population and environmental variables, such as diet and lifestyle
conditions (8). A previous study
demonstrated that the underweight population had a 19.7% higher
risk of developing cardiovascular diseases (CVDs) compared with the
normal weight population, whereas the overweight and obese
populations had a 50% and a 96% elevated risk, respectively
(9).
Concentrations of cholesterol and serum
triglycerides (TGs) are closely related to obesity (10). A higher BMI has been conclusively
linked to higher levels of total cholesterol, low-density
lipoprotein cholesterol (LDL) and TGs, and is inversely associated
with high-density lipoprotein cholesterol (HDL), according to
epidemiological and clinical research. It has been suggested that
the association between BMI and lipoprotein levels, particularly
LDL, plays a role in the development of CVDs linked to obesity
(11,12).
Human fat cells are a major source of endogenous
TNF-α production. TNF-α is a cytokine released mainly by
macrophages in response to inflammation, endotoxemia and cancer
(13). Research has consistently
demonstrated that serum TNF-α levels are markedly elevated in
overweight and obese individuals compared to those with a normal
BMI. A positive association exists between the TNF-α concentration
and BMI, waist-to-hip ratio and other anthropometric indicators of
obesity (14,15).
The aim of the present study was to determine
whether any change in the normal value of BMI has an effect on
biochemical and immunological parameters in adult males. This was
analyzed by measuring the complete blood count, lipid profiles,
leptin levels and TNF-α levels. In order to evaluate and improve
therapies for obesity-related diseases, the present study
emphasizes that it is crucial to evaluate these markers and their
association with BMI. The present study also provides key insight
that may aid in the development of more focused and efficient
therapeutic approaches.
Patients and methods
Patient selection
The present study was carried out on 120 Iraqi male
participants of various BMI categories (16 to >43
kg/m2) with an age range of 30-60 years and a median age
of 42 years, who attended the obesity medical center at Al-Kindy
Teaching Hospital and the Nutrition Research Institute (Baghdad,
Iraq) during the period extended between June, 2024 to May, 2025.
These sites were selected as they are major centers for handling
the target samples. The BMI of the volunteers was calculated by
dividing the weight by square height; the participants were then
divided into six groups according to the BMI value. The study
participants were categorized as ‘apparently healthy’ based on a
comprehensive clinical screening process. This included: i) A
detailed medical questionnaire to exclude individuals with
self-reported chronic diseases; ii) the measurement of vital signs
(Blood Pressure); and iii) routine blood tests (including fasting
blood glucose and a lipid profile) to objectively exclude
individuals with subclinical metabolic abnormalities. Only
individuals with all screening results within normal clinical
ranges were included. The exclusion criteria included individuals
with major metabolic diseases, chronic or acute infections, those
who recently underwent major surgery, and the use of medications
that could alter body composition or metabolic profiles. All
volunteers gave their informed consent for the use of their
clinical data for scientific purposes. The Research Ethics
Committee of Mustansiriyah University (Baghdad, Iraq) reviewed and
approved the present study (approval no. BCSMU/162 4/00070Z).
Ethics approval was obtained from the authors' institution as the
governing document. This approval was presented to the
administrations of Al-Kindy Teaching Hospital and the Nutrition
Research Institute. As these are affiliated teaching hospitals with
established inter-institutional collaboration protocols, they
granted administrative site permission for sample collection based
on the university's central ethics clearance.
Blood samples
A total of 5 ml peripheral venous blood was
collected from each of the 120 male participants with varying BMI
categories using a sterile syringe. A total of 2 ml blood samples
were transferred into anticoagulated tubes containing ethylene
diamine tetra acetic acid (EDTA) and used immediately to determine
the complete blood count (CBC) using a Sysmex XP-300 automated
hematology analyzer (Sysmex Corporation). The remaining 3 ml blood
samples were transferred into a Gel Activator tube and left for 15
min, followed by centrifugation at 1,006 x g at 4˚C for 10 min to
separate serum. The serum obtained from each individual was
dispended into several sterilized Eppendorf tubes and stored at
-20˚C until use for the analysis of various parameters using the
Reader automated ELISA system and an automated microplate reader at
450 nm (Human GmbH) according to the manufacturer's instructions.
Each sample was designated by a serial number and the name of the
individual.
Statistical analysis
Data are presented as the mean ± standard error
(SE). All analyses were performed using IBM SPSS Statistics for
Windows, Version 25.0 (IBM Corp). The normality of data
distribution was assessed using the Shapiro-Wilk test. For normally
distributed data, differences between the six BMI groups were
analyzed using one-way analysis of variance (ANOVA), followed by
the Tukey's honestly significant difference (HSD) test post hoc
test for multiple comparisons to identify significant differences
among group means. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
The distribution of the 120 male participants in the
present study according to BMI categorization is demonstrated in
Table I.
 | Table IDistribution of the study
participants according to BMI categories. |
Table I
Distribution of the study
participants according to BMI categories.
| BMI groups | BMI
(kg/m2) | No. of
participants | Range of BMI sample
(kg/m2) |
|---|
| Underweight | <18.5 | 20 | 16-18.2 |
| Normal | 18.5-24.9 | 22 | 18.8-24.5 |
| Overweight | 25-29.9 | 21 | 25.1-29.2 |
| Obese I | 30-34.9 | 19 | 30.2-34.2 |
| Obese II | 35-39.9 | 20 | 35.3-38.5 |
| Obese III | ≥40 | 18 | 40.3-42.9 |
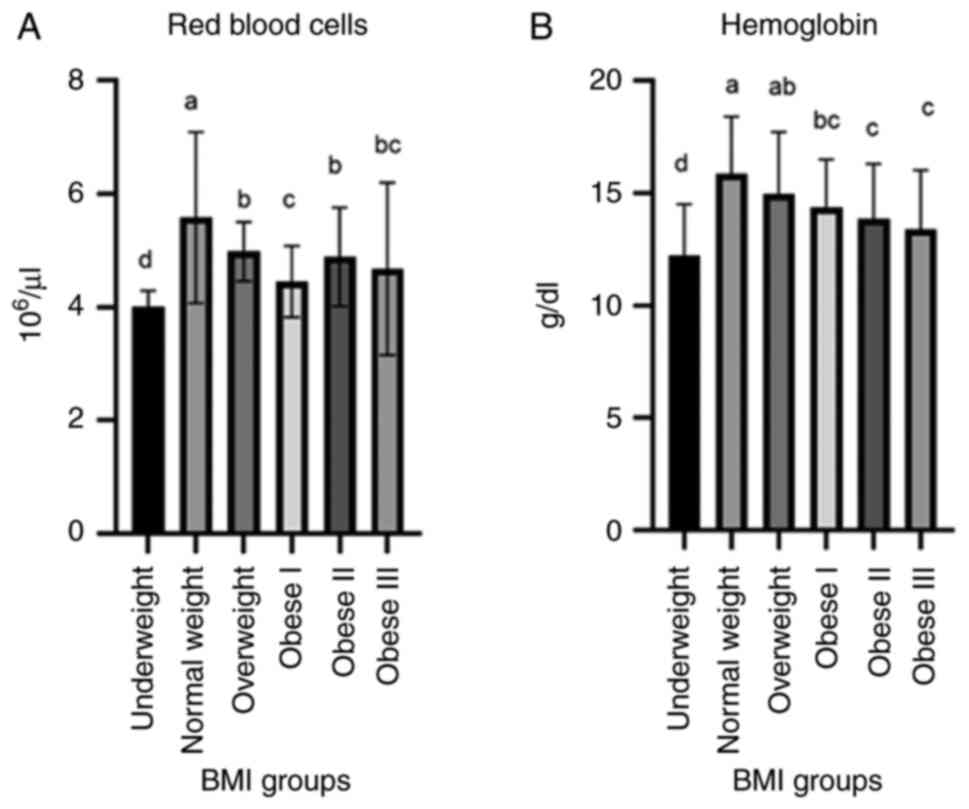
The level of hemoglobin (g/dl) was significantly
increased in the overweight, obese I and obese II groups; however,
the lowest value was observed in the underweight and obese III
group. The values for red blood cells (RBCs, 106/µl)
were decreased in the underweight and obese (class I-III) groups;
these values differed significantly from those of the normal weight
group (Fig. 1).
The values for white blood cells (WBCs,
103/µl) were significantly increased (P<0.003) in the
obese II and obese III groups in comparison to the WBC counts in
the underweight and normal weight groups (Fig. 2A). Lymphocyte counts exhibited
highly significant differences across all study groups (P=0.029).
Lymphocyte counts were significantly decreased in the underweight
group (1516.49±4.2) in comparison with the obese II and obese III
groups (2437.11±4.1 and 3068.58±3.3, respectively). In the normal,
overweight and obese I groups, the lymphocyte counts were
1649.80±2.1, 2017.13±2.1 and 2097.31±3.1, respectively (Fig. 2B). However, for the monocyte and
granulocyte levels, the post hoc analysis indicated no significant
differences between any of the study groups (P>0.05) (Fig. 2C and D). Furthermore, the platelet count was
significantly increased (P<0.05) in the obese II and obese III
groups (274±9.15 and 278±8.12, respectively) in comparison with the
underweight group (198±11.13) (Fig.
2E).
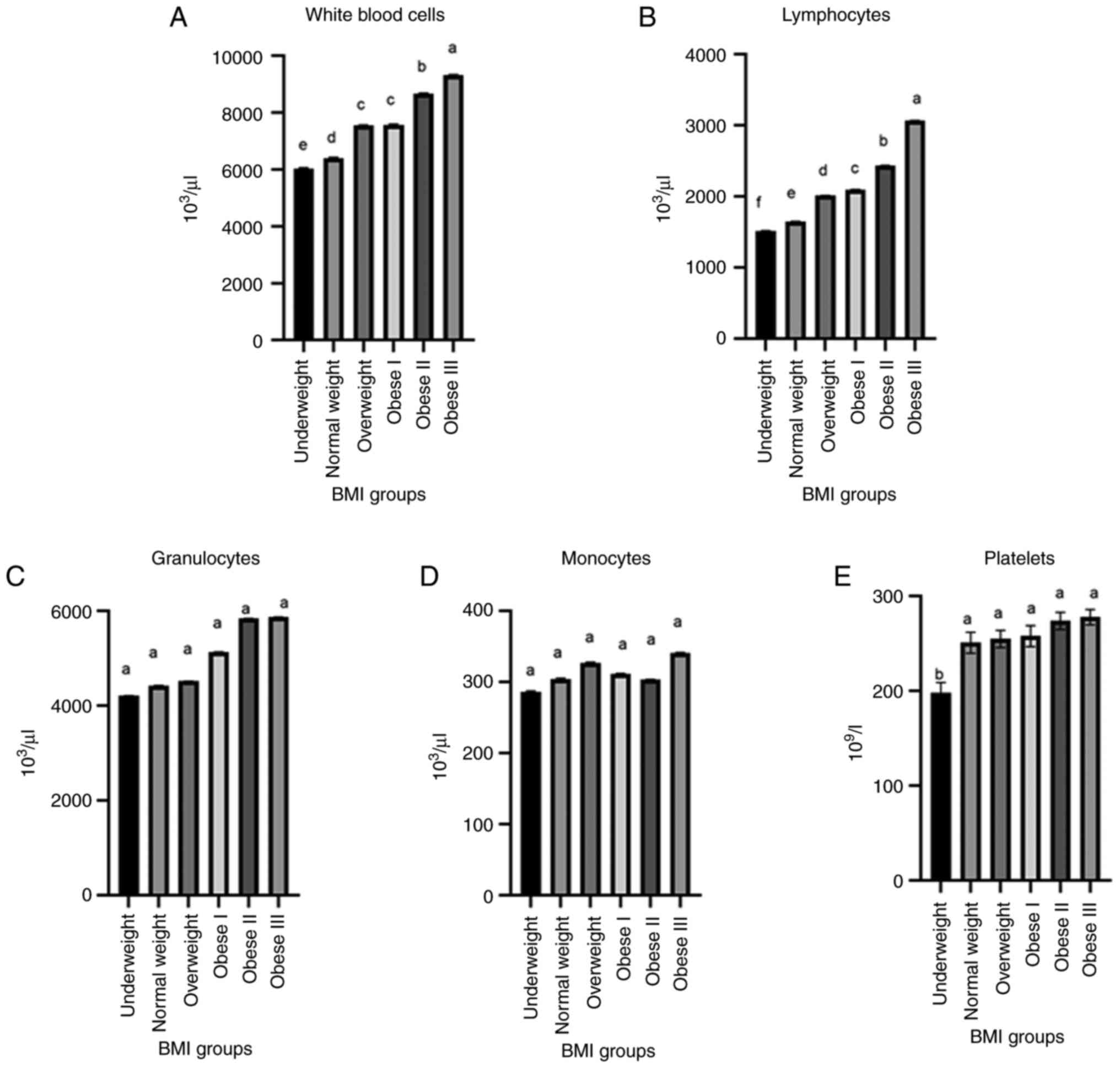 | Figure 2Comparison between (A) white blood
cells, (B) lymphocytes, (C) granulocytes, (D) monocyte, and (E)
platelets according to the BMI categories of the study
participants. In the graphs, bars marked by different lowercase
letters (a, b, c, d, e, f) indicate statistically significant
differences (P<0.05). The different BMI categories are explained
in Table I. BMI, body mass
index. |
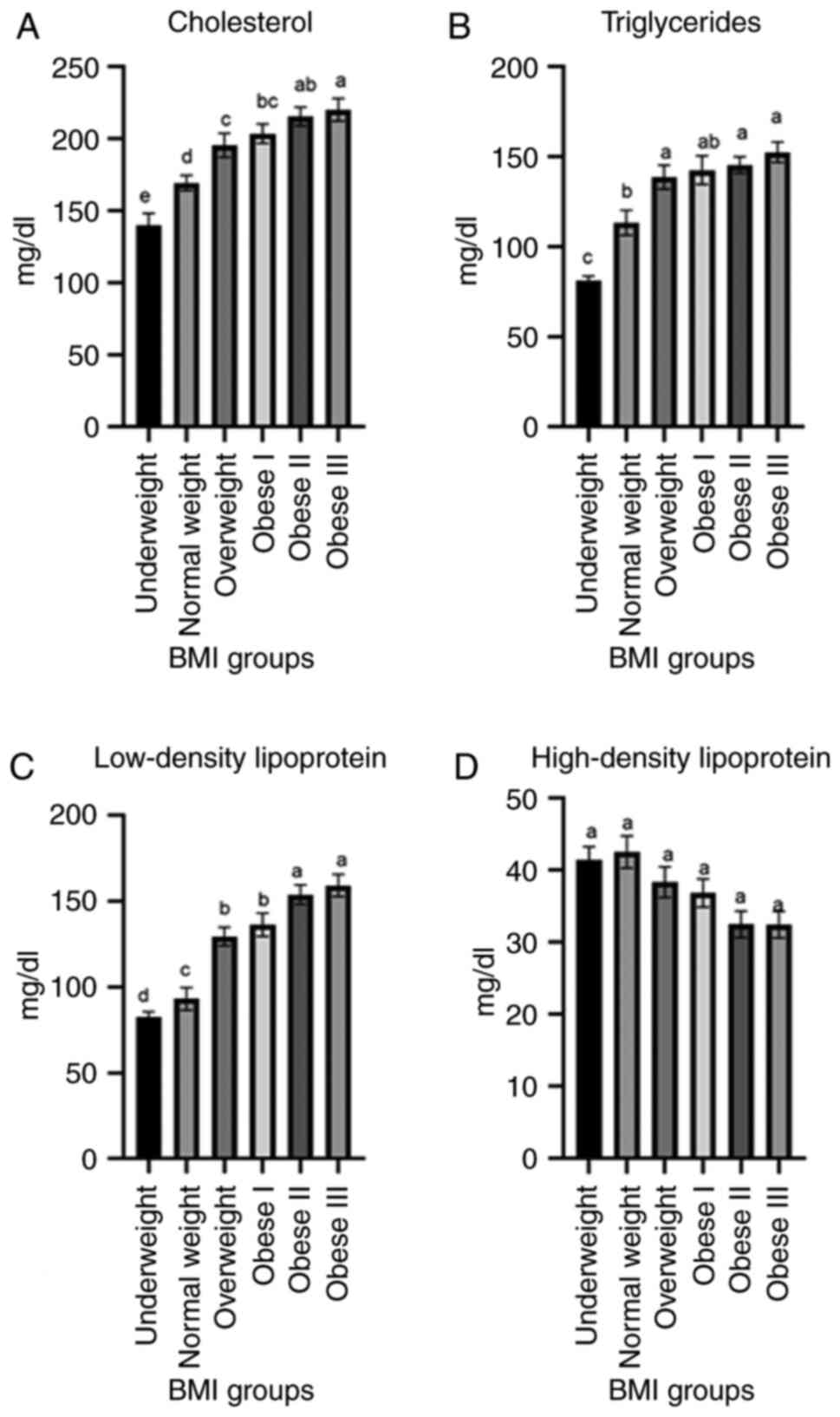
The results of the analyses of lipid profiles are
presented in Fig. 3. The
cholesterol level (mg/dl) was significantly increased (P<0.003)
in the obese (classes I-III) groups in comparison to the
underweight and normal weight groups. The levels of TGs (mg/dl)
exhibited a highly significant (P<0.0031) increase in the obese
III group (152.55±5.65) and obese II group (145.50±4.65) in
comparison to the underweight group (81.20±2.48) and normal group
(113.30±6.87). The LDL levels (mg/dl) also exhibited a significant
(P<0.04) increase in the obese III group (159.24±6.55) and obese
II group (153.90±5.70) in comparison to the underweight group
(82.54±3.24), normal weight group (93.18±6.62) and overweight group
(129.45±5.45). The levels of HDL (mg/dl) were decreased in the
obese III (30.45±1.85), obese II (32.50±1.85) and obese I
(36.85±1.95) groups in comparison to the underweight (41.42±1.85)
and normal weight (53.56±2.23) groups.
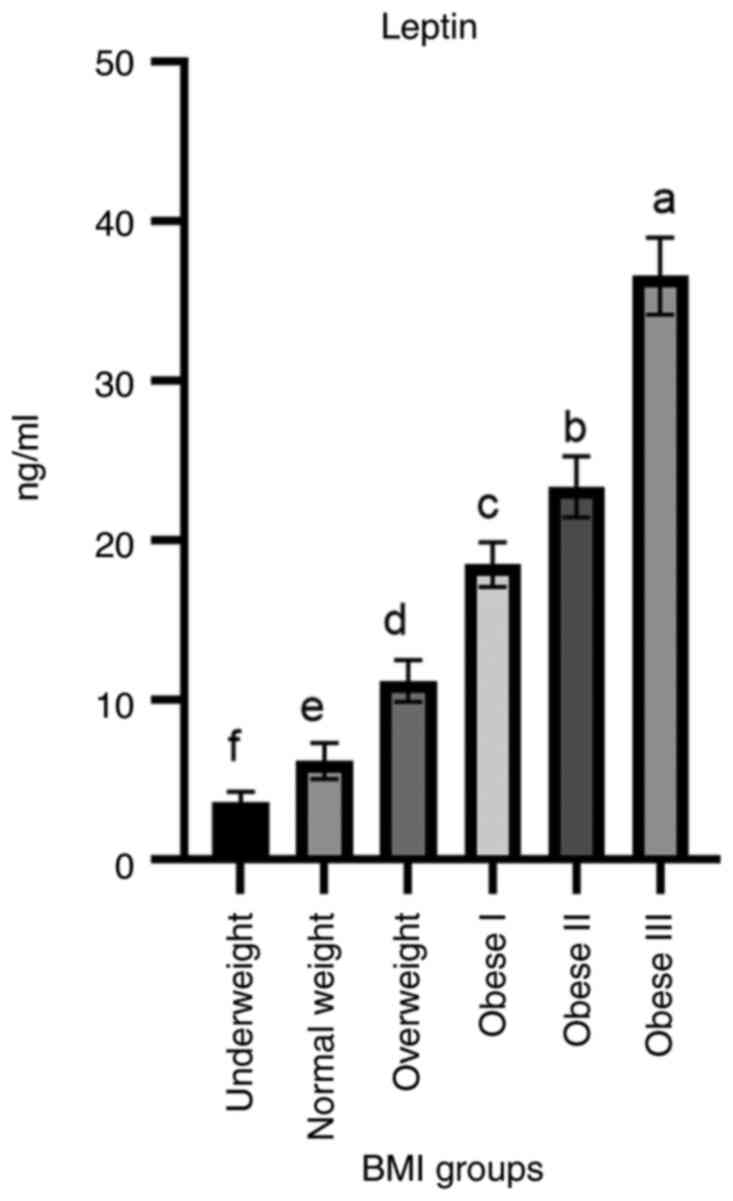
Leptin hormone levels (ng/ml) exhibited a highly
significant (P<0.003) increase in the obese III group
(36.6±2.41) in comparison to the underweight and normal BMI groups
(3.6±0.67, 6.2±1.12 respectively). In addition, the obese II
(23.4±1.92) group exhibited a high level of leptin followed by the
obese I (18.5±1.41) and overweight groups (Fig. 4).
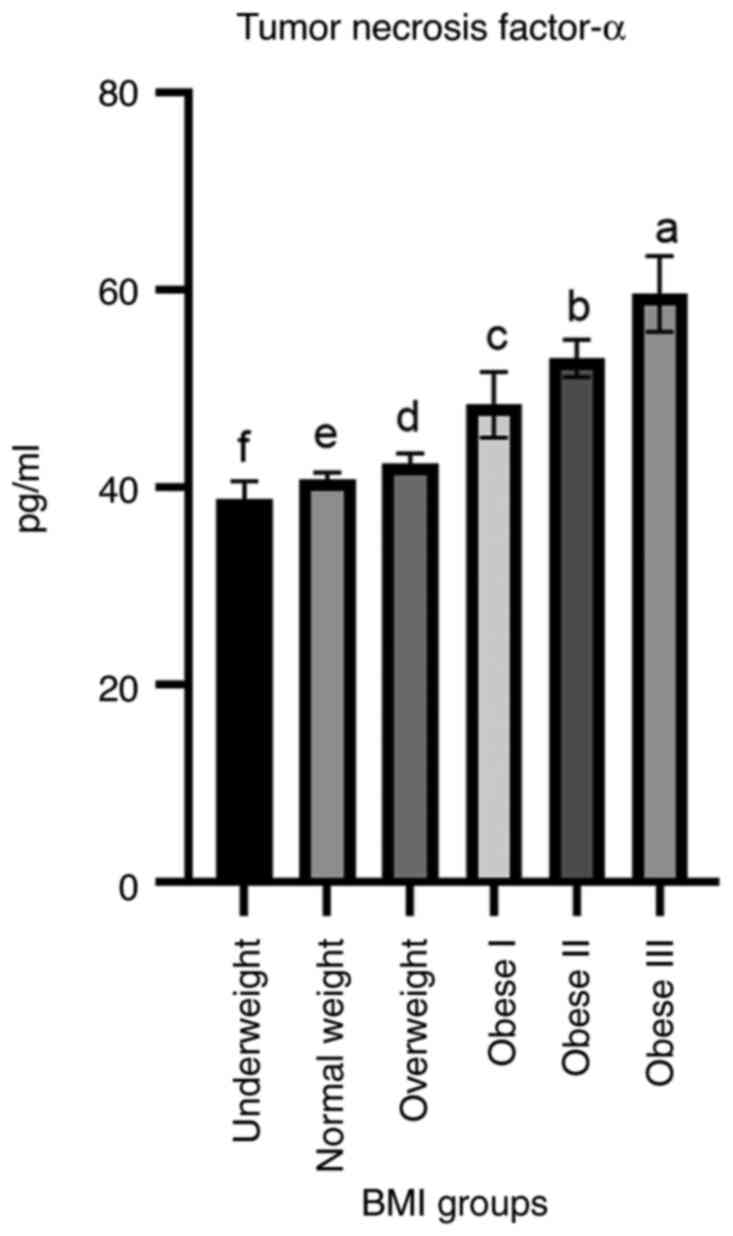
As demonstrated in Fig.
5, the TNF-α levels were significantly increased in the obese
I, obese II and obese III groups compared to other groups of study
participants. The mean level of this cytokine was 48.43±3.34,
53.14±1.91 and 59.69±3.83 pg/ml in the obese I, obese II and obese
III groups, respectively, compared to the other groups.
Discussion
The present study revealed significant associations
between BMI and the examined immunological and biochemical
variables of the study participants, highlighting the importance of
monitoring these markers in high-risk BMI groups to prevent
potential metabolic and immune-related complications. From these
results, it was noted that the underweight group had the lowest
mean hemoglobin concentration; however, this was increased in the
overweight, obese I and obese II groups, with low values also
observed in the obese III group. Hemoglobin is an iron-rich protein
in the blood that imparts a red hue. It constitutes 95% of an RBC,
fulfilling the average adults requirement of 250 oxygen each minute
(16). In the condition of anemia,
there is a lack of hemoglobin, and this deficiency reduces the
number of RBCs carrying oxygen, which prevents the fat burning
process in an obese state (17).
Therefore, an increased in the amount of body fat may be
interpreted as a sign of a decreased hemoglobin level (18). Moreover, compared to individuals
with a normal weight, those who are overweight or obese are at a
higher risk of developing anemia (19). In the present study, the lowest
values of hemoglobin were found in the obese II and obese III
groups (Fig. 1). This result is in
accordance with the findings reported in the study by Alshwaiyat
et al (20), who
demonstrated that an increased in BMI had an adverse effect on the
iron status. The etiology of iron deficiency anemia in obese
individuals may stem from an inadequate iron intake due to an
unbalanced diet. Additionally, other hypothesized factors
contributing to iron deficiency in individuals with an elevated BMI
include diminished iron absorption in the small intestine,
heightened iron demands resulting from an increased blood volume,
and the association of obesity with a chronic low-grade
inflammatory state. Moreover, Alshwaiyat et al (21) further supported the hypothesis that
hemoglobin levels and a high BMI are negatively associated. As
obese individuals may consume a higher amount of fats or
carbohydrates in their diet rather than nutrient-dense foods high
in vitamins and minerals, their hemoglobin levels decrease (i.e.,
more macronutrients and less micronutrients) (22). In addition, the association of
obesity with a chronic low inflammatory condition may therefore be
one of the proposed causes of iron deficiency in the obesity state
through inflammatory-mediated mechanisms (23). Furthermore, Nasif et al
(24) demonstrated that obesity is
an excessive accumulation of energy as adipose tissue, which
adversely affects health.
The decrease in the level of hemoglobin in the
present study observed in both the underweight and obese III groups
may be related to the low level of RBCs, as the RBCs are comprised
of hemoglobin, which is a metal protein that contains heme groups
whose atoms are temporarily bounds to oxygen molecules in the
lungs. A reduction in the hemoglobin content in RBBs results in a
diminished oxygen-carrying ability of the blood, leading to
insufficient cardiac output. Consequently, individuals with anemia
experience dyspnea, palpitations and angina-like symptoms following
intensive activity (25).
Moreover, iron deficiency anemia is prevalent among urban
populations due to inadequate dietary habits and diminished
physical activity. Conversely, nutritional anemia is observed in
medical students who possess superior nutritional understanding and
reside in healthier environments (26).
Furthermore, in present study, it was found that the
levels of WBCs were increased in the obese groups compared to the
normal group (Fig. 2). These
results are in accordance with those reported by Li et al
(27) who found strong evidence of
an association between obesity and WBCs counts. Obesity is a
chronic inflammatory condition, and an elevated WBC count has
widely recognized associations with inflammatory conditions
(27). There is mounting evidence
to indicate that obesity is a chronic inflammatory disease, and
that the inflammatory features of the condition may be connected to
certain comorbidities and risks associated with being overweight
(28). There is also evidence to
indicate that weight loss may cause the WBC count to decrease
(29).
Indeed, a study conducted on an Indian population
indicated an association among body fat mass, leptin and WBC
counts; however, the association between total WBC counts and
leptin levels was more pronounced than that with fat mass (30). Naturally, there is a substantial
association between elevated levels of pro-inflammatory substances
and obesity, particularly central obesity. Numerous bioactive
proteins, or adipokines, including cytokines that encourage
inflammation such as TNF-α, are secreted by adipocytes (31). Furthermore, baseline WBC,
neutrophils and lymphocyte counts increase with an increasing BMI
and decrease with age (32). In
addition, as granulocytes and monocytes may release substances into
the bloodstream, including free radicals and proteolytic enzymes
that can be detrimental to the health of an individual, the
increase in leukocyte counts associated with obesity is clinically
significant (33).
In the present study, lymphocyte counts were
significantly lower in the underweight group (Fig. 2B). Lymphocyte counts are used as
indicators of nutritional status or potential malnutrition
(34). When BMI decreases, the
lymphocyte counts also decrease; as demonstrated in a previous
study among young Japanese women, the mean lymphocyte count was
higher in the obese group and lower in the underweight group than
in the normal weight group (35).
Another study by Rumińska et al (36), demonstrated that compared to
individuals whose body fat was distributed naturally, obese
individuals had a higher levels of inflammatory markers, including
a 17% higher WBC count.
The results of the present study demonstrated that
although the number of platelets was increased in the obese II and
obese III groups, there were no significant differences between the
obese II and obese III groups as regards the number of platelets
compared to the normal, overweight and obese I group. However, the
lowest value of platelets was observed in the -underweight group
(Fig. 2E). A previous study by
Anık et al (37),
demonstrated an association between obesity and higher platelet
counts in patients used as an inflammatory factor. However, the
majority of diseases may be prevented by resolving behavioral risk
factors by national campaigns, such as tobacco use, an unhealthy
diet, obesity, the lack of physical exercise and alcohol intake. In
fact, the continuous exposure of obese individuals to a
pro-inflammatory and pro-thrombotic condition, in which platelets
play a crucial role, has been linked to CVDs on numerous occasions
(38). Nevertheless, the exact
mechanisms via which obesity results in platelet dysfunction are
not yet fully understood (39).
In the present study, an analysis was carried out to
compare lipid profiles, including cholesterol, TGs, HDL and LDL
levels among the participants belonging to the different BMI
categories. The results revealed the existence of significant
variations between the different groups of BMI categories (Fig. 3). The term dyslipidemia refers to
altered lipid profiles. The increase in the cholesterol, TC and LDL
levels in the overweight and obese groups in the present study was
in agreement with the findings of a previous study (40), in which obesity was linked to
numerous detrimental alterations in lipid metabolism, characterized
by elevated serum levels of total cholesterol, LDL and TGs,
alongside a decrease in the serum HDL concentration.
The elevation of TGs in lipid particles alters their
metabolism; TG-rich HDL particles are hydrolyzed more swiftly,
resulting in a decrease in HDL levels (41). Moreover, Turki et al
(42) revealed that individuals
who were obese had lower serum levels of HDL than subjects who were
not, while subjects who were overweight had greater levels of total
cholesterol and LDL. In addition, Shamai et al (43) demonstrated that an elevated BMI
exhibited an inverse correlation with HDL and a direct correlation
with TGs. In the previous study by Kawamoto et al (44), it was suggested that a high BMI
status attenuated the associations of alcohol intake with lower
LDL, cholesterol, a higher HDL cholesterol and a lower LDL/HDL
ratio in Japanese males. Moreover, Raja et al (45) found an association of BMI with
cholesterol and TGs in obese and non-obese subjects; they found a
significant association between cholesterol and TGs with BMI.
Cholesterol and TGs levels were strongly associated with obesity;
therefore, obesity may be considered a risk for the development of
hypertension, CVDs (45). Weight
gain is caused by the consumption of junk food, poor nutrition, a
sedentary lifestyle and the lack of physical activity. Hundreds of
individuals suffer from coronary heart disease, hypertension and
diabetes as a result of elevated lipid and blood sugar levels,
which are caused by obesity. Insulin resistance may be the cause of
the association between BMI, and both HDL and TGs; however, in the
present study, the lack of a significant association between BMI
and LDL is an unexpected finding that warrants for further
research.
The present study demonstrated a higher level of
leptin in the obese II and obese III groups (Fig. 4); this finding was in agreement
with that observed in the study by Obradovic et al (10), who demonstrated that the serum
leptin levels significantly increased with BMI, particularly with a
BMI ≥30 kg/m2, which aligns with obesity categories II
and III. Obese patients have excessive levels of leptin; while
leptin is known as the satiety hormone, and its levels are
supposedly low in obese patients, leptin stimulates the release of
gonadotropin releasing hormone; in obesity, excess levels of leptin
cause a resistance later in on life (46). Moreover, Alaamri et al
(47) examined the serum leptin
levels of young Saudi students and demonstrated a substantial and
independent association between elevated serum leptin levels and
BMI. The higher prevalence of infertility in obese males would
undoubtedly be caused by the large amounts of leptin released by
the adipose tissue and its higher circulating levels (48).
Serum leptin concentrations are significantly
associated with body fat percentage and weight. Obesity is linked
to various lifestyle-related diseases and is frequently regarded as
a contributing factor to male infertility. While adipocytes produce
numerous other adipokines, previous research has indicated that
leptin may serve as a crucial connection between obesity and
obesity-related diseases (49).
The finding of the present study are in agreement with those of the
study by Kazmi et al (50),
who demonstrated a positive association between BMI and leptin
levels in obese subjects. Additionally, the structural and
functional resemblance between leptin and its receptor with
inflammatory cytokines suggests that leptin can be classified as a
cytokine. Despite its significance in the normal immune response,
leptin deficiency heightens vulnerability to infectious and
inflammatory stimuli, potentially resulting in the dysregulation of
cytokine production (51). Leptin
also has immunological effects; its signaling can control innate
inflammatory responses, adaptive immunity and even suppress
regulatory T-cell differentiation (52). In addition to its metabolic and
endocrine functions, leptin controls energy consumption and food
intake by a direct effect on the hypothalamus (53). It may also modulate hematopoiesis,
innate and adaptive immunological responses and inflammation,
particularly in the presence of pro-inflammatory activities
(54). Hyperleptinemia,
characterized by an excess amount of leptin, is frequently
associated with obesity and may greatly contribute to the
development of severe health issues, including CVDs and rheumatoid
arthritis (55).
The present study demonstrated that the serum levels
of TNF-α were significantly increased in parallel with the increase
in the BMI value (Fig. 5).
Previous research has confirmed a positive association between the
serum TNF-α concentration and BMI, particularly in overweight and
obese patients. A previous study on an obese population revealed
that increased BMI values were associated with increased serum
TNF-α concentrations (56).
Another clinical study (14),
demonstrated an association between the amount of fat tissue and
obesity and the increase in the TNF-α concentration in blood. TNF-α
leads to brown adipocyte regression and insulin resistance in
adipocytes, which results in an aberrant energy consumption
(57).
Furthermore, in a large comparative study on obese
adults, the serum TNF-α levels were found to be considerably higher
in overweight and obese individuals, and strongly correlated with
BMI (r=0.65, P<0.001), alongside other metabolic parameters such
as glucose and lipids (14). In
addition, the plasma concentrations of TNF-α have been positively
associated with elevated TG levels, which may be explained by its
ability to induce the overproduction of very-low-density
lipoprotein particles (58). The
mechanistic basis lies in TNF-α being a proinflammatory cytokine
secreted predominantly by macrophages in adipose tissue. It
contributes to metabolic disturbances by promoting insulin
resistance and altering lipid metabolism, which is reflected by
high serum TNF-α levels in parallel with an increased BMI (59). Nonetheless, it is undeniable that
optimal immune functions are inextricably related to nutritional
status; this may be as a dietary fatty acid can affect cytokine
formation (60). This has given
rise to the alternative, but not exclusive notion that, at least in
diet-induced obesity, dietary components, particularly fats, may be
crucial in controlling the inflammatory profile of adipose tissue
(61).
In conclusion, the present study demonstrates that
deviations from normal BMI significantly affect hematological,
metabolic and inflammatory pathways in men, underscoring the
critical role of BMI assessment in developing targeted therapeutic
strategies for obesity-related comorbidities. The present study
performed a power analysis test which confirmed that the sample
size (n=120/group) provided adequate statistical power (99.9%) to
detect clinically meaningful effects for the primary endpoints,
based on an effect size of 0.5 and α=0.05. It is important to
emphasize that the present study wa exploratory in nature and did
not intend to establish a diagnostic tool. The findings presented
herein, should be regarded as a preliminary foundation for future,
more comprehensive research. However, the present study has several
limitations that should be mentioned. Firstly, as the present study
was cross-sectional, it was impossible to establish a causal
association between the variables that were observed. Second, the
results are limited in their capacity to be applied to women as
they are based solely on a cohort of adult men. Additionally, even
when key variables were taken into account, the results may still
have been affected by confounding variables that were not taken
into account, such as dietary habits, specific amounts of physical
activity and genetic predispositions. Despite these limitations,
however, the present study provides new information about the
association between adult male obesity, and indicators of immune
function and systemic physiology. In order to validate these
associations and clarify their underlying causative mechanisms,
further longitudinal studies that involve a variety of populations
and include more accurate assessments of adiposity and potential
confounders are warranted.
Acknowledgements
The authors would like to thank Dr Ali A. Mahdi, at
the College of Health and Medical Technology, Middle Technical
University in Baghdad, Iraq, for providing academic assistance and
guidance.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TJT and MMB contributed to the conception and design
of the study, and wrote and edited the manuscript. HQM and YFF
collected and analyzed data. MQM reviewed and edited the
manuscript, and was also involved in data curation, and supervised
the study All authors have read and approved the final manuscript.
TJT and MMB confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Research Ethics Committee of Mustansiriyah University (approval no.
BCSMU/162 4/00070Z), and subsequently authorized by Obesity Medical
Center at al-Kindy Teaching Hospital, and Nutrition Research
Institute administration per standard institutional collaboration
procedures. These sites were selected as they are major centers for
handling the target samples. All patients who participated in the
present study provided written informed consent for the publication
of their data. The consent form emphasized that participation was
entirely voluntary, and the participants could withdraw at any time
without facing any repercussions. Anonymity and confidentiality
were safeguarded by assigning coded identifiers rather than names
or medical record numbers. In adherence to international ethical
guidelines, including the Declaration of Helsinki, the study
maintained strict ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed SK and Mohammed RA: Obesity:
Prevalence, causes, consequences, management, preventive strategies
and future research directions. Metab Open.
27(100375)2025.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khanna D, Peltzer C, Kahar P and Parmar
MS: Body mass index (BMI): A screening tool analysis. Cureus.
14(e22119)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin X and Li H: Obesity: Epidemiology,
Pathophysiology, and Therapeutics. Front Endocrinol (Lausanne).
12(706978)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mohajan D and Mohajan HK: Body mass index
(BMI) is a popular anthropometric tool to measure obesity among
adults. J Innov Med Res. 2:25–33. 2023.
|
|
5
|
Yang WS, Chang YC, Chang CH, Wu LC, Wang
JL and Lin HH: The association between body mass index and the risk
of hospitalization and Mortality due to infection: A prospective
cohort study. Open Forum Infect Dis. 8(ofaa545)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ghilotti F, Bellocco R, Ye W, Adami HO and
Trolle Lagerros Y: Obesity and risk of infections: results from men
and women in the Swedish National March Cohort. Int J Epidemiol.
48:1783–1794. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pugliese G, Liccardi A, Graziadio C,
Barrea L, Muscogiuri G and Colao A: Obesity and infectious
diseases: Pathophysiology and epidemiology of a double pandemic
condition. Int J Obes. 46:449–465. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Carey IM, Harris T, Chaudhry UAR, DeWilde
S, Limb ES, Bowen L, Audi S, Cook DG, Whincup PH, Sattar N, et al:
Body mass index and infection risks in people with and without type
2 diabetes: A cohort study using electronic health records:
Epidemiology and Population Health. Int J Obes (Lond).
49:1800–1809. 2025.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pozorska K, Baranowska-Bosiacka I, Raducha
D, Kupnicka P, Bosiacki M, Bosiacka B, Szmit-Domagalska J,
Ratajczak J, Horodnicka-Józwa A, Walczak M, et al: The assessment
of anthropometric measures and changes in selected biochemical
parameters in obese children in relation to blood lead level.
Metabolites. 14(540)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Obradovic M, Sudar-Milovanovic E, Soskic
S, Essack M, Arya S, Stewart AJ, Gojobori T and Isenovic ER: Leptin
and obesity: Role and clinical implication. Front Endocrinol
(Lausanne). 12(585887)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu H, Han Y, Cao C and He Y: The
triglyceride glucose-body mass index: A non-invasive index that
identifies non-alcoholic fatty liver disease in the general
Japanese population. J Transl Med. 20(398)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Veghari G, Sedaghat M, Maghsodlo S,
Banihashem S, Moharloei P, Angizeh A, Tazik E, Moghaddami A and
Joshaghani H: The association between abdominal obesity and serum
cholesterol level. Int J Appl basic Med Res. 5:83–86.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aladhami AK, Unger CA, Ennis SL, Altomare
D, Ji H, Hope MC III, Velázquez KT and Enos RT: Macrophage tumor
necrosis factor-alpha deletion does not protect against
obesity-associated metabolic dysfunction. FASEB J.
35(e21665)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eswar S, Rajagopalan B, Ete K and
Nageswara Rao Gattem S: Serum tumor necrosis factor alpha (TNF-α)
levels in obese and overweight adults: Correlations with metabolic
syndrome and inflammatory markers. Cureus.
16(e64619)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ameen EM and Mohammed HY: Correlation
between tumor necrosis factor-alfa and anti-tyrosine phosphatase
with obesity and diabetes type 2. Iraqi J Sci. 3322–3331. 2022.
|
|
16
|
Al-Shura AN: Advanced Hematology in
Integrated Cardiovascular Chinese Medicine: Volume 3. Academic
Press, 2019.
|
|
17
|
Salman MS, Shwaish H, Jassim MN and Hasan
W: The relationship between hemoglobin and BMI. Acta Sci Microbiol.
3:20–22. 2020.
|
|
18
|
Acharya S, Patnaik M, Mishra SP and
Panigrahi AK: Correlation of hemoglobin versus body mass index and
body fat in young adult female medical students. Natl J Physiol
Pharm Pharmacol. 8(1371)2018.
|
|
19
|
Hamed M, Zaghloul A, Halawani SH, Fatani
BA, Alshareef B, Almalki A, Alsharif E, ALhothaly QA, Alhadhrami S
and Abd Elmoneim HM: Prevalence of Overweight/obesity associated
with anemia among female medical students at Umm Al-Qura University
in Makkah, Saudi Arabia: A Cross-Sectional Study. Cureus.
16(e57081)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alshwaiyat NM, Ahmad A, Wan Hassan WMR and
Al-Jamal HAN: Association between obesity and iron deficiency. Exp
Ther Med. 22(1268)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alshwaiyat NM, Ahmad A and Al-Jamal HAN:
Effect of diet-induced weight loss on iron status and its markers
among young women with overweight/obesity and iron deficiency
anemia: A randomized controlled trial. Front Nutr.
10(1155947)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahire ED, Pathan AS, Pandagale PM,
Khairnar SJ, Surana KR, Kshirsagar SJ, et al: Role of Dietary in
the Prevention and Management of Obesity. In: The Metabolic
Syndrome. Apple Academic, pp139-161, 2023.
|
|
23
|
Clark P: Iron deficiency related to
obesity. J Infus Nurs. 47:163–174. 2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nasif ZN, Eltayef EM, Al-Janabi NM and
Niseaf AN: Relationship of obesity to serum ferritin, lipid
profile, uric acid and urea at obesity medical center in Iraq.
Al-Mustansiriyah J Sci. (29)2018.
|
|
25
|
Turner J, Parsi M and Badireddy M: Anemia.
[Updated 2023 Aug 8]. StatPearls [Internet] Treasure Isl StatPearls
Publ, 2024.
|
|
26
|
Vibhute NA, Shah U, Belgaumi U, Kadashetti
V, Bommanavar S and Kamate W: Prevalence and awareness of
nutritional anemia among female medical students in Karad,
Maharashtra, India: A cross-sectional study. J Fam Med Prim care.
8:2369–2372. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Z, Yao Z and Liu Q: The association
between white blood cell counts and metabolic health obesity among
US adults. Front Nutr. 12(1458764)2025.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ellulu MS, Patimah I, Khaza'ai H, Rahmat A
and Abed Y: Obesity and inflammation: The linking mechanism and the
complications. Arch Med Sci. 13:851–863. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Purdy JC and Shatzel JJ: The hematologic
consequences of obesity. Eur J Haematol. 106:306–319.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wilson CA, Bekele G, Nicolson M, Ravussin
E and Pratley RE: Relationship of the white blood cell count to
body fat: Role of leptin. Br J Haematol. 99:447–451.
1997.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wen X, Zhang B, Wu B, Xiao H, Li Z, Li R,
Xu X and Li T: Signaling pathways in obesity: Mechanisms and
therapeutic interventions. Signal Transduct Target Ther.
7(298)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hsieh CY, Lee WH, Liu YH, Lu CC, Chen SC
and Su HM: Significant impact of body mass index on the
relationship between increased white blood cell count and new-onset
diabetes. Int J Med Sci. 20(359)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Caiati C, Stanca A and Lepera ME: Free
radicals and Obesity-related chronic inflammation contrasted by
antioxidants: A new perspective in coronary artery disease.
Metabolites. 13(712)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Takele Y, Adem E, Getahun M, Tajebe F,
Kiflie A, Hailu A, Raynes J, Mengesha B, Ayele TA, Shkedy Z, et al:
Malnutrition in healthy individuals results in increased mixed
cytokine profiles, altered neutrophil subsets and function. PLoS
One. 11(e0157919)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Alrubaie A, Majid S, Alrubaie R and Kadhim
FAZB: Effects of body mass index (BMI) on complete blood count
parameters. Inflammation. (8)2019.
|
|
36
|
Rumińska M, Witkowska-Sędek E,
Artemniak-Wojtowicz D, Krajewska M, Majcher A, Sobol M and Pyrżak
B: Changes in leukocyte profile and C-reactive protein
concentration in overweight and obese adolescents after reduction
of body weight. Cent Eur J Immunol. 44:307–315. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Anık A, Çelik E and Anık A: The relation
of complete blood count parameters with metabolic and clinical
parameters in overweight and obese children. J Pediatr Res.
8:161–170. 2021.
|
|
38
|
Jamshidi L and Seif A: Association between
obesity, white blood cell and platelet count. Zahedan J Res Med
Sci. 19(e4955)2017.
|
|
39
|
Vilahur G, Ben-Aicha S and Badimon L: New
insights into the role of adipose tissue in thrombosis. Cardiovasc
Res. 113:1046–1054. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hussain A, Ali I, Kaleem WA and Yasmeen F:
Correlation between body mass index and lipid profile in patients
with type 2 Diabetes attending a tertiary care hospital in
Peshawar. Pakistan J Med Sci. 35:591–597. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Akcan T and Kraemer FB: HDL meets
triglyceride. J Lipid Res. 66(100796)2025.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Turki KM, Hadi Alosami M and Hasan
Abdul-Qahar Z: The effect of obesity on serum leptin and lipid
profile. Iraqi Postgrad Med J. 1:27–32. 2009.
|
|
43
|
Shamai L, Lurix E, Shen M, Novaro GM,
Szomstein S, Rosenthal R, Hernandez AV and Asher CR: Association of
body mass index and lipid profiles: Evaluation of a broad spectrum
of body mass index patients including the morbidly obese. Obes
Surg. 21:42–47. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kawamoto R, Tabara Y, Kohara K, Miki T,
Kusunoki T, Takayama S, Abe M, Katoh T and Ohtsuka N: Low-density
lipoprotein cholesterol to High-density lipoprotein cholesterol
ratio is the best surrogate marker for insulin resistance in
non-obese Japanese adults. Lipids Health Dis. 9(138)2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Raja AWK, Liaquat HK, Abdul RK, Kamran A
and Khawaja AY: An Association of Blood Sugar. Cholest
Triglycerides with Obes Hum Subj from Muzaffarabad Azad Kashmir,
Pakistan. Curr Res Diabetes Obesity J. 13(555861)2020.
|
|
46
|
Cabler S, Agarwal A, Flint M and Du
Plessis SS: Obesity: Modern man's fertility nemesis. Asian J
Androl. 12(480)2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Alaamri S, Serafi AS, Hussain Z, Alrooqi
MM, Bafail MA and Sohail S: Blood pressure correlates with serum
leptin and body mass index in overweight male Saudi students. J
Pers Med. 13(828)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Almabhouh FA, Md Mokhtar AH, Malik IA,
Aziz NAAA, Durairajanayagam D and Singh HJ: Leptin and reproductive
dysfunction in obese men. Andrologia. 52(e13433)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Malik IA, Durairajanayagam D and Singh HJ:
Leptin and its actions on reproduction in males. Asian J Androl.
21:296–299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kazmi A, Sattar A, Hashim R, Khan SP,
Younus M and Khan FA: Serum leptin values in the healthy obese and
non-obese subjects of Rawalpindi. J Pak Med Assoc. 63:245–248.
2013.PubMed/NCBI
|
|
51
|
Bernotiene E, Palmer G and Gabay C: The
role of leptin in innate and adaptive immune responses. Arthritis
Res Ther. 8(217)2006.PubMed/NCBI View
Article : Google Scholar
|
|
52
|
Naylor C and Petri WA: Leptin regulation
of immune responses. Trends Mol Med. 22:88–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Erusan RR, Nalini D, Manohar G and Malathi
R: Correlation between obesity and inflammation in cardiovascular
diseases-evaluation of leptin and inflammatory cytokines. Open J
Endocr Metab Dis. 2:7–15. 2012.
|
|
54
|
Paz-Filho G, Mastronardi C, Franco CB,
Wang KB, Wong ML and Licinio J: Leptin: Molecular mechanisms,
systemic pro-inflammatory effects, and clinical implications. Arq
Bras Endocrinol Metabol. 56:597–607. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Docanto MM, Ham S, Corbould A and Brown
KA: Obesity-associated inflammatory cytokines and prostaglandin E2
stimulate glucose transporter mRNA expression and glucose uptake in
primary human adipose stromal cells. J Interf Cytokine Res.
35:600–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ateia YA, Saleh EM, Abdullah TN and Al
Musawee Z: Levels of some proinflammatory cytokines in obese women
with polycystic ovary syndrome after metformin therapy. Al-Kindy
Coll Med J. 9:45–48. 2013.
|
|
57
|
Enayati S, Seifirad S, Amiri P, Abolhalaj
M and Mohammad-Amoli M: Interleukin-1 beta, interferon-gamma, and
tumor necrosis factor-alpha gene expression in peripheral blood
mononuclear cells of patients with coronary artery disease. ARYA
Atheroscler. 11(267)2015.PubMed/NCBI
|
|
58
|
Lim J, Yang EJ and Chang JH: Upregulation
of TNF-α by triglycerides is mediated by MEK1 activation in jurkat
T cells. Biomed Sci Lett. 24:213–220. 2018.
|
|
59
|
Ullah MI, Alzahrani B, Alsrhani A, Atif M,
Alameen AAM and Ejaz H: Determination of serum tumor necrosis
factor-alpha (TNF-α) levels in metabolic syndrome patients from
Saudi population. Pakistan J Med Sci. 37(700)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mallick R, Basak S, Das RK, Banerjee A,
Paul S, Pathak S and Duttaroy AK: Fatty acids and their proteins in
adipose tissue inflammation. Cell Biochem Biophys. 82:35–51.
2024.PubMed/NCBI View Article : Google Scholar
|