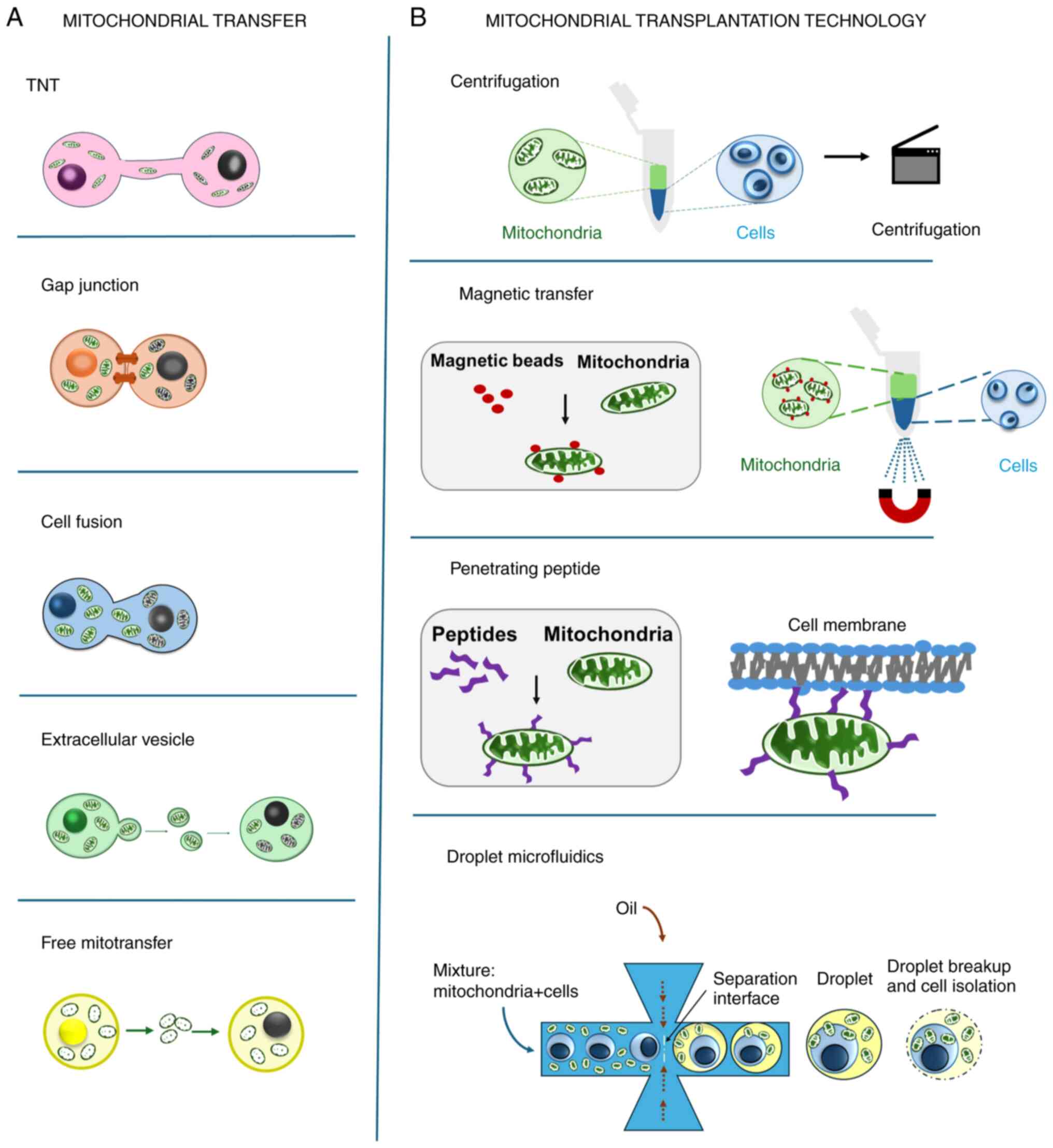
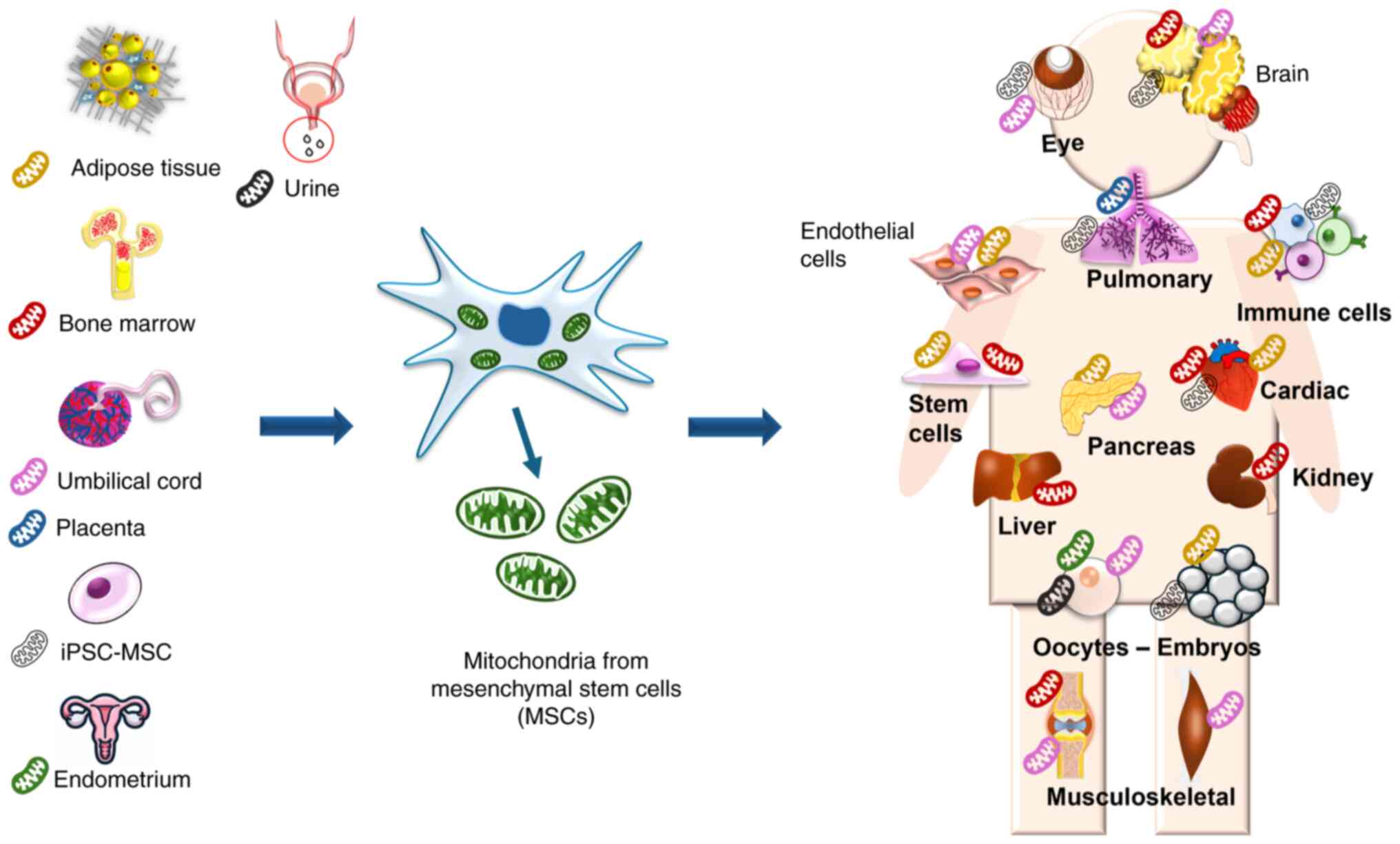
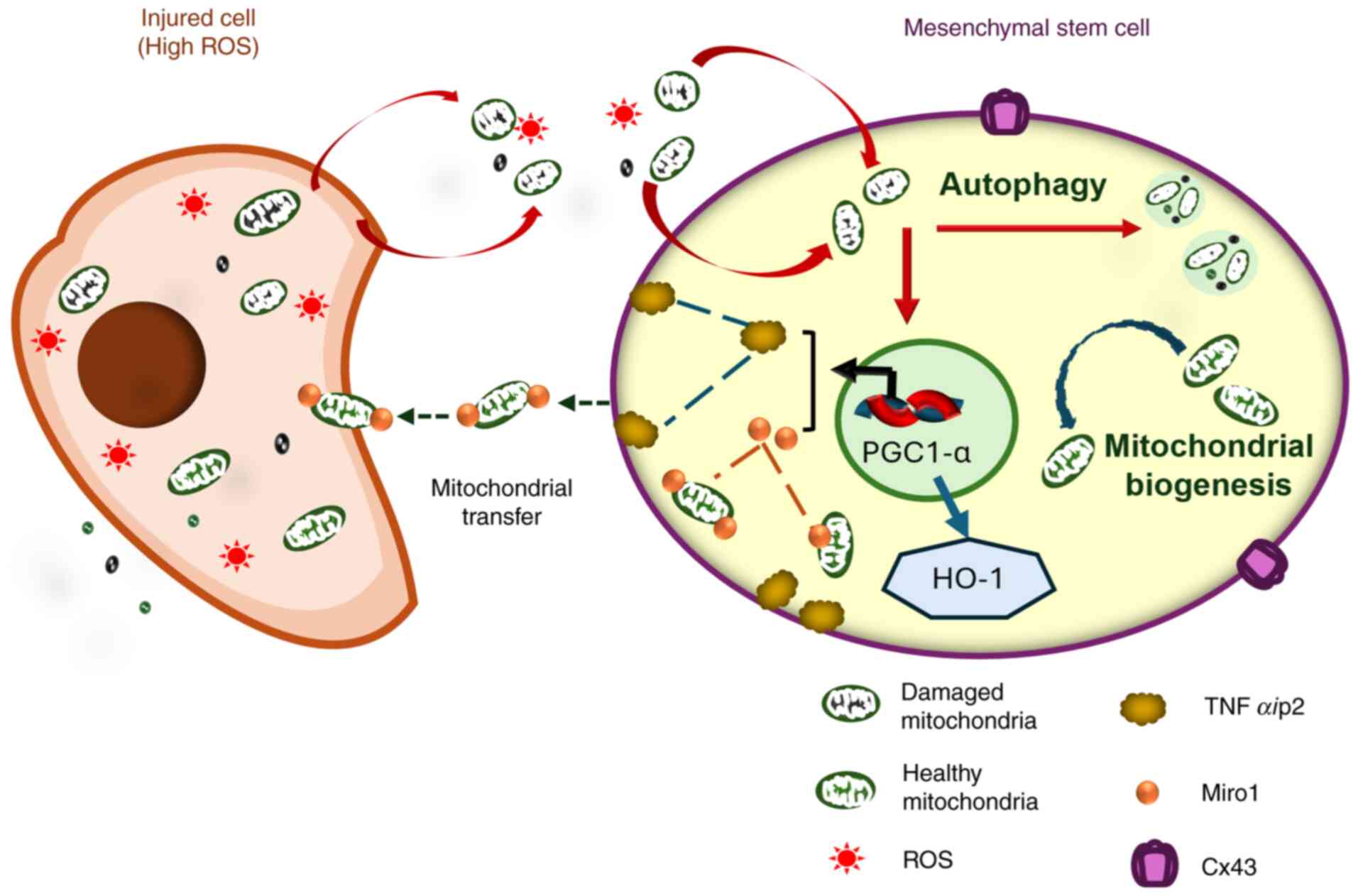
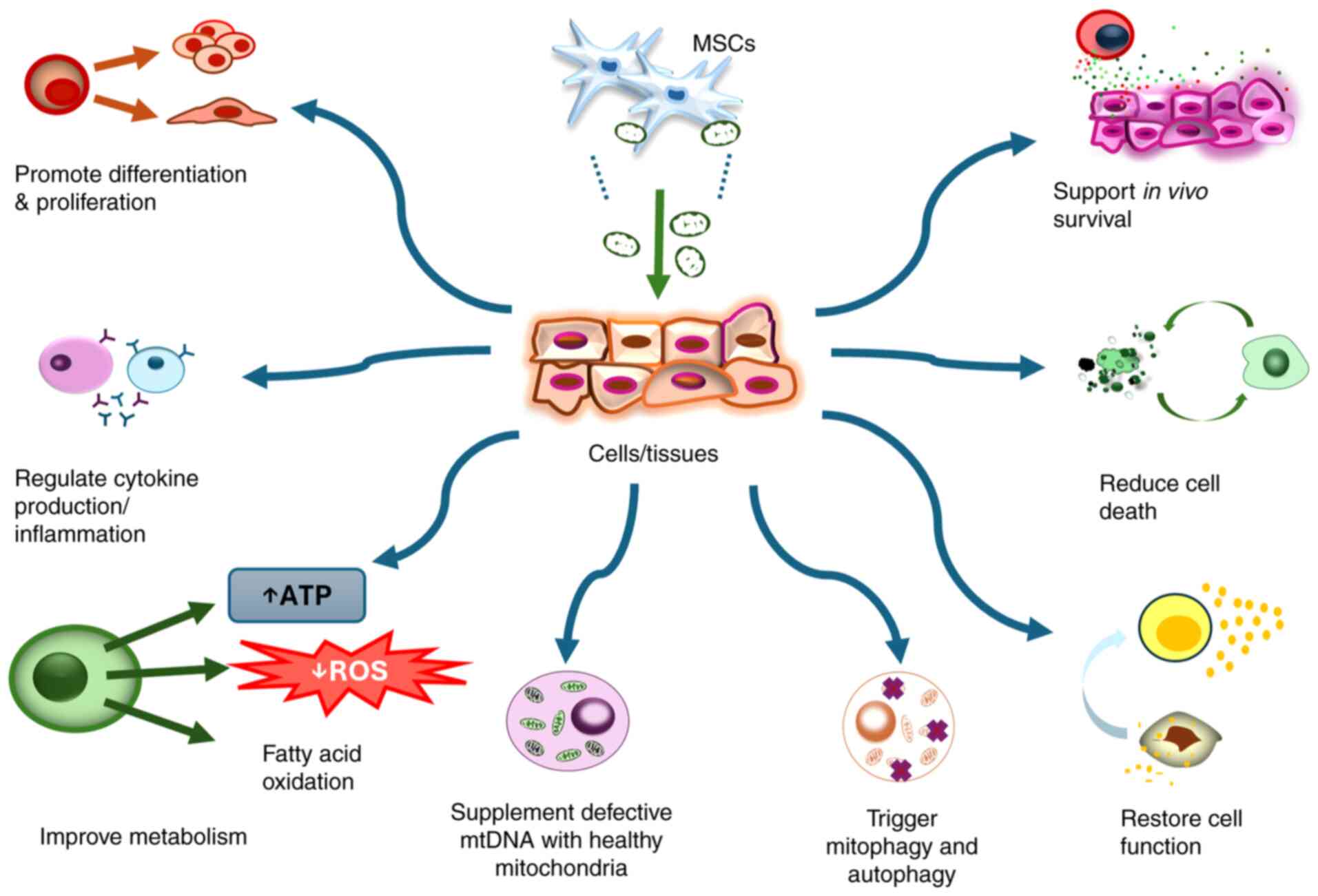
|
1
|
Amorim JA, Coppotelli G, Rolo AP, Palmeira
CM, Ross JM and Sinclair DA: Mitochondrial and metabolic
dysfunction in ageing and age-related diseases. Nat Rev Endocrinol.
18:243–258. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hyatt H, Deminice R, Yoshihara T and
Powers SK: Mitochondrial dysfunction induces muscle atrophy during
prolonged inactivity: A review of the causes and effects. Arch
Biochem Biophys. 662:49–60. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dagda RK: Role of mitochondrial
dysfunction in degenerative brain diseases, an overview. Brain Sci.
8(178)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kaarniranta K, Uusitalo H, Blasiak J,
Felszeghy S, Kannan R, Kauppinen A, Salminen A, Sinha D and
Ferrington D: Mechanisms of mitochondrial dysfunction and their
impact on age-related macular degeneration. Prog Retin Eye Res.
79(100858)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zilio E, Piano V and Wirth B:
Mitochondrial dysfunction in spinal muscular atrophy. Int J Mol
Sci. 23(10878)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Weiss SL, Zhang D, Bush J, Graham K, Starr
J, Murray J, Tuluc F, Henrickson S, Deutschman CS, Becker L, et al:
Mitochondrial dysfunction is associated with an immune paralysis
phenotype in pediatric sepsis. Shock. 54:285–293. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
McBride MA, Owen AM, Stothers CL,
Hernandez A, Luan L, Burelbach KR, Patil TK, Bohannon JK, Sherwood
ER and Patil NK: The metabolic basis of immune dysfunction
following sepsis and trauma. Front Immunol. 11(1043)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Prasun P: Mitochondrial dysfunction in
metabolic syndrome. Biochim Biophys Acta Mol Basis Dis.
1866(165838)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bhatti JS, Bhatti GK and Reddy PH:
Mitochondrial dysfunction and oxidative stress in metabolic
disorders-a step towards mitochondria based therapeutic strategies.
Biochim Biophys Acta Mol Basis Dis. 1863:1066–1077. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Montgomery MK: Mitochondrial dysfunction
and diabetes: Is mitochondrial transfer a friend or foe? Biology
(Basel). 8(33)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Feichtinger RG, Sperl W, Bauer JW and
Kofler B: Mitochondrial dysfunction: A neglected component of skin
diseases. Exp Dermatol. 23:607–614. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mizumura K, Cloonan SM, Nakahira K,
Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko
GR, et al: Mitophagy-dependent necroptosis contributes to the
pathogenesis of COPD. J Clin Invest. 124:3987–4003. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li X, Zhang W, Cao Q, Wang Z, Zhao M, Xu L
and Zhuang Q: Mitochondrial dysfunction in fibrotic diseases. Cell
Death Discov. 6(80)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ryter SW, Rosas IO, Owen CA, Martinez FJ,
Choi ME, Lee CG, Elias JA and Choi AMK: Mitochondrial dysfunction
as a pathogenic mediator of chronic obstructive pulmonary disease
and idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 15 (Suppl
4):S266–S272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kandasamy J, Olave N, Ballinger SW and
Ambalavanan N: Vascular endothelial mitochondrial function predicts
death or pulmonary outcomes in preterm infants. Am J Respir Crit
Care Med. 196:1040–1049. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rizzuto R, De Stefani D, Raffaello A and
Mammucari C: Mitochondria as sensors and regulators of calcium
signalling. Nat Rev Mol Cell Biol. 13:566–578. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matuz-Mares D, González-Andrade M,
Araiza-Villanueva MG, Vilchis-Landeros MM and Vázquez-Meza H:
Mitochondrial calcium: Effects of its imbalance in disease.
Antioxidants (Basel). 11(801)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Belosludtsev KN, Talanov EY, Starinets VS,
Agafonov AV, Dubinin MV and Belosludtseva NV: Transport of
Ca2+ and Ca2+-dependent permeability
transition in rat liver mitochondria under the
streptozotocin-induced type I diabetes. Cells.
8(1014)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sundaramoorthy P, Sim JJ, Jang YS, Mishra
SK, Jeong KY, Mander P, Chul OB, Shim WS, Oh SH, Nam KY and Kim HM:
Modulation of intracellular calcium levels by calcium lactate
affects colon cancer cell motility through calcium-dependent
calpain. PLoS One. 10(e0116984)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Spees JL, Olson SD, Whitney MJ and Prockop
DJ: Mitochondrial transfer between cells can rescue aerobic
respiration. Proc Natl Acad Sci USA. 103:1283–1288. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rodriguez AM, Nakhle J, Griessinger E and
Vignais ML: Intercellular mitochondria trafficking highlighting the
dual role of mesenchymal stem cells as both sensors and rescuers of
tissue injury. Cell Cycle. 17:712–721. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Plotnikov EY, Babenko VA, Silachev DN,
Zorova LD, Khryapenkova TG, Savchenko ES, Pevzner IB and Zorov DB:
Intercellular transfer of mitochondria. Biochemistry (Mosc).
80:542–548. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hayashida K, Takegawa R, Shoaib M, Aoki T,
Choudhary RC, Kuschner CE, Nishikimi M, Miyara SJ, Rolston DM,
Guevara S, et al: Mitochondrial transplantation therapy for
ischemia reperfusion injury: A systematic review of animal and
human studies. J Transl Med. 19(214)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang C, Yokomori R, Chua LH, Tan SH, Tan
DQ, Miharada K, Sanda T and Suda T: Mitochondria transfer mediates
stress erythropoiesis by altering the bioenergetic profiles of
early erythroblasts through CD47. J Exp Med.
219(e20220685)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu D, Gao Y, Liu J, Huang Y, Yin J, Feng
Y, Shi L, Meloni BP, Zhang C, Zheng M and Gao J: Intercellular
mitochondrial transfer as a means of tissue revitalization. Signal
Transduct Target Ther. 6(65)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Emani SM, Piekarski BL, Harrild D, Del
Nido PJ and McCully JD: Autologous mitochondrial transplantation
for dysfunction after ischemia-reperfusion injury. J Thorac
Cardiovasc Surg. 154:286–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guariento A, Piekarski BL, Doulamis IP,
Blitzer D, Ferraro AM, Harrild DM, Zurakowski D, Del Nido PJ,
McCully JD and Emani SM: Autologous mitochondrial transplantation
for cardiogenic shock in pediatric patients following
ischemia-reperfusion injury. J Thorac Cardiovasc Surg.
162:992–1001. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Michaeloudes C, Li X, Mak JCW and Bhavsar
PK: Study of mesenchymal stem cell-mediated mitochondrial transfer
in in vitro models of oxidant-mediated airway epithelial and smooth
muscle cell injury. Methods Mol Biol. 2269:93–105. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Konari N, Nagaishi K, Kikuchi S and
Fujimiya M: Mitochondria transfer from mesenchymal stem cells
structurally and functionally repairs renal proximal tubular
epithelial cells in diabetic nephropathy in vivo. Sci Rep.
9(5184)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moschoi R, Imbert V, Nebout M, Chiche J,
Mary D, Prebet T, Saland E, Castellano R, Pouyet L, Collette Y, et
al: Protective mitochondrial transfer from bone marrow stromal
cells to acute myeloid leukemic cells during chemotherapy. Blood.
128:253–264. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lambrecht BN and Hammad H: The immunology
of asthma. Nat Immunol. 16:45–56. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chan TK, Tan WSD, Peh HY and Wong WSF:
Aeroallergens induce reactive oxygen species production and DNA
damage and dampen antioxidant responses in bronchial epithelial
cells. J Immunol. 199:39–47. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhao L, Gao J, Chen G, Huang C, Kong W,
Feng Y and Zhen G: Mitochondria dysfunction in airway epithelial
cells is associated with type 2-low asthma. Front Genet.
14(1186317)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yao Y, Fan XL, Jiang D, Zhang Y, Li X, Xu
ZB, Fang SB, Chiu S, Tse HF, Lian Q and Fu QL: Connexin 43-mediated
mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation.
Stem Cell Reports. 11:1120–1135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Malsin ES and Kamp DW: The mitochondria in
lung fibrosis: Friend or foe? Transl Res. 202:1–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Takahashi M, Mizumura K, Gon Y, Shimizu T,
Kozu Y, Shikano S, Iida Y, Hikichi M, Okamoto S, Tsuya K, et al:
Iron-dependent mitochondrial dysfunction contributes to the
pathogenesis of pulmonary fibrosis. Front Pharmacol.
12(643980)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huang T, Lin R, Su Y, Sun H, Zheng X,
Zhang J, Lu X, Zhao B, Jiang X, Huang L, et al: Efficient
intervention for pulmonary fibrosis via mitochondrial transfer
promoted by mitochondrial biogenesis. Nat Commun.
14(5781)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li CL, Liu JF and Liu SF: Mitochondrial
dysfunction in chronic obstructive pulmonary disease: Unraveling
the molecular nexus. Biomedicines. 12(814)2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Canton M, Sánchez-Rodríguez R, Spera I,
Venegas FC, Favia M, Viola A and Castegna A: Reactive oxygen
species in macrophages: Sources and targets. Front Immunol.
12(734229)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aridgides DS, Mellinger DL, Armstrong DA,
Hazlett HF, Dessaint JA, Hampton TH, Atkins GT, Carroll JL and
Ashare A: Functional and metabolic impairment in cigarette
smoke-exposed macrophages is tied to oxidative stress. Sci Rep.
9(9624)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tulen CBM, Wang Y, Beentjes D, Jessen PJJ,
Ninaber DK, Reynaert NL, van Schooten FJ, Opperhuizen A, Hiemstra
PS and Remels AHV: Dysregulated mitochondrial metabolism upon
cigarette smoke exposure in various human bronchial epithelial cell
models. Dis Model Mech. 15(dmm049247)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li X, Michaeloudes C, Zhang Y, Wiegman CH,
Adcock IM, Lian Q, Mak JCW, Bhavsar PK and Chung KF: Mesenchymal
stem cells alleviate oxidative stress-induced mitochondrial
dysfunction in the airways. J Allergy Clin Immunol.
141:1634–1645.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li X, Zhang Y, Yeung SC, Liang Y, Liang X,
Ding Y, Ip MS, Tse HF, Mak JC and Lian Q: Mitochondrial transfer of
induced pluripotent stem cell-derived mesenchymal stem cells to
airway epithelial cells attenuates cigarette smoke-induced damage.
Am J Respir Cell Mol Biol. 51:455–465. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu BB, Leung KT and Poon EN:
Mitochondrial-targeted therapy for doxorubicin-induced
cardiotoxicity. Int J Mol Sci. 23(1912)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
He H, Wang L, Qiao Y, Zhou Q, Li H, Chen
S, Yin D, Huang Q and He M: Doxorubicin induces endotheliotoxicity
and mitochondrial dysfunction via ROS/eNOS/NO pathway. Front
Pharmacol. 10(1531)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tang H, Tao A, Song J, Liu Q, Wang H and
Rui T: Doxorubicin-induced cardiomyocyte apoptosis: Role of
mitofusin 2. Int J Biochem Cell Biol. 88:55–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang Y, Yu Z, Jiang D, Liang X, Liao S,
Zhang Z, Yue W, Li X, Chiu SM, Chai YH, et al: iPSC-MSCs with high
intrinsic MIRO1 and sensitivity to TNF-α yield efficacious
mitochondrial transfer to rescue anthracycline-induced
cardiomyopathy. Stem Cell Reports. 7:749–763. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jin N, Zhang M, Zhou L, Jin S, Cheng H, Li
X, Shi Y, Xiang T, Zhang Z, Liu Z, et al: Mitochondria
transplantation alleviates cardiomyocytes apoptosis through
inhibiting AMPKα-mTOR mediated excessive autophagy. FASEB J.
38(e23655)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tanaka-Esposito C, Chen Q and Lesnefsky
EJ: Blockade of electron transport before ischemia protects
mitochondria and decreases myocardial injury during reperfusion in
aged rat hearts. Transl Res. 160:207–216. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Huang J, Li R and Wang C: The role of
mitochondrial quality control in cardiac ischemia/reperfusion
injury. Oxid Med Cell Longev. 2021(5543452)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bouhamida E, Morciano G, Perrone M, Kahsay
AE, Della Sala M, Wieckowski MR, Fiorica F, Pinton P, Giorgi C and
Patergnani S: The interplay of hypoxia signaling on mitochondrial
dysfunction and inflammation in cardiovascular diseases and cancer:
From molecular mechanisms to therapeutic approaches. Biology
(Basel). 11(300)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cselenyák A, Pankotai E, Horváth EM, Kiss
L and Lacza Z: Mesenchymal stem cells rescue cardiomyoblasts from
cell death in an in vitro ischemia model via direct cell-to-cell
connections. BMC Cell Biol. 11(29)2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Han H, Hu J, Yan Q, Zhu J, Zhu Z, Chen Y,
Sun J and Zhang R: Bone marrow-derived mesenchymal stem cells
rescue injured H9c2 cells via transferring intact mitochondria
through tunneling nanotubes in an in vitro simulated
ischemia/reperfusion model. Mol Med Rep. 13:1517–1524.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mori D, Miyagawa S, Kawamura T, Yoshioka
D, Hata H, Ueno T, oda K, Kuratani T, Oota M, Kawai K, et al:
Mitochondrial transfer induced by adipose-derived mesenchymal stem
cell transplantation improves cardiac function in rat models of
ischemic cardiomyopathy. Cell Transplant.
32(9636897221148457)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Mahrouf-Yorgov M, Augeul L, Da Silva CC,
Jourdan M, Rigolet M, Manin S, Ferrera R, Ovize M, Henry A, Guguin
A, et al: Mesenchymal stem cells sense mitochondria released from
damaged cells as danger signals to activate their rescue
properties. Cell Death Differ. 24:1224–1238. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang ZH, Zhu W, Ren HZ, Zhao X, Wang S,
Ma HC and Shi XL: Mesenchymal stem cells increase expression of
heme oxygenase-1 leading to anti-inflammatory activity in treatment
of acute liver failure. Stem Cell Res Ther. 8(70)2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Tang Q, Zheng G, Feng Z, Chen Y, Lou Y,
Wang C, Zhang X, Zhang Y, Xu H, Shang P and Liu H: Trehalose
ameliorates oxidative stress-mediated mitochondrial dysfunction and
ER stress via selective autophagy stimulation and autophagic flux
restoration in osteoarthritis development. Cell Death Dis.
8(e3081)2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liu H, Li Z, Cao Y, Cui Y, Yang X, Meng Z
and Wang R: Effect of chondrocyte mitochondrial dysfunction on
cartilage degeneration: A possible pathway for osteoarthritis
pathology at the subcellular level. Mol Med Rep. 20:3308–3316.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sanchez-Lopez E, Coras R, Torres A, Lane
NE and Guma M: Synovial inflammation in osteoarthritis progression.
Nat Rev Rheumatol. 18:258–275. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zheng L, Zhang Z, Sheng P and Mobasheri A:
The role of metabolism in chondrocyte dysfunction and the
progression of osteoarthritis. Ageing Res Rev.
66(101249)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang R, Maimaitijuma T, Ma YY, Jiao Y and
Cao YP: Mitochondrial transfer from bone-marrow-derived mesenchymal
stromal cells to chondrocytes protects against cartilage
degenerative mitochondrial dysfunction in rats chondrocytes. Chin
Med J (Engl). 134:212–218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wu Z, Korntner SH, Mullen AM and Zeugolis
DI: Collagen type II: From biosynthesis to advanced biomaterials
for cartilage engineering. Biomater Biosyst.
4(100030)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Fahey M, Bennett M, Thomas M, Montney K,
Vivancos-Koopman I, Pugliese B, Browning L, Bonassar LJ and Delco
M: Mesenchymal stromal cells donate mitochondria to articular
chondrocytes exposed to mitochondrial, environmental, and
mechanical stress. Sci Rep. 12(21525)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Michelacci YM, Baccarin RYA and Rodrigues
NNP: Chondrocyte homeostasis and differentiation: Transcriptional
control and signaling in healthy and osteoarthritic conditions.
Life (Basel). 13(1460)2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Fearon U, Canavan M, Biniecka M and Veale
DJ: Hypoxia, mitochondrial dysfunction and synovial invasiveness in
rheumatoid arthritis. Nat Rev Rheumatol. 12:385–397.
2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang X, Eliasberg CD and Rodeo SA:
Mitochondrial dysfunction and potential mitochondrial protectant
treatments in tendinopathy. Ann N Y Acad Sci. 1490:29–41.
2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zhang X, Wada S, Zhang Y, Chen D, Deng XH
and Rodeo SA: Assessment of mitochondrial dysfunction in a murine
model of supraspinatus tendinopathy. J Bone Joint Surg Am.
103:174–183. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Liu YC, Wang HL, Huang YZ, Weng YH, Chen
RS, Tsai WC, Yeh TH, Lu CS, Chen YL, Lin YW, et al: Alda-1, an
activator of ALDH2, ameliorates Achilles tendinopathy in cellular
and mouse models. Biochem Pharmacol. 175(113919)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Dex S, Alberton P, Willkomm L, Söllradl T,
Bago S, Milz S, Shakibaei M, Ignatius A, Bloch W, Clausen-Schaumann
H, et al: Tenomodulin is required for tendon endurance running and
collagen I fibril adaptation to mechanical load. EBioMedicine.
20:240–254. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wei B, Ji M, Lin Y, Wang S, Liu Y, Geng R,
Hu X, Xu L, Li Z, Zhang W and Lu J: Mitochondrial transfer from
bone mesenchymal stem cells protects against tendinopathy both in
vitro and in vivo. Stem Cell Res Ther. 14(104)2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lee JM, Hwang JW, Kim MJ, Jung SY, Kim KS,
Ahn EH, Min K and Choi YS: Mitochondrial transplantation modulates
inflammation and apoptosis, alleviating tendinopathy both in vivo
and in vitro. Antioxidants (Basel). 10(696)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kubat GB, Bouhamida E, Ulger O, Turkel I,
Pedriali G, Ramaccini D, Ekinci O, Ozerklig B, Atalay O, Patergnani
S, et al: Mitochondrial dysfunction and skeletal muscle atrophy:
Causes, mechanisms, and treatment strategies. Mitochondrion.
72:33–58. 2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hyatt HW and Powers SK: Mitochondrial
dysfunction is a common denominator linking skeletal muscle wasting
due to disease, aging, and prolonged inactivity. Antioxidants
(Basel). 10(588)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Matsumoto C, Sekine H, Nahata M, Mogami S,
Ohbuchi K, Fujitsuka N and Takeda H: Role of mitochondrial
dysfunction in the pathogenesis of cisplatin-induced myotube
atrophy. Biol Pharm Bull. 45:780–792. 2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Shen S, Liao Q, Liu J, Pan R, Lee SMY and
Lin L: Myricanol rescues dexamethasone-induced muscle dysfunction
via a sirtuin 1-dependent mechanism. J Cachexia Sarcopenia Muscle.
10:429–444. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Yang B, Yang X, Sun X, Shi J, Shen Y and
Chen R: IL-6 deficiency attenuates skeletal muscle atrophy by
inhibiting mitochondrial ROS production through the upregulation of
PGC-1 α in septic mice. Oxid Med Cell Longev.
2022(9148246)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Xiao Y, Karam C, Yi J, Zhang L, Li X, Yoon
D, Wang H, Dhakal K, Ramlow P, Yu T, et al: ROS-related
mitochondrial dysfunction in skeletal muscle of an ALS mouse model
during the disease progression. Pharmacol Res. 138:25–36.
2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Moore TM, Lin AJ, Strumwasser AR, Cory K,
Whitney K, Ho T, Ho T, Lee JL, Rucker DH, Nguyen CQ, et al:
Mitochondrial dysfunction is an early consequence of partial or
complete dystrophin loss in mdx mice. Front Physiol.
11(690)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Mohiuddin M, Choi JJ, Lee NH, Jeong H,
Anderson SE, Han WM, Aliya B, Peykova TZ, Verma S, García AJ, et
al: Transplantation of muscle stem cell mitochondria rejuvenates
the bioenergetic function of dystrophic muscle. bioRxiv:
2020.04.17.017822, 2020.
|
|
80
|
Wang L, Jiao XF, Wu C, Li XQ, Sun HX, Shen
XY, Zhang KZ, Zhao C, Liu L, Wang M, et al: Trimetazidine
attenuates dexamethasone-induced muscle atrophy via inhibiting
NLRP3/GSDMD pathway-mediated pyroptosis. Cell Death Discov.
7(251)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kim MJ, Lee JM, Min K and Choi YS:
Xenogeneic transplantation of mitochondria induces muscle
regeneration in an in vivo rat model of dexamethasone-induced
atrophy. J Muscle Res Cell Motil. 45:53–68. 2024.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Xu S, Li Y, Chen JP, Li DZ, Jiang Q, Wu T
and Zhou XZ: Oxygen glucose deprivation/re-oxygenation-induced
neuronal cell death is associated with Lnc-D63785 m6A methylation
and miR-422a accumulation. Cell Death Dis. 11(816)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Juntunen M, Hagman S, Moisan A, Narkilahti
S and Miettinen S: In vitro oxygen-glucose deprivation-induced
stroke models with human neuroblastoma cell- and induced
pluripotent stem cell-derived neurons. Stem Cells Int.
2020(8841026)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Liu F, Lu J, Manaenko A, Tang J and Hu Q:
Mitochondria in ischemic stroke: New insight and implications.
Aging Dis. 9:924–937. 2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Norat P, Soldozy S, Sokolowski JD, Gorick
CM, Kumar JS, Chae Y, Yağmurlu K, Prada F, Walker M, Levitt MR, et
al: Mitochondrial dysfunction in neurological disorders: Exploring
mitochondrial transplantation. NPJ Regen Med. 5(22)2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Babenko VA, Silachev DN, Zorova LD,
Pevzner IB, Khutornenko AA, Plotnikov EY, Sukhikh GT and Zorov DB:
Improving the post-stroke therapeutic potency of mesenchymal
multipotent stromal cells by cocultivation with cortical neurons:
The role of crosstalk between cells. Stem Cells Transl Med.
4:1011–1020. 2015.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Jian Z, Ding S, Deng H, Wang J, Yi W, Wang
L, Zhu S, Gu L and Xiong X: Probenecid protects against
oxygen-glucose deprivation injury in primary astrocytes by
regulating inflammasome activity. Brain Res. 1643:123–129.
2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Babenko VA, Silachev DN, Popkov VA, Zorova
LD, Pevzner IB, Plotnikov EY, Sukhikh GT and Zorov DB: Miro1
enhances mitochondria transfer from multipotent mesenchymal stem
cells (MMSC) to neural cells and improves the efficacy of cell
recovery. Molecules. 23(687)2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Li J, Li H, Cai S, Bai S, Cai H and Zhang
X: CD157 in bone marrow mesenchymal stem cells mediates
mitochondrial production and transfer to improve neuronal apoptosis
and functional recovery after spinal cord injury. Stem Cell Res
Ther. 12(289)2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Yang Y, Ye G, Zhang YL, He HW, Yu BQ, Hong
YM, You W and Li X: Transfer of mitochondria from mesenchymal stem
cells derived from induced pluripotent stem cells attenuates
hypoxia-ischemia-induced mitochondrial dysfunction in PC12 cells.
Neural Regen Res. 15:464–472. 2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Tseng N, Lambie SC, Huynh CQ, Sanford B,
Patel M, Herson PS and Ormond DR: Mitochondrial transfer from
mesenchymal stem cells improves neuronal metabolism after oxidant
injury in vitro: The role of Miro1. J Cereb Blood Flow Metab.
41:761–770. 2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Alizadeh A, Dyck SM and Karimi-Abdolrezaee
S: Traumatic spinal cord injury: An overview of pathophysiology,
models and acute injury mechanisms. Front Neurol.
10(282)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Schmidt J and Quintá HR: Mitochondrial
dysfunction as a target in spinal cord injury: Intimate correlation
between pathological processes and therapeutic approaches. Neural
Regen Res. 18:2161–2166. 2023.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Rong Y, Fan J, Ji C, Wang Z, Ge X, Wang J,
Ye W, Yin G, Cai W and Liu W: USP11 regulates autophagy-dependent
ferroptosis after spinal cord ischemia-reperfusion injury by
deubiquitinating beclin 1. Cell Death Differ. 29:1164–1175.
2022.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Li D, Lu X, Xu G, Liu S, Gong Z, Lu F, Xia
X, Jiang J, Wang H, Zou F and Ma X: Dihydroorotate dehydrogenase
regulates ferroptosis in neurons after spinal cord injury via the
P53-ALOX15 signaling pathway. CNS Neurosci Ther. 29:1923–1939.
2023.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Yao S, Pang M, Wang Y, Wang X, Lin Y, Lv
Y, Xie Z, Hou J, Du C, Qiu Y, et al: Mesenchymal stem cell
attenuates spinal cord injury by inhibiting mitochondrial quality
control-associated neuronal ferroptosis. Redox Biol.
67(102871)2023.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Jain R, Begum N, Tryphena KP, Singh SB,
Srivastava S, Rai SN, Vamanu E and Khatri DK: Inter and
intracellular mitochondrial transfer: Future of mitochondrial
transplant therapy in Parkinson's disease. Biomed Pharmacother.
159(114268)2023.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Poewe W, Seppi K, Tanner CM, Halliday GM,
Brundin P, Volkmann J, Schrag AE and Lang AE: Parkinson disease.
Nat Rev Dis Primers. 3(17013)2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
van der Bliek AM, Sedensky MM and Morgan
PG: Cell biology of the mitochondrion. Genetics. 207:843–871.
2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Ahn EH, Lei K, Kang SS, Wang ZH, Liu X,
Hong W, Wang YT, Edgington-Mitchell LE, Jin L and Ye K:
Mitochondrial dysfunction triggers the pathogenesis of Parkinson's
disease in neuronal C/EBPβ transgenic mice. Mol Psychiatry.
26:7838–7850. 2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Xiao B, Kuruvilla J and Tan EK: Mitophagy
and reactive oxygen species interplay in Parkinson's disease. NPJ
Parkinsons Dis. 8(135)2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Eo H, Yu SH, Choi Y, Kim Y, Kang YC, Lee
H, Kim JH, Han K, Lee HK, Chang MY, et al: Mitochondrial
transplantation exhibits neuroprotective effects and improves
behavioral deficits in an animal model of Parkinson's disease.
Neurotherapeutics. 21(e00355)2024.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Thakur P and Nehru B: Inhibition of
neuroinflammation and mitochondrial dysfunctions by carbenoxolone
in the rotenone model of Parkinson's disease. Mol Neurobiol.
51:209–219. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Cheng XY, Biswas S, Li J, Mao CJ,
Chechneva O, Chen J, Li K, Li J, Zhang JR, Liu CF and Deng WB:
Human iPSCs derived astrocytes rescue rotenone-induced
mitochondrial dysfunction and dopaminergic neurodegeneration in
vitro by donating functional mitochondria. Transl Neurodegener.
9(13)2020.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Chiu GS, Maj MA, Rizvi S, Dantzer R,
Vichaya EG, Laumet G, Kavelaars A and Heijnen CJ: Pifithrin-μ
prevents cisplatin-induced chemobrain by preserving neuronal
mitochondrial function. Cancer Res. 77:742–752. 2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Kleih M, Böpple K, Dong M, Gaißler A,
Heine S, Olayioye MA, Aulitzky WE and Essmann F: Direct impact of
cisplatin on mitochondria induces ROS production that dictates cell
fate of ovarian cancer cells. Cell Death Dis.
10(851)2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Boukelmoune N, Chiu GS, Kavelaars A and
Heijnen CJ: Mitochondrial transfer from mesenchymal stem cells to
neural stem cells protects against the neurotoxic effects of
cisplatin. Acta Neuropathol Commun. 6(139)2018.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Méthot S, Proulx S, Brunette I and
Rochette PJ: Rescuing cellular function in Fuchs endothelial
corneal dystrophy by healthy exogenous mitochondrial
internalization. Sci Rep. 13(3380)2023.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Jiang D, Chen FX, Zhou H, Lu YY, Tan H, Yu
SJ, Yuan J, Liu H, Meng W and Jin ZB: Bioenergetic crosstalk
between mesenchymal stem cells and various ocular cells through the
intercellular trafficking of mitochondria. Theranostics.
10:7260–7272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Vallabh NA, Romano V and Willoughby CE:
Mitochondrial dysfunction and oxidative stress in corneal disease.
Mitochondrion. 36:103–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Numa K, Ueno M, Fujita T, Ueda K, Hiramoto
N, Mukai A, Tokuda Y, Nakano M, Sotozono C, Kinoshita S and Hamuro
J: Mitochondria as a Platform for dictating the cell fate of
cultured human corneal endothelial cells. Invest Ophthalmol Vis
Sci. 61(10)2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Jiang D, Gao F, Zhang Y, Wong DS, Li Q,
Tse HF, Xu G, Yu Z and Lian Q: Mitochondrial transfer of
mesenchymal stem cells effectively protects corneal epithelial
cells from mitochondrial damage. Cell Death Dis.
7(e2467)2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Hanna SJ, McCoy-Simandle K, Leung E, Genna
A, Condeelis J and Cox D: Tunneling nanotubes, a novel mode of
tumor cell-macrophage communication in tumor cell invasion. J Cell
Sci. 132(jcs223321)2019.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Wang L, Klingeborn M, Travis AM, Hao Y,
Arshavsky VY and Gospe SM III: Progressive optic atrophy in a
retinal ganglion cell-specific mouse model of complex I deficiency.
Sci Rep. 10(16326)2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Yu AK, Song L, Murray KD, van der List D,
Sun C, Shen Y, Xia Z and Cortopassi GA: Mitochondrial complex I
deficiency leads to inflammation and retinal ganglion cell death in
the Ndufs4 mouse. Hum Mol Genet. 24:2848–2860. 2015.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Warwick AM, Bomze HM, Wang L, Hao Y,
Stinnett SS and Gospe SM III: Hypoxia-mediated rescue of retinal
ganglion cells deficient in mitochondrial complex I is independent
of the hypoxia-inducible factor pathway. Sci Rep.
14(24114)2024.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Jiang D, Xiong G, Feng H, Zhang Z, Chen P,
Yan B, Chen L, Gandhervin K, Ma C, Li C, et al: Donation of
mitochondria by iPSC-derived mesenchymal stem cells protects
retinal ganglion cells against mitochondrial complex I
defect-induced degeneration. Theranostics. 9:2395–2410.
2019.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Navneet S, Wilson K and Rohrer B: Muller
glial cells in the macula: Their activation and cell-cell
interactions in age-related macular degeneration. Invest Ophthalmol
Vis Sci. 65(42)2024.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Xue B, Xie Y, Xue Y, Hu N, Zhang G, Guan H
and Ji M: Involvement of P2X7 receptors in retinal
ganglion cell apoptosis induced by activated Müller cells. Exp Eye
Res. 153:42–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Hu X, Zhao GL, Xu MX, Zhou H, Li F, Miao
Y, Lei B, Yang XL and Wang Z: Interplay between Muller cells and
microglia aggravates retinal inflammatory response in experimental
glaucoma. J Neuroinflammation. 18(303)2021.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Qiu AW, Bian Z, Mao PA and Liu QH: IL-17A
exacerbates diabetic retinopathy by impairing Müller cell function
via Act1 signaling. Exp Mol Med. 48(e280)2016.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Natoli R, Fernando N, Madigan M, Chu-Tan
JA, Valter K, Provis J and Rutar M: Microglia-derived IL-1β
promotes chemokine expression by Müller cells and RPE in focal
retinal degeneration. Mol Neurodegener. 12(31)2017.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Marques E, Alves Teixeira M, Nguyen C,
Terzi F and Gallazzini M: Lipocalin-2 induces mitochondrial
dysfunction in renal tubular cells via mTOR pathway activation.
Cell Rep. 42(113032)2023.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Chen Y, Yang Y, Liu Z and He L:
Adiponectin promotes repair of renal tubular epithelial cells by
regulating mitochondrial biogenesis and function. Metabolism.
128(154959)2022.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Ji X, Yang X, Gu X, Chu L, Sun S, Sun J,
Song P, Mu Q, Wang Y, Sun X, et al: CUL3 induces mitochondrial
dysfunction via MRPL12 ubiquitination in renal tubular epithelial
cells. FEBS J. 290:5340–5352. 2023.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Cai F, Li D, Xie Y, Wang X, Ma H, Xu H,
Cheng J, Zhuang H and Hua ZC: Sulfide:quinone oxidoreductase
alleviates ferroptosis in acute kidney injury via ameliorating
mitochondrial dysfunction of renal tubular epithelial cells. Redox
Biol. 69(102973)2024.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Xiong YB, Huang WY, Ling X, Zhou S, Wang
XX, Li XL and Zhou LL: Mitochondrial calcium uniporter promotes
kidney aging in mice through inducing mitochondrial
calcium-mediated renal tubular cell senescence. Acta Pharmacol Sin.
45:2149–2162. 2024.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Wang J, Yue X, Meng C, Wang Z, Jin X, Cui
X, Yang J, Shan C, Gao Z, Yang Y, et al: Acute hyperglycemia may
induce renal tubular injury through mitophagy inhibition. Front
Endocrinol (Lausanne). 11(536213)2020.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Aluksanasuwan S, Plumworasawat S, Malaitad
T, Chaiyarit S and Thongboonkerd V: High glucose induces
phosphorylation and oxidation of mitochondrial proteins in renal
tubular cells: A proteomics approach. Sci Rep.
10(5843)2020.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Nahdi AMTA, John A and Raza H: Elucidation
of molecular mechanisms of streptozotocin-induced oxidative stress,
apoptosis, and mitochondrial dysfunction in Rin-5F pancreatic
β-cells. Oxid Med Cell Longev. 2017(7054272)2017.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Bhargava P and Schnellmann RG:
Mitochondrial energetics in the kidney. Nat Rev Nephrol.
13:629–646. 2017.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Kapetanaki S, Kumawat AK, Persson K and
Demirel I: TMAO suppresses megalin expression and albumin uptake in
human proximal tubular cells via PI3K and ERK signaling. Int J Mol
Sci. 23(8856)2022.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Peruchetti DB, Silva-Aguiar RP, Siqueira
GM, Dias WB and Caruso-Neves C: High glucose reduces
megalin-mediated albumin endocytosis in renal proximal tubule cells
through protein kinase B O-GlcNAcylation. J Biol Chem.
293:11388–11400. 2018.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Kupriyanova Y, Zaharia OP, Bobrov P,
Karusheva Y, Burkart V, Szendroedi J, Hwang JH and Roden M: GDS
group. Early changes in hepatic energy metabolism and lipid content
in recent-onset type 1 and 2 diabetes mellitus. J Hepatol.
74:1028–1037. 2021.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Tilg H, Moschen AR and Roden M: NAFLD and
diabetes mellitus. Nat Rev Gastroenterol Hepatol. 14:32–42.
2017.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Nassir F and Ibdah JA: Role of
mitochondria in nonalcoholic fatty liver disease. Int J Mol Sci.
15:8713–8742. 2014.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Zheng P, Ma W, Gu Y, Wu H, Bian Z, Liu N,
Yang D and Chen X: High-fat diet causes mitochondrial damage and
downregulation of mitofusin-2 and optic atrophy-1 in multiple
organs. J Clin Biochem Nutr. 73:61–76. 2023.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Bi Y, Guo X, Zhang M, Zhu K, Shi C, Fan B,
Wu Y, Yang Z and Ji G: Bone marrow derived-mesenchymal stem cell
improves diabetes-associated fatty liver via mitochondria
transformation in mice. Stem Cell Res Ther. 12(602)2021.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Hsu MJ, Karkossa I, Schäfer I, Christ M,
Kühne H, Schubert K, Rolle-Kampczyk UE, Kalkhof S, Nickel S, Seibel
P, et al: Mitochondrial transfer by human mesenchymal stromal cells
ameliorates hepatocyte lipid load in a mouse model of NASH.
Biomedicines. 8(350)2020.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Gaspers LD, Pierobon N and Thomas AP:
Intercellular calcium waves integrate hormonal control of glucose
output in the intact liver. J Physiol. 597:2867–2885.
2019.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Barreto SG, Habtezion A, Gukovskaya A,
Lugea A, Jeon C, Yadav D, Hegyi P, Venglovecz V, Sutton R and
Pandol SJ: Critical thresholds: key to unlocking the door to the
prevention and specific treatments for acute pancreatitis. Gut.
70:194–203. 2021.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Liu W, Ren Y, Wang T, Wang M, Xu Y, Zhang
J, Bi J, Wu Z, Zhang Y and Wu R: Blocking CIRP protects against
acute pancreatitis by improving mitochondrial function and
suppressing pyroptosis in acinar cells. Cell Death Discov.
10(156)2024.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Mukherjee R, Mareninova OA, Odinokova IV,
Huang W, Murphy J, Chvanov M, Javed MA, Wen L, Booth DM, Cane MC,
et al: Mechanism of mitochondrial permeability transition pore
induction and damage in the pancreas: Inhibition prevents acute
pancreatitis by protecting production of ATP. Gut. 65:1333–1346.
2016.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Hu Z, Wang D, Gong J, Li Y, Ma Z, Luo T,
Jia X, Shi Y and Song Z: MSCs deliver hypoxia-treated mitochondria
reprogramming acinar metabolism to alleviate severe acute
pancreatitis injury. Adv Sci (Weinh). 10(e2207691)2023.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Shapiro AM, Pokrywczynska M and Ricordi C:
Clinical pancreatic islet transplantation. Nat Rev Endocrinol.
13:268–277. 2017.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Kin T, Senior P, O'Gorman D, Richer B,
Salam A and Shapiro AMJ: Risk factors for islet loss during culture
prior to transplantation. Transpl Int. 21:1029–1035.
2008.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Jimenez-Sánchez C, Brun T and Maechler P:
Mitochondrial carriers regulating insulin secretion profiled in
human islets upon metabolic stress. Biomolecules.
10(1543)2020.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Zhang K, Bao R, Huang F, Yang K, Ding Y,
Lauterboeck L, Yoshida M, Long Q and Yang Q: ATP synthase
inhibitory factor subunit 1 regulates islet β-cell function via
repression of mitochondrial homeostasis. Lab Invest. 102:69–79.
2022.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Rackham CL, Hubber EL, Czajka A, Malik AN,
King AJF and Jones PM: Optimizing beta cell function through
mesenchymal stromal cell-mediated mitochondria transfer. Stem
Cells. 38:574–584. 2020.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Shoop WK, Lape J, Trum M, Powell A,
Sevigny E, Mischler A, Bacman SR, Fontanesi F, Smith J, Jantz D, et
al: Efficient elimination of MELAS-associated m.3243G mutant
mitochondrial DNA by an engineered mitoARCUS nuclease. Nat Metab.
5:2169–2183. 2023.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Ikeda T, Osaka H, Shimbo H, Tajika M,
Yamazaki M, Ueda A, Murayama K and Yamagata T: Mitochondrial DNA
3243A>T mutation in a patient with MELAS syndrome. Hum Genome
Var. 5(25)2018.PubMed/NCBI View Article : Google Scholar
|
|
154
|
El-Hattab AW, Adesina AM, Jones J and
Scaglia F: MELAS syndrome: Clinical manifestations, pathogenesis,
and treatment options. Mol Genet Metab. 116:4–12. 2015.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Lin TK, Chen SD, Chuang YC, Lan MY, Chuang
JH, Wang PW, Hsu TY, Wang FS, Tsai MH, Huang ST, et al:
Mitochondrial transfer of wharton's jelly mesenchymal stem cells
eliminates mutation burden and rescues mitochondrial bioenergetics
in rotenone-stressed MELAS fibroblasts. Oxid Med Cell Longev.
2019(9537504)2019.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Liu L, Yang J, Otani Y, Shiga T, Yamaguchi
A, Oda Y, Hattori M, Goto T, Ishibashi S, Kawashima-Sonoyama Y, et
al: MELAS-derived neurons functionally improve by mitochondrial
transfer from highly purified mesenchymal stem cells (REC). Int J
Mol Sci. 24(17186)2023.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Tuppen HA, Blakely EL, Turnbull DM and
Taylor RW: Mitochondrial DNA mutations and human disease. Biochim
Biophys Acta. 1797:113–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Chuang YC, Liou CW, Chen SD, Wang PW,
Chuang JH, Tiao MM, Hsu TY, Lin HY and Lin TK: Mitochondrial
transfer from wharton's jelly mesenchymal stem cell to MERRF cybrid
reduces oxidative stress and improves mitochondrial bioenergetics.
Oxid Med Cell Longev. 2017(5691215)2017.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Capristo M, Del Dotto V, Tropeano CV,
Fiorini C, Caporali L, La Morgia C, Valentino ML, Montopoli M,
Carelli V and Maresca A: Rapamycin rescues mitochondrial
dysfunction in cells carrying the m.8344A > G mutation in the
mitochondrial tRNALys. Mol Med. 28(90)2022.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Jacoby E, Blumkin M, Anikster Y,
Varda-Bloom N, Pansheen J, Bar Yoseph O, Gruber N, Lahav E, Besser
MJ, Schachter J, et al: First-in-human mitochondrial augmentation
of hematopoietic stem cells in pearson syndrome. Blood.
132(1024)2018.
|
|
161
|
Jacoby E, Bar-Yosef O, Gruber N, Lahav E,
Varda-Bloom N, Bolkier Y, Bar D, Blumkin MB, Barak S, Eisenstein E,
et al: Mitochondrial augmentation of hematopoietic stem cells in
children with single large-scale mitochondrial DNA deletion
syndromes. Sci Transl Med. 14(eabo3724)2022.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Fu Y, Ni J and Chen J, Ma G, Zhao M, Zhu
S, Shi T, Zhu J, Huang Z, Zhang J and Chen J: Dual-functionalized
MSCs that express CX3CR1 and IL-25 exhibit enhanced therapeutic
effects on inflammatory bowel disease. Mol Ther. 28:1214–1228.
2020.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Van Nguyen TT, Vu NB and Van Pham P:
Mesenchymal stem cell transplantation for ischemic diseases:
Mechanisms and challenges. Tissue Eng Regen Med. 18:587–611.
2021.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Zhu XY, Lerman A and Lerman LO: Concise
review: Mesenchymal stem cell treatment for ischemic kidney
disease. Stem Cells. 31:1731–1736. 2013.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Baldari S, Di Rocco G, Piccoli M, Pozzobon
M, Muraca M and Toietta G: Challenges and strategies for improving
the regenerative effects of mesenchymal stromal cell-based
therapies. Int J Mol Sci. 18(2087)2017.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Hu C and Li L: Preconditioning influences
mesenchymal stem cell properties in vitro and in vivo. J Cell Mol
Med. 22:1428–1442. 2018.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Li CJ, Chen PK, Sun LY and Pang CY:
Enhancement of mitochondrial transfer by antioxidants in human
mesenchymal stem cells. Oxid Med Cell Longev.
2017(8510805)2017.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Lee DS and Kim JE: PDI-mediated
S-nitrosylation of DRP1 facilitates DRP1-S616 phosphorylation and
mitochondrial fission in CA1 neurons. Cell Death Dis.
9(869)2018.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Yao X, Ma Y, Zhou W, Liao Y, Jiang Z, Lin
J, He Q, Wu H, Wei W, Wang X, et al: In-cytoplasm mitochondrial
transplantation for mesenchymal stem cells engineering and tissue
regeneration. Bioeng Transl Med. 7(e10250)2021.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Guo Y, Chi X, Wang Y, Heng BC, Wei Y,
Zhang X, Zhao H, Yin Y and Deng X: Mitochondria transfer enhances
proliferation, migration, and osteogenic differentiation of bone
marrow mesenchymal stem cell and promotes bone defect healing. Stem
Cell Res Ther. 11(245)2020.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Lin RZ, Im GB, Luo AC, Zhu Y, Hong X,
Neumeyer J, Tang HW, Perrimon N and Melero-Martin JM: Mitochondrial
transfer mediates endothelial cell engraftment through mitophagy.
Nature. 629:660–668. 2024.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Akhter W, Nakhle J, Vaillant L, Garcin G,
Le Saout C, Simon M, Crozet C, Djouad F, Jorgensen C, Vignais ML
and Hernandez J: Transfer of mesenchymal stem cell mitochondria to
CD4+ T cells contributes to repress Th1 differentiation
by downregulating T-bet expression. Stem Cell Res Ther.
14(12)2023.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Court AC, Le-Gatt A, Luz-Crawford P, Parra
E, Aliaga-Tobar V, Bátiz LF, Contreras RA, Ortúzar MI, Kurte M,
Elizondo-Vega R, et al: Mitochondrial transfer from MSCs to T cells
induces Treg differentiation and restricts inflammatory response.
EMBO Rep. 21(e48052)2020.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Romano M, Tung SL, Smyth LA and Lombardi
G: Treg therapy in transplantation: A general overview. Transpl
Int. 30:745–753. 2017.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Li LZ, Zhang Z and Bhoj VG: Conventional T
cell therapies pave the way for novel Treg therapeutics. Cell
Immunol. 359(104234)2021.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Piekarska K, Urban-Wójciuk Z, Kurkowiak M,
Pelikant-Małecka I, Schumacher A, Sakowska J, Spodnik JH,
Arcimowicz Ł, Zielińska H, Tymoniuk B, et al: Mesenchymal stem
cells transfer mitochondria to allogeneic Tregs in an HLA-dependent
manner improving their immunosuppressive activity. Nat Commun.
13(856)2022.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Espinosa-Carrasco G, Le Saout C, Fontanaud
P, Stratmann T, Mollard P, Schaeffer M and Hernandez J:
CD4+ T helper cells play a key role in maintaining
diabetogenic CD8+ T cell function in the pancreas. Front
Immunol. 8(2001)2018.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Luz-Crawford P, Hernandez J, Djouad F,
Luque-Campos N, Caicedo A, Carrère-Kremer S, Brondello JM, Vignais
ML, Pène J and Jorgensen C: Mesenchymal stem cell repression of
Th17 cells is triggered by mitochondrial transfer. Stem Cell Res
Ther. 10(232)2019.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Lazarevic V, Glimcher LH and Lord GM:
T-bet: A bridge between innate and adaptive immunity. Nat Rev
Immunol. 13:777–789. 2013.PubMed/NCBI View Article : Google Scholar : Ji L, Chen Y, Wang
H, Zhang W, He L, Wu J and Liu Y: Overexpression of Sirt6 promotes
M2 macrophage transformation, alleviating renal injury in diabetic
nephropathy. Int J Oncol 55: 103-115, 2019.
|
|
180
|
Xu L, Yan X, Zhao Y, Wang J, Liu B, Yu S,
Fu J, Liu Y and Su J: Macrophage polarization mediated by
mitochondrial dysfunction induces adipose tissue inflammation in
obesity. Int J Mol Sci. 23(9252)2022.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Ran L, Zhang S, Wang G, Zhao P, Sun J,
Zhou J, Gan H, Jeon R, Li Q, Herrmann J and Wang F: Mitochondrial
pyruvate carrier-mediated metabolism is dispensable for the
classical activation of macrophages. Nat Metab. 5:804–820.
2023.PubMed/NCBI View Article : Google Scholar
|
|
182
|
De Santa F, Vitiello L, Torcinaro A and
Ferraro E: The role of metabolic remodeling in macrophage
polarization and its effect on skeletal muscle regeneration.
Antioxid Redox Signal. 30:1553–1598. 2019.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Yuan Y, Chen Y, Peng T, Li L, Zhu W, Liu
F, Luo R, Cheng J, Liu J and Lu Y: Mitochondrial ROS-induced
lysosomal dysfunction impairs autophagic flux and contributes to M1
macrophage polarization in a diabetic condition. Clin Sci (Lond).
133:1759–1777. 2019.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Yuan Y, Yuan L, Li L, Liu F, Liu J, Chen
Y, Cheng J and Lu Y: Mitochondrial transfer from mesenchymal stem
cells to macrophages restricts inflammation and alleviates kidney
injury in diabetic nephropathy mice via PGC-1α activation. Stem
Cells. 39:913–928. 2021.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Hussell T and Bell TJ: Alveolar
macrophages: Plasticity in a tissue-specific context. Nat Rev
Immunol. 14:81–93. 2014.PubMed/NCBI View Article : Google Scholar
|
|
186
|
Jackson MV, Morrison TJ, Doherty DF,
McAuley DF, Matthay MA, Kissenpfennig A, O'Kane CM and
Krasnodembskaya AD: Mitochondrial transfer via tunneling nanotubes
is an important mechanism by which mesenchymal stem cells enhance
macrophage phagocytosis in the in vitro and in vivo models of ARDS.
Stem Cells. 34:2210–2223. 2016.PubMed/NCBI View Article : Google Scholar
|
|
187
|
Jackson MV and Krasnodembskaya AD:
Analysis of mitochondrial transfer in direct Co-cultures of human
monocyte-derived macrophages (MDM) and mesenchymal stem cells
(MSC). Bio Protoc. 7(e2255)2017.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Phinney DG, Di Giuseppe M, Njah J, Sala E,
Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, et
al: Mesenchymal stem cells use extracellular vesicles to outsource
mitophagy and shuttle microRNAs. Nat Commun. 6(8472)2015.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Xia L, Zhang C, Lv N, Liang Z, Ma T, Cheng
H, Xia Y and Shi L: AdMSC-derived exosomes alleviate acute lung
injury via transferring mitochondrial component to improve
homeostasis of alveolar macrophages. Theranostics. 12:2928–2947.
2022.PubMed/NCBI View Article : Google Scholar
|
|
190
|
West AP, Khoury-Hanold W, Staron M, Tal
MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff
DA, et al: Mitochondrial DNA stress primes the antiviral innate
immune response. Nature. 520:553–557. 2015.PubMed/NCBI View Article : Google Scholar
|
|
191
|
Morrison TJ, Jackson MV, Cunningham EK,
Kissenpfennig A, McAuley DF, O'Kane CM and Krasnodembskaya AD:
Mesenchymal stromal cells modulate macrophages in clinically
relevant lung injury models by extracellular vesicle mitochondrial
transfer. Am J Respir Crit Care Med. 196:1275–1286. 2017.PubMed/NCBI View Article : Google Scholar
|
|
192
|
Kang MH, Kim YJ and Lee JH: Mitochondria
in reproduction. Clin Exp Reprod Med. 50:1–11. 2023.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Dadarwal D, Pfeifer L, Cervantes M, Adams
GP and Singh J: Effect of maternal age on ATP content and
distribution of mitochondria in bovine oocytes. PLoS One.
19(e0302444)2024.PubMed/NCBI View Article : Google Scholar
|
|
194
|
Zhao J and Li Y: Adenosine triphosphate
content in human unfertilized oocytes, undivided zygotes and
embryos unsuitable for transfer or cryopreservation. J Int Med Res.
40:734–739. 2012.PubMed/NCBI View Article : Google Scholar
|
|
195
|
May-Panloup P, Chrétien MF, Jacques C,
Vasseur C, Malthièry Y and Reynier P: Low oocyte mitochondrial DNA
content in ovarian insufficiency. Hum Reprod. 20:593–597.
2005.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Zhang D, Keilty D, Zhang ZF and Chian RC:
Mitochondria in oocyte aging: Current understanding. Facts Views
Vis Obgyn. 9:29–38. 2017.PubMed/NCBI
|
|
197
|
Cozzolino M, Marin D and Sisti G: New
frontiers in IVF: mtDNA and autologous germline mitochondrial
energy transfer. Reprod Biol Endocrinol. 17(55)2019.PubMed/NCBI View Article : Google Scholar
|
|
198
|
Takeda K: Functional consequences of
mitochondrial mismatch in reconstituted embryos and offspring. J
Reprod Dev. 65:485–489. 2019.PubMed/NCBI View Article : Google Scholar
|
|
199
|
Tang S, Yang N, Yu M, Wang S, Hu X, Ni H
and Cai W: Noninvasive autologous mitochondria transport improves
the quality and developmental potential of oocytes from aged mice.
F S Sci. 3:310–321. 2022.PubMed/NCBI View Article : Google Scholar
|
|
200
|
Wang ZB, Hao JX, Meng TG, Guo L, Dong MZ,
Fan LH, Ouyang YC, Wang G, Sun QY, Ou XH and Yao YQ: Transfer of
autologous mitochondria from adipose tissue-derived stem cells
rescues oocyte quality and infertility in aged mice. Aging (Albany
NY). 9:2480–2488. 2017.PubMed/NCBI View Article : Google Scholar
|
|
201
|
Kankanam Gamage US, Hashimoto S, Miyamoto
Y, Nakano T, Yamanaka M, Koike A, Satoh M and Morimoto Y:
Mitochondria transfer from adipose stem cells improves the
developmental potential of cryopreserved oocytes. Biomolecules.
12(1008)2022.PubMed/NCBI View Article : Google Scholar
|
|
202
|
Zhang Q, Hao JX, Liu BW, Ouyang YC, Guo
JN, Dong MZ, Wang ZB, Gao F and Yao YQ: Supplementation of
mitochondria from endometrial mesenchymal stem cells improves
oocyte quality in aged mice. Cell Prolif. 56(e13372)2023.PubMed/NCBI View Article : Google Scholar
|
|
203
|
Zhang C, Tao L, Yue Y, Ren L, Zhang Z,
Wang X, Tian J and An L: Mitochondrial transfer from induced
pluripotent stem cells rescues developmental potential of in vitro
fertilized embryos from aging females†. Biol Reprod. 104:1114–1125.
2021.PubMed/NCBI View Article : Google Scholar
|
|
204
|
Tilly J and Woods D: Compositions and
methods for autologous germline mitochondrial energy transfer.
United States Patent Number. 8,642,329, 2014.
|
|
205
|
Fakih MH, Shmoury ME, Szeptycki J, Cruz
DBD, Lux C, Verjee S, Burgess CM, Cohn GM and Casper RF: The
AUGMENTSM treatment: Physician reported outcomes of the
initial global patient experience. JFIV Reprod Med Genet. 3:1–7.
2015.
|
|
206
|
Oktay K, Baltaci V, Sonmezer M, Turan V,
Unsal E, Baltaci A, Aktuna S and Moy F: Oogonial precursor
cell-derived autologous mitochondria injection to improve outcomes
in women with multiple IVF failures due to low oocyte quality: A
clinical translation. Reprod Sci. 22:1612–1617. 2015.PubMed/NCBI View Article : Google Scholar
|
|
207
|
Jiang Z, Shi C, Han H, Fu M, Zhu H, Han T,
Fei J, Huang Y, Jin Z, He J, et al: Autologous non-invasively
derived stem cells mitochondria transfer shows therapeutic
advantages in human embryo quality rescue. Biol Res.
56(60)2023.PubMed/NCBI View Article : Google Scholar
|
|
208
|
Morimoto Y, Gamage USK, Yamochi T, Saeki
N, Morimoto N, Yamanaka M, Koike A, Miyamoto Y, Tanaka K, Fukuda A,
et al: Mitochondrial transfer into human oocytes improved embryo
quality and clinical outcomes in recurrent pregnancy failure cases.
Int J Mol Sci. 24(2738)2023.PubMed/NCBI View Article : Google Scholar
|
|
209
|
Acquistapace A, Bru T, Lesault PF, Figeac
F, Coudert AE, le Coz O, Christov C, Baudin X, Auber F, Yiou R, et
al: Human mesenchymal stem cells reprogram adult cardiomyocytes
toward a progenitor-like state through partial cell fusion and
mitochondria transfer. Stem Cells. 29:812–824. 2011.PubMed/NCBI View Article : Google Scholar
|
|
210
|
Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y,
Li X, Sho T, Wang X, Li Y, et al: Mitocytosis, a migrasome-mediated
mitochondrial quality-control process. Cell. 184:2896–2910.e13.
2021.PubMed/NCBI View Article : Google Scholar
|
|
211
|
Sadeghsoltani F, Avci ÇB, Hassanpour P,
Haiaty S, Rahmati M, Mota A, Rahbarghazi R, Nemati M, Mahdipour M,
Talebi M, et al: Autophagy modulation effect on homotypic transfer
of intracellular components via tunneling nanotubes in mesenchymal
stem cells. Stem Cell Res Ther. 15(189)2024.PubMed/NCBI View Article : Google Scholar
|
|
212
|
Dai L, Wu Z, Yin L, Cheng L, Zhou Q and
Ding F: Exogenous functional mitochondria derived from bone
mesenchymal stem cells that respond to ROS can rescue neural cells
following ischemic stroke. J Inflamm Res. 17:3383–3395.
2024.PubMed/NCBI View Article : Google Scholar
|
|
213
|
Mistry JJ, Marlein CR, Moore JA, Hellmich
C, Wojtowicz EE, Smith JGW, Macaulay I, Sun Y, Morfakis A,
Patterson A, et al: ROS-mediated PI3K activation drives
mitochondrial transfer from stromal cells to hematopoietic stem
cells in response to infection. Proc Natl Acad Sci USA.
116:24610–24619. 2019.PubMed/NCBI View Article : Google Scholar
|
|
214
|
Li Y, Wang Y, Yang W, Wu Z, Ma D, Sun J,
Tao H, Ye Q, Liu J, Ma Z, et al: ROS-responsive exogenous
functional mitochondria can rescue neural cells post-ischemic
stroke. Front Cell Dev Biol. 11(1207748)2023.PubMed/NCBI View Article : Google Scholar
|
|
215
|
Saito K, Zhang Q, Yang H, Yamatani K, Ai
T, Ruvolo V, Baran N, Cai T, Ma H, Jacamo R, et al: Exogenous
mitochondrial transfer and endogenous mitochondrial fission
facilitate AML resistance to OxPhos inhibition. Blood Adv.
5:4233–4255. 2021.PubMed/NCBI View Article : Google Scholar
|
|
216
|
Ahmad T, Mukherjee S, Pattnaik B, Kumar M,
Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, et
al: Miro1 regulates intercellular mitochondrial transport &
enhances mesenchymal stem cell rescue efficacy. EMBO J.
33:994–1010. 2014.PubMed/NCBI View Article : Google Scholar
|
|
217
|
Novak J, Nahacka Z, Oliveira GL, Brisudova
P, Dubisova M, Dvorakova S, Miklovicova S, Dalecka M, Puttrich V,
Grycova L, et al: The adaptor protein Miro1 modulates horizontal
transfer of mitochondria in mouse melanoma models. Cell Rep.
44(115154)2025.PubMed/NCBI View Article : Google Scholar
|
|
218
|
Barutta F, Corbetta B, Bellini S, Gambino
R, Bruno S, Kimura S, Hase K, Ohno H and Gruden G: Protective
effect of mesenchymal stromal cells in diabetic nephropathy: The In
vitro and In vivo role of the M-Sec-tunneling nanotubes. Clin Sci
(Lond). 138:1537–1559. 2024.PubMed/NCBI View Article : Google Scholar
|
|
219
|
Barutta F, Kimura S, Hase K, Bellini S,
Corbetta B, Corbelli A, Fiordaliso F, Barreca A, Papotti MG,
Ghiggeri GM, et al: Protective role of the M-Sec-tunneling nanotube
system in podocytes. J Am Soc Nephrol. 32:1114–1130.
2021.PubMed/NCBI View Article : Google Scholar
|
|
220
|
Kastl L, Sauer SW, Ruppert T, Beissbarth
T, Becker MS, Süss D, Krammer PH and Gülow K: TNF-α mediates
mitochondrial uncoupling and enhances ROS-dependent cell migration
via NF-κB activation in liver cells. FEBS Lett. 588:175–183.
2014.PubMed/NCBI View Article : Google Scholar
|
|
221
|
Wang K, Zhou L, Mao H, Liu J, Chen Z and
Zhang L: Intercellular mitochondrial transfer alleviates pyroptosis
in dental pulp damage. Cell Prolif. 56(e13442)2023.PubMed/NCBI View Article : Google Scholar
|
|
222
|
Kimura S, Yamashita M, Yamakami-Kimura M,
Sato Y, Yamagata A, Kobashigawa Y, Inagaki F, Amada T, Hase K,
Iwanaga T, et al: Distinct roles for the N- and C-terminal regions
of M-Sec in plasma membrane deformation during tunneling nanotube
formation. Sci Rep. 6(33548)2016.PubMed/NCBI View Article : Google Scholar
|
|
223
|
Gao C, Dai Y, Spezza PA, Boasiako P, Tang
A, Rasquinha G, Zhong H, Shao B, Liu Y, Shi PA, et al:
Megakaryocytes transfer mitochondria to bone marrow mesenchymal
stromal cells to lower platelet activation. J Clin Invest.
135(e189801)2025.PubMed/NCBI View Article : Google Scholar
|
|
224
|
Irwin RM, Thomas MA, Fahey MJ, Mayán MD,
Smyth JW and Delco ML: Connexin 43 regulates intercellular
mitochondrial transfer from human mesenchymal stromal cells to
chondrocytes. Stem Cell Res Ther. 15(359)2024.PubMed/NCBI View Article : Google Scholar
|
|
225
|
Huang T, Zhang T, Jiang X, Li A, Su Y,
Bian Q, Wu H, Lin R, Li N, Cao H, et al: Iron oxide nanoparticles
augment the intercellular mitochondrial transfer-mediated therapy.
Sci Adv. 7(eabj0534)2021.PubMed/NCBI View Article : Google Scholar
|
|
226
|
Qiao X, Huang N, Meng W, Liu Y, Li J, Li
C, Wang W, Lai Y, Zhao Y, Ma Z, et al: Beyond mitochondrial
transfer, cell fusion rescues metabolic dysfunction and boosts
malignancy in adenoid cystic carcinoma. Cell Rep.
43(114652)2024.PubMed/NCBI View Article : Google Scholar
|
|
227
|
Powell AE, Anderson EC, Davies PS, Silk
AD, Pelz C, Impey S and Wong MH: Fusion between intestinal
epithelial cells and macrophages in a cancer context results in
nuclear reprogramming. Cancer Res. 71:1497–1505. 2011.PubMed/NCBI View Article : Google Scholar
|
|
228
|
Nahacka Z, Novak J, Zobalova R and Neuzil
J: Miro proteins and their role in mitochondrial transfer in cancer
and beyond. Front Cell Dev Biol. 10(937753)2022.PubMed/NCBI View Article : Google Scholar
|
|
229
|
Zhang H, Yu X, Ye J, Li H, Hu J, Tan Y,
Fang Y, Akbay E, Yu F, Weng C, et al: Systematic investigation of
mitochondrial transfer between cancer cells and T cells at
single-cell resolution. Cancer Cell. 41:1788–1802.e10.
2023.PubMed/NCBI View Article : Google Scholar
|
|
230
|
Goliwas KF, Libring S, Berestesky E,
Gholizadeh S, Schwager SC, Frost AR, Gaborski TR, Zhang J and
Reinhart-King CA: Mitochondrial transfer from cancer-associated
fibroblasts increases migration in aggressive breast cancer. J Cell
Sci. 136(jcs260419)2023.PubMed/NCBI View Article : Google Scholar
|
|
231
|
Xie Q, Zeng J, Zheng Y, Li T, Ren J, Chen
K, Zhang Q, Xie R, Xu F and Zhu J: Mitochondrial transplantation
attenuates cerebral ischemia-reperfusion injury: Possible
involvement of mitochondrial component separation. Oxid Med Cell
Longev. 2021(1006636)2021.PubMed/NCBI View Article : Google Scholar
|
|
232
|
Peruzzotti-Jametti L, Bernstock JD, Willis
CM, Manferrari G, Rogall R, Fernandez-Vizarra E, Williamson JC,
Braga A, van den Bosch A, Leonardi T, et al: Neural stem cells
traffic functional mitochondria via extracellular vesicles. PLoS
Biol. 19(e3001166)2021.PubMed/NCBI View Article : Google Scholar
|
|
233
|
Zheng D, Zhou H, Wang H, Zhu Y, Wu Y, Li
Q, Li T and Liu L: Mesenchymal stem cell-derived microvesicles
improve intestinal barrier function by restoring mitochondrial
dynamic balance in sepsis rats. Stem Cell Res Ther.
12(299)2021.PubMed/NCBI View Article : Google Scholar
|
|
234
|
Cowan DB, Yao R, Thedsanamoorthy JK,
Zurakowski D, del Nido PJ and McCully JD: Transit and integration
of extracellular mitochondria in human heart cells. Sci Rep.
7(17450)2017.PubMed/NCBI View Article : Google Scholar
|
|
235
|
Fu A: Mitotherapy as a novel therapeutic
strategy for mitochondrial diseases. Curr Mol Pharmacol. 13:41–49.
2020.PubMed/NCBI View Article : Google Scholar
|
|
236
|
Chen J, Zhong J, Wang LL and Chen YY:
Mitochondrial transfer in cardiovascular disease: From mechanisms
to therapeutic implications. Front Cardiovasc Med.
8(771298)2021.PubMed/NCBI View Article : Google Scholar
|
|
237
|
Wang ZH, Chen L, Li W, Chen L and Wang YP:
Mitochondria transfer and transplantation in human health and
diseases. Mitochondrion. 65:80–87. 2022.PubMed/NCBI View Article : Google Scholar
|
|
238
|
Walker M, Levitt MR, Federico EM, Miralles
FJ, Levy SH, Lynne Prijoles K, Winter A, Swicord JK and Sancak Y:
Autologous mitochondrial transplant for acute cerebral ischemia:
Phase 1 trial results and review. J Cereb Blood Flow Metab: Dec 4,
2024 (Epub ahead of print).
|
|
239
|
Nakai R, Varnum S, Field RL, Shi H, Giwa
R, Jia W, Krysa SJ, Cohen EF, Borcherding N, Saneto RP, et al:
Mitochondria transfer-based therapies reduce the morbidity and
mortality of Leigh syndrome. Nat Metab. 6:1886–1896.
2024.PubMed/NCBI View Article : Google Scholar
|
|
240
|
Li C, Cheung MKH, Han S, Zhang Z, Chen L,
Chen J, Zeng H and Qiu J: Mesenchymal stem cells and their
mitochondrial transfer: A double-edged sword. Biosci Rep.
39(BSR20182417)2019.PubMed/NCBI View Article : Google Scholar
|
|
241
|
Hosseinian S, Ali Pour P and Kheradvar A:
Prospects of mitochondrial transplantation in clinical medicine:
Aspirations and challenges. Mitochondrion. 65:33–44.
2022.PubMed/NCBI View Article : Google Scholar
|
|
242
|
Caicedo A, Aponte PM, Cabrera F, Hidalgo C
and Khoury M: Artificial mitochondria transfer: Current challenges,
advances, and future applications. Stem Cells Int.
2017(7610414)2017.PubMed/NCBI View Article : Google Scholar
|
|
243
|
Ishikawa K, Toyama-Sorimachi N, Nakada K,
Morimoto M, Imanishi H, Yoshizaki M, Sasawatari S, Niikura M,
Takenaga K, Yonekawa H and Hayashi J: The innate immune system in
host mice targets cells with allogenic mitochondrial DNA. J Exp
Med. 207:2297–2305. 2010.PubMed/NCBI View Article : Google Scholar
|
|
244
|
Deuse T, Hu X, Agbor-Enoh S, Koch M,
Spitzer MH, Gravina A, Alawi M, Marishta A, Peters B,
Kosaloglu-Yalcin Z, et al: De novo mutations in mitochondrial DNA
of iPSCs produce immunogenic neoepitopes in mice and humans. Nat
Biotechnol. 37:1137–1144. 2019.PubMed/NCBI View Article : Google Scholar
|
|
245
|
Klopstock T, Klopstock B and Prokisch H:
Mitochondrial replacement approaches: Challenges for clinical
implementation. Genome Med. 8(126)2016.PubMed/NCBI View Article : Google Scholar
|
|
246
|
Yamada Y, Ito M, Arai M, Hibino M,
Tsujioka T and Harashima H: Challenges in promoting mitochondrial
transplantation therapy. Int J Mol Sci. 21(6365)2020.PubMed/NCBI View Article : Google Scholar
|
|
247
|
Yuan J, Chen F, Jiang D, Xu Z, Zhang H and
Jin ZB: ROCK inhibitor enhances mitochondrial transfer via
tunneling nanotubes in retinal pigment epithelium. Theranostics.
14:5762–5777. 2024.PubMed/NCBI View Article : Google Scholar
|
|
248
|
Chi A, Yang B, Dai H, Li X, Mo J, Gao Y,
Chen Z, Feng X, Ma M, Li Y, et al: Stem Leydig cells support
macrophage immunological homeostasis through mitochondrial transfer
in mice. Nat Commun. 15(2120)2024.PubMed/NCBI View Article : Google Scholar
|
|
249
|
Játiva S, Calle P, Torrico S, Muñoz Á,
García M, Martinez I, Sola A and Hotter G: Mitochondrial
transplantation enhances phagocytic function and decreases lipid
accumulation in foam cell macrophages. Biomedicines.
10(329)2022.PubMed/NCBI View Article : Google Scholar
|