Introduction
Oral squamous cell carcinoma (OSCC), accounting for
~90% of oral cancer cases, and poses a global health challenge with
a 5-year survival rate of 35-50%, primarily due to late diagnosis
driven by non-specific symptoms and invasive diagnostics (1,2).
Fanconi anemia (FA), a rare genetic disorder affecting 1 in 130,000
individuals, markedly increases susceptibility to squamous cell
carcinomas (SCC), with OSCC among the most prevalent, exhibiting a
500-700-fold elevated risk, often at younger ages (3,4).
This necessitates robust surveillance strategies that are
sensitive, comprehensive and sustainable for high-risk patients
with FA (5). Non-invasive
biomarkers, such as microRNAs (miRNAs/miRs) and proteins, enable
patient-friendly monitoring through biofluids, such as saliva and
serum, thereby enhancing early detection (6).
miR-34a, a potent tumor suppressor, regulates the
Wnt and PI3K pathways by targeting genes [e.g., Wnt family member 1
(WNT1), phosphatase and tensin homolog (PTEN) and baculoviral IAP
repeat containing 5 (BIRC5)] and promotes apoptosis (7). However, the downregulation of its
expression has been reported in SCCs (head and neck, lung and
cervical) and other types of cancer (leukemia and glioma), with
this being associated with tumor progression (8). Survivin, an apoptosis inhibitor,
suppresses caspase 3/9 activity and is overexpressed in OSCC, head
and neck SCC, and cancers such as lung and gastric cancer, is
detectable in serum and saliva, and is linked to an aggressive
tumor behavior (9). Despite their
established roles in SCCs, the combined utility of salivary miR-34a
and serum survivin for OSCC surveillance in FA remains
underexplored, particularly in a comparative setting with patients
with OSCC and healthy controls. Thus, the present pilot study
evaluated these biomarkers in patients with FA (n=24), patients
with OSCC (n=24) and healthy controls (n=40) cohorts to establish
non-invasive tools for early detection of OSCC in FA, aiming to
improve clinical outcomes in this high-risk population.
Patients and methods
Study population
The present study included 24 patients with OSCC
(mean age, 54.52±10.49 years; 58.3% male; TNM stages I-IV), 24
patients with FA (mean age, 20.03±5.87 years; 58.3% male) and 40
healthy controls (mean age, 42.23±10.22 years, 50% male). OSCC was
diagnosed via histopathological biopsy, and FA was confirmed by
chromosomal breakage tests (using mitomycin C and diepoxybutane
tests). Genetic analysis for specific germline mutations (e.g.,
FANCA and FANCC) was not performed in the present study due to
resource limitations.
Exclusion criteria included other malignancies,
autoimmune disorders, or recent infections. The patients with FA
had no history of OSCC at the time of sampling. The demographic
characteristics of the study participants (age, sex, oral hygiene
and oral lesions) are presented in Table I.
 | Table IDemographic characteristics of
patients with OSCC ad FA, and the controls. |
Table I
Demographic characteristics of
patients with OSCC ad FA, and the controls.
| Feature | Patients with OSCC
(n=24) | Patients with FA
(n=24) | Control group
(n=40) |
|---|
| Age range, years | 31-67 | 14-32 | 25-53 |
| Age, years (mean ±
SD) | 54.52±10.49 | 20.03±5.87 | 42.13±10.92 |
| Sex, n (%) | | | |
|
Male | 14 (58.3) | 14 (58.3) | 20(50) |
|
Female | 10 (41.7) | 10 (41.6) | 20(50) |
| Oral hygiene | | | |
|
Poor | 10 (41.6) | 14 | 21(55) |
|
Good | 14 (58.3) | 10 | 19(45) |
| Oral lesions | | | |
|
Present | 19 (79.1) | 9 (37.5) | 14(20) |
|
Absent | 5 (20.9) | 15 (67.5%) | 26(80) |
Ethical approval and consent
The present study was approved by the Istanbul
University Clinical Research Ethics Committee (Approval no. 1307,
November 13, 2019) and conducted in accordance with the Declaration
of Helsinki. Written informed consent was obtained from all
participants, with parental consent being obtained for those <18
years of age.
Sample collection
Saliva (4 ml) and serum (6 ml in EDTA tubes) were
collected under standardized conditions. Saliva was centrifuged at
3,000 x g for 10 min at 4˚C to remove debris, and serum was
separated at 1,500 x g for 10 min at 4˚C. Supernatants were stored
at -80˚C until analysis.
miRNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Salivary miRNA was extracted using the miRNeasy Mini
kit (Qiagen GmbH). RNA purity and concentration were verified using
a NanoDrop spectrophotometer (A260/A280 ratio: 1.8-2.0). cDNA was
synthesized using the miRCURY LNA RT kit (Qiagen GmbH).
Quantitative PCR (qPCR) was performed using SYBR-Green Master Mix
(Qiagen GmbH) on a LightCycler 480 (Roche Diagnostics) under the
following cycling conditions: Initial activation at 95˚C for 2 min,
followed by 40 cycles of denaturation at 95˚C for 10 sec and
annealing/extension at 56˚C for 60 sec, and a melt curve analysis
from 65˚C to 95˚C.Primers for hsa-miR-34a-5p (forward,
5'-GCAGTGGCAGTGTCTTAG-3'; reverse,
5´-GGTCCAGTTTTTTTTTTTTTTTACAAC-3') and U6 snRNA (human; forward,
5'-CTCGCTTCGGCAGCACA-3'; reverse, 5' AACGCTTCACGAATTTGCT-3' were
purchased from Qiagen GmbH. Relative expression levels were
calculated using the 2-ΔΔCq method (10).
Protein analysis
Serum survivin levels were quantified using a
commercial ELISA kit (Cat. No: E3904Hu, Human Survivin ELISA kit,
BT-Lab). Standard curves were generated according to the
manufacturer's instructions, and the optical density was measured
at 450 nm using a microplate reader (BioTek; Agilent Technologies,
Inc.).
Statistical analysis
Data normality was assessed using the Shapiro-Wilk
test for continuous variables (age, salivary miR-34a expression and
serum survivin levels). Age satisfied the normality assumption
(Shapiro-Wilk, P=0.37) and data were compared between groups using
one-way ANOVA with the Bonferroni correction for post hoc pairwise
comparisons. Salivary miR-34a and serum survivin distributions
deviated from normality (Shapiro-Wilk, P<0.05) and are therefore
are presented as the median and interquartile range (IQR). Group
comparisons for these biomarkers were performed using the
Kruskal-Wallis test, followed by Dunn's post hoc test for multiple
comparisons. Categorical variables were compared using the
Chi-squared test or Fisher's exact test, as appropriate. All
correlation analyses were performed using Spearman's rank
correlation. Receiver operating characteristic (ROC) analyses were
performed to calculate the area under the curve (AUC), 95%
confidence intervals (CIs), cut-off values, sensitivity and
specificity. All tests were two-sided, and a value of P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS v25 (IBM Corp.).
Results
Demographic and clinical
characteristics of the study participants
The present study comprised 24 patients with OSCC
(mean age, 54.52±10.7 years; 58.3% male), 24 patients with FA (mean
age, 20.03±5.87 years; 58.3% male) and 40 healthy controls (mean
age, 42.13±10.92 years; 50% male). Age followed a normal
distribution (Shapiro-Wilk, P=0.37), while salivary miR-34a and
serum survivin did not (Shapiro-Wilk, P<0.05). Therefore,
non-parametric tests were applied for these biomarkers. Sex
distribution was also comparable (P>0.05). The demographic
characteristics of the patient and control groups are summarized in
Table I.
miR-34a expression levels
As salivary miR-34a and serum survivin values
deviated from normality (Shapiro-Wilk, P<0.05), they are
presented as the median (IQR) and were compared using the
Kruskal-Wallis test with Dunn's post-hoc (Table II). Salivary miR-34a expression,
quantified using RT-qPCR with the 2-ΔΔCq method with U6
normalization, was significantly lower in the patients with OSCC
[median (IQR), 1.33 (0.68-3.69); P=0.012] and FA [median (IQR),
0.72 (0.05-3.19); P=0.014] compared to the controls [median (IQR),
3.63 (0.32-14.77)]. No significant difference was observed between
the OSCC and FA (P=0.78) groups. These results are presented in
Fig. 1 and Table II.
 | Table IImiR-34a and survivin levels in the
OSCC, FA and control groups. |
Table II
miR-34a and survivin levels in the
OSCC, FA and control groups.
| Group | miR-34a
(2-ΔΔCq) median (IQR) | P-value vs.
control | Survivin (ng/ml)
median (IQR) | P-value vs.
control |
|---|
| OSCC (n=24) | 1.33 (0.68-3.69) | 0.012 | 196.19
(165.83-298.75) | 0.01 |
| FA (n=24) | 0.72 (0.05-3.19) | 0.014 | 216.38
(102.89-858.87) | 0.001 |
| Control (n=40) | 3.63
(0.32-14.77) | | 121.90
(103.85-182.03) | |
In the patients OSCC, the miR-34a levels tended to
be lower in those with advanced tumor stages (T3-T4) and lymph node
metastasis; however, these associations were not statistically
significant (P=0.34 and P=0.87, respectively; Table III). No significant associations
were found with other clinical parameters, including depth of
invasion, differentiation, or treatment response (P>0.05;
Table III).
 | Table IIIAssociation of miR-34a and survivin
expression with clinicopathological features of patients with
OSCC. |
Table III
Association of miR-34a and survivin
expression with clinicopathological features of patients with
OSCC.
| | | miR-34a | Survivin |
|---|
| Clinical
characteristics of patients with OSCC | No. of patients
(%) | High expression | Low expression | P-value | High expression | Low expression | P-value |
|---|
| Tumor size | | | | | | | 0.31 |
|
<2
cm | 3 (12.5) | 1 (33.3) | 2 (66.7) | | 1 (33.3) | 2 (66.7) | |
|
2-3 cm | 15 (62.5) | 4 (26.6) | 11 (73.3) | | 10 (66.7) | 5 (33.3) | |
|
>4
cm | 6(25) | 2 (33.3) | 4 (66.7) | 0.08 | 4 (66.7) | 2 (33.3) | |
| Tumor stage | | | | | | | 0.23 |
|
T1, T2 | 11 (45.8) | 3 (37.5) | 8 (62.5) | | 10 (58.8) | 7 (41.2) | |
|
T3, T4 | 13 (54.2) | 4 (30.8) | 9 (69.2) | 0.34 | 8 (61.5) | 5 (38.5) | |
| Lymph node
metastasis | | | | | | | 0.72 |
|
Present | 9 (37.5) | 2 (22.2) | 7 (77.7) | 0.87 | 6 (66.7) | 3 (33.3) | |
|
Absent | 15 (62.5) | 5 (33.3) | 10 (66.7) | | 8 (53.3) | 7 (46.7) | |
| Degree of
differentiation | | | | | | | 0.84 |
|
Poor | 2 (8.3) | 0 (0) | 2(100) | | 1(50) | 1(50) | |
|
Moderate | 17 (70.9) | 6 (37.5) | 10 (62.5) | 1.56 | 10 (58.8) | 7 (29.2) | |
|
Well | 5 (20.8) | 1(20) | 4(80) | | 3(60) | 2(40) | |
| Depth of
invasion | | | | | | | 0.95 |
|
<10
mm | 8 (33.3) | 4(50) | 4(50) | | 8(100) | 0 (0) | |
|
>10mm | 16 (66.7) | 5 (31.3) | 11 (68.7) | 0.058 | 4(25) | 12(75) | |
| Perineural
invasion | | | | | | | 0.45 |
|
Present | 14 (58.3) | 4 (28.5) | 5 (71.5) | 0.08 | 7(50) | 7(50) | |
|
Absent | 10 (41.6) | 3(30) | 7(70) | | 7(70) | 3(30) | |
| Lymphovascular
invasion | | | | | | | 0.22 |
|
Present | 9 (37.5) | 2 (22.2) | 7 (77.8) | 0.21 | 5 (55.6) | 4 (44.4) | |
|
Absent | 15 (62.5) | 5 (33.3) | 10 (66.7) | | 11 (73.3) | 4 (26.7) | |
| Chemotherapy
response | | | | | | | 0.08 |
|
Present | 15 (62.5) | 5 (33.3) | 10 (66.7) | 0.07 | 9(60) | 6(40) | |
|
Absent | 9 (37.5) | 2 (22.2) | 7 (77.8) | | 1 (11.1) | 8 (88.9) | |
| Radiotherapy
response | | | | | | | 0.09 |
|
Present | 16 (66.6) | 4(25) | 12(75) | 0.99 | 5 (31.3) | 11 (68.7) | |
|
Absent | 8 (33.4) | 4(50) | 4(50) | | 2(25) | 6(75) | |
| Progression | | | | | | | 0.14 |
|
Present | 7 (29.2) | 1 (14.3) | 6 (85.7) | 0.16 | 7(100) | 0 (0) | |
|
Absent | 17 (70.8) | 6 (35.3) | 11 (64.7) | | 7 (41.2) | 10 (58.8) | |
Survivin levels
The serum survivin levels, measured using ELISA,
were significantly elevated in patients with OSCC [median (IQR),
196.19 (165.83-298.75) ng/ml; P=0.01) and FA [median (IQR), 216.38
(102.89-858.87); P=0.001] compared to the controls [median (IQR),
121.90 (103.85-182.03)]. The survivin levels were higher in
patients with FA than in patients with OSCC (P=0.028). These
results are presented in Fig. 2
and Table II.
In OSCC, the survivin levels did not exhibit a
significant association with clinical parameters, including tumor
stage, lymph node metastasis, or progression (P>0.05; Table III).
Correlation analysis
ROC analysis was used to evaluate the diagnostic
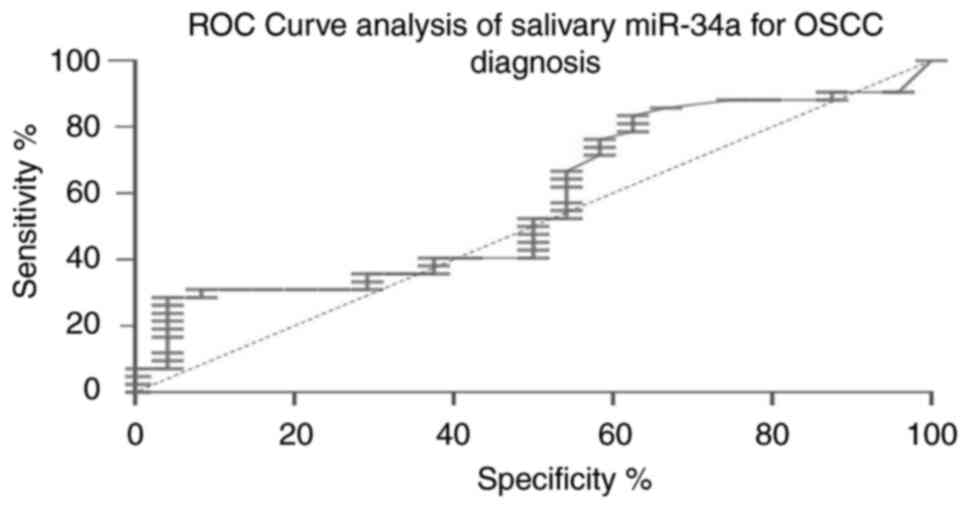
accuracy of miR-34a and survivin in OSCC. Salivary miR-34a
exhibited poor discriminatory power (AUC, 0.575; 95% CI,
0.439-0.719; cut-off value, >24.53; sensitivity, 24%;
specificity, 95%; Fig. 3), while
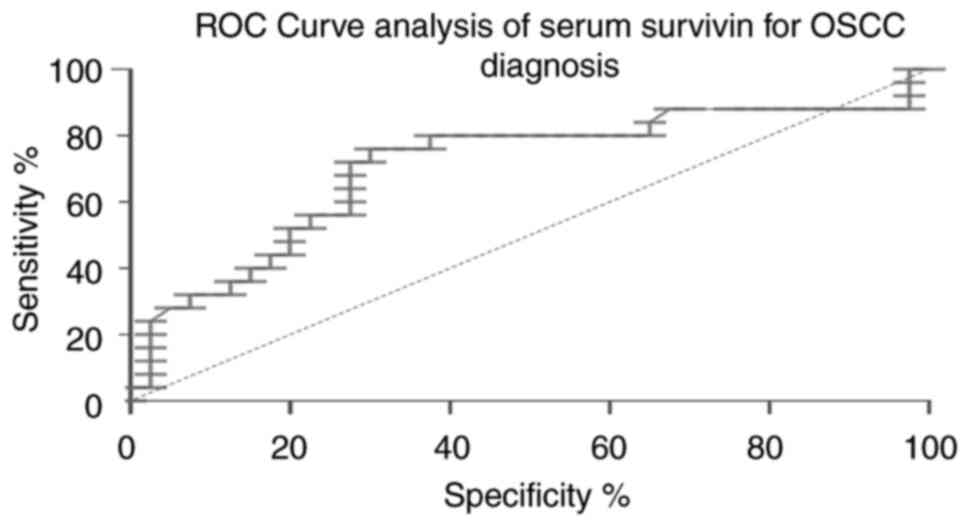
serum survivin exhibited a moderate discriminatory power (AUC,
0.710; 95% CI, 0.657-0.934; cut-off value, >191.1; sensitivity,
70%; specificity, 97%; Fig. 4). In
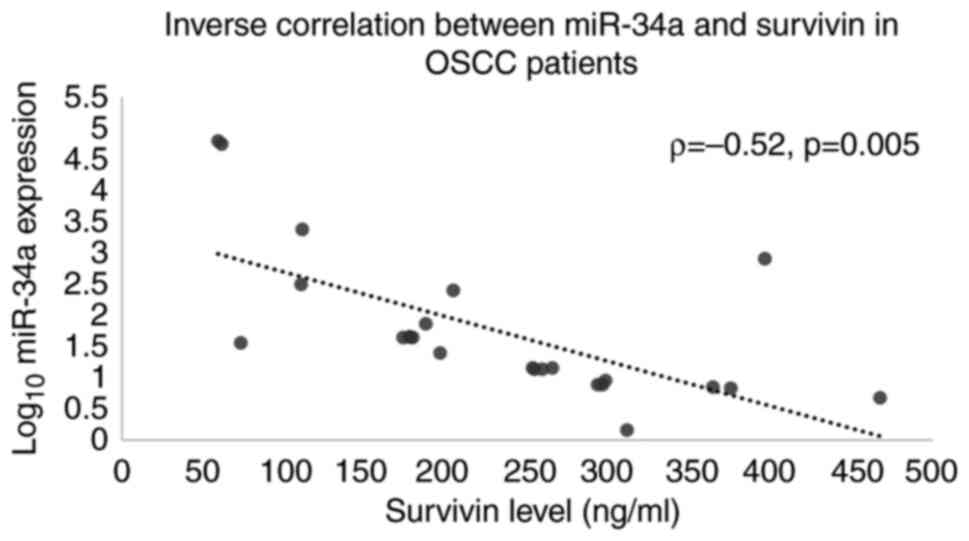
patients with OSCC, salivary miR-34a expression inversely
correlated with serum survivin levels (Spearman's Rho=-0.52;
P=0.005; Table IV and Fig. 5).
 | Table IVInverse correlation between miR-34a
and survivin expression in OSCC patients. |
Table IV
Inverse correlation between miR-34a
and survivin expression in OSCC patients.
| Group | Correlation
coefficient (Rho) | P-value |
|---|
| OSCC (n=24) | -0.52 | 0.005 |
| FA (n=24) | - | >0.05 |
| Control (n=40) | - | >0.05 |
Discussion
The present pilot study was the first to evaluate
salivary miR-34a and serum survivin as non-invasive biomarkers for
OSCC surveillance in patients with FA and OSCC, with a focus on
their roles in cancer pathways. The findings revealed significant
alterations in miR-34a (P=0.012 in OSCC and P=0.014 in FA) and
survivin (P=0.04 in OSCC and P=0.01 in FA) compared to the
controls, providing molecular insight into the risk of developing
OSCC in patients with FA. Another key finding and limitation is the
marked age disparity between patients with FA and OSCC, which
reflects the biology of FA-related carcinogenesis, but introduces
potential age-related confounding factors.
The marked reduction of miR-34a levels in both OSCC
and FA aligns with its tumor-suppressive role, as it regulates the
Wnt and PI3K pathways by targeting genes, such as WNT1, PTEN and
BIRC5(11). However, miR-34a did
not exhibit a significant association with clinical parameters,
such as T stage or lymph node metastasis (P=0.34 and P=0.87,
respectively). It exhibited poor diagnostic performance (AUC,
0.575), indicating limitations as a standalone early predictive
marker for OSCC. Future studies are thus required to investigate
the use of combined biomarkers to improve the diagnostic accuracy.
In OSCC, a decreased miR-34a expression was associated with
enhanced tumor proliferation and metastasis, consistent with
studies reporting its downregulation in head and neck SCCs and an
association with advanced tumor stages (12). In the present study, the findings
of a reduced miR-34a level in OSCC and FA are consistent with those
of the study by Kalfert et al (8), who reported the downregulation of
miR-34a in head and neck cancers, emphasizing its potential as a
salivary biomarker for early detection.
In FA, the reduction of miR-34a expression, despite
elevated p53 activity, suggests alternative pathways that suppress
tumor-suppressive mechanisms, potentially indicating early
malignant transformation. This finding is in contrast to that of
reports of miR-34a upregulation in acute graft-vs.-host disease in
FA, highlighting the context-specific regulation of this miRNA
(13).
Elevated survivin levels in OSCC and FA underscore
its anti-apoptotic function via the PI3K/AKT signaling pathway,
promoting tumor survival (9).
Overexpressed in SCCs (e.g., head and neck, lung) and detectable in
biofluids, survivin is associated with an aggressive tumor behavior
and a poor prognosis (9,14). In the present study, in patients
with FA, significantly higher survivin levels (P=0.001 vs. controls
and P=0.028 vs. OSCC) may reflect the evasion of apoptosis under
cellular stress. However, its lack of specificity limits its
utility as an OSCC-specific marker in FA. These findings align with
those in the study by Xie et al (15), who identified survivin as a
prognostic biomarker in oral cancers through a meta-analysis,
highlighting its role in tumor progression. The inverse correlation
observed between salivary miR-34a and serum survivin (Spearman's
Rho=-0.52, P=0.005) in patients with OSCC supports a potential
regulatory axis, which may be disrupted in FA. Although the present
study did not directly investigate mechanistic regulation, previous
studies suggest that miR-34a can downregulate survivin directly via
the PI3K/AKT pathway (16). This
possible regulatory axis warrants functional validation in future
in vitro and in vivo studies.
The consistency of miR-34a and survivin alterations
in saliva and serum supports non-invasive sampling, which is
particularly beneficial for patients with FA who are intolerant to
invasive procedures. Salivary biomarkers, such as miR-34a, could
enhance annual OSCC screening protocols for high-risk populations
such as patients with FA by enabling non-invasive monitoring during
routine dental check-ups. Although limited by sample size (FA,
n=24; OSCC, n=24; controls, n=40), which prevents the
identification of significant associations with clinical
parameters, the trends of lower miR-34a expression in advanced OSCC
stages align with previous research (8,12).
The ROC analysis for survivin (AUC, 0.710; sensitivity, 70%;
specificity, 97%) indicates moderate diagnostic potential, which is
lower than that reported in other cancer types (e.g., AUC, 0.729;
sensitivity, 57%; specificity, 82.6% in colon cancer) (17). By contrast, miR-34a exhibited poor
diagnostic performance (AUC, 0.575), further indicating that it
cannot be considered a reliable standalone marker and supporting
the need for multi-marker approaches.
The findings of the present study align with those
in the study by Chen et al (18), who linked miR-34a suppression to
cutaneous SCC progression, and studies identifying survivin as a
prognostic marker in head and neck cancers (9,14,15).
By demonstrating alterations in miR-34a and survivin in FA and
OSCC, the present study lays the groundwork for non-invasive
biomarker strategies to enhance early detection of OSCC in FA.
However, larger studies are warranted to validate their clinical
utility.
The present study has certain limitations which
should be mentioned. The key limitations of the present study
include the small sample size, the genotypic heterogeneity of FA
(e.g., FANCA mutations) and demographic variability between groups.
In particular, the significant age disparity between the patients
with FA (mean age, 20.03±5.87 years) and patients with OSCC (mean
age, 54.52±10.49 years) reflects the biology of FA-related
carcinogenesis, but also introduces potential age-related
confounding factors. The recruitment of age-matched patients with
FA and sporadic OSCC was not feasible in the current single-center
sample, and the limited cohort size precluded adequately powered
age-adjusted multivariable analyses. Additionally, the lack of
tumor stage stratification in OSCC further limits the
generalizability of the findings. Consequently, the biomarker
differences observed herein should be interpreted cautiously and
validated in larger, multicenter cohorts that are age-balanced or
stratified and prospectively followed for OSCC development and
treatment outcomes.
In conclusion, the present study demonstrates that
miR-34a and survivin are promising non-invasive biomarkers for the
surveillance of OSCC in patients with FA and OSCC. Their
integration into clinical screening protocols could enhance early
detection and improve survival outcomes in patients with FA and
OSCC.
Acknowledgements
The authors would like to thank the organizing
committee of the European Hematology Oncology Congress (EHOC) 2021,
held as a hybrid event on November 10-13, 2021, at the Hilton
Istanbul Bomonti Hotel and Conference Center, for providing a
platform to present a preliminary version of this study (Abstract,
published with DOI: 10.1016/j.htct.2021.10.1085), which helped
refine the research through valuable feedback.
Funding
Funding: The present study was supported by the Scientific
Research Projects Coordination Unit of Istanbul University (Project
no. 34946) as part of a doctoral thesis project.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZAK conceptualized and designed the study, performed
the experiments, analyzed the data, drafted the manuscript and
supervised the project. NY contributed to the study design,
performed the clinical examinations of patients with Fanconi
anemia, and contributed to the writing of the manuscript and
evaluation of the results. TTC conducted the clinical examinations
of patients with Fanconi anemia and evaluated the study outcomes.
BB, HMY and OK performed clinical the examinations of patients with
oral squamous cell carcinoma and contributed to the evaluation of
the study outcomes. MGG conducted the statistical analyses and
contributed to data interpretation. ZAK and NY confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Istanbul
University Clinical Research Ethics Committee (Approval no. 1307;
November 13, 2019) and conducted in accordance with the Declaration
of Helsinki. Written informed consent was obtained from all
participants, with parental consent being obtained for those <18
years of age.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Rivera C and Venegas B: Histological and
molecular aspects of oral squamous cell carcinoma (Review). Oncol
Lett. 8:7–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mehrotra R and Yadav S: Oral squamous cell
carcinoma: Etiology, pathogenesis, and prognostic value of genomic
alterations. Indian J Cancer. 43:60–66. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kutler DI, Auerbach AD, Satagopan J,
Giampietro PF, Batish SD, Huvos AG, Goberdhan A, Shah JP and Singh
B: High incidence of head and neck squamous cell carcinoma in
patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg.
129:106–112. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Singh T and Andi K: Fanconi anemia and
oral squamous cell carcinoma: Management considerations. N Z Med J.
130:92–95. 2017.PubMed/NCBI
|
|
6
|
Smetsers SE, Velleuer E, Dietrich R, Wu T,
Brink A, Buijze M, Deeg DJ, Soulier J, Leemans CR, Braakhuis BJ and
Brakenhoff RH: Noninvasive molecular screening for oral precancer
in Fanconi anemia patients. Cancer Prev Res (Phila). 8:1102–1111.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mazumder S, Datta S, Ray JG, Chaudhuri K
and Chatterjee R: Liquid Biopsy: miRNA as a potential biomarker in
oral cancer. Cancer Epidemiol. 58:137–145. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kalfert D, Ludvikova M, Pesta M, Ludvik J,
Dostalova L and Kholová I: Multifunctional Roles of miR-34a in
cancer: A review with the emphasis on head and neck squamous cell
carcinoma and thyroid cancer with clinical implications.
Diagnostics (Basel). 10(563)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pickhard A, Gröber S, Haug AK, Piontek G,
Wirth M, Straßen U, Rudelius M and Reiter R: Survivin and pAkt as
potential prognostic markers in squamous cell carcinoma of the head
and neck. Oral Surg Oral Med Oral Pathol Oral Radiol. 117:733–742.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fu J, Imani S, Wu MY and Wu RC:
MicroRNA-34 family in cancers: Role, mechanism, and therapeutic
potential. Cancers (Basel). 15(4723)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kumar B, Yadav A, Lang J, Teknos TN and
Kumar P: Dysregulation of microRNA-34a expression in head and neck
squamous cell carcinoma promotes tumor growth and tumor
angiogenesis. PLoS One. 7(e37601)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang L, Romero M, Ratajczak P, Lebœuf C,
Belhadj S, Peffault de Latour R, Zhao WL, Socié G and Janin A:
Increased apoptosis is linked to severe acute GVHD in patients with
Fanconi anemia. Bone Marrow Transplant. 48:849–853. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khan Z, Khan AA, Yadav H, Prasad GBKS and
Bisen PS: Survivin, a molecular target for therapeutic
interventions in squamous cell carcinoma. Cell Mol Biol Lett.
22(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie S, Xu H, Shan X, Liu B, Wang K and Cai
Z: Clinicopathological and prognostic significance of survivin
expression in patients with oral squamous cell carcinoma: Evidence
from a meta-analysis. PLoS One. 10(e0116517)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shen Z, Zhan G, Ye D, Ren Y, Cheng L, Wu Z
and Guo J: MicroRNA-34a affects the occurrence of laryngeal
squamous cell carcinoma by targeting the antiapoptotic gene
survivin. Med Oncol. 29:2473–2480. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jakubowska K, Pryczynicz A,
Dymicka-Piekarska V, Famulski W and Guzińska-Ustymowicz K:
Immunohistochemical expression and serum level of survivin protein
in colorectal cancer patients. Oncol Lett. 12:3591–3597.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen S, Yuan M, Chen H, Wu T, Wu T, Zhang
D, Miao X and Shi J: MiR-34a-5p suppresses cutaneous squamous cell
carcinoma progression by targeting SIRT6. Arch Dermatol Res.
316(299)2024.PubMed/NCBI View Article : Google Scholar
|















![Salivary miR-34a expression levels in
the FA, OSCC and control groups. Bar graph illustrating the median
(IQR) miR-34a expression levels (quantified by RT-qPCR using the
2-ΔΔCq method with U6 normalization) across the groups
[OSCC, 1.33 (0.68-3.69); FA, 0.72 (0.05-3.19); and control, 3.63
(0.32-14.77)]. P-values indicate significant differences: P=0.012
for OSCC vs. control and P=0.014 for FA vs. control. Error bars
represent IQR. IQR, interquartile range; OSCC, oral squamous cell
carcinoma; FA, Fanconi anemia.](/article_images/wasj/8/1/wasj-08-01-00422-g00.jpg)
![Serum survivin levels in the FA, OSCC
and control groups. Bar graph illustrating the median (IQR) serum
survivin levels (measured using ELISA) in the different groups
[OSCC, 196.19 (165.83-298.75) ng/ml; FA, 216.38 (102.89-858.87)
ng/ml; and control, 121.90 (103.85-182.03) ng/ml]. P-values were as
follows: P=0.01 for OSCC vs. control, P=0.001 for FA vs. control,
and P=0.028 for FA vs. OSCC. Error bars represent IQR. IQR,
interquartile range; OSCC, oral squamous cell carcinoma; FA,
Fanconi anemia.](/article_images/wasj/8/1/wasj-08-01-00422-g01.jpg)