Introduction
Desmoid-type fibromatosis (DTF) is a benign, locally
infiltrating mesenchymal tumor that causes fibroblast and
myofibroblast proliferation, and increased collagen production in
deep musculoaponeurotic structures. It has intermediate biological
characteristics between benign fibrous lesions and fibrosarcoma
(1). The disease is extremely
rare, accounting for only 0.03% of all neoplasms (2). Additionally, a notable predilection
towards females has been observed (3). The etiology of DTF is multifaceted,
possibly influenced by a combination of genetic predisposition,
traumatic events and hormonal factors (4). DTF is classified into three subtypes
based on their anatomical location: Abdominal wall,
intra-abdominal, and extra-abdominal. Extra-abdominal desmoid
tumors account for approximately one-third of all desmoid cases and
most commonly arise in the shoulder, pelvic girdle and limbs.
However, only 10-25% of cases occur in the head and neck region
(5). Unlike other benign neoplasms
in this area, desmoid tumors exhibit infiltrative growth and a
marked propensity for local recurrence following surgery,
underscoring the need for accurate diagnosis and appropriate
management. Although complete resection with negative microscopic
margins is considered the standard of care for DTF which is
unresponsive to other treatments, achieving such margins in the
head and neck is challenging due to the density of vital
structures. This difficulty is even more pronounced in pediatric
patients, where surgeons may hesitate to perform wide resections
owing to the substantial risk of post-operative functional
morbidity (3,4).
The present study describes the case of a male
pediatric patient diagnosed with DTF involving the level II. The
present case report was written in accordance with the CaReL
guidelines, with filtering references to prevent citing contents in
blacklisted journals (6,7).
Case report
Patient information
On December 20, 2023, a 2-year-old male patient was
brought to Smart Health Tower, Sulaymaniyah, Iraq, presenting with
a gradually progressing swelling in the left submandibular region
that had developed insidiously over the previous 3 months.
Clinical findings
The neck mass was large, firm and non-tender, below
the angle of the mandible on the left side of the neck.
Diagnostic assessment
The hematological test results of the patient were
normal. A neck ultrasound revealed a well-defined, lobulated,
predominantly solid hypoechoic left subcutaneous mass, measuring
38x31x27 mm, with mild vascularity, mild surrounding inhomogeneity
and features suggestive of a suppurative or necrotic lymph node.
There was bilateral cervical lymphadenopathy, with nodes of
variable size, well-defined, with a cortical thickness <2 mm,
and a preserved shape and hilar echotexture; the largest node
measured 15x6 mm in the left level II. The thyroid, submandibular
and parotid glands were normal, with no focal lesions (data not
shown). A contrast-enhanced computed tomography (CT) scan of the
chest and abdomen yielded normal findings (data not shown).
Therapeutic intervention
A surgical cervical exploration with lymph node
biopsy was performed under general anesthesia with the patient
placed in the supine position. A post-operative histopathological
examination (HPE) was performed. The biopsy specimen was placed
into tissue cassettes and processed using the DiaPath Donatello
automated processor with a standard 11-h protocol involving graded
alcohols, xylene and paraffin. Following paraffin embedding and
trimming, blocks were sectioned at a thickness of 4-6 µm onto
standard glass slides, incubated in an oven at 60˚C overnight, and
stained with hematoxylin and eosin (H&E) on the DiaPath Giotto
automated stainer using Gill II hematoxylin (1% for 10 min). The
slides were then dried, coverslipped, and examined under a light
microscope (Leica Microsystems GmbH). For immunohistochemistry,
paraffin-embedded tissues were sectioned at 4-6 µm and mounted onto
charged glass slides, followed by overnight incubation at 60˚C.
Antigen retrieval was performed using the Dako PT Link system
(Agilent Technologies, Inc.) by heating the sections to 100˚C for
5-10 min in either pH 6.0 or pH 9.0 retrieval solution, depending
on the target antibody. The slides were then washed for 1 min in a
20 ml buffer solution (0.05 mol/l Tris/HCl, 0.15 mol/l NaCl, 0.05%
Tween-20, pH 7.6) at room temperature. Hydrophobic wells were
created using the Dako Pen (Agilent Technologies, Inc.). Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide. Primary
antibodies [smooth muscle actin (mouse monoclonal, clone 1A4,
1:100, cat. no. M0851) and β-catenin (mouse monoclonal clone
β-catenin-1, 1:100, cat. no. M3539) both from Dako; Agilent
Technologies, Inc.] were applied at room temperature for 80 min,
followed by incubation with a horseradish peroxidase-conjugated
secondary antibody (1:200, cat. no. P0447, Dako; Agilent
Technologies, Inc.) and diaminobenzidine (DAB) chromogen for 15 min
each at room temperature. Counterstaining was performed with Gill
II hematoxylin for 30 sec, after which the slides were dried and
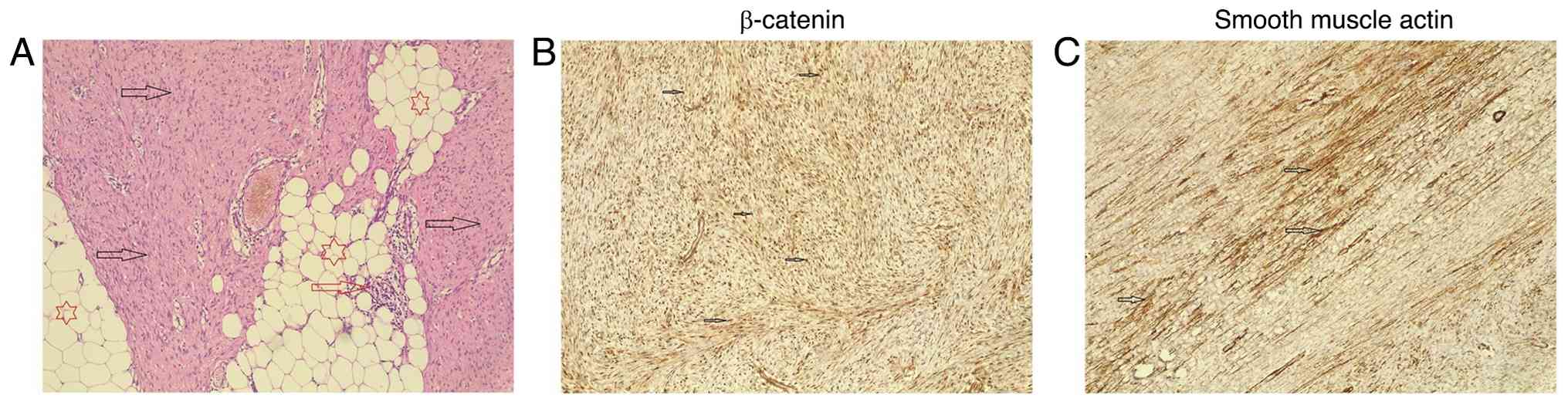
coverslipped. The post-operative HPE revealed a 4-cm, well-defined
mass with a rubbery tan-white fibrotic texture. It was diagnosed as
a low-grade spindle cell neoplasm, specifically a DTF, aided by
immunohistochemical staining, which yielded positive results for
smooth muscle actin and β-catenin (Fig. 1).
Follow-up and outcome
The post-operative period was uneventful, and the
patient was discharged from the hospital in good health after 2
days. The follow-up treatment plan included regular clinical
assessments and imaging as needed to monitor for recurrence, as
part of standard care for DTF, which has a risk of local
recurrence. Following a 6-month follow-up period, no recurrence was
reported.
Discussion
Herein, a literature review was also conducted to
identify reports on DTF of the head and neck through a Google
Scholar search using the key word ‘neck desmoid fibromatosis’. A
total of 7 cases of DTF in the head and neck region were reviewed
and these are summarized in Table
I. Of the 7 cases, 4 patients were female and 3 patients were
male, with ages ranging from 9 months to 42 years. The tumor in 4
cases was located in the neck, while the remaining tumors were
found in the mandible (n=2) and tongue (n=1). Among the cases
reviewed herein, the neck tumors generally progressed gradually,
occasionally causing symptoms, such as numbness, pain and weakness,
whereas tumors in other locations exhibited a more rapid
progression. The preferred imaging modalities for these tumors were
a CT scan and magnetic resonance imaging (MRI). Surgical
exploration with mass excision and clear safety margins was the
standard approach for managing these tumors. The majority of
patients experienced uneventful outcomes following surgery, apart
from 1 patient who developed temporary partial facial nerve
paralysis.
 | Table ISummary of seven reported cases of
desmoid fibromatosis of the head and neck region identified in the
literature. |
Table I
Summary of seven reported cases of
desmoid fibromatosis of the head and neck region identified in the
literature.
| Authors | Age | Sex of included
patient | Presenting
compliant | Examination | Location | Imaging used for
diagnosis (CT/MRI) | Management | Outcome | (Refs.) |
|---|
| Kant et
al | 22 years | Female | Intermittent neck
pain, numbness, and weakness in her left shoulder | Weakness was evident
in her left shoulder and left elbow, and sensory loss was noted in
the left C3, C4, and C5 dermatomes | Neck | Well-defined
lobulated multicompartmental mass along the left brachial plexus
(C2-C6), occupying the paraspinal, prevertebral, and parapharyngeal
spaces, displacing the trachea and esophagus rightward and the left
carotid sheath anterolaterally. | Cervical exploration
and subtotal decompression of the tumor were performed | Unknown | (4) |
| | 36 years | Male | Gradually progressing
swelling on the left side of his neck | Neck mass was large,
firm, and non-tender, extending from the angle of the mandible to
the clavicle on the left side of the neck | Neck | Multilobulated
isointense mass on the left neck extending along the left brachial
plexus, medially into the neural foramina, and inferiorly into the
mediastinum. | Unknown | Unknown | (4) |
| Miyashita et
al | 9 months | Female | A tumor rapidly
enlarged, with part protruding from the tongue | The patient was
unable to fully close her mouth, and swallowing was aided by
compensatory motion of the unaffected side | Tongue | Whole-body CT
examination revealed no signs of any other tumoral lesions. MRI
revealed a large mass with contrast enhancement on the right side
of the tongue. MRI of the sagittal plane did not show the
possibility of infiltration into the root of the tongue. | A partial glossectomy
with a 5-mm safety margin and simultaneous reconstruction with a
local flap were performed | Good outcome without
recurrence after 1 year | (8) |
| Bouatay et
al | 38 years | Female | Recurrent desmoid
fibromatosis after 1 month of management | Painless, hard,
non-tender right laterocervical swelling (8 cm), fixed to the skin
and deep structures | Neck | Cervical CT scan
revealed a soft tissue mass infiltrating the deep lateral cervical
muscles and surrounding superficial and deep fat. | Complete tumor
resection | No local recurrence
was observed after 15 months | (11) |
| Alherabi et
al | 42 years | Female | Neck mass gradually
increasing in size | Hard, non-tender
swelling in the left posterolateral upper neck, fixed to the skin
and deep structures | Neck | Neck CT scan revealed
a soft tissue mass in the upper neck, lateral to the
sternocleidomastoid muscle, infiltrating deep lateral cervical
muscles and adjacent superficial and deep fat. | Mass widely excised
with a small remnant at the carotid bifurcation; 16-month CT showed
slow regrowth, treated with 60 Gy radiotherapy. The case
encountered temporary partial facial nerve paralysis | Good after a 2-year
follow-up with significant regression of the tumor | (14) |
| Sato et
al | 3 years | Male | Swelling of
submandibular region for 1 month | Inspection and
palpation revealed a 50x35 mm bony-hard, poorly mobile mass arising
from the left mandibular angle, expanding anteroinferiorly | Mandible | MRI revealed a
50x45x32 mm left mandibular tumor with unclear margins and high T2
signal; CT scan revealed moth-eaten mandibular destruction. | Surgical excision was
performed | Good without
recurrence after 25 months | (5) |
| Said-Al-Naief et
al | 8 years | Male | A rapidly enlarging,
painless mass along the right mandibular border for two
months. | Slight expansion of
the right mandibular border with overlying soft tissue
fullness. | Mandible | CT scan revealed an 8
mm right mandibular body mass with outer cortical destruction. | Mass resection with
wide margins of the right inferior mandibular border was
performed. | Good without
recurrence after 4.5 years | (15) |
DTF of the head and neck is a rare benign
mesenchymal tumor with an annual incidence of 2-4 cases per million
individuals (2,8). In the case series study by Hoos et
al (9), a sex predilection for
females was noted, whereas in the literature review performed in
the study by Miyashita et al (8), including 141 pediatric patients with
DTF of the head and neck aged birth to 18 years, no sex
predominance was observed. In the literature review performed in
the study by Miyashita et al (8), the mandible was the most common site
of involvement, accounting for 25% of cases. Additional reported
sites included the submandibular region, the infratemporal fossa,
the neck, the peritracheal area and the paraspinal region (8).
Empirical evidence indicates that among DTFs
involving the neck, the anterolateral aspect is a common site, with
the majority of patients presenting with a painless mass (60-94%).
However, neurogenic symptoms, such as pain or focal motor deficit
may be observed in a smaller proportion of cases (16-36%) (10). Compression of the brachial plexus
can result in symptoms, such as tingling, paresthesia, numbness and
weakness in hand muscles (1). A
rapidly enlarging DTF was also reported in a pediatric case in the
study by Miyashita et al (8). The case in the present study was a
2-year-old male presenting with a painless, slow-growing swelling
in the left submandibular region, which progressed over a period of
3 months.
The diagnosis of DTF in the head and neck region is
primarily based on a clinical presentation, imaging studies and
biopsy. While radiographs may be a useful initial diagnostic tool,
their findings can vary, and a definitive diagnosis usually
requires HPE (10,11). The accurate assessment of the
extent of the tumor and its relation to surrounding structures is
crucial for effective management. Advancing imaging techniques,
such as neck CT scans and MRI, play a key role in such an
assessment. MRI, in particular, is preferred for its superior soft
tissue contrast, which aids in more precise tumor delineation and
reduces the risk of damaging critical structures during surgery
(11). Typically, DTF exhibits
intermediate signal intensity on T1-weighted images (T1WIs),
similar to muscle tissue, and on T2-weighted images (T2WIs), where
the signal is lower than that of fat, but higher than that of
adjacent muscle. Fat-suppressed T2-weighted images often exhibit
increased signal intensity within these lesions. Areas with a low
signal intensity within DTF correspond to regions of higher
collagen content. Post-contrast imaging usually reveals moderate to
marked heterogeneous enhancement, reflecting the vascularity of the
lesion. In the case in the present study, a neck ultrasound
revealed a well-defined, lobulated, predominantly solid hypoechoic
left subcutaneous mass, measuring 38x31x27 mm, with mild
vascularity. Chest and abdominal contrasted CT scans yielded normal
findings.
The primary clinical differential diagnoses include
soft-tissue sarcoma, lymphoma, myositis ossificans and
arteriovenous malformation. Imaging often rules out the latter
conditions; however, distinguishing desmoid tumors from soft tissue
sarcomas can be challenging (12).
Desmoid tumors exhibit a spectrum of clinical behaviors, ranging
from indolent growth to aggressive local infiltration, which
significantly influences management strategies. Aggressive
variants, characterized by rapid growth, local invasion and a
higher risk of recurrence, often require multimodal treatment.
Post-operative radiation therapy (RT) is particularly considered in
cases where achieving negative surgical margins is challenging or
where tumors are located near critical structures, making complete
excision infeasible without undue morbidity (1,13).
Despite this, the role of RT remains controversial due to the
benign histological nature of desmoid tumors and the potential for
significant complications, coupled with a lack of prospective
clinical trials assessing its efficacy in reducing recurrence rates
(11,14,15).
Current evidence is derived from retrospective studies, which
suggest that combining RT with surgery may improve local control
rates, particularly in cases where wide margins are difficult to
achieve (8). Surgical resection
has traditionally been the primary treatment for primary and
recurrent desmoid tumors, aiming for tumor-free margins. A 5-year
local control rate of 80% with negative-margin resection has been
reported. However, some experts argued that achieving negative
margins may lead to unnecessary complications and may not prevent
local recurrence (11). The
recurrence rate among pediatric patients undergoing surgery has
been reported as 27.2% (8).
For benign or less aggressive variants, which
demonstrate slow growth and minimal recurrence risk, a more
conservative approach, such as watchful waiting, is gaining
acceptance, particularly for asymptomatic cases or tumors in
anatomically challenging locations. Given the challenges of
balancing treatment efficacy with minimizing overtreatment or
unnecessary morbidity, the management of desmoid tumors underscores
the need for a multidisciplinary approach. In pediatric patients
with DF that is unresponsive to non-surgical therapies, surgeons
may hesitate to pursue wide resections due to the substantial risk
of postoperative morbidity. When a large tumor is located near
vital structures, achieving adequate surgical margins can be
particularly challenging (8). The
literature review performed in the study by Miyashita et al
(8) revealed post-operative
complications in 13 patients (10.4%). Trismus was the most frequent
(n=6). A total of 2 patients developed secondary papillary
carcinoma following radiation therapy. Additional complications
included osteomyelitis (n=2), mild ptosis resulting from facial
nerve sectioning (n=1), restricted neck mobility (n=1) and
Claude-Bernard-Horner syndrome (n=1) (8). Individualized treatment planning,
guided by the biological behavior of the tumor, is essential to
optimize patient outcomes while addressing the limitations of
current evidence and therapeutic options (10,15).
In the present case report, the mass was successfully excised under
general anesthesia with no complications. No recurrence was
reported following 6 months of follow-up. The limitations of the
present case report include the inability to retrieve the
ultrasound figures due to poor archiving and CT scan figures for
the case, as they were conducted at an external facility.
In conclusion, DTF of the head and neck presents a
complex clinical scenario. Its rarity and the challenges associated
with diagnosis, location, and treatment necessitate careful
consideration, particularly in pediatric cases, in order to prevent
unnecessary interventions and complications. Ongoing research is
thus warranted to define standardized treatment protocols and
improve therapeutic outcomes for this challenging condition.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FHK and AMS were major contributors to the
conception of the study, as well as to the literature search for
related studies. WNS, AAQ and SHH contributed to the clinical
management of the patient, assisted in data acquisition and
interpretation, and participated in the literature review and
manuscript preparation. HOB, HMD, ROM, ASM and KMS contributed to
the conception and design of the study, the literature review, the
critical revision of the manuscript, and in the processing of the
table. SHT was the radiologist who performed the assessment of the
case. AMA was the pathologist who performed the diagnosis of the
case. FHK and AMS confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient's parents for participation in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient's parents for the publication of the present and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kasper B, Ströbel P and Hohenberger P:
Desmoid tumors: Clinical features and treatment options for
advanced disease. Oncologist. 16:682–693. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nuyttens JJ, Rust PF, Thomas CR Jr and
Turrisi AT III: Surgery versus radiation therapy for patients with
aggressive fibromatosis or desmoid tumors: A comparative review of
22 articles. Cancer. 88:1517–1523. 2000.PubMed/NCBI
|
|
3
|
Wang CP, Chang YL, Ko JY, Cheng CH, Yeh CF
and Lou PJ: Desmoid tumor of the head and neck. Head Neck.
28:1008–1013. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kant S, Charan BD, Goel V, Das S, Sahu S,
Sharma R, Borkar S, Sebastian LJD and Garg A: Desmoid-type
fibromatosis of neck masquerading as nerve sheath tumors: two case
reports. Egyptian J Radiol Nuclear Med. 54(176)2023.
|
|
5
|
Sato K, Kawana M, Nonomura N and Takahashi
S: Desmoid-type infantile fibromatosis in the mandible: A case
report. Am J Otolaryngol. 21:207–212. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Prasad S, Nassar M, Azzam AY,
García-Muro-San José F, Jamee M, Sliman RK, Evola G, Mustafa AM,
Abdullah HO, Abdalla B, et al: CaReL Guidelines: A Consensus-based
guideline on case reports and literature review (CaReL). Barw Med
J. 2:13–19. 2024.
|
|
7
|
Abdullah HO, Abdalla BA, Kakamad FH, Ahmed
JO, Baba HO, Hassan MN, Bapir R, Rahim HM, Omar DA, Kakamad SH, et
al: Predatory publishing lists: A review on the ongoing battle
against fraudulent actions. Barw Med J. 2:26–30. 2024.
|
|
8
|
Miyashita H, Asoda S, Soma T, Munakata K,
Yazawa M, Nakagawa T and Kawana H: Desmoid-type fibromatosis of the
head and neck in children: A case report and review of the
literature. J Med Case Rep. 10(173)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hoos A, Lewis JJ, Urist MJ, Shaha AR,
Hawkins WG, Shah JP and Brennan MF: Desmoid tumors of the head and
neck-a clinical study of a rare entity. Head Neck. 22:814–821.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou MY, Bui NQ, Charville GW, Ghanouni P
and Ganjoo KN: Current management and recent progress in desmoid
tumors. Cancer Treat Res Commun. 31(100562)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bouatay R, Bouaziz N, Harrathi K and
Koubaa J: Aggressive and recurrent desmoid tumor of the head and
neck: A therapeutic challenge. Otolaryngol Case Rep.
30(100578)2024.
|
|
12
|
de Bree E, Zoras O, Hunt JL, Takes RP,
Suárez C, Mendenhall WM, Hinni ML, Rodrigo JP, Shaha AR, Rinaldo A,
et al: Desmoid tumors of the head and neck: A therapeutic
challenge. Head Neck. 36:1517–1526. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guadagnolo BA, Zagars GK and Ballo MT:
Long-term outcomes for desmoid tumors treated with radiation
therapy. Int J Radiat Oncol Biol Phys. 71:441–447. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alherabi AZ, Marglani OA, Bukhari DH and
Al-Khatib TA: Desmoid tumor (fibromatosis) of the head and neck.
Saudi Med J. 36(101)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Said-Al-Naief N, Fernandes R, Louis P,
Bell W and Siegal GP: Desmoplastic fibroma of the jaw: A case
report and review of literature. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 101:82–94. 2006.PubMed/NCBI View Article : Google Scholar
|