Introduction
Intracranial multiple dural arteriovenous fistulas
(MDAVFs) are difficult to treat and pose a challenge for
neurosurgeons. Intracranial MDAVFs are also rare, with an incidence
rate of 6–9% among all cranial DAVFs reported in Korea, Canada and
the USA (1–3). As few cases have been reported,
understanding of intracranial MDAVFs is limited. At present, the
pathogenesis underlying MDAVF development is not well understood,
though there is a consensus that three mechanisms are possible: i)
MDAVFs may develop following establishment of a sinus thrombosis
involving several sinuses; ii) pre-existing DAVFs may induce sinus
thrombosis or venous hypertension, resulting in the formation of
MDAVFs; and iii) MDAVFs may be caused by increased angiogenic
activity and technical problems that are associated with
transvenous embolization (4). In
addition, angiogenic factors, hemodynamic disruption and congenital
factors may be involved in MDAVF pathogenesis (1–3,5,6).
In clinical terms, intracranial MDAVFs are
considered to follow a relatively malignant evolution, and their
hemodynamics, angioarchitecture and imaging manifestations are
complex (7,8). Affected patients exhibited higher
incidence rates of hemorrhage and neurological deficits, and
aggressive treatments should therefore be used for therapy
(2,9).
At present, a number of approaches are used to treat intracranial
MDAVFs. These include endovascular embolization, surgical
resection, radiosurgery and conservative treatment, although in
more aggressive cases, combined treatments are required (10,11).
However, it is currently unknown which treatment is
most effective or which therapeutic principle should be followed.
Although progress has been made in diagnosing and evaluating
MDAVFs, generally through hemodynamic studies and improved imaging
techniques, understanding of DAVFs remains limited. As the majority
of reports on intracranial MDAVFs are confined to case reports and
small case series, the present review sought to assess the
available literature published to date. ‘Multiple dural
arteriovenous fistulas’ and ‘multiple dural arteriovenous
malformations’ were used as search terms in PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and Web of
Science (https://www.isiknowledge.com)
databases to identify relevant English-language studies. Reports
among this relevant literature were presently reviewed to highlight
the extent of progress in research into intracranial MDAVFs.
Definition and classification
Multiple intracranial MDAVFs may occur
simultaneously or develop over time in a single patient (1). The type of each MDAVF should therefore
be defined with regard to its time of onset and location (7). Multiple MDAVFs that occur simultaneously
are referred to as ‘synchronous’, these types of MDAVF may overlap,
leading to difficulty in distinguishing them from other lesions
(2). Conversely, when independent
MDAVFs develop over time in the same patient, for instance, de
novo fistulas that develop in different locations in a temporal
sequence, they are referred to as ‘metachronous’ (2).
A previous literature review noted that the majority
of reports on intracranial MDAVFs described synchronous-type
fistulas, while those on metachronous MDAVFs were rare. One report
that included increased cases of metachronous MDAVFs was published
by Ha et al (2) in 2012, in
which 14 intracranial MDAVFs were described, including 7 cases of
metachronous-type MDAVF. The specific causes of synchronous-type
MDAVFs are unknown, though they are all induced by a single
initiating process (12). Conversely,
for metachronous-type MDAVFs, the first DAVF may induce development
of the others (13). However, in
diagnosing intracranial MDAVFs, it is frequently difficult to
determine which type of intracranial MDAVFs the patient is
presenting with, as is not possible to distinguish whether
synchronous-type lesions developed from the metachronous-type
(14).
Pathogenesis
The exact pathogenesis underlying the development of
single intracranial MDAVFs remains unknown. It has been suggested
that MDAVFs share the same pathogenesis as single DAVFs, and that
venous sinus thrombosis, congenital development, head trauma and
surgical procedures may therefore be involved in the development of
MDAVFs (15). In addition, conditions
including infections, postpartum status and coagulopathies may
provide a conducive environment for MDAVFs (16,17). These
incidents cause closed preexisting arteriovenous channels to become
enlarged (18). However, in MDAVFs,
multiple regions are simultaneously involved, and thus the
mechanisms likely differ from those underlying the development of
single DAVFs.
Venous sinus thrombosis
Of all potential causes, venous hypertension
following venous sinus thrombosis has been proposed to be a
critical pathogenic factor (19–21). If
sinus thromboses are simultaneous and extensive, they may form
MDAVFs that can be identified following recanalization of the
thrombosed sinuses (22). For
instance, in the report by Ha et al (2), 71.4% of intracranial MDAVFs exhibited
extensive dural sinus thrombosis. The mechanisms involved in venous
sinus thrombosis leading to MDAVFs are complex. Inflammation may
serve an important role by upregulating the release of angiogenic
growth factors to cause neovascularization of the affected sinus
wall (16).
Angiogenic factors
Numerous angiogenic factors, including
platelet-derived endothelial-cell growth factor, fibroblast growth
factor and transforming growth factor-β may also provide a
conducive environment for the development of intracranial single
DAVFs (5). The production of
angiogenic factors may be induced by infections, postpartum status,
a state of hypercoagulability, a vascular proliferative state,
arthritis, psoriasis and hemangiomatosis, in which strong
angiogenic stimuli overcome homeostatic barriers, resulting in
unabated vascular proliferation and eventually DAVFs (23). Additionally, angiogenic factors affect
the dural venous sinuses, which may explain why certain patients
present with numerous individual DAVFs (24). The hypothesis that angiogenic factors
are a causative factor is particularly convincing when considering
patients with MDAVFs that simultaneously involve cranial and spinal
areas (11). In such a pathological
state, targeted anti-angiogenic therapy may promote the spontaneous
obliteration of MDAVFs and prevent their recurrence following
successful treatment (5,25).
Disturbance of hemodynamics
When the first intracranial DAVF develops, the
hemodynamics of the venous system in the brain is disturbed, and
this may induce the development of new DAVFs, eventually resulting
in the formation of metachronous-type MDAVFs (1,2). It has
been hypothesized that the following two mechanisms are involved in
this process: i) Venous drainage caused by an established DAVF may
cause turbulent flow or stagnation in the distant venous sinus,
resulting in thrombosis of the sinus and development of additional
DAVFs; ii) venous hypertension may cause the development of a DAVF,
and the elevation in sinus pressure caused by the initial DAVF may
result in the formation of multiple new DAVFs at other sites
(1–3).
For instance, Kubota et al (13) described a 43-year-old woman presenting
with an cavernous DAVF following transvenous embolization, who
subsequently developed a new DAVF around the jugular valve. A
change in hemodynamics was considered the cause, as venous pressure
was elevated and prolonged by the shunted venous flow. Thus, a
transvenous approach for DAVF may result in the formation of a new
DAVF (13). In addition to treatment
for DAVF, treatments for other intracranial vascular diseases may
also cause the formation of new intracranial DAVFs. For instance,
Bai et al (26) in 2012
treated a pediatric case of high-flow pial AVF using embolization,
after which a de novo DAVF and a small arteriovenous
malformation developed as a result of changes in hemodynamics that
occurred following the embolization.
Congenital factors
In addition to MDAVFs caused by acquired factors,
certain intracranial MDAVFs may be congenital in origin,
particularly those observed in children. In these cases, MDAVFs are
often associated with a developmental malformation in the venous
sinus (6). For instance, Vilela et
al (27) described a 5-year-old
patient with MDAVFs who also presented with status epilepticus
resulting from severe venous congestive encephalopathy, occlusion
of the right sigmoid sinus, absence of cavernous sinuses and
stenosis in the left sigmoid sinus-jugular bulb. In another case
reported by Ushikoshi et al (28), a 5-year-old boy presented with an
infantile-type DAVF in a dilated anterior part of the superior
sagittal sinus and two other adult-type DAVFs. In addition to
MDAVFs that are caused by developmental malformation of the venous
sinus, intracranial MDAVFs may be accompanied by other congenital
diseases. For instance, on assessment of a 46-year-old man with
Cowden syndrome, Prats-Sánchez et al (29) suggested that phosphatase and tensin
homolog gene mutations were the underlying cause for intracranial
MDAVFs.
In summary, the exact pathogenesis underlying the
development of intracranial MDAVFs remains unclear, though venous
sinus thrombosis, angiogenic factors, disturbed hemodynamics and
congenital factors are considered to be potential causes. However,
for the majority of intracranial MDAVFs, there is no evidence of an
underlying pathogenesis (16,30,31). Thus,
identification of the causes of intracranial MDAVFs is required to
aid prevent their progression.
Angioarchitecture and hemodynamics
At present, understanding of the angioarchitecture
of intracranial MDAVFs is based on single DAVFs (7). However, MDAVFs present with more complex
angioarchitecture and hemodynamics; when intracranial MDAVFs
develop, they may overlap, which causes the architecture of the
feeding arteries to become more complex (9). Venous hypertension caused by a single
DAVF may be enhanced by the presence of the other DAVFs, and
compensatory blood flow throughout the brain may be disturbed,
causing the condition of the patient to rapidly deteriorate
(32). Under these circumstances, it
is important to distinguish which DAVFs are the major implicated
fistulas, which should be the DAVFs with a higher Borden/Cognard
classification (33,34). Determining which are the responsible
DAVFs requires selective artery angiography.
For intracranial MDAVFs, the angioarchitecture and
hemodynamics of the MDAVFs may be more dependent on the pattern of
involvement of the venous system (35). In MDAVFs, retrograde leptomeningeal
venous drainage serves a critical role, MDAVFs increase the
pressure in the venous sinus and the resistance to blood flow to
the sinus (15). Thus, in patients
with more than a single DAVF, the rate of cortical venous drainage
reflux is higher. For instance, Van Dijk et al (3) in 2002 reported that cortical venous
reflux was present in 84% of MDAVF patients. Therefore, MDAVFs may
be associated with a high risk of intracranial hemorrhage or venous
ischemia.
Furthermore, in MDAVFs, venous hypertension occurs
more frequently in the deep venous system. The deep white matter is
therefore vulnerable to venous congestion, which can cause
leukoaraiosis (36). Additionally, in
children with intracranial MDAVFs, developmental malformations are
often observed in the venous sinus, and these may increase
complexity of the hemodynamics (37).
Therapeutic decisions, such as whether the feeding arteries are of
sufficient thickness to perform an embolization via a transarterial
approach or whether a draining sinus with stenosis can be dilated
using stenting angioplasty via a transvenous approach, should be
determined based on a complete understanding of the
angioarchitecture and hemodynamics of the intracranial MDAVFs.
Clinical features
Single intracranial DAVFs typically present as a
spectrum of benign symptoms, including headache, murmur, pulsatile
tinnitus and eye symptoms, though they may occasionally present
with increased intracranial pressure or even fatal hemorrhage
(38). However, intracranial MDAVFs
differ markedly from single lesions, as MDAVFs may alter the
dynamics of venous flow throughout the brain, which impairs
cerebral circulation by causing severe venous hypertensive
encephalopathy (14). Thus, MDAVFs
have greater probability of presenting with hemorrhage, infarction
or neurological deficit and to run a malignant course (30). The clinical features of intracranial
MDAVFs are subsequently described.
General characteristics
As current understanding of intracranial MDAVFs is
derived from data on DAVFs, it may be speculated that the clinical
characteristic of MDAVFs are similar to those commonly observed in
DAVFs. Martinez-Burbano et al (9) reviewed the literature and identified
that intracranial MDAVFs were slightly more predominant in females,
at a ratio of 1.65:1 (women: men), and that the average age of
onset was approximately 60 years old. Similar results have been
reported previously. For instance, Fujita et al (4) observed that in patients with MDAVFs,
ages ranged from 43 to 75-years-old (mean, 57.4-years-old), and
that the population distribution had a female predominance.
Additionally, DAVFs occurred primarily in the cavernous sinuses,
while other locations included the transverse and sigmoid sinuses
(4). These results are similar to
those obtained in studies of single DAVFs (39,40).
However, to date, few cases of intracranial MDAVFs have been
reported. Thus, evaluations of their general characteristics may be
inaccurate.
Rapid progression of symptoms
As there is severe cortical venous reflux in MDAVFs,
the compensation of blood flow tends to cause disequilibrium, which
leads to venous hypertension (41).
When venous hypertensive encephalopathy develops alongside MDAVFs,
this has been associated with aggressive initial symptoms,
including neurological deficits, seizures and hemorrhage (2). This rapid progression of symptoms is a
characteristic of intracranial MDAVFs that distinguishes them from
single DAVFs. In addition to the aforementioned common symptoms,
intracranial MDAVFs may present with higher-order brain
dysfunctions, including progressive dementia, cognitive decline and
progressive memory loss, and are an indicator of dysfunction
throughout the brain (42,43). For instance, Abe et al
(44) in 2014 described a 67-year-old
female presenting with intracranial MDAVFs that manifested as
dementia, which rapidly progressed over 2 months. In certain cases,
rapidly progressive dementia has been associated with
extrapyramidal motor symptoms, including parkinsonism (45).
Symptoms in the spinal cord
DAVFs may occur in the cranial dura and also in the
spinal dura, while MDAVFs occur in the spinal dura (1). Van Dijk et al (3) reported that spinal MDAVFs comprised 2%
of all spinal DAVFs. Therefore, when intracranial MDAVFs develop,
if a patient presents with myelopathy and this symptom cannot be
explained by an intracranial MDAVF, spinal MDAVFs should be
considered as they may be caused by venous congestion of the spinal
cord (46). Shankar et al
(11) reported a 61-year-old man with
two intracranial DAVFs that were associated with four cervical
DAVFs.
Symptoms of pediatric MDAVFs
Intracranial pediatric MDAVFs differ from adult
intracranial MDAVFs; their clinical manifestations are distinct and
may be summarized as symptoms that are caused by high-flow dural
arteriovenous fistulas (47).
Affected patients often present with symptoms including
hyperdynamic heart failure, increased intracranial pressure,
macrocrania, neurocognitive delay and seizures (6). The symptoms of retrograde venous
drainage include hemorrhage and neurological deficits, and symptoms
associated with cavernous sinus involvement and hydrocephalus,
among other indications (6).
Imaging examinations
Computed tomography (CT) imaging
Brain CT is advantageous as it enables the presence
of hemorrhaging to be evaluated in a patient, unlike other
examination techniques (48). It may
also be used to identify venous hypertension and sinus thrombosis
(49). Flat panel CT analysis and
three-dimensional angiographic reconstructions are particularly
useful for increasing understanding of the complex anatomy and
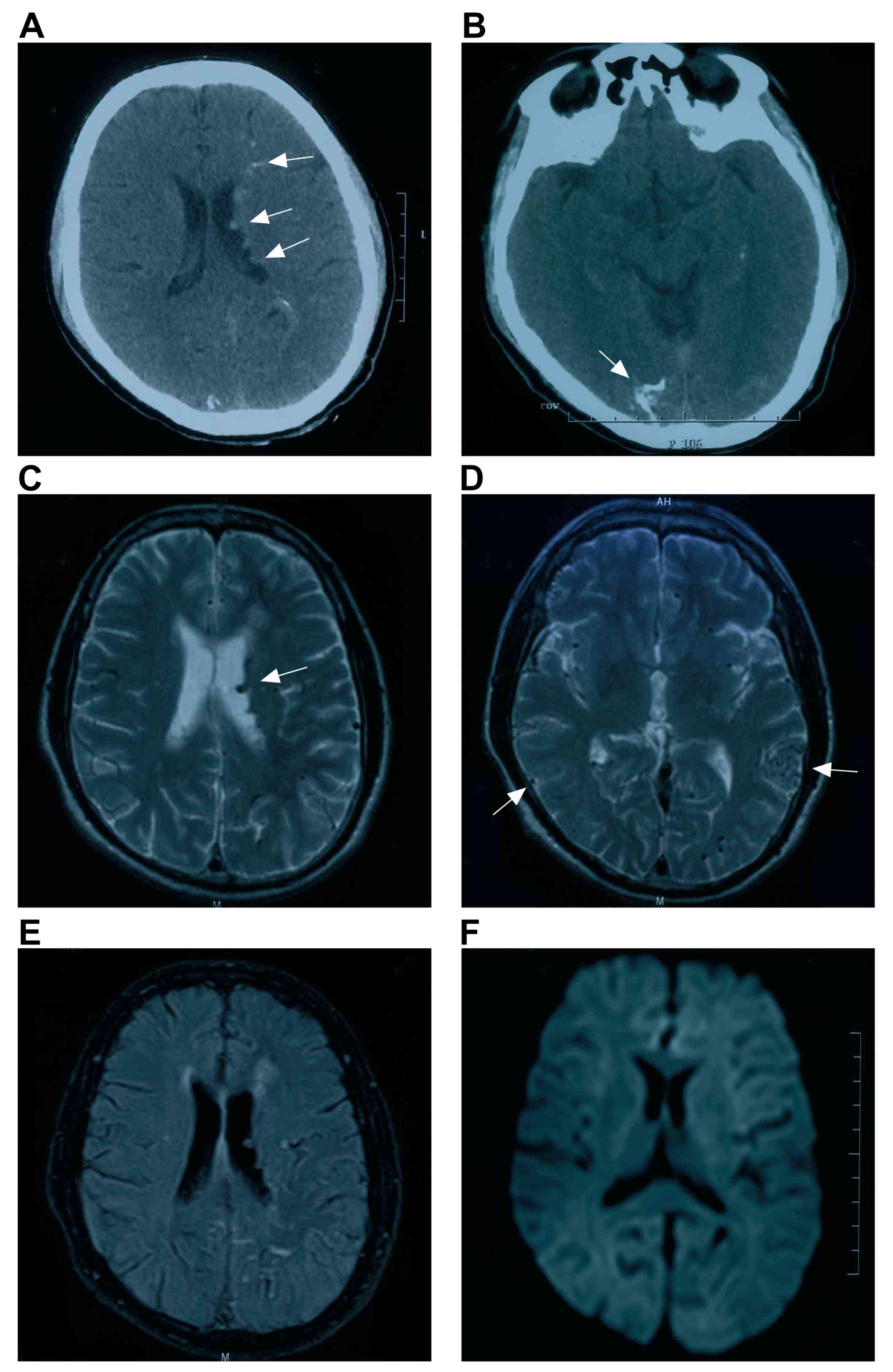
relationships of intracranial MDAVFs (50). In Fig. 1A
and B, typical images of MDAVF imaging with brain CT are
presented.
Magnetic resonance imaging (MRI)
In assessing intracranial MDAVFs, MRI is primarily
used to evaluate changes in brain structures, and identifies a
reduction in diffuse white matter fluid based on inversion recovery
time and signal abnormalities, hyperintense changes (leukoaraiosis)
and restricted diffusion in the bilateral corona radiata, and
extensive enlarged serpentine vascular flow voids, which are caused
by venous thrombosis and venous hypertension (44). In addition, MRI may be used to
determine the progression of intracranial MDAVFs. For instance, if
signal abnormality or hyperintensity improves, it indicates
improvement in intracranial venous hypertension. This may implicate
MRI as a more convenient technique in these cases (30). In Fig.
1C-F, typical images of MDAVF detection by brain MRI are
presented.
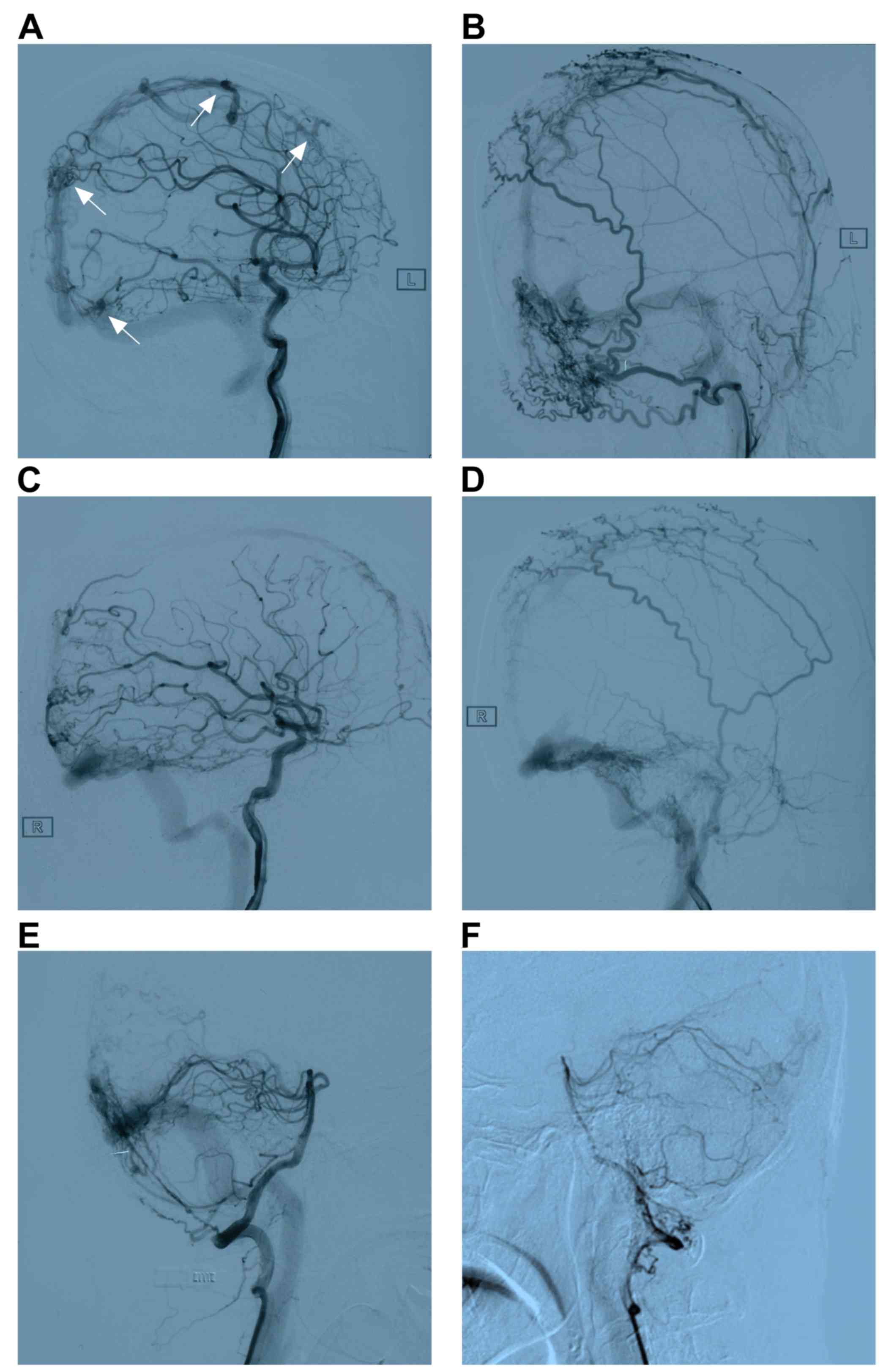
Digital subtraction angiography (DSA)
imaging
A DSA examination is considered the gold standard
imaging technique and may be used to identify the site of a
cerebral parenchyma, fistula or arterial feeders, the pattern and
direction of venous drainage, and the morphology and diameter of
the venous sinus in MDAVFs (51,52). In
certain cases, pseudophlebitic patterns of venous drainage that
typically indicate severe venous hypertension have also been
observed (53). As intracranial
MDAVFs are more complex, may overlap and are difficult to visualize
or distinguish from other DAVFs, selective injections of different
feeding arteries are often necessary during DSA to identify each
DAVF (52). Typical images of MDAVF
imaging with DSA are presented in Fig.
2.
Blood flow examination
When intracranial MDAVFs develop as a result of
venous hypertension, the atrial blood flow of the whole brain is
decreased. During this time, it is recommended that brain blood
perfusion should be examined in affected patients using
single-photon emission computed tomography (SPECT). This technique
has previously been demonstrated to identify marked decreases in
cerebral blood flow throughout the majority of the brain (44).
Treatment
Intracranial MDAVFs may critically disturb blood
flow to the brain, and therefore treatment is crucial (16). Radical treatment is considered more
appropriate, as partial obliteration of the DAVFs increases the
difficulty of subsequent therapy (54). In the treatment of MDAVFs, a number of
principles are generally followed: One priority of treatment is to
target fistulas with cortical venous reflux with a higher
Borden/Cognard classification; additionally, treatment for a DAVF
is performed in multiple stages, and focuses on decreasing venous
hypertension and improving cerebral hemodynamics rather than
completely obliterating all fistulas (9,11). It is
important to obtain a precise understanding of each DAVF's vascular
anatomy, as not all fistulas contribute to venous hypertension
(24,30,55). At
present, treatment for intracranial MDAVFs may include endovascular
treatment, microsurgery, stereotactic radiosurgery or a combination
of several methods(3).
Endovascular embolization
Intracranial MDAVFs are not typically located in the
same or adjacent regions, and thus it is difficult to expose them
in a single operating field. Therefore, an endovascular approach is
advantageous as it allows access to all MDAVFs. Endovascular
embolization is currently the first-line standard of care for
intracranial MDAVFs. The target of this treatment regimen is
complete occlusion of the fistula with cortical venous reflux
(24). During endovascular
embolization, if the embolic agent completely occludes the fistula
by crossing into the immediate receptive venous structure, an
adequate outcome can be achieved (56). Endovascular embolization may be
performed using different types of embolic agents and a variety of
routes. Different embolic agents include coils, n-butyl
cyanoacrylate and Onyx, and routes of access include transarterial
and transvenous approaches; which agent and route are chosen
depends on the angioarchitecture of the intracranial MDAVFs
(40,57).
When the main feeding arteries are of sufficient
thickness, high-grade MDAVFs are limited within a region and do not
become involved in anastomosis, and transarterial approaches are
therefore recommended (58). In cases
when the feeding arteries are substantially thinner and associated
with venous sinus stenosis, or when multiple DAVFs are involved in
the same sinus, a transvenous embolization is an appropriate method
(59). For instance, Saito et
al (19) treated a 55-year-old
man with two isolated DAVFs that were located in the anterior
superior sagittal sinus and transverse sinus, and achieved complete
embolization by directly packing the isolated sinuses via the
superior sagittal sinus.
However, when treating multiple MDAVFs, it should be
considered whether multiple sinus occlusion can be tolerated,
particularly when both the sagittal and transverse-sigmoid sinuses,
which are the most appropriate candidates for transvenous
embolization, are affected in patients with cortical venous
drainage and normal veins though which the brain tissues are not
draining into the affected sinus (54). When treating intracranial MDAVFs, a
multi-stage intervention may be effective for embolizing the
multiple shunts and cortical refluxes. For instance, Abe et
al (44) successfully treated
patients with two intracranial MDAVFs by performing four
endovascular procedures at approximate 1-week intervals over 5
weeks.
Microsurgical resection
At present, although endovascular embolization is
considered a first-line standard for intracranial MDAVFs, for
certain dysplastic MDAVFs and in those in which the main feeding
arteries are thin or the venous sinus has a thrombosis or stenosis,
endovascular treatments are difficult (54). For these cases, microsurgical
resection is considered an appropriate method of treatment,
microsurgery may be used to remove or clip the shunt between the
feeding artery and draining vein (60). During microsurgical resection and
clipping, the following two operative techniques can be used:
First, an en bloc DAVF and parent sinus resection can be performed
to treat a sinus DAVF; second, selective arteriovenous
disconnection may be used to treat cortical DAVFs with direct
leptomeningeal venous drainage (24).
When treatments are performed that do not include resection of the
involved sinuses, they do not consistently cure the pathology,
thus, the first method is recommended (23).
Microsurgical resection is a higher risk method
compared with endovascular embolization as it is difficult to
expose the DAVF and may result in blood loss (60). For DAVFs with a deep-seated location,
such as the tentorium or sigmoid sinus, the operation may be
complex and associated with high risk (61). Although the operation is difficult, a
detailed operating plan and appropriate case choice may achieve a
satisfactory outcome. Indeed, success with this method has been
observed in previous decades: In 1986, Al-Mefty et al
(62) treated a pediatric case of
extensive DAVF of the sigmoid sinus and bilateral occlusion of the
transverse sinus using microsurgery, and the prognosis was
acceptable.
Radiation therapy
Radiation therapy is considered a safe and effective
method, and may serve substantial role, in the treatment if DAVFs
that involve a large dural sinus. In these cases, isolated use of
radiosurgery has been described (63). Thus, for intracranial MDAVFs, certain
DAVFs with a low Borden/Cognard classification or residual lesions
following a prior resection or endovascular treatment may be
treated using radiation therapy (64). As using radiation to obliterate DAVFs
generally requires a 1 to 3-year treatment regimen, it is important
to treat the most unstable fistulas first using endovascular or
surgical approaches to avoid intracranial hemorrhage (65).
Combined therapy
As several locations are involved in MDAVFs, which
have a complex angioarchitecture and hemodynamics and may be
associated with pial AVFs, it is difficult to resolve all fistulas
using a single method (15). A
combination of approaches including endovascular treatment,
microsurgery and stereotactic radiosurgery typically achieves the
most effective outcomes (3). For
instance, a combined strategy was used by Mitsuhara et al
(10) to treat a 70-year-old man with
MDAVFs involving the superior sagittal sinus and bilateral
transverse-sigmoid sinuses, and an occlusion of the right jugular
vein. They first surgically isolated the superior sagittal sinus,
then performed a transvenous embolization in the right
transverse-sigmoid sinus DAVF, and finally performed Gamma Knife
radiosurgery to remove the residual DAVFs. The patient's symptoms
including headache and tinnitus improved following the treatments
(10). Therefore, a combined
treatment regimen should be recommended.
Other approaches
When aggressively treating intracranial MDAVFs, it
is important to consider that certain low-flow DAVFs may
spontaneously heal or exhibit changed patterns on follow-up DSA
(30). Thus, persistent low-risk
lesions without retrograde cortical venous drainage do not
consistently require treatment (66).
Furthermore, in addition to blocking the fistulas using a
transarterial or transvenous route, dredging is also a viable
strategy. Vilela et al (27)
described a 5-year-old patient with MDAVFs presenting with status
epilepticus resulting from severe venous congestive encephalopathy,
as well as an occlusion in the right sigmoid sinus, an absence of
cavernous sinuses and substantial stenosis in the left sigmoid
sinus-jugular bulb. Venous sinus angioplasty and stent placement
were performed, and the child recovered without neurological
deficit (27). For intracranial
MDAVFs with sinus thrombosis, anticoagulatory therapy may also be
attempted (42).
Prognosis
If intracranial MDAVFs are left untreated, the
angiographic and clinical prognoses are poor (9). Generally, appropriate treatment leads to
marked improvement or even complete resolution of encephalopathy
and neurological deficits and improved cognition (30). For instance, Abe et al
(44) reported a 67-year-old female
who presented with intracranial MDAVFs that manifested as dementia,
which rapidly progressed over 2 months. Following treatment, the
dementia had been resolved, and the patient remained in stable
condition without recurrence. However, when brain circulation
decompensates or the MDAVFs are resistant to treatment, even the
most appropriate treatments are unable to block progression, and
satisfactory outcomes are unattainable. This was demonstrated by
Friedman et al (67), who
treated a 31-year-old man presenting with intracranial MDAVFs after
trauma, the patient underwent more than 20 treatments, including
transarterial embolization, transvenous embolization, stereotactic
radiosurgery and craniotomy; however, the MDAVFs continued to
progress, and the patient succumbed to the disease following a
course of almost 5 years. In certain cases, despite treatments for
intracranial MDAVFs achieving satisfactory effects, MDAVFs may
recur. For instance, Mirza and Fraser (17) treated a 24-year-old patient presenting
with MDAVFs, and after 2 months of radiation therapy, one of the
DAVFs recurred; however, after 6 months of therapy, no recurrence
was detected. When considering the potential recurrence of a DAVF,
a transvenous approach may be effective, as sinus occlusion may be
associated with the progression of DAVFs (68).
Conclusion
Intracranial MDAVFs are a challenge for
neurosurgeons due to potential undetermined factors involved.
Intracranial MDAVFs may be divided into synchronous-type and
metachronous-type. At present, the pathogenesis underlying MDAVF
development is not well understood. Intracranial MDAVFs run a
malignant clinical course, and patients generally experience
symptoms that rapidly progress following onset. A number of imaging
techniques may be used to detect MDAVFs, including CT, MRI, DSA and
SPECT. Of these, CT and MRI provide information regarding brain
morphology, SPECT provides information regarding brain blood flow,
and DSA is currently the gold standard and may be used to evaluate
angioarchitecture and hemodynamics. MDAVFs should be treated
aggressively, and treatment should include endovascular
embolization, surgical resection, radiosurgery and conservative
methods, as combined treatments are typically required to achieve
sufficient clinical outcome. Through administering appropriate and
aggressive treatment regimens, neurological deficits and cognitive
functions may be markedly improved.
References
|
1
|
Barnwell SL, Halbach VV, Dowd CF,
Higashida RT, Hieshima GB and Wilson CB: Multiple dural
arteriovenous fistulas of the cranium and spine. AJNR Am J
Neuroradiol. 12:441–445. 1991.PubMed/NCBI
|
|
2
|
Ha SY, Kwon YS, Kim BM, Kim DI and Kim DJ:
Clinical and angiographic characteristics of multiple dural
arteriovenous shunts. AJNR Am J Neuroradiol. 33:1691–1695. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Dijk JM, TerBrugge KG, Willinsky RA
and Wallace MC: Multiplicity of dural arteriovenous fistulas. J
Neurosurg. 96:76–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujita A, Nakamura M and Tamaki N:
Multiple dural arteriovenous fistulas involving both the cavernous
sinus and the posterior fossa: Report of two cases and review of
the literature. No Shinkei Geka. 29:1065–1072. 2001.PubMed/NCBI
|
|
5
|
Folkman J: Successful treatment of an
angiogenic disease. N Engl J Med. 320:1211–1212. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu J, Lv X, Li Y and Wu Z: Therapeutic
progress in pediatric intracranial dural arteriovenous shunts: A
review. Interv Neuroradiol. 22:548–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fudaba H, Kubo T, Goda M, Sugita K,
Morishige M, Onishi K, Ishii K, Anan M, Nagai Y and Fujiki M: The
Potentiality for development of multiple dural arteriovenous
fistulas after ligation of the internal jugular vein. A case
report. NMC Case Rep J. 4:71–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oh SJ, Chon YI, Kong SK and Goh EK:
Multiple dural arteriovenous fistulas presenting as pulsatile
tinnitus treated with external manual compression. J Audiol Otol.
21:156–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinez-Burbano B, Correa Diaz EP and
Jácome Sánchez C: Evolutionary history of multiple dural fistula. J
Investig Med High Impact Case Rep. 4: View Article : Google Scholar : 2016.PubMed/NCBI
|
|
10
|
Mitsuhara T, Ikawa F, Ohbayashi N, Shirozu
H, Abiko M and Ichinose N: A case of multiple dural arteriovenous
fistulas treated by multiple modalities. No Shinkei Geka.
39:575–580. 2011.PubMed/NCBI
|
|
11
|
Shankar JJ, Terbrugge Karel and Krings T:
Multiple spinal and cranial dural arteriovenous fistulas. J
Neurosurg Spine. 15:113–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spittau B, Millán DS, El-Sherifi S, Hader
C, Singh TP, Motschall E, Vach W, Urbach H and Meckel S: Dural
arteriovenous fistulas of the hypoglossal canal: Systematic review
on imaging anatomy, clinical findings, and endovascular management.
J Neurosurg. 122:883–903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kubota Y, Ueda T, Kaku Y and Sakai N:
Development of a dural arteriovenous fistula around the jugular
valve after transvenous embolization of cavernous dural
arteriovenous fistula. Surg Neurol. 51:174–176. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rahmanian A, Farrokhi MR, Alibai EA and
Masoudi MS: Multiple intracranial dural arteriovenous fistula. J
Res Med Sci. 18:360–362. 2013.PubMed/NCBI
|
|
15
|
Minamide H, Hayashi Y and Uchiyama N:
Multiple tentorial dural arteriovenous fistulas with acquired pial
arteriovenous fistula presented with unilateral eye symptoms
successfully treated by transarterial embolization and direct
surgery. A case report. No Shinkei Geka. 45:239–245.
2017.PubMed/NCBI
|
|
16
|
Kusaka N, Sugiu K, Katsumata A, Nakashima
H, Tamiya T and Ohmoto T: The importance of venous hypertension in
the formation of dural arteriovenous fistulas: A case report of
multiple fistulas remote from sinus thrombosis. Neuroradiology.
43:980–984. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mirza FA and Fraser JF: Multiple dural and
pial arteriovenous fistulae in a twenty-four-year-old woman in the
setting of superior sagittal sinus thrombosis: Case report and
review of literature. J Stroke Cerebrovasc Dis. 25:e192–e199. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishijima M, Takaku A, Endo S, Kuwayama N,
Koizumi F, Sato H and Owada K: Etiological evaluation of dural
arteriovenous malformations of the lateral and sigmoid sinuses
based on histopathological examinations. J Neurosurg. 76:600–606.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saito A, Takahashi N, Furuno Y, Kamiyama
H, Nishimura S, Midorikawa H and Nishijima M: Multiple isolated
sinus dural arteriovenous fistulas associated with antithrombin III
deficiency. A case report. Neurol Med Chir (Tokyo). 48:455–459.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Desal HA, Lee SK, Kim BS, Raoul S,
Tymianski M and TerBrugge KG: Multiple de novo vascular
malformations in relation to diffuse venous occlusive disease. A
case report. Neuroradiology. 47:38–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsubara S, Satoh K, Satomi J, Shigekiyo
T, Kinouchi T, Miyake H and Nagahiro S: Acquired pial and dural
arteriovenous fistulae following superior sagittal sinus thrombosis
in patients with protein S deficiency: A report of two cases.
Neurol Med Chir (Tokyo). 54:245–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugiura Y, Miyamoto T, Takehara S, Sumiya
K and Nozaki T: Multiple dural arteriovenous fistulas following
extensive sinus thrombosis. A case report. No Shinkei Geka.
24:379–383. 1996.PubMed/NCBI
|
|
23
|
Aoun SG, Bendok BR and Batjer HH: Acute
management of ruptured arteriovenous malformations and dural
arteriovenous fistulas. Neurosurg Clin N Am. 23:87–103. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russell SM, Woo HH and Nelson PK:
Transarterial wedged-catheter, flow-arrest, N-butyl cyanoacrylate
embolization of three dural arteriovenous fistulae in a single
patient. Interv Neuroradiol. 9:283–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chaloupka JC, Marx WF and Kallmes DF:
Dural arteriovenous fistulas. J Neurosurg. 94:858–861.
2001.PubMed/NCBI
|
|
26
|
Bai Y, He C, Zhang H and Ling F: De novo
multiple dural arteriovenous fistulas and arteriovenous
malformation after embolization of cerebral arteriovenous fistula.
A case report. Childs Nerv Syst. 28:1981–1983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vilela P, Willinsky R and Terbrugge K:
Treatment of intracranial venous occlusive disease with sigmoid
sinus angioplasty and stent placement in a case of infantile
multifocal dural arteriovenous shunts. Interv Neuroradiol. 7:51–60.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ushikoshi S, Kikuchi Y and Miyasaka K:
Multiple dural arteriovenous shunts in a 5-year-old boy. AJNR Am J
Neuroradiol. 20:728–730. 1999.PubMed/NCBI
|
|
29
|
Prats-Sánchez LA, Hervás-García JV,
Becerra JL, Lozano M, Castaño C, Munuera J, Escudero D and
García-Esperón C: Multiple intracranial arteriovenous fistulas in
Cowden syndrome. J Stroke Cerebrovasc Dis. 25:e93–e94. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gist TL, Rangel-Castilla L, Krishna C,
Roman GC, Cech DA and Diaz O: Endovascular management of six
simultaneous intracranial dural arteriovenous fistulas in a single
patient. J Neurointerv Surg. 6:e162014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li M, Lin N, Wu J, Liang J and He W:
Multiple intracranial aneurysms associated with multiple dural
arteriovenous fistulas and cerebral arteriovenous malformation.
World Neurosurg. 77:3982012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pascoe HM, Lui EH, Mitchell P and Gaillard
F: Progressive subcortical calcifications secondary to venous
hypertension in an intracranial dural arteriovenous fistula. J Clin
Neurosci. 39:98–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Borden JA, Wu JK and Shucart WA: A
proposed classification for spinal and cranial dural arteriovenous
fistulous malformations and implications for treatment. J
Neurosurg. 82:166–179. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cognard C, Gobin YP, Pierot L, Bailly AL,
Houdart E, Casasco A, Chiras J and Merland JJ: Cerebral dural
arteriovenous fistulas: Clinical and angiographic correlation with
a revised classification of venous drainage. Radiology.
194:671–680. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dogan M, Kahraman AS, Firat C, Ak M,
Yildirim O and Dogan DG: Multiple dural arteriovenous fistulas
involving the cavernous sinus, transverse sinus, sigmoid sinus and
spinal drainage: CT angiography findings in 14-year-old boy. Eur
Rev Med Pharmacol Sci. 16:1305–1306. 2012.PubMed/NCBI
|
|
36
|
Zeidman SM, Monsein LH, Arosarena O,
Aletich V, Biafore JA, Dawson RC, Debrun GM and Hurko O:
Reversibility of white matter changes and dementia after treatment
of dural fistulas. AJNR Am J Neuroradiol. 16:1080–1083.
1995.PubMed/NCBI
|
|
37
|
Iizuka Y, Rodesch G, Garcia-Monaco R,
Alvarez H, Burrows P, Hui F and Lasjaunias P: Multiple cerebral
arteriovenous shunts in children: Report of 13 cases. Childs Nerv
Syst. 8:437–444. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Signorelli F, Gory B, Maduri R, Guyotat J,
Pelissou-Guyotat I, Chirchiglia D, Riva R and Turjman F:
Intracranial dural arteriovenous fistulas: A review of their
current management based on emerging knowledge. J Neurosurg Sci.
61:193–206. 2017.PubMed/NCBI
|
|
39
|
Serulle Y, Miller TR and Gandhi D: Dural
arteriovenous fistulae: Imaging and management. Neuroimaging Clin N
Am. 26:247–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsai LK, Liu HM and Jeng JS: Diagnosis and
management of intracranial dural arteriovenous fistulas. Expert Rev
Neurother. 16:307–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Awad IA, Little JR, Akarawi WP and Ahl J:
Intracranial dural arteriovenous malformations: Factors
predisposing to an aggressive neurological course. J Neurosurg.
72:839–850. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mendonça N, Santos G, Duro D, Machado E,
Goulão A and Santana I: Multiple dural arteriovenous fistulas
presenting as rapidly progressive dementia. Neurologist.
18:130–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Netravathi M, Pal PK, Bharath RD and
Ravishankar S: Intracranial dural arteriovenous fistula presenting
as parkinsonism and cognitive dysfunction. J Clin Neurosci.
18:138–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abe K, Okuda O, Ohishi H, Sonobe M and
Arai H: Multiple dural arteriovenous fistulas causing rapid
progressive dementia successfully treated by endovascular surgery.
A case report. Neurol Med Chir (Tokyo). 54:145–149. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mejia P, Piedra LM and Merchan-Del Hierro
X: Rapidly progressive dementia and parkinsonism associated to
multiple dural arteriovenous fistulas. Rev Neurol. 64:214–218.
2017.PubMed/NCBI
|
|
46
|
Takai K, Komori T and Taniguchi M:
Microvascular anatomy of spinal dural arteriovenous fistulas:
Arteriovenous connections and their relationships with the dura
mater. J Neurosurg Spine. 23:526–533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hetts SW, Moftakhar P, Maluste N,
Fullerton HJ, Cooke DL, Amans MR, Dowd CF, Higashida RT and Halbach
VV: Pediatric intracranial dural arteriovenous fistulas:
Age-related differences in clinical features, angioarchitecture,
and treatment outcomes. J Neurosurg Pediatr. 18:602–610. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Alerhand S and Lay C: Spontaneous
iIntracerebral hemorrhage. Emerg Med Clin North Am. 35:825–845.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kunz WG, Schuler F, Sommer WH, Fabritius
MP, Havla L, Meinel FG, Reiser MF, Ertl-Wagner B and Thierfelder
KM: Wavelet-based angiographic reconstruction of computed
tomography perfusion data: Diagnostic value in cerebral venous
sinus thrombosis. Invest Radiol. 52:302–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Okamura A, Nakaoka M, Ohbayashi N, Yahara
K and Nabika S: Intraoperative cone-beam computed tomography
contributes to avoiding hypoglossal nerve palsy during transvenous
embolization for dural arteriovenous fistula of the anterior
condylar confluence. Interv Neuroradiol. 22:584–589. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lescher S, Gehrisch S, Klein S and
Berkefeld J: Time-resolved 3D rotational angiography: Display of
detailed neurovascular anatomy in patients with intracranial
vascular malformations. J Neurointerv Surg. 9:887–894. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Azuma M, Hirai T, Shigematsu Y, Kitajima
M, Kai Y, Yano S, Nakamura H, Makino K, Iryo Y and Yamashita Y:
Evaluation of intracranial dural arteriovenous fistulas: Comparison
of unenhanced 3T 3D time-of-flight MR angiography with digital
subtraction angiography. Magn Reson Med Sci. 14:285–293. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Willinsky R, Terbrugge K, Montanera W,
Mikulis D and Wallace MC: Venous congestion: An MR finding in dural
arteriovenous malformations with cortical venous drainage. AJNR Am
J Neuroradiol. 15:1501–1507. 1994.PubMed/NCBI
|
|
54
|
Ushikoshi S, Kikuchi Y, Houkin K, Saito H
and Abe H: Multiple dural arteriovenous fistulas. Neurol Med Chir
(Tokyo). 38:478–484. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fiumara E, Tumbiolo S, Bellomonte ML,
Savatteri P, Finazzo F and La Gattuta F: Resection of the
transverse sinuses and confluence of sinuses for treatment of
multiple dural arteriovenous fistulas. A case report. J Neurosurg.
100:348–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sato K, Matsumoto Y, Endo H and Tominaga
T: A hemorrhagic complication after Onyx embolization of a
tentorial dural arteriovenous fistula: A caution about subdural
extension with pial arterial supply. Interv Neuroradiol.
23:307–312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Luo CB, Chang FC and Teng MM: Update of
embolization of intracranial dural arteriovenous fistula. J Chin
Med Assoc. 77:610–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Watanabe T, Matsumaru Y, Sonobe M, Asahi
T, Onitsuka K, Sugita K, Takahashi S and Nose T: Multiple dural
arteriovenous fistulae involving the cavernous and sphenoparietal
sinuses. Neuroradiology. 42:771–774. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nakamura M, Tamaki N, Hara Y and Nagashima
T: Two unusual cases of multiple dural arteriovenous fistulas.
Neurosurgery. 41:288–292; discussion 292–283. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin N, Brouillard AM, Mokin M, Natarajan
SK, Snyder KV, Levy EI and Siddiqui AH: Direct access to the middle
meningeal artery for embolization of complex dural arteriovenous
fistula: A hybrid treatment approach. J Neurointerv Surg.
7:e242015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vougioukas VI, Coulin CJ, Shah M, Berlis
A, Hubbe U and Van Velthoven V: Benefits and limitations of image
guidance in the surgical treatment of intracranial dural
arteriovenous fistulas. Acta Neurochir (Wien). 148:145–153,
discussion 153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Al-Mefty O, Jinkins JR and Fox JL:
Extensive dural arteriovenous malformation. A case report. J
Neurosurg. 65:417–420. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yen CP, Lanzino G and Sheehan JP:
Stereotactic radiosurgery of intracranial dural arteriovenous
fistulas. Neurosurg Clin N Am. 24:591–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dmytriw AA, Schwartz ML, Cusimano MD,
Mendes Pereira V, Krings T, Tymianski M, Radovanovic I and Agid R:
Gamma Knife radiosurgery for the treatment of intracranial dural
arteriovenous fistulas. Interv Neuroradiol. 23:211–220. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bertalanffy A, Dietrich W, Kitz K and
Bavinzski G: Treatment of dural arteriovenous fistulae (dAVF's) at
the superior sagittal sinus (SSS) using embolisation combined with
micro- or radiosurgery. Minim Invasive Neurosurg. 44:205–210. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kim DJ, terBrugge K, Krings T, Willinsky R
and Wallace C: Spontaneous angiographic conversion of intracranial
dural arteriovenous shunt: Long-term follow-up in nontreated
patients. Stroke. 41:1489–1494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Friedman JA, Meyer FB, Nichols DA, Coffey
RJ, Hopkins LN, Maher CO, Meissner ID and Pollock BE: Fatal
progression of posttraumatic dural arteriovenous fistulas
refractory to multimodal therapy. A case report. J Neurosurg.
94:831–835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Torok CM, Nogueira RG, Yoo AJ,
Leslie-Mazwi TM, Hirsch JA, Stapleton CJ, Patel AB and Rabinov JD:
Transarterial venous sinus occlusion of dural arteriovenous
fistulas using ONYX. Interv Neuroradiol. 22:711–716. 2016.
View Article : Google Scholar : PubMed/NCBI
|