Introduction
Hereditary plasminogen activator inhibitor-1 (PAI-1)
deficiency is considered an extremely rare disorder characterized
by hyperfibrinolysis due to decreased PAI-1 activity that results
in frequent bleeding episodes. Five variants of PAI-1 polymorphisms
have been identified: two common polymorphisms −765 4G/5G and −844
A>G in the promoter; and three less common, Ala15Thr, Val17Ile
at the signal peptide and Asn195Ile (1). To date, only two types of mutations
are clearly associated with PAI-1 deficiency. One is the Ala15Thr
mutation (2), and the second is
the frame-shift mutation in exon 4 of the PAI-1 gene, which results
in complete PAI-1 deficiency (3).
In this study, we present the case of a woman with PAI-1 functional
deficiency having a heterozygous Ala15Thr mutation.
Materials and methods
All blood work was routinely carried out by
different hospital laboratories.
Analysis of plasma clot formation with
thromboelastography
Thromboelastography (TEG) not only allows for the
measurement of the global coagulation profile, but also yields data
on the kinetics and dynamics of clot formation and clot lysis in
whole blood or in plasma (4,5). The
critical part of this instrument is a pin hanging on a torsion wire
suspended in a cup holding a sample (360 μl). When plasma changes
viscosity during clot formation the pin motion is progressively
restrained by the clot and the cup. The strength of the clot
determines the degree of force on the pin. Sodium-citrated blood
was used for TEG assays by mixing 1 ml of blood with 20 μl of
kaolin (Haemoscope Co., Neils, IL) to which a constant amount of
tPA was added [10 μl of tPA (2.1 mg/ml in 0.4 M HEPES, 0.1 M NaCl,
pH 7.4)] as a fibrinolytic agent (6,7) to
measure proteolysis under controlled conditions (5,8,9).
Subsequently, 320 μl of the mixture was transferred to each TEG cup
containing 20 μl of CaCl2 (0.2 M) and an activity assay
buffer (50 mM HEPES, 150 mM NaCl, 1% human serum albumin, 0.05%
Tween-20 buffer, pH 6.6) with i) VLHL PAI-1 to prevent lysis by
tPA, or ii) VLHL PAI-1 plus the tested compound to check its
inhibitory action demonstrated by lysis of the clot when tPA is
unopposed by PAI-1 activity.
DNA sequencing
The PAI-1 gene was analyzed by PCR, and sequencing
of both DNA strands of the entire coding region and the highly
conserved exon-intron splice junction was carried out. Analysis was
carried out by Centrogen GmbH, Rostock, Germany.
Results
We report the case of a 60-year-old woman of
American Indian descent with a bleeding diathesis. She had a
life-long history of recurrent and prolonged bleeding such as mild
epistaxis, gingival bleeding and microscopic hematuria. Prior to
this study, she experienced severe bleeding after surgery. The
patient had a large family with a history of bleeding tendency
including menorrhagia in several female family members. She
describes one case of severe bleeding lasting for three months
while the female patient was in a coma and treated with multiple
transfusions. The bleeding tendency was related to her mother's
side of the family.
Her symptoms and family history suggested a
hereditary bleeding disorder. Therefore, she insisted on multiple
blood analyses, which were performed over the years to eliminate
common bleeding conditions. Screening studies revealed no
abnormalities that could explain the bleeding tendency: hematocrit,
37.8–41.4% (range 34.0–46.0%); hemoglobin levels, 12.9–13.5 gm/dl
(range 11.5–15.0 gm/dl); an elevated platelet count in some blood
work, 381–470×103/μl (range
150–400×103/μl).
Coagulation tests were conducted as well:
prothrombin time, 17 sec (range 16–23 sec); fibrinogen levels,
3.26–3.51 g/l (range 1.70–4.50 g/l); factor VIII coagulant
activity, 84–87% (range 50–150%); von Willebrand factor levels,
65–70% (range 46–155%); ristocetin cofactor activity, 81% (range
50–150%); factor IX, 110%, (range 50–150%); factor XI, 163% (range
50–150%); factor XIII, 196% (range 57–192%). Euglobulin clot lysis
time test was carried out twice; the first for 45 min and the
second for >60 min (normal >60 min). α2-antiplasmin was 120%
(range 85–156%) and PAI-1 antigen, 8.9–14.7 ng/ml (range 4.0–43.0
ng/ml). Tests of PAI-1 activity were carried out; these were the
most controversial due to the lack of standardized and uniform
methods. PAI-1 activity tested by amidolytic assays was determined
as <6 IU/ml (range 15.0–40.7 IU/ml). Using ELISA based on
binding active PAI-1 to immobilized uPA was determined as 5.2 U/ml
[range 1–15 U/ml (10) or 0–36.7
U/ml (11)] clearly being on the
lower end of the normal range.
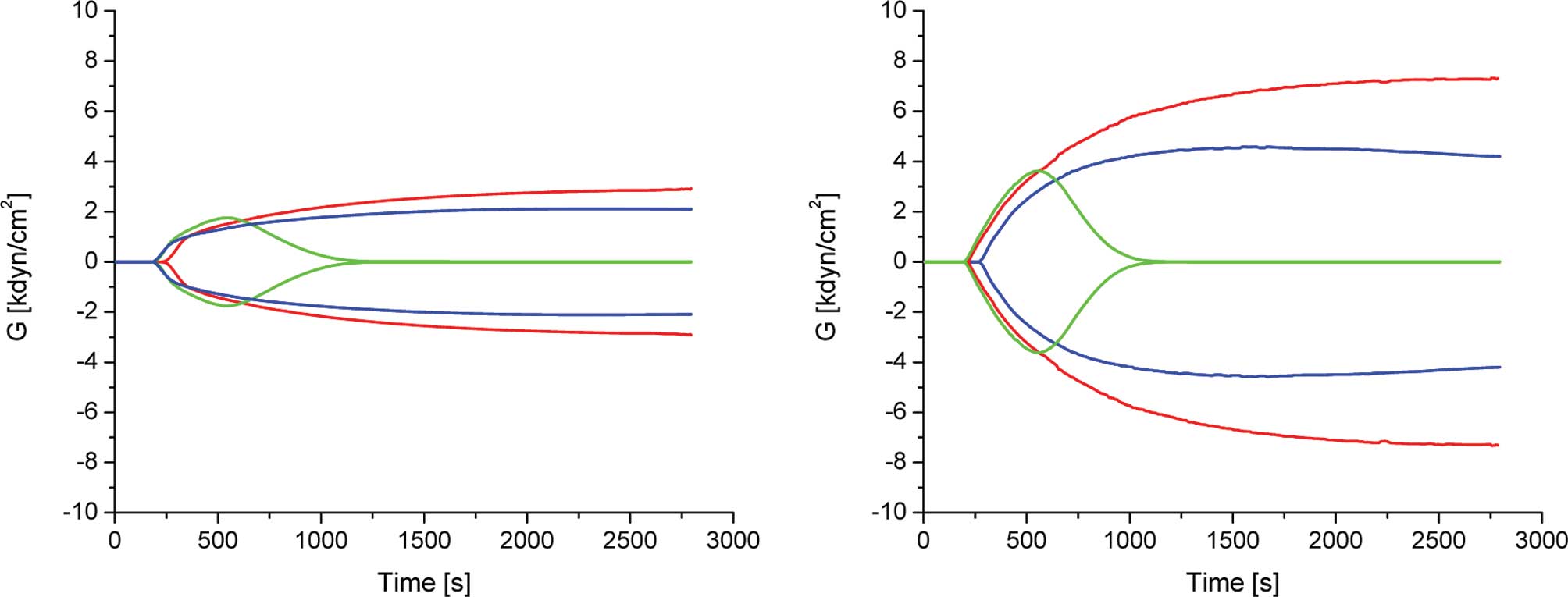
Clot formation of sodium-citrated blood was analyzed
by TEG, which allows for the measurement of the overall coagulation
profile. TEG also yields data on the kinetics and dynamics of clot
formation and clot lysis in whole blood. Most of the parameters
were within the normal range with the exception of A (amplitude),
which was used to derive the elastic modulus strength: G =
[5000A/(100-A)]/1000). As shown in Fig. 1 and Table I, the mean of G of 2.2±0.4
dyn/cm2 was almost 2-fold less than the reported normal
range (4.6–10.9 dyn/cm2) (12). A similar pattern was observed for
TEG of PAI-1-deficient and normal mouse blood (13).
 | Table I.Thromboelastogram parameters of the
blood of the studied patient. |
Table I.
Thromboelastogram parameters of the
blood of the studied patient.
| R (min) | K (min) | An (°) | MA (mm) | MG
(dyn/cm2) | LY30 (min) |
|---|
| Normal | 2–8 | 1–3 | 55–78 | 51–69 | 4.6–10.9 | 0–8 |
| Control | 4.2±0.1 | 3.0±0.2 | 65.6±1.2 | 34.7±1.3 | 2.7±0.2 | 0 |
| tPA | 3.4±0.1 | 2.1±0.4 | 69.0±2.8 | 27.6±2.3 | 1.9±0.2 | 86.4±0.8 |
| tPA+PAI-1 | 3.3±0.1 | 4.2±0.6 | 70.5±5.2 | 29.3±1.6 | 2.1±0.2 | 0 |
Therefore, the PAI-1 gene was sequenced for possible
mutations. PAI-1 gene sequencing revealed that the patient had a
heterozygous mutation G to A at nucleotide position 4497 in exon 2,
resulting in the replacement of alanine 15 (GCC) to threonine (ACC)
at the signal peptide.
Discussion
PAI-1 deficiency was first reported in 1989, when
undetectable PAI-1 activity and antigen levels were noted in a
76-year-old man with a life-long bleeding tendency (14,15).
At that time this condition was considered extremely rare. Since
then, more cases have been reported in the literature (2,3,14).
PAI-1 deficiency seems to be more common than previously thought,
possibly due to misdiagnosis or lack of awareness of this condition
by primary physicians.
Initially, PAI-1 deficiency and its related bleeding
diathesis was defined as a very low PAI-1 antigen (less than 4
ng/ml) or activity (lower than 1 IU/ml) (3,16).
However, hereditary PAI-1 deficiency and severe menorrhagia have
been reported in patients with a PAI-1 antigen level of 11.4 ng/ml
(4.0–43 ng/ml) and PAI-1 activity less than 5 AU/ml (5–37 AU/ml)
(17). The levels of PAI-1 in our
reported case were similar, which initially challenged our
assumption of PAI-1 deficiency. The TEG analysis revealed weak
blood clotting, which indicated a low platelet count contradicting
PAI-1 deficiency. However, her platelet count was slightly
elevated. For this reason sequencing of the PAI-1 gene was carried
out. A mutation found in the signal peptide strongly suggested that
this type of deficiency was related to the secretory dynamics of
PAI-1 secretion rather than to its low levels or activity as
originally suggested by Zhang at el, who described the first
case of an Ala15Thr mutation (2).
To conclude, we considered the PAI-1 mutation to be
the likely etiology of the bleeding diathesis in this patient.
Acknowledgements
We thank Dr R. Hart (President,
PharmaIP LLC, Greenwich, CT, USA) for the helpful remarks,
discussions and support. This work was supported, in part, by
grants from PharmaIP LLC and the Frank D. Stranahan Endowment Fund
for Oncological Research. Special thanks to the patient for her
extraordinary involvement and willingness to provide multiple
samples of her blood.
References
|
1.
|
Lopes C, Dina C, Durand E and Froguel P:
PAI-1 polymorphisms modulate phenotypes associated with the
metabolic syndrome in obese and diabetic Caucasian population.
Diabetologia. 46:1284–1290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zhang ZY, Wang ZY, Dong NZ, Bai X, Zhang W
and Ruan CG: A case of deficiency of plasma plasminogen activator
inhibitor-1 related to Ala15Thr mutation in its signal peptide.
Blood Coagul Fibrinolysis. 16:79–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fay WP, Parker AC, Condrey LR and Shapiro
AD: Human plasminogen activator inhibitor-1 (PAI-1) deficiency:
characterization of a large kindred with a null mutation in the
PAI-1 gene. Blood. 90:204–208. 1997.PubMed/NCBI
|
|
4.
|
Evans PA, Hawkins K, Lawrence M, Barrow
MS, Williams PR and Williams RL: Studies of whole blood coagulation
by oscillatory shear, thromboelastography and free oscillation
rheometry. Clin Hemorheol Microcirc. 38:267–277. 2008.PubMed/NCBI
|
|
5.
|
Gallimore MJ, Harris SL, Tappenden KA,
Winter M and Jones DW: Urokinase induced fibrinolysis in
thromboelastography: a model for studying fibrinolysis and
coagulation in whole blood. J Thromb Haemost. 3:2506–2513. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Carr ME Jr, Krishnamurti C and Alving BM:
Effect of plasminogen activator inhibitor-1 on tissue-type
plasminogen activator-induced fibrinolysis. Thromb Haemost.
67:106–110. 1992.PubMed/NCBI
|
|
7.
|
Sugiki M, Maruyama M, Yoshida E, Mihara H,
Kamiguti AS and Theakston DG: Enhancement of plasma fibrinolysis in
vitro by jararhagin, the main haemorrhagic metalloproteinase in
Bothrops jararaca venom. Toxicon. 33:1605–1617. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jankun J, Aleem AM, Selman SH, et al:
Highly stable plasminogen activator inhibitor type one (VLHL PAI-1)
protects fibrin clots from tissue plasminogen activator-mediated
fibrinolysis. Int J Mol Med. 20:683–687. 2007.
|
|
9.
|
Kohro S, Yamakage M, Omote T and Namiki A:
In vitro effects of propofol on blood coagulability and
fibrinolysis by the use of thromboelastograph technique. Acta
Anaesthesiol Scand. 43:217–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Agren A, Wiman B and Schulman S:
Laboratory evidence of hyperfibrinolysis in association with low
plasminogen activator inhibitor type 1 activity. Blood Coagul
Fibrinolysis. 18:657–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Perkowska A, Elhasade A, Durlik M, et al:
The effect of chronic allograft rejection on plasma regulators of
fibrinolysis. Ann Transplant. 7:44–51. 2002.PubMed/NCBI
|
|
12.
|
TEG 5000 User's Manual Version 4.2
Software. Haemoscope Corporation 2006.
|
|
13.
|
Jankun J, Aleem AM, Struniawski R,
Lysiak-Szydlowska W, Selman SH and Skrzypczak-Jankun E: Accelerated
thrombus lysis in the blood of plasminogen activator inhibitor
deficient mice is inhibited by PAI-1 with a very long half-life.
Pharmacol Rep. 61:673–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mehta R and Shapiro AD: Plasminogen
activator inhibitor type 1 deficiency. Haemophilia. 14:1255–1260.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Schleef RR, Higgins DL, Pillemer E and
Levitt LJ: Bleeding diathesis due to decreased functional activity
of type 1 plasminogen activator inhibitor. J Clin Invest.
83:1747–1752. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jankun J and Skrzypczak-Jankun E: Yin and
yang of the plasminogen activator inhibitor. Pol Arch Med Wewn.
119:410–417. 2009.PubMed/NCBI
|
|
17.
|
Repine T and Osswald M: Menorrhagia due to
a qualitative deficiency of plasminogen activator inhibitor-1: case
report and literature review. Clin Appl Thromb Hemost. 10:293–296.
2004. View Article : Google Scholar : PubMed/NCBI
|