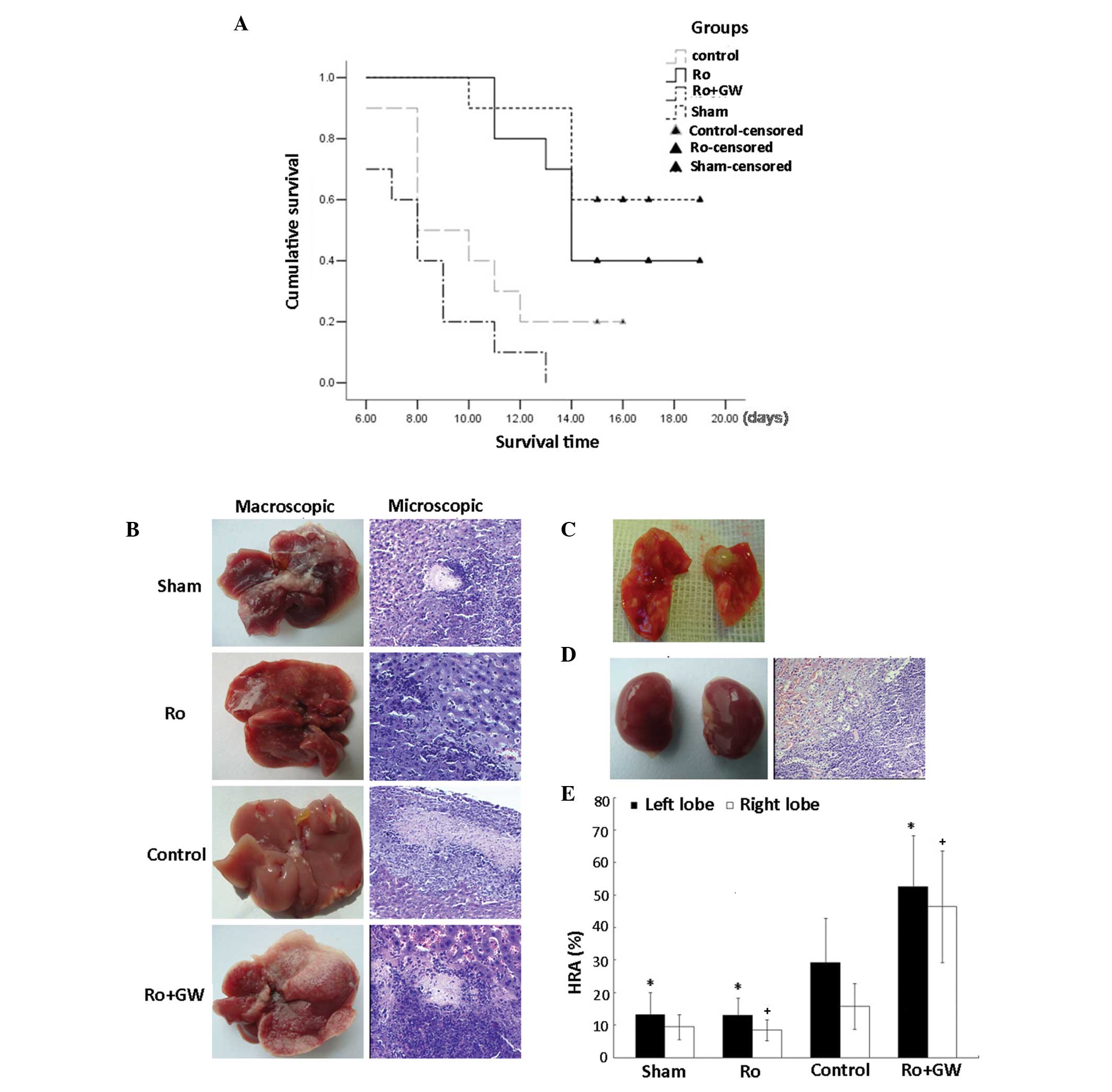
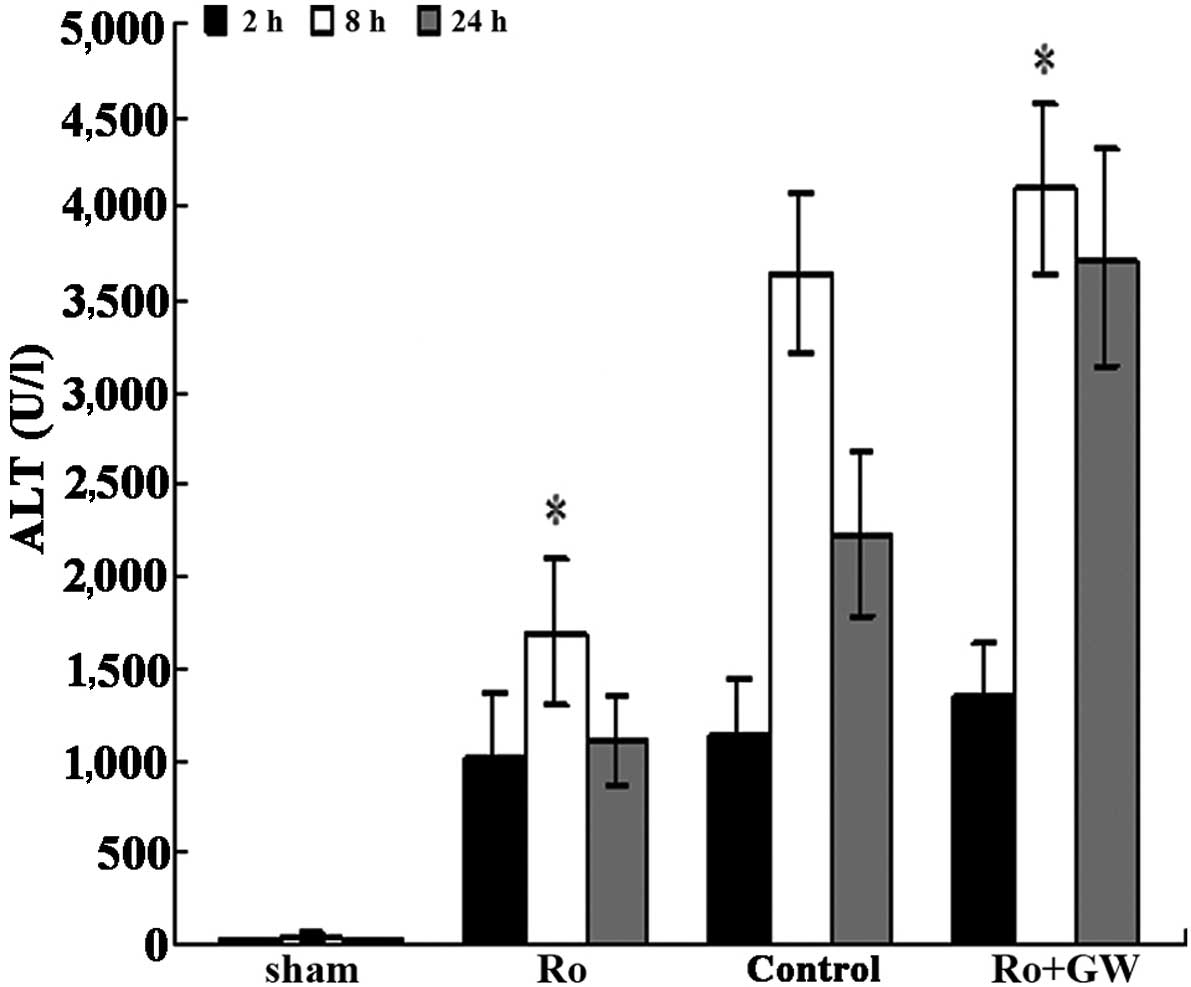
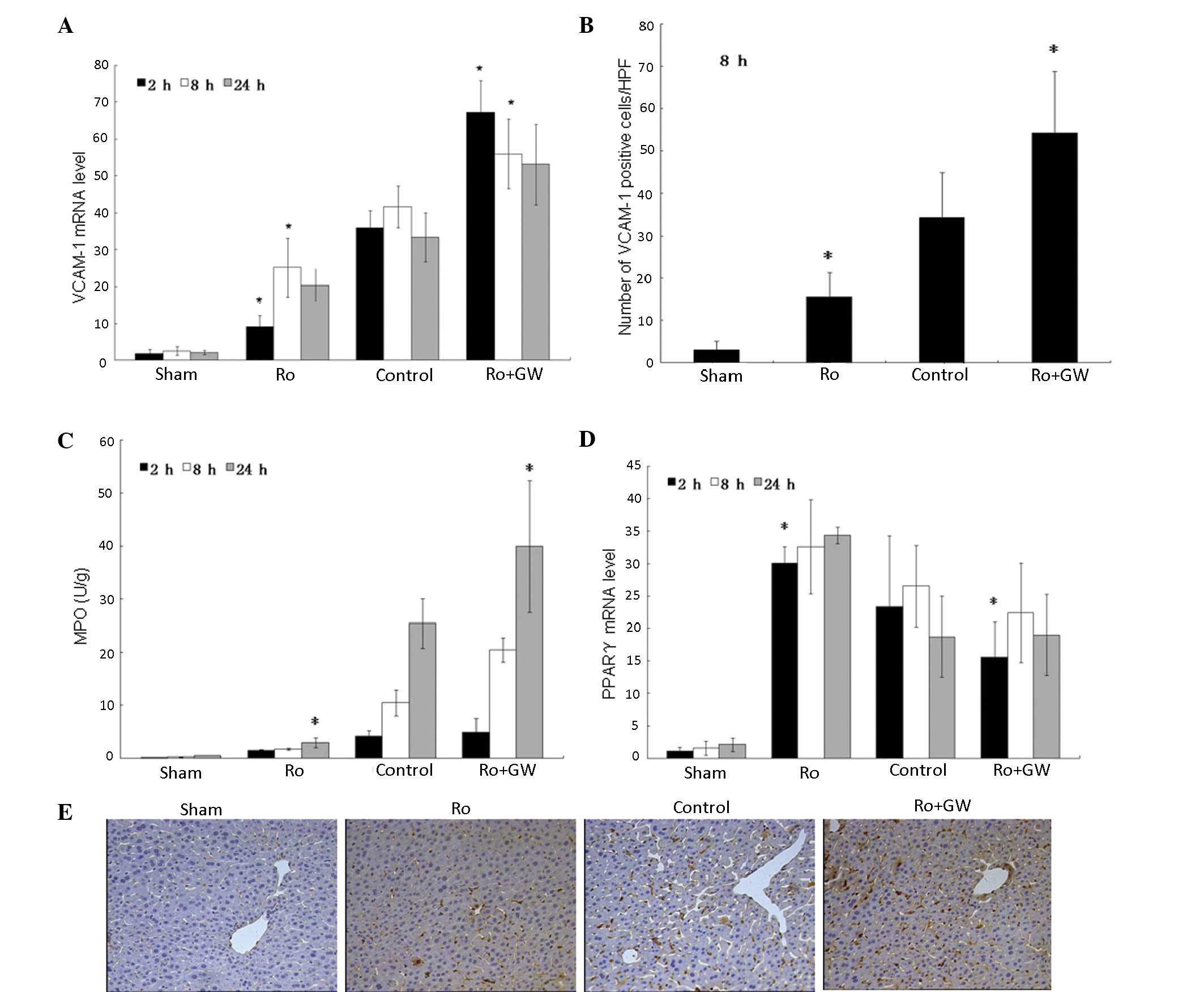
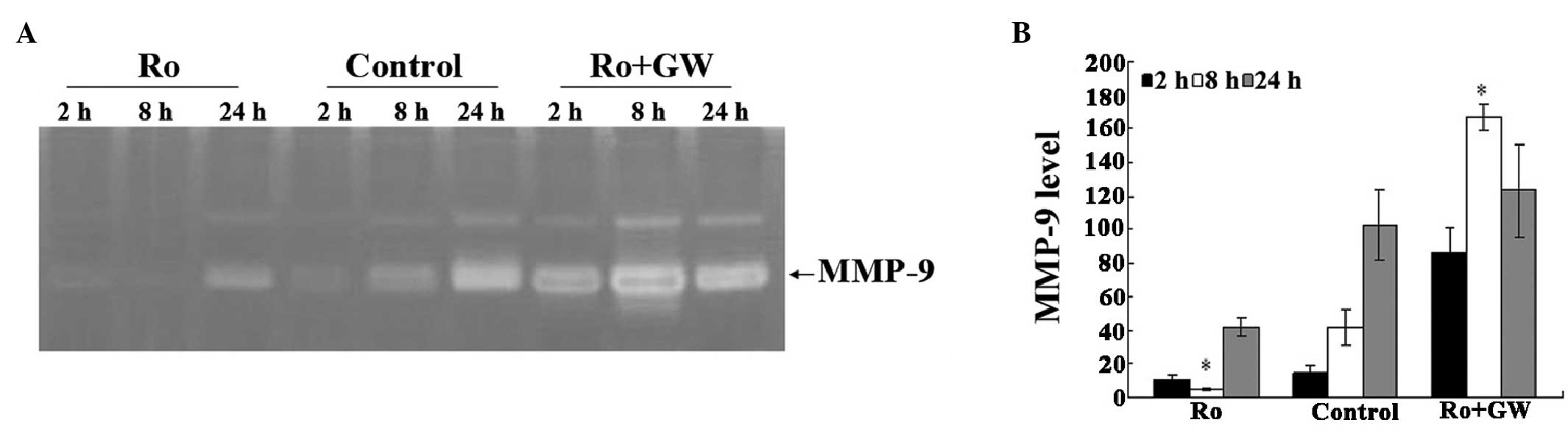
|
1
|
Fu SY, Lau WY, Li AJ, Yang Y, Pan ZY, Sun
YM, Lai EC, Zhou WP and Wu MC: Liver resection under total vascular
exclusion with or without preceding Pringle manoeuvre. Br J Surg.
97:50–55. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Makuuchi M, Takayama T, Kubota K, Kimura
W, Midorikawa Y, Miyagawa S and Kawasaki S: Hepatic resection for
hepatocellular carcinoma - Japanese experience.
Hepatogastroenterology. 45(Suppl 3): 1267–1274. 1998.PubMed/NCBI
|
|
3
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuchiya Y, Sawada S, Yoshioka I, Ohashi
Y, Matsuo M, Harimaya Y, Tsukada K and Saiki I: Increased surgical
stress promotes tumor metastasis. Surgery. 133:547–555. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uotani H, Yamashita I, Nagata T, Kishimoto
H, Kashii Y and Tsukada K: Induction of E-selectin after partial
hepatectomy promotes metastases to liver in mice. J Surg Res.
96:197–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kooby DA, Stockman J, Ben-Porat L, Gonen
M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH and
Fong Y: Influence of transfusions on perioperative and long-term
outcome in patients following hepatic resection for colorectal
metastases. Ann Surg. 237:860–869; discussion 869–870. 2003.
View Article : Google Scholar
|
|
7
|
Jarrar D, Chaudry IH and Wang P: Organ
dysfunction following hemorrhage and sepsis, Mechanisms and
therapeutic approaches (Review). Int J Mol Med. 4:575–583.
1999.PubMed/NCBI
|
|
8
|
Selzner M and Clavien PA: Failure of
regeneration of the steatotic rat liver: Disruption at two
different levels in the regeneration pathway. Hepatology. 31:35–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaeschke H, Bautista AP, Spolarics Z and
Spitzer JJ: Superoxide generation by Kupffer cells and priming of
neutrophils during reperfusion after hepatic ischemia. Free Radic
Res Commun. 15:277–284. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higashiyama A, Watanabe H, Okumura K and
Yagita H: Involvement of tumor necrosis factor alpha and very late
activation antigen 4/vascular cell adhesion molecule 1 interaction
in surgical-stress-enhanced experimental metastasis. Cancer Immunol
Immunother. 42:231–236. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nicoud IB, Jones CM, Pierce JM, Earl TM,
Matrisian LM, Chari RS and Gorden DL: Warm hepatic
ischemia-reperfusion promotes growth of colorectal carcinoma
micrometastases in mouse liver via matrix metalloproteinase-9
induction. Cancer Res. 67:2720–2728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miles FL and Pruitt FL: vanG olen KL and
Cooper CR: Stepping out of the flow: Capillary extravasation in
cancer metastasis. Clin Exp Metastasis. 25:305–324. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Da Costa ML, Redmond HP, Finnegan N, Flynn
M and Bouchier-Hayes D: Laparotomy and laparoscopy differentially
accelerate experimental flank tumour growth. Br J Surg.
85:1439–1442. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Konstantopoulos K and Thomas SN: Cancer
cells in transit: The vascular interactions of tumor cells. Annu
Rev Biomed Eng. 11:177–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdelrahman M, Sivarajah A and Thiemermann
C: Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion
injury, inflammation and shock. Cardiovasc Res. 65:772–781. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuzzocrea S: Peroxisome
proliferator-activated receptors gamma ligands and ischemia and
reperfusion injury. Vascul Pharmacol. 41:187–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuboki S, Shin T, Huber N, Eismann T,
Galloway E, Schuster R, Blanchard J, Zingarelli B and Lentsch AB:
Peroxisome proliferator-activated receptor-gamma protects against
hepatic ischemia/reperfusion injury in mice. Hepatology.
47:215–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kohn EC: Invasion and metastasis: B iology
and clinical potential. Pharmacol Ther. 52:235–244. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tantivejkul K, Kalikin LM and Pienta KJ:
Dynamic process of prostate cancer metastasis to bone. J Cell
Biochem. 91:706–717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Bilt JD, Kranenburg O, Nijkamp MW,
Smakman N, Veenendaal LM, Te Velde EA, Voest EE, van Diest PJ and
Borel Rinkes IH: Ischemia/reperfusion accelerates the outgrowth of
hepatic micrometastases in a highly standardized murine model.
Hepatology. 42:165–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herron GS, Banda MJ, Clark EJ, Gavrilovic
J and Werb Z: Secretion of metalloproteinases by stimulated
capillary endothelial cells. Histopathology. J Biol Chem.
261:2814–2818. 1986.PubMed/NCBI
|
|
22
|
Tamagawa K, Horiuchi T, Uchinami M, Doi K,
Yoshida M, Nakamura T, Sasaki H, Taniguchi M and Tanaka K: Hepatic
ischemia-reperfusion increases vascular endothelial growth factor
and cancer growth in rats. J Surg Res. 148:158–163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akahori1 T, Sho M, Hamada K, Suzaki Y,
Kuzumoto Y, Nomi T, Nakamura S, Enomoto K, Kanehiro H and Nakajima
Y: Importance of peroxisome proliferator-activated receptor γ in
hepatic ischemia/reperfusion injury in mice. J Hepatol. 47:784–792.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu J, Qiao L, Zimmermann L, Ebert MP,
Zhang H, Lin W, Röcken C, Malfertheiner P and Farrell GC:
Troglitazone inhibits tumor growth in hepatocellular carcinoma in
vitro and in vivo. Hepatology. 43:134–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang S and Dong C: Integrin VLA-4
enhances sialyl-Lewisx/a-negative melanoma adhesion to and
extravasation through the endothelium under low flow conditions. Am
J Physiol Cell Physiol. 295:C701–C707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conrad R, Remberger M, Cederlund K,
Hentschke P, Sundberg B, Ringdén O and Barkholt L: Inflammatory
cytokines predominate in cases of tumor regression after
hematopoietic stem cell transplantation for solid cancer. Biol
Blood Marrow Transplant. 12:346–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirai T, Matsumoto H, Yamashita K, Urakami
A, Iki K, Yamamura M and Tsunoda T: Surgical oncotaxis - excessive
surgical stress and postoperative complications contribute to
enhancing tumor metastasis, resulting in a poor prognosis for
cancer patients. Ann Thorac Cardiovasc Surg. 11:4–6.
2005.PubMed/NCBI
|
|
28
|
McGary EC, Lev DC and Bar-Eli M: Cellular
adhesion pathways and metastatic potential of human melanoma.
Cancer Biol Ther. 1:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mendoza L, Carrascal T, De Luca M, Fuentes
AM, Salado C, Blanco J and Vidal-Vanaclocha F: Hydrogen peroxide
mediates vascular cell adhesion molecule-1 expression from
interleukin-18-activated hepatic sinusoidal endothelium
Implications for circulating cancer cell arrest in the murine
liver. Hepatology. 34:298–310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mendoza L, Valcárcel M, Carrascal T,
Egilegor E, Salado C, Sim BK and Vidal-Vanaclocha F: Inhibition of
cytokine-induced microvascular arrest of tumor cells by recombinant
endostatin prevents experimental hepatic melanoma metastasis.
Cancer Res. 64:304–310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grommes C, Landreth GE, Sastre M, Beck M,
Feinstein DL, Jacobs AH, Schlegel U and Heneka MT: Inhibition of in
vivo glioma growth and invasion by peroxisome
proliferator-activated receptor gamma agonist treatment. Mol
Pharmacol. 70:1524–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishikawa M, Tamada A, Hyoudou K, Umeyama
Y, Takahashi Y, Kobayashi Y, Kumai H, Ishida E, Staud F, Yabe Y, et
al: Inhibition of experimental hepatic metastasis by targeted
delivery of catalase in mice. Clin Exp Metastasis. 21:213–221.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janani P, Sivakumari K, Geetha A, Yuvaraj
S and Parthasarathy C: Bacoside A downregulates matrix
metalloproteinases 2 and 9 in DEN-induced hepatocellular carcinoma.
Cell Biochem Funct. 28:164–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshida M, Horiuchi T, Uchinami M, Tabo T,
Kimura N, Yokomachi J, Doi K, Nakamura T, Tamagawa K and Tanaka K:
Intermittent hepatic ischemia-reperfusion minimizes liver
metastasis in rats. J Surg Res. 111:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doi K, Horiuchi T, Uchinami M, Tabo T,
Kimura N, Yokomachi J, Yoshida M and Tanaka K: Hepatic
ischemia-reperfusion promotes liver metastasis of colon cancer. J
Surg Res. 105:243–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:12493–12498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orr FW and Warner DJ: Effects of systemic
complement activation and neutrophil-mediated pulmonary injury on
the retention and metastasis of circulating cancer cells in mouse
lungs. Lab Invest. 62:331–338. 1990.PubMed/NCBI
|
|
38
|
Wu QD, Wang JH, Condron C, Bouchier-Hayes
D and Redmond HP: Human neutrophils facilitate tumor cell
transendothelial migration. Am J Physiol Cell Physiol.
280:C814–C822. 2001.PubMed/NCBI
|
|
39
|
Rogers AB and Fox JG: Inflammation and
Cancer. Histopathology. Am J Physiol Gastrointest Liver Physiol.
286:G361–G366. 2004.PubMed/NCBI
|
|
40
|
Elsharkawy AM and Mann DA: Nuclear
factor-kappaB and the hepatic inflammation-fibrosis-cancer axis.
Hepatology. 46:590–597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vainer GW, Pikarsky E and Ben-Neriah Y:
Contradictory functions of NF-kappaB in liver physiology and
cancer. Cancer Lett. 267:182–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baud V and Karin M: Is NF-kappaB a good
target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov.
8:33–40. 2009. View Article : Google Scholar : PubMed/NCBI
|