Introduction
Although itch, also known as alloknesis, and pain
are unpleasant sensations, they are two distinct sensations
(1); however, recent studies have
identified various interactions between itch and pain transmission
and sensitization pathways (2–4). For
example, when painful stimuli is applied to lesional skin of
patients with atopic dermatitis an itch sensation is evoked rather
than pain, which is thought to be the result of central
sensitization for itching (5,6).
Similarly, intradermal injection of algogenic substances capsaicin
or bradykinin has been demonstrated to induce a scratching reflex,
rather than pain-related behavior, in inflamed skin of mice
(7,8). The authors of the present study have
previously reported that activation of bradykinin receptor B1 (B1R)
contributes to alloknesis in mouse skin inflamed by treatment with
complete Freund's adjuvant (CFA) (9). Moreover, B1R agonists are known to
effectively induce scratch in inflamed lesioned skin (7). Although these previous studies have
improved understanding of this phenomenon, the underlying
mechanisms are yet to elucidated.
It has been well-documented that CFA is capable of
activating ERK1/2 in primary sensory and secondary order dorsal
horn neurons, and that pERK1/2 is associated with the generation
and maintenance of inflammatory pain (10,11).
ERK1/2 activation has previously been described in the spinal cord
during histamine- and DNFB-induced alloknesis (12), in DRG neurons during ET-1-induced
acute alloknesis (13), during
chronic alloknesis in dry skin and in allergic contact dermatitis
models (14). However, its role in
B1R agonist-induced alloknesis in an animal model of CFA
inflammation has not been described. Therefore, we hypothesize that
prolonged activation of ERK following CFA-induced inflammation may
mediate the abnormal scratching response to B1R agonists. ERK1/2
are two closely related members of the mitogen-activated protein
kinase family. Upon activation, phosphorylated ERK1/2 (pERK1/2)
transduce extracellular stimuli into intracellular signaling and
subsequently trigger the expression of a plethora of nuclear
transcription factors to regulate numerous cellular functions
(15,16). Following peripheral nerve injury,
phosphorylation and activation of ERK1/2 is induced in the dorsal
root ganglia (DRG) (17–20) and dorsal horn of the spinal cord
(21–23), and inhibition of ERK1/2 activation
prevents behavioral pain sensitization (11,24). At
the molecular level, previous studies have demonstrated that
activation of ERK1/2 signaling in the spinal cord or sensory
neurons may be responsible for the relay of distinct types of
stimulus-evoked acute itch and spontaneous scratching responses in
chronic itch models (12,14,25). It
has also been demonstrated that ERK1/2 activation is required at
the spinal level for itch responses, which are induced by histamine
or dinitrofluorobenzene (DNFB) (12). Furthermore, it has been reported that
pruritogenic molecules, including endothelin 1 (ET-1) and cytokine
interleukin 31 (IL-31), stimulated alloknesis in cultured primary
sensory neurons and induced phosphorylation of ERK1/2, whereas
inhibition of ERK1/2 activation blocked ET-1- and cytokine
IL-31-induced scratching behavior in vivo (13,25).
When taken together, these findings demonstrate that ERK1/2
activation in the spinal cord or sensory neurons may have an
important role in itch transmission.
To our knowledge, there is a lack of previous
studies investigating the mechanisms mediating itch-related
scratching in response to algesic chemical stimuli delivered to
inflamed tissue in mice. The aim of the present study was to
investigate the potential role of ERK1/2 signaling in B1R
agonist-induced alloknesis, using a CFA-induced mouse model of
inflammation.
Materials and methods
Reagents and antibodies
CFA and MEK1/2 inhibitor (PD0325901) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). ERK1/2 inhibitor (328006)
and kinin B1 receptor agonist [des-Arg(9)-bradykinin] were purchased from EMD
Millipore (Billerica, MA, USA) and Tocris Bioscience (Bristol, UK),
respectively. Rabbit anti-pERK1/2 (cat. no. 4370) and anti-ERK1/2
(cat. no. 9102) monoclonal antibodies, goat anti-rabbit horseradish
peroxidase (HRP)-conjugated secondary antibody (cat. no. 7074) and
rabbit anti-tubulin monoclonal antibody (cat. no. 2128) were
obtained from Cell Signaling Technology, Inc., (Danvers, MA,
USA).
Mice
A total of 98 male C57BL/6J mice, weighing 20–22 g
and aged ~6 weeks, were obtained from the Center for Laboratory
Animals, Sun Yat-Sen University (Guangzhou, China). Mice were
maintained at 22±1°C on a 12/12 h light/dark cycle with ad
libitum access to food and water. A mouse model of skin
inflammation was established via intradermal injection of 50 µl CFA
into the nape of the neck, as previously described (7,8). In the
control group, an identical volume of normal saline was
administered instead of CFA. The experimental procedures and animal
use and care protocols were approved by the Committee on Ethical
Use of Animals at the Guangdong Academy of Medical Sciences
(Guangzhou, China), and were performed according to the National
Institutes of Health guidelines for the care and use of
animals.
Behavioral tests
Seven mice from each of the CFA and control groups
were used for the behavioral tests. Behavioral studies were
conducted at approximately the same time each day between 9:00 a.m.
and 4:00 p.m. in order to reduce circadian effects. Four days after
injection with CFA, the mice were placed into individual small
plastic chambers (22×12×10 cm) for 30 min prior to the experiment
for acclimation. From video recordings over a period of 30 min,
scratching behavior was quantified by the number of hind limb
scratches directed to the area surrounding the drug injection site
on the neck. Off-site scratches, such as to the cheek, were
excluded from the counts.
Treatment with BIR agonist
As peripheral noxious stimuli is capable of inducing
phosphorylation of ERK1/2 (26,27),
western blot analysis was used to determine whether increased
expression of pERK1/2 could be induced in the spinal cord by
stimulation with a B1R agonist. Four days after inflammation was
induced with CFA, mice (n=15) were injected with des-Arg(9)-bradykinin B1R agonist (0.4 mmol/l) in
the nape of the neck. The control group (n=15) were administered
normal saline stead of the B1R agonist. Mice were anesthetized with
1% sevoflurane (Wanshi Company, Osaka, Japan) and sacrificed by
cervical dislocation at various time points (5, 15, 30, 45 and 60
min) post-injection. These time points were selected as they
correspond with the time-dependent induction of pERK1/2 expression
in the spinal cord by histamine (12). Cervical spinal cord samples were
collected and preserved at −80°C for analysis of pERK1/2 levels by
western blotting.
Treatment with ERK1/2 and MEK1/2
inhibitors
CFA-treated mice were divided into four groups, as
follows: i) 328006 group, in which mice were intraperitoneally
injected with 30 mg/kg ERK1/2 inhibitor (100 µl 328006 in 10% DMSO;
n=7); ii) PD0325901 group, in which mice were intraperitoneally
injected with 10 mg/kg MEK1/2 inhibitor (100 µl PD0325901 in 10%
DMSO; n=7); and iii) control group, in which mice were administered
an identical volume of 10% DMSO and normal saline into the nape of
the neck (n=14). The mice were treated with 328006, PD0325901 or
vehicle 30 min prior to intradermal administration of the
des-Arg(9)-bradykinin B1R agonist
(0.4 mmol/l in 50 µl) into the nape of the neck. Dosages of ERK1/2
and MEK1/2 inhibitors were calculated based the study conducted by
Kido-Nakahara et al (13).
Western blotting
Mice (n=3/group) were anesthetized with sevoflurane
and sacrificed by cervical dislocation. Cervical cord segments and
DRG neurons were dissected and preserved at −80°C. Lysates of DRG
neurons or spinal cord sections were prepared by homogenization in
lysis buffer containing a protease inhibitor mixture (Roche
Diagnostics, Basel, Switzerland) and a phosphatase inhibitor
cocktail (Thermo Fisher Scientific Inc., Waltham, MA, USA). Cell
debris was removed by centrifugation at 10,000 × g for 5 min at
4°C. Samples were boiled for 5 min in sodium dodecyl sulfate (SDS)
sample buffer, containing 1.0 mol/l Tris-HCL, 8% SDS, 0.1%
bromophenol blue, 10% glycerol and 2.5% 2-mercaptoethanol (pH 6.8).
Proteins were separated by SDS-polyacrylamide gel electrophoresis
and transferred to polyvinylidene fluoride membranes (GE Healthcare
Life Sciences, Chalfont, UK). Membranes were blocked with 5%
low-fat milk and incubated overnight at 4°C with rabbit
anti-pERK1/2, anti-ERK1/2 (1:1,000) and anti-tubulin (1:2,000)
monoclonal antibodies. Subsequently, the membranes were washed with
phosphate-buffered saline and incubated with HRP-conjugated
anti-rabbit secondary antibody (1:5,000) for 1 h at 37°C. Labeled
proteins were detected by incubation with enhanced
chemiluminescence solution (Thermo Fisher Scientific, Inc.) for 1
min and visualized with an ImageQuant Las 4000 imager (GE
Healthcare Biosciences, Pittsburgh, PA, USA). Tubulin was used as
an internal control. Band intensities were quantified using ImageJ
software, version 1.45 (https://imagej.nih.gov/ij/).
Statistical analysis
All results are expressed as the mean ± standard
error of the mean. Between-group comparisons of scratching
responses were performed using unpaired t-tests. Two-factorial
analysis of variance was used for all other comparisons.
Statistical analyses were performed using SPSS software, version
19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
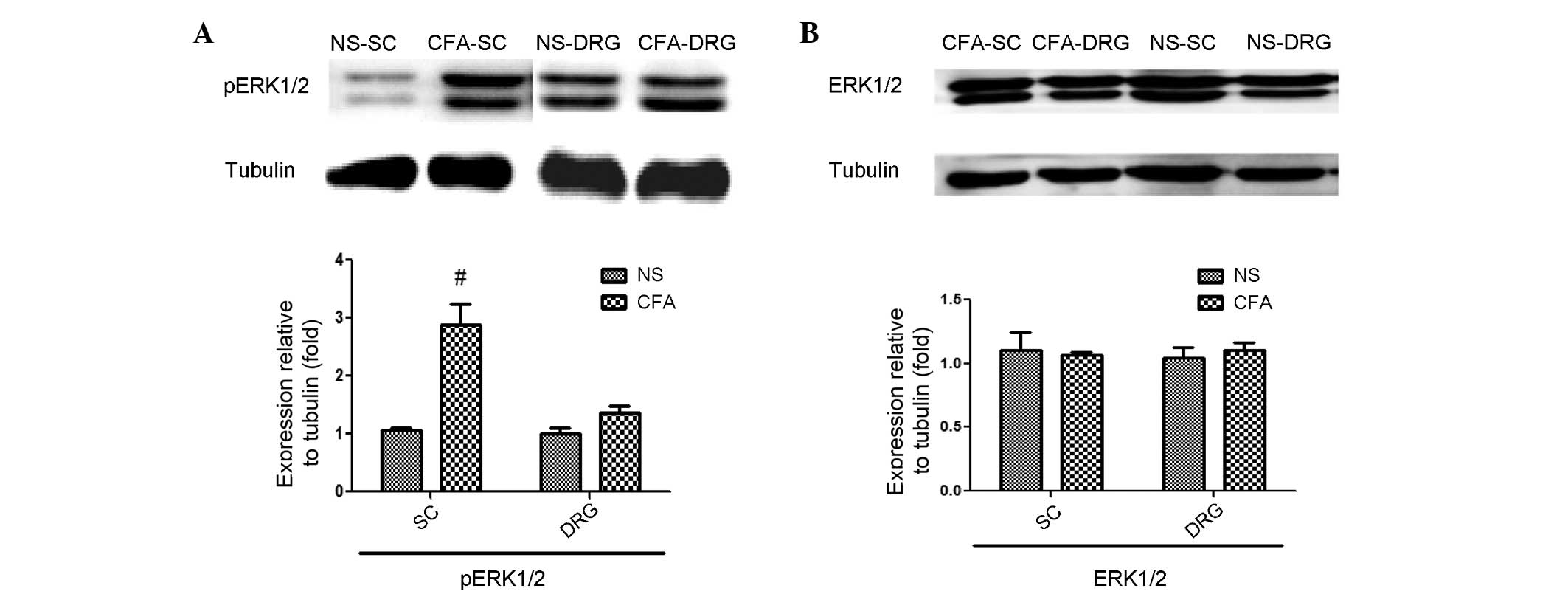
pERK1/2 expression levels are
significantly increased in the cervical spinal cord of CFA-inflamed
mice
To explore the potential role of ERK1/2 signaling as
a mediator of alloknesis in CFA-treated mice, the present study
investigated whether the expression levels of pERK1/2, which is a
well-established indicator of ERK1/2 signaling activation, were
increased in the spinal cord or DRG neurons of CFA-induced mice, as
compared with control mice. Western blot analysis indicated that
pERK1/2 were significantly increased in the cervical spinal cord 4
days after CFA-induced inflammation (P=0.0217), whereas pERK1/2
expression levels in DRG neuron extracts and total ERK1/2 levels
were unchanged (Fig. 1). These
results indicate that there was sustained ERK1/2 activation in the
spinal cord for at least 4 days following the injection of CFA in
the nape region, which was not localized to DRG neurons.
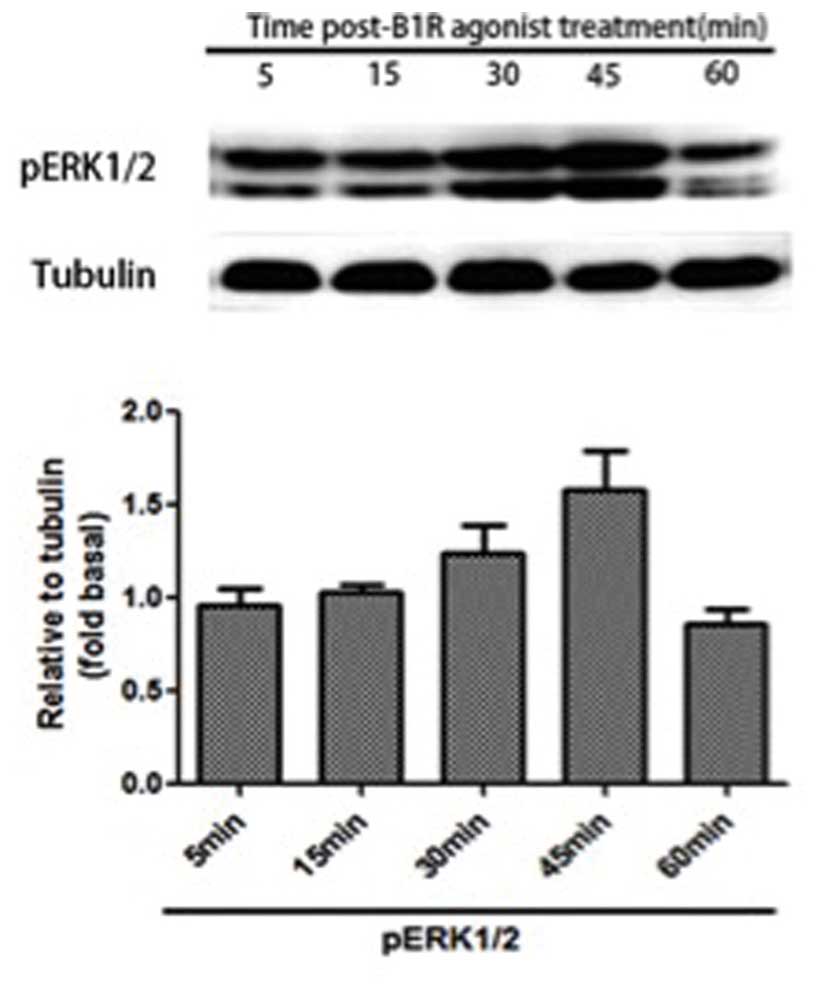
pERK1/2 is upregulated following
treatment with B1R agonist
As shown in Fig. 2,
pERK1/2 expression levels in the cervical spinal cord increased 30
min post-B1R agonist administration, peaked at 45 min, and then
returned to control levels by 60 min post-administration. These
results suggest that B1R agonist treatment is able to stimulate
greater ERK1/2 activation in CFA-inflamed mice, as compared with
the persistent activation induced by CFA treatment alone.
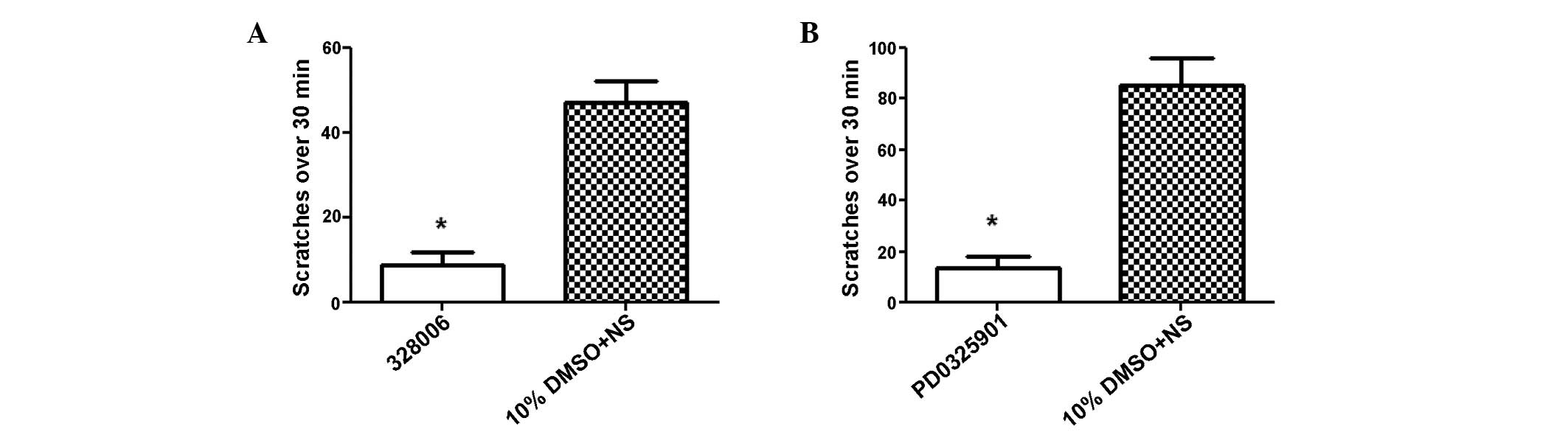
Inhibiting ERK1/2 signaling decreases
B1R agonist-evoked scratching behavior
Western blot analysis indicated a potential role for
ERK1/2 signaling in B1R agonist-induced pruritus in vivo. To
further examine this hypothesis, a pharmacological approach was
used to determine whether ERK1/2 activation is associated with the
scratching response in vivo. PD0325901 MEK1/2 inhibitor,
which targets ERK1/2 phosphorylation but is inactive against
various other MAP kinase pathway kinases (28), was intraperitoneally administered 30
min prior to intradermal B1R agonist injection in the nape of neck.
As compared with the control group, behavioral analyses
demonstrated that B1R agonist-induced scratching behaviors were
significantly reduced (>4-fold) by inhibiting ERK1/2 via
systemic application of the PD0325901 MEK1/2 inhibitor (P<0.001;
Fig. 3A). Consistent with this
result, treatment with the 328006 ERK1/2 inhibitor prior to B1R
agonist intradermal injection also significantly decreased
scratching (to a similar extent), as compared with the control
group (P<0.001; Fig. 3B). These
results suggested that the ERK1/2 signaling pathway may be
associated with the transmission of itch sensations in response to
B1R agonist treatment in inflamed skin and indicated the
contribution of ERK1/2 activation in the spinal cord as a mediator
of this process.
Discussion
The present study demonstrated two major findings.
Firstly, CFA-induced inflammation in the nape of the mouse neck
produced a sustained increase in ERK1/2 activation in the cervical
spinal cord, which was associated with increased scratching
behavior in response to intradermal injection of the
des-Arg(9)-bradykinin B1R agonist
into the inflamed skin. Secondly, the upregulated levels of pERK1/2
in the CFA-induced mouse model of inflammation were further
enhanced by treatment with the B1R agonist. This enhancement of
pERK1/2 expression in the spinal cord may account for the
abnormally elevated scratching behavior that was observed in
response to B1R agonist treatment following CFA-induced
inflammation.
Previous studies have demonstrated that ERK1/2
signaling in the spinal cord may contribute to the sensation of
pain (11,18,23).
However, additional evidence has also suggested that the ERK1/2
signaling pathway is associated with itch transduction (12,13,25). A
previous study has demonstrated that pruritogens are capable of
activating ERK1/2 signaling in the spinal cord, which is associated
with the transduction of acute alloknesis (12). It has also been demonstrated that
ERK1/2 phosphorylation in DRG neurons is critical for acute
alloknesis, which are induced by cytokines IL-31 and ET-1 (13,25).
Furthermore, ERK1/2 signaling in chronic alloknesis was also
demonstrated by Zhao et al (14), who showed that pERK1/2 is a key
mediator of itch sensations in the sensory neurons of mice with
chronic alloknesis, since transient pERK1/2 activation in the
spinal cord was absent when spontaneous scratching was observed.
Therefore, these studies suggested that ERK1/2 activation in the
spinal cord or DRG neurons is associated with neural transmission
mediation of the itch sensation.
The present study investigated whether pERK1/2
expression increased in the spinal cord or DRG neurons n response
to B1R agonist stimulation during CFA-induced inflammation, and
whether this increase was capable of mediating scratching behavior.
The results of the present study demonstrated a persistent
enhancement of pERK1/2 in the spinal cord of CFA-inflamed mice, but
not in DRG neurons. These data are consistent with a recent study
by Zhang et al (12), which
showed that intradermal injection of pruritogen histamine into the
nape or cheek of mice induced phosphorylation of ERK1/2 in the
spinal cord but not in DRG neurons, and that ERK1/2 activation was
crucial for the initiation and maintenance of the itch sensation.
Conversely, our findings differ from a study published by Zhao
et al (14), which indicated
that persistent activation of ERK1/2 signaling in DRG neurons, but
not in the spinal cord, was required for the maintenance of
spontaneous scratching in allergic contact dermatitis and dry skin
models of chronic alloknesis. It should be noted that scratching
behaviour was detected in the present study, therefore the
possibility that increases in pERK1/2 were elicited by the
scratching itself and not by the pruritus produced by model
treatments cannot be excluded. This may also account for the
increased activation of ERK1/2 in DRG neurons that was observed in
the chronic itch model (12,29); whereas the present study demonstrated
that enhanced levels of pERK1/2 were only required in the spinal
cord for the B1R agonist-evoked itch sensation.
To elucidate whether B1R agonist treatment is
capable of triggering alterations in the phosphorylation status of
ERK1/2, similar to those observed following injection with
histamine, the time-dependent induction of pERK1/2 in the cervical
spinal cord was assessed in CFA-inflamed mice after B1R agonist
injection (9,30). Western blot analysis demonstrated
that treatment with a B1R agonist induced further enhancement of
ERK1/2 activation, which was already increased due to CFA
inflammation. Furthermore, blocking ERK1/2 signal activation via
systemic application of MEK1/2 or ERK1/2 inhibitors significantly
decreased scratching behavior, indicating that the ERK1/2 signaling
pathway may have a role in the itch sensation following B1R agonist
application.
Phosphorylation of ERK1/2 in the spinal cord
following peripheral inflammation by CFA contributes to persistent
inflammatory pain (10,21). The results of the present study
suggest that activated ERK1/2 may have a role in the abnormal itch
sensation following B1R activation in CFA-inflamed mice. It has
previously been reported that the enhancement of nocifensive
responses to metabotropic glutamate receptor agonist (RS)-3,
5-dihydroxyphenylglycine was associated with sustained ERK
activation in dorsal horn neurons, which persisted for seven days
following injection with CFA (31).
It is possible that sustained ERK activation regulates plasticity
in the spinal cord and underlies a component of central and
peripheral sensitization, which leads to scratching in response to
B1R agonist stimulation in CFA-inflamed skin (32,33).
The results of the present study suggested that the
persistence of ERK1/2 activation in the CFA inflammation model, and
further upregulation by B1R agonist treatment in the spinal cord,
underlies a change in the behavioral response to B1R activation in
inflamed mice. Previous studies have demonstrated the involvement
of B1R in pruriceptive processing following CFA-induced
inflammation (8,9) and B1R blockade has been shown to
ameliorate CFA-induced inflammatory hyperalgesia (34,35). A
modulatory function for MAP kinase pathways has been demonstrated
to regulate B1R expression during tissue injury (36) and control its function in
inflammatory disorders (37). In
addition, in previous studies B1R was upregulated in the airways of
mice treated with TNF-a and IL-4, which also demonstrated the
involvement of the MAPK pathway in this process (38,39).
Therefore, in light of these finding and the results of the present
study, we hypothesize that there is an association between
sustained ERK1/2 activation and B1R in CFA-inflamed mice. The
present data suggested that the persistence of ERK1/2 activity
itself is important for B1R agonist-mediated itch signaling
following inflammation. However, it still remains to be
investigated whether there is increased expression of B1R in the
CFA-induced inflammation model and if sustained ERK1/2 activity
upregulates B1R levels.
The authors of the present study have previously
reported that a painful bradykinin stimulus evokes an itch
sensation in CFA-inflamed skin, which was substantially blocked by
a specific B1R antagonist (8).
Therefore, CFA-induced inflammation may induce novel pruriceptive
properties in B1R, resulting in B1R becoming responsive to
algogenic stimuli. This previous study and the present results
demonstrated that ERK1/2 activation in the spinal cord may be a
prerequisite for the abnormal itch sensation stimulated by the B1R
agonist during chronic inflammation. Therefore, the ERK1/2
signaling pathway may have a pivotal role in itch processing,
providing novel insight into the molecular mechanisms that mediate
itch responses to algogenic stimuli.
In conclusion, the present study demonstrated that
CFA-induced inflammation results in persistent ERK1/2 activation in
the spinal cord, which can be further upregulated by B1R agonist
treatment, and is associated with itch processing in the spinal
cord. As the cellular mechanisms that regulate itch sensations
evoked by pain stimuli in inflamed skin remain poorly understood,
further examination of the role of ERK1/2 signaling pathways in
this process may yield novel mechanistic insights.
Acknowledgements
The present study was supported by the National
Natural Science Foundation (grant nos. 81171040 and 81371236) and
the Natural Science Foundation of Guangdong Province, China (grant
no. 10151008002000005).
References
|
1
|
Ständer S and Schmelz M: Chronic itch and
pain-Similarities and differences. Eur J Pain. 10:4732006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma C, Nie H, Gu Q, Sikand P and Lamotte
RH: In vivo responses of cutaneous C-mechanosensitive neurons in
mouse to punctate chemical stimuli that elicit itch and nociceptive
sensations in humans. J Neurophysiol. 107:357–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu T and Ji R: New insights into the
mechanisms of itch: Are pain and itch controlled by distinct
mechanisms? Pflügers Arch. 465:1671–1685. 2013. View Article : Google Scholar
|
|
4
|
Davidson S and Giesler GJ: The multiple
pathways for itch and their interactions with pain. Trends
Neurosci. 33:550–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hosogi M, Schmelz M, Miyachi Y and Ikoma
A: Bradykinin is a potent pruritogen in atopic dermatitis: A switch
from pain to itch. Pain. 126:16–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sikand P, Shimada SG, Green BG and LaMotte
RH: Similar itch and nociceptive sensations evoked by punctate
cutaneous application of capsaicin, histamine and cowhage. Pain.
144:66–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang J, Xiao G and Ji W: Capsaicin
induces reflex scratching in inflamed skin. Pharmacology. 88:82–87.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang J, He Y and Ji W: Bradykinin-evoked
scratching responses in complete Freund's adjuvant-inflamed skin
through activation of B1 receptor. Exp Biol Med (Maywood).
237:318–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng J, Chen Y, Xiong J, Chen X, Liang J
and Ji W: The kinin B1 receptor mediates alloknesis in a murine
model of inflammation. Neurosci Lett. 560:31–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang JQ, Fang JF, Liang Y and Du JY:
Electroacupuncture mediates extracellular signal-regulated kinase
1/2 pathways in the spinal cord of rats with inflammatory pain. BMC
Complement Altern Med. 14:2852014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji RR, Befort K, Brenner GJ and Woolf CJ:
ERK MAP kinase activation in superficial spinal cord neurons
induces prodynorphin and NK-1 upregulation and contributes to
persistent inflammatory pain hypersensitivity. J Neurosci.
22:478–485. 2002.PubMed/NCBI
|
|
12
|
Zhang L, Jiang GY, Song NJ, Huang Y, Chen
JY, Wang QX and Ding YQ: Extracellular signal-regulated kinase
(ERK) activation is required for itch sensation in the spinal cord.
Mol Brain. 7:252014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kido-Nakahara M, Buddenkotte J, Kempkes C,
Ikoma A, Cevikbas F, Akiyama T, Nunes F, Seeliger S, Hasdemir B,
Mess C, et al: Neural peptidase endothelin-converting enzyme 1
regulates endothelin 1-induced pruritus. J Clin Invest.
124:2683–2695. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao ZQ, Huo FQ, Jeffry J, Hampton L,
Demehri S, Kim S, Liu XY, Barry DM, Wan L, Liu ZC, et al: Chronic
itch development in sensory neurons requires BRAF signaling
pathways. J Clin Invest. 123:4769–4780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pearson G, Robinson F, Gibson Beers T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaul YD and Seger R: The MEK/ERK cascade:
From signaling specificity to diverse functions. Biochim Biophys
Acta. 1773:1213–1226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seino D, Tokunaga A, Tachibana T, Yoshiya
S, Dai Y, Obata K, Yamanaka H, Kobayashi K and Noguchi K: The role
of ERK signaling and the P2X receptor on mechanical pain evoked by
movement of inflamed knee joint. Pain. 123:193–203. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Obata K and Noguchi K: MAPK activation in
nociceptive neurons and pain hypersensitivity. Life Sci.
74:2643–2653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Obata K, Yamanaka H, Dai Y, Mizushima T,
Fukuoka T, Tokunaga A and Noguchi K: Differential activation of
MAPK in injured and uninjured DRG neurons following chronic
constriction injury of the sciatic nerve in rats. Eur J Neurosci.
20:2881–2895. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Obata K, Yamanaka H, Dai Y, Mizushima T,
Fukuoka T, Tokunaga A and Noguchi K: Activation of extracellular
signal-regulated protein kinase in the dorsal root ganglion
following inflammation near the nerve cell body. Neuroscience.
126:1011–1021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obata K, Yamanaka H, Dai Y, Tachibana T,
Fukuoka T, Tokunaga A, Yoshikawa H and Noguchi K: Differential
activation of extracellular signal-regulated protein kinase in
primary afferent neurons regulates brain-derived neurotrophic
factor expression after peripheral inflammation and nerve injury. J
Neurosci. 23:4117–4126. 2003.PubMed/NCBI
|
|
22
|
Zhang X, Zhang H, Shao H, Xue Q and Yu B:
ERK MAP kinase activation in spinal cord regulates phosphorylation
of cdk5 at serine 159 and contributes to peripheral inflammation
induced pain/hypersensitivity. PLoS One. 9:e877882014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YL, Shi XD, Fu D, Xu JM and Dai RP:
Activation of spinal ERK1/2 contributes to mechanical allodynia in
a rat model of postoperative pain. Molecular Medicine Reports.
7:1661–1665. 2013.PubMed/NCBI
|
|
24
|
Sanna MD, Mello T, Ghelardini C and
Galeotti N: Inhibition of spinal ERK1/2-c-JUN signaling pathway
counteracts the development of low doses morphine-induced
hyperalgesia. Eur J Pharmacol. 764:271–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cevikbas F, Wang X, Akiyama T, Kempkes C,
Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, et
al: A sensory neuron-expressed IL-31 receptor mediates T helper
cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin
Immunol. 133:448–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji RR, Baba H, Brenner GJ and Woolf CJ:
Nociceptive-specific activation of ERK in spinal neurons
contributes to pain hypersensitivity. Nat Neurosci. 2:1114–1119.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karim F, Hu HJ, Adwanikar H, Kaplan D and
Gereau RW IV: Impaired inflammatory pain and thermal hyperalgesia
in mice expressing neuron-specific dominant negative mitogen
activated protein kinase kinase (MEK). Mol Pain. 2:22006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Favata MF, Horiuchi KY, Manos EJ, Daulerio
AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F,
et al: Identification of a novel inhibitor of mitogen-activated
protein kinase kinase. J Biol Chem. 273:18623–18632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song XS, Cao JL, Xu YB, He JH, Zhang LC
and Zeng YM: Activation of ERK/CREB pathway in spinal cord
contributes to chronic constrictive injury-induced neuropathic pain
in rats. Acta Pharmacol Sin. 26:789–798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kilstein Y, Nowak W, Errasti AE, Feás AA,
Armesto AR, Pelorosso FG and Rothlin RP: Involvement of
Extracellular Signal-Regulated Kinase 5 in Kinin B1 Receptor
Upregulation in Isolated Human Umbilical Veins. J Pharmacol Exp
Ther. 357:114–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adwanikar H, Karim F and Gereau RW IV:
Inflammation persistently enhances nocifensive behaviors mediated
by spinal group I mGluRs through sustained ERK activation. Pain.
111:125–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao YJ and Ji RR: Light touch induces ERK
activation in superficial dorsal horn neurons after inflammation:
Involvement of spinal astrocytes and JNK signaling in touch-evoked
central sensitization and mechanical allodynia. J Neurochem.
115:505–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu YM, Wang XT, Zhang ZY, Suo ZW, Yang X
and Hu XD: Noradrenergic α2 receptor attenuated inflammatory pain
through STEP61/ERK signalling. Eur J Pain. 19:1298–1307. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferreira J, Campos MM, Pesquero JB, Araujo
RC, Bader M and Calixto JB: Evidence for the participation of
kinins in Freund's adjuvant-induced inflammatory and nociceptive
responses in kinin B1 and B2 receptor knockout mice.
Neuropharmacology. 41:1006–1012. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo MC, Chen Q, Ossipov MH, Rankin DR,
Porreca F and Lai J: Spinal dynorphin and bradykinin receptors
maintain inflammatory hyperalgesia. J Pain. 9:1096–1105. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Larrivee JF, Bachvarov DR, Houle F, Landry
J, Huot J and Marceau F: Role of the mitogen-activated protein
kinases in the expression of the kinin B1 receptors induced by
tissue injury. J Immunol. 160:1419–1426. 1998.PubMed/NCBI
|
|
37
|
Ehrenfeld P, Matus CE, Pavicic F, Toledo
C, Nualart F, Gonzalez CB, Burgos RA, Bhoola KD and Figueroa CD:
Kinin B1receptor activation turns on exocytosis of matrix
metalloprotease-9 and myeloperoxidase in human neutrophils:
Involvement of mitogen-activated protein kinase family. J Leukoc
Biol. 86:1179–1189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Adner M and Cardell LO:
Up-regulation of bradykinin receptors in a murine in-vitro model of
chronic airway inflammation. Eur J Pharmacol. 489:117–126. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bryborn M, Adner M and Cardell LO:
Interleukin-4 increases murine airway response to kinins, via
up-regulation of bradykinin B1-receptors and altered signalling
along mitogen-activated protein kinase pathways. Clin Exp Allergy.
34:1291–1298. 2004. View Article : Google Scholar : PubMed/NCBI
|