Introduction
Osteoporosis is a metabolic bone disease, which
results from a disturbance in normal bone remodeling such that the
balance shifts from bone formation to resorption; this in turn may
lead to bone loss and the occurrence of fractures following mineral
flux (1). Patients with osteoporosis
exhibit a high frequency of fractures, and hip fractures in senile
patients are particularly serious since they often limit the life
of a patient (2). At present,
osteoporosis affects ~25 million Americans (3). It has been estimated that a 50-year-old
woman in the United States has an 11–18% lifetime risk of suffering
a hip fracture (3).
Numerous attempts have been made to develop novel
agents for the prevention and treatment of bone diseases (4). At present, anti-resorptive agents,
including biophosphonate, estrogen and calcitonin, are widely used
(5); however, there is a requirement
for highly effective resorptive inhibitors with improved safety and
efficacy profiles. Anabolic agents, which are able to stimulate
bone formation, are less well-known (6); however, trials to develop anabolic
agents have been conducted, and have improved current understanding
of the mechanisms underlying osteoblast differentiation and bone
formation (7).
The estrogen-deficient, ovariectomized (OVX) rat
model of osteoporosis has been considered useful for the evaluation
of osteoporotic drugs, since various parameters associated with
osteoporosis have been shown to be markedly decreased within 4–6
weeks following an ovariectomy (8).
The OVX rat model of osteoporosis was selected for the present
study as it approximates postmenopausal bone loss, as demonstrated
by previous studies (8–10). A rat model was selected in particular
as a 4-month study of rats is considered to be comparable to a
1-year study of humans (11). The
effects of a drug in the OVX rat model may be assessed by detecting
histomorphometrical alterations in bone mass, formation and
resorption, and changes in bone weight, blood chemistry,
urinalysis, bone mineral content (BMC), bone mineral density (BMD)
and failure load (FL) (12).
Bone remodeling by bone cells is crucial in
determining and increasing the bone mass during pathological
conditions, including bone disorders (13). There are two major therapeutic
strategies for patients with osteoporosis: Reducing bone turnover
or increasing mineral deposition at bones. Alendronate and other
bisphosphonates, which inhibit bone-turnover, have been prescribed
to millions of patients worldwide, and have exhibited good safety
profiles, with reports of only minor side effects (14,15).
Alendronate is a nitrogen-containing bisphosphonate and a potent
inhibitor of bone resorption, which has been used to treat and
prevent osteoporosis, in addition to exhibiting anti-osteoporosis
effects in OVX animals and postmenopausal women (16,17).
Conversely, the parathyroid hormone, which has been developed to
treat osteoporosis, is able to stimulate osteoblastic bone
formation, increase bone mass and prevent vertebral fractures
(18). Similarly, bone formation may
be induced by treatment with strontium ranelate (19). Our previous mouse and in vitro
studies demonstrated that Polycan was able to prevent bone loss by
reducing net bone resorption and bone turnover (20,21). In
addition, an increase in bone-specific alkaline phosphatase (bALP)
expression levels was indicative of an increase in bone formation
following treatment with Polycan (20,21).
β-glucan is a fiber-type complex polysaccharide
derived from the cell wall of baker's yeast, oat and barley fiber,
and numerous medicinal mushrooms (22). β-glucan is primarily used to enhance
the immune system (23) and lower
blood cholesterol levels (24).
Polycan, which is purified β-glucan derived from Aureobasidium
pullulans SM-2001, predominantly consists of β-1,3/1,6-glucan,
as well as other organic materials, including amino acids, mono- or
di-unsaturated fatty acids (linoleic and linolenic acids) and
fibrous polysaccharide (25).
Previous studies have demonstrated that Polycan was able to exert
anti-osteoporosis effects, including inhibiting bone loss and
accelerating bone formation in vitro and in OVX mice
(20,21), and promoting the healing of fractures
(26). However, to the best of our
knowledge, the effects of Polycan in a rat model of osteoporosis
have yet to be evaluated. Therefore, the present study aimed to
investigate the effects of Polycan (31.25, 62.5 and 125 mg/kg), in
comparison with the effects of alendronate, in a rat model of
ovariectomy-induced osteoporosis.
Materials and methods
Rats
A total of 96 virgin Sprague-Dawley pathogen-free
female rats (age, 6 weeks; weight, 137–173 g; Charles River
Laboratories, Inc., Yokohama, Japan) were used in the present
study, following a 7-day acclimatization period. The rats were
maintained in polycarbonate cages at 20–25°C and 30–35% humidity,
under a 12-h light/dark cycle and with access to food (Samyang
Foods Co., Ltd., Wonju, Korea) and water ad libitum. A total
of 80 rats were assigned to the experimental group (OVX-induced
osteoporosis) and 16 rats were assigned to the sham control group.
Following the ovariectomy procedure, 48 rats were selected for
inclusion in the study, based on their body weight and behavior,
according to the Guide for the Care and Use of Laboratory Animals
from the Institute for Laboratory Animal Research (Washington,
D.C., USA). The present study was approved by the Institute of
Laboratory Animal Resources at Daegu Haany University (DHU2011-015;
Gyeongsan, Korea).
Test articles, grouping and
dosing
Polycan derived from A. pullulans SM-2001
(Glucan Co., Ltd., Busan, Korea) was stored in a refrigerator at
4°C. The Polycan consisted of 13% β-1,3/1,6-glucan and 40%
β-glucan, as demonstrated using analytical methods described in a
previous study (25). The 48 rats
were grouped as follows (n=8 rats/group): A sham control group, an
OVX control group, an alendronate group, and 3 groups of rats
treated with 31.25, 62.5 and 125 mg/kg polycan, respectively. The
Polycan was diluted in distilled water to 62.5, 31.25 and 125
mg/kg, and was administered by gastric gavage using a 3-ml syringe.
The Polycan was administered daily for 126 days, commencing one
week following the ovariectomy procedure. A 10-mg/kg dose of
alendronate (Merck & Co., Inc., Whitehouse Station, NJ, USA)
was generated by dissolving the alendronate in distilled water.
Surgical procedure
The rats in the experimental group underwent a
bilateral ovariectomy following intraperitoneal injection with 25
mg/kg Zoletile (Virbac S.A., Carros, France), as described in a
previous study (27). In the sham
control group, the bilateral ovariectomy procedure was conducted
without removal of the ovaries. Following the surgical procedure,
the rats were divided into 6 groups (8 rats/group; a sham control
group, an OVX control group, an alendronate group, and 3 polycan
groups with various dosages).
Bone labeling
For dynamic histomorphometry, all rats were treated
subcutaneously with 30 mg/kg/ml tetracycline (Sigma-Aldrich, St.
Louis, MO, USA) 13 days prior to sacrifice, and 8 mg/kg/ml calcein
(Sigma-Aldrich) 3 days prior to sacrifice. Briefly, tetracycline
binds to newly formed bone at the bone/osteoid (unmineralized bone)
interface where it exhibits linear fluorescence. Fluorescence was
observed under a UV light microscope (model Eclipse 80i; Nikon
Corporation, Tokyo, Japan). Animal sacrifice was carried out under
anesthesia with 0.05 ml/kg Zoletile by exsanguination from the
caudal vena cava. Animals with unintended problems (including
cachexia and abnormal clinical signs) were sacrificed by cervical
dislocation.
Body weight alterations
The body weight of all rats was measured prior to
the initial treatment and once per week during the experimental
period, concluding at sacrifice as described above. Blood was
collected from the caudal vena cava and centrifuged at 720 × g for
30 min. The supernatant was collected using a micropipette. Prior
to the body weight measurements at the initial dosing and at
sacrifice, the experimental animals were fasted overnight in order
to reduce the occurrence of errors due to feeding. Alterations in
the body weight were calculated as the body weight at sacrifice
minus the body weight at the initial treatment, in order to
eliminate individual differences.
Serum biochemistry
Blood samples (10 ml) were collected from the vena
cava at sacrifice, and the serum was separated. All serum samples
were frozen at −40°C until required for further experimentation.
Serum levels of osteocalcin (ng/ml) were detected using an
Osteocalcina Myria kit (Technogenetics Srl, Milan, Italy) and a
Packard Cobra II γ-counter (GMI, Inc., Ramsey, MN, USA). Serum
levels of bALP (U/L) were detected using the commercially available
Enzyme-Immunoassay kit (Metra™ bALP kit; cat. no. 8012; Quidel
Corporation, San Diego, CA, USA). The serum calcium (Ca) levels
(mg/ml) were detected using the Orthocresolphthalein Complexone
(OCPC) method and an automated blood analyzer (TBA 200FR; Toshiba,
Tokyo, Japan). Briefly, the OCPC complexone (P5631; Sigma-Aldrich)
method is based on the reaction of Ca2+ with
o-cresolphthalein complexone in an alkaline solution, which forms
an intense violet fluorescence which maximally absorbs at 577 nm.
8-hydroxyquionline is added to prevent interference by magnesium
and iron. The serum phosphorus (P) levels were detected using the
kinetic ultraviolet (UV) method using a blood biochemistry
autoanalyzer (Dri-Chem NX500i; Fujifilm Medical System Co., Ltd.,
Tokyo, Japan).
Urinalysis
Urine from individual rats was collected over a 24-h
period following the final treatment, and was centrifuged at 720 ×
g for 10 min to remove sediments. The levels of deoxypyridinoline
(Dpd; nM) in the urine were detected using a commercial
Enzyme-Immunoassay kit (Metra™ Dpd kit; cat. no. 8007; Quidel
Corporation) and an enzyme-linked immunosorbent assay plate reader
(Tecan Schweiz AG, Männedorf, Switzerland). The levels of
creatinine (g/day) in the urine were detected using the Jaffe
reaction and an automated urine analyzer (model TBA-2000FR;
Toshiba). In addition, the Dpd/creatinine ratios were measured.
BMC
Following sacrifice, the right side of the femur and
tibia, as well as the 4th lumbar vertebrae
(L4), were harvested and dried at 120°C for 8 h. The
dried tibiae underwent carbonization at 800°C for 6 h in a furnace,
after which they were dissolved in nitric acid. In a dissolved
solution, the Ca and P concentrations (mg/g) were calculated using
the OCPC and kinetic UV methods, respectively. In addition, the
Ca/P ratio was calculated using the following formula: Ca/P ratio =
(bone Ca content/bone P content) × 100.
BMD and FL
The BMD (g/cm2) of the epiphyseal plates
and body of the right femur and tibia was measured using
dual-energy X-ray absorption (Lunar PIXImus; GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA). In addition, the BMD of the
articulate regions and body of the L4 was determined
prior to BMC measurement. The bone strength was measured in terms
of FL. The FL (newtons, N) of the mid-shaft regions of the
right femur and tibia was measured using a three-point bending test
to failure on a computerized testing machine (Instron 6022;
Instron, Norwood, MA, USA; speed, 20 mm/min). In addition, the FL
of the L4 body was measured.
Histology and histomorphometry
The left side of the femur and tibia, and the
5th lumbar vertebrae (L5) of each rat were
separated, dehydrated in a graded series of ethanol and xylene, and
embedded undecalcified in modified methyl methacrylate
(Araldite/Embed Embedding kit; cat. no. 13940; Electron Microscopy
Sciences, Hatfield, PA, USA). Subsequently, the samples were
sectioned (3–4 µm) and stained with hematoxylin and eosin staining.
A section was left unstained for dynamic histomorphometry. For each
prepared sample, the histological profiles (osteoporotic hole) were
observed under a microscope (Carl Zeiss AG, Oberkochen,
Germany).
Bone histomorphometry was conducted using automated
image analysis processing software (analySIS® AUTO; SiS
Sensoren Instrumente Systeme GmbH, Schwentinental, Germany), as
outlined in a previous report (28).
Briefly, in order to determine the bone mass and structure, the
trabecular bone volume (Tb.Ar), thickness (Tb.Wi), number (N.Tb)
and length (Tb.Pm), and the cortical bone thickness (Ct.Wi), were
measured. In order to determine the extent of bone resorption, the
osteoclast cell number (N.Oc) in uniform regions of the epiphyseal
plates (N/epiphyseal), and the osteoclast cell surface/bone surface
(Oc/BS%), were determined. In order to measure the rate of bone
formation (µm/day), the mineral appositional rate (MAR) in the
trabecular and cortical regions was determined by dividing the
distance between the midpoints of the two labels by the time
interval between the labeling periods (10 days). The single labeled
surface (sL.Pm) corresponded to the trabecular or cortical surface
covered with single label (tetracycline only), and was expressed as
a percentage of the bone surface. The double labeled surface
(dL.Pm) corresponded to the bone surface covered with both labels
(tetracycline and calcein) and was expressed as a percentage of the
bone surface. The mineralizing surface (Md.Pm) was calculated using
the following equation: Md.Pm (%) = [(1/2 sL.PM) + dL.Pm]. The
surface referent bone formation rate (BFR/BS), which corresponded
to the amount of new bone mineralized at the tissue level per
mm2 of bone surface area per day, was calculated using
the following equation: BFR/BS (µm3/µm2/day)
= Md.Pm × MAR.
Statistical analysis
Data are presented as the mean ± standard deviation.
Data was analyzed using one-way analysis of variance, followed by a
least-significant difference multiple comparison test, in order to
determine which pairs of group comparisons were significantly
different. When a significant difference was detected using the
Kruskal-Wallis H test, a Mann-Whitney U test was conducted, in
order to determine the specific pairs of group comparisons that
were significantly different. Statistical analyses were performed
using SPSS software version 14.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 and P<0.01 were considered to indicate statistically
significant and highly statistically significant differences,
respectively.
Results
Alterations in body weight
A significant increase in body weight was detected
in the OVX rats compared with the sham control group at 7 and 126
days following the initial treatment (P<0.01). There was no
significant difference in body weight alterations between the
treated groups (alendronate and Polycan-treated groups) and the OVX
control group (Table I).
 | Table I.Rat body weight in the various
groups. |
Table I.
Rat body weight in the various
groups.
|
| Body weight |
|
|---|
|
|
|
|
|---|
| Group | Day 0 (A) | Day 7 | Day 126 (B) | Gain (B - A) |
|---|
| Sham control |
181.63±5.48 |
207.75±3.92 |
283.13±13.05 |
101.50±12.81 |
| OVX control |
182.25±4.89 |
227.63±6.82a |
337.88±25.95a |
155.63±22.03a |
| Alendronate |
178.75±10.59 |
225.25±16.15b |
318.38±37.75 |
139.63±31.37a |
| Polycan |
|
|
|
|
| 31.25
mg/kg |
176.75±11.42 |
223.10±18.02b |
329.88±41.27b |
153.13±33.36a |
| 62.5
mg/kg |
178.25±4.65 |
227.88±8.63a |
338.25±22.93a |
160.00±21.73a |
| 125
mg/kg |
177.75±6.45 |
221.63±9.46a |
333.00±32.26a |
125.25±30.41 |
Alterations in serum biochemistry
The serum levels of osteocalcin were significantly
increased in the OVX control group compared with the sham control
group (P<0.01; Table II). A
significant reduction in the serum levels of bALP, Ca and P was
detected in the OVX control group compared with the sham control
group (P<0.01). Conversely, marked dose-dependent reductions in
the serum levels of osteocalcin, and elevations in the serum levels
of bALP, Ca and P were detected in the Polycan-treated groups
compared with the OVX control group (P<0.05). The serum levels
of osteocalcin were significantly decreased in the
alendronate-treated group compared with the OVX control group
(P<0.01); however, no significant differences in the serum
levels of bALP, Ca and P were detected between the
alendronate-treated group and the OVX control group (Table II).
 | Table II.Alterations in serum
biochemistry. |
Table II.
Alterations in serum
biochemistry.
| Group | Osteocalcin
(ng/ml) | bALP (U/l) | Ca (mg/dl) | P (mg/dl) |
|---|
| Sham control |
1.349±0.185 |
1.425±0.238 |
9.338±0.774 |
6.650±0.960 |
| OVX control |
2.031±0.205a |
0.963±0.160a |
8.400±0.288a |
5.570±0.289a |
| Alendronate |
1.621±0.276b |
1.000±0.273a |
8.738±0.667 |
5.286±0.576a |
| Polycan |
|
|
|
|
| 31.25
mg/kg |
1.908±0.086a |
1.213±0.181c |
8.863±0.555c |
5.595±0.452a |
| 62.5
mg/kg |
1.801±0.141a,c |
1.238±0.220b |
8.963±0.504c |
6.028±0.339c |
| 125
mg/kg |
1.760±0.167a,b |
1.300±0.141b |
9.013±0.344b |
6.044±0.417 |
Alterations in urinalysis
Significantly increased urine Dpd levels and
Dpd/creatinine ratios were detected in the OVX control group
compared with the sham control group (P<0.01). Conversely,
dose-dependent reductions in the urine Dpd levels and
Dpd/creatinine ratios were detected in the Polycan-treated groups
compared with the OVX control group (Table III).
 | Table III.Alterations in urinalysis. |
Table III.
Alterations in urinalysis.
| Group | Dpd (nM) | Creatinine
(g/day) | Dpd/creatinine
(nM/g/day) |
|---|
| Sham control |
37.13±6.36 |
0.0061±0.0016 |
6,589.14±2,065.59 |
| OVX control |
57.53±4.99a |
0.0059±0.0009 |
9,948.74±1,681.29a |
| Alendronate |
35.94±12.75b |
0.0057±0.0013 |
6,344.94±1,898.94c |
| Polycan |
|
|
|
| 31.25
mg/kg |
49.73±10.15d |
0.0068±0.0019 |
7,858.53±2,496.99 |
| 62.5
mg/kg |
49.73±5.33a,c |
0.0065±0.0006 |
7,781.32±1,156.59b |
| 125
mg/kg |
49.69±8.70a,b |
0.0065±0.0006 |
7,751.24±1,556.92b |
Alterations in BMC
Significantly decreased levels of Ca and P were
detected in the femur, tibia and L4 of the OVX control
group compared with the sham control group (P<0.01). Conversely,
significant increases in the BMC were detected in all treated
groups (P<0.05). No significant alterations in Ca/P ratios were
detected in any of the groups (Table
IV).
 | Table IV.Alterations in bone mineral
contents. |
Table IV.
Alterations in bone mineral
contents.
|
|
|
|
| Polycan-treated
groups |
|---|
|
|
|
|
|
|
|---|
| Bone | Sham control | OVX control | Alendronate | 31.25 mg/kg | 62.5 mg/kg | 125 mg/kg |
|---|
| Femur |
|
|
|
|
|
|
| Ca mg/g
bone |
170.86±13.75 |
95.82±17.78a |
120.95±12.40a,b |
106.05±14.02c |
125.12±9.03a,b |
138.07±16.11a,b |
| P mg/g
bone |
98.71±7.56 |
57.29±11.04a |
73.60±13.18a,b |
63.19±9.20c |
76.48±11.47a,d |
83.30±10.28c,d |
| Ca/P
ratio |
1.74±0.16 |
1.70±0.34 |
1.69±0.34 |
1.70±0.28 |
1.67±0.29 |
1.68±0.29 |
| Tibia |
|
|
|
|
|
|
| Ca mg/g
bone |
196.13±9.74 |
103.82±15.02a |
133.37±21.61a,d |
110.14±14.66c |
128.77±10.45a,d |
140.72±15.00a,d |
| P mg/g
bone |
99.66±11.83 |
52.67±7.66a |
67.93±7.47a,d |
55.54±6.04c |
67.11±6.52a,d |
70.00±7.12a,d |
| Ca/P
ratio |
1.99±0.22 |
1.99±0.31 |
1.99±0.42 |
2.00±0.31 |
1.93±0.17 |
2.03±0.28 |
| L4 |
|
|
|
|
|
|
| Ca mg/g
bone |
210.14±8.35a |
124.41±12.48a |
138.50±9.19a |
138.10±13.87c |
146.84±16.78a,b |
155.79±11.09a,d |
| P mg/g
bone |
113.50±13.77a |
66.91±8.53a |
74.12±7.24a |
75.61±12.46c |
78.77±10.05a,b |
83.25±6.81a,d |
| Ca/P
ratio |
1.87±0.23 |
1.90±0.45 |
1.88±0.20 |
1.87±0.38 |
1.88±0.23 |
1.88±0.16 |
Alterations in BMD and FL
The BMD and FL of the tibia, femur and L4
were significantly decreased in the OVX control group compared with
the sham control group (P<0.01). Conversely, significantly
increased BMD and FL were detected in all three types of bone in
the majority of the Polycan-treated groups compared with the OVX
control group (P<0.05). However, the rats treated with 31.25
mg/kg Polycan did not exhibit significant increases in BMD and FL
compared with the OVX control group (Table V).
 | Table V.Alterations in BMD and FL in the rat
femur, tibia and L4 verterae. |
Table V.
Alterations in BMD and FL in the rat
femur, tibia and L4 verterae.
|
|
|
|
| Polycan-treated
groups |
|---|
|
|
|
|
|
|
|---|
| Bone | Sham control | OVX control | Alendronate | 31.25 mg/kg | 62.5 mg/kg | 125 mg/kg |
|---|
| Femur |
|
|
|
|
|
|
|
Epiphyseal BMD |
0.378±0.040 |
0.213±0.032a |
0.513±0.107a,b |
0.253±0.026a,c |
0.286±0.030a,b |
0.293±0.021a,b |
|
Mid-shaft BMD |
0.336±0.036 |
0.189±0.025a |
0.224±0.027a,c |
0.228±0.032a,c |
0.251±0.037a,b |
0.265±0.029a,b |
| FL |
119.03±8.68 |
70.48±11.39a |
83.50±7.70a,c |
78.03±6.39a |
86.30±6.08b,c |
88.33±10.02a,c |
| Tibia |
|
|
|
|
|
|
|
Epiphyseal BMD |
0.305±0.023 |
0.199±0.017a |
0.385±0.066a,b |
0.221±0.034a |
0.249±0.026a,b |
0.268±0.046b |
|
Mid-shaft BMD |
0.291±0.015 |
0.179±0.023a |
0.195±0.024a |
0.209±0.020a,c |
0.224±0.025a,b |
0.243±0.042a,b |
| FL |
79.61±2.44 |
50.96±6.27a |
55.69±11.18a |
57.25±4.78a |
62.06±3.52a,b |
63.41±5.05a,b |
| L4 |
|
|
|
|
|
|
|
Epiphyseal BMD |
0.338±0.034 |
0.225±0.023a |
0.255±0.030a,c |
0.249±0.026a |
0.258±0.025a,c |
0.293±0.015a,b |
|
Mid-shaft BMD |
0.305±0.021 |
0.186±0.028a |
0.199±0.022a |
0.205±0.016a |
0.238±0.019a,b |
0.256±0.033a,b |
| FL |
66.20±5.35 |
42.20±8.25a |
50.06±11.14a |
48.93±5.85a |
50.60±6.03a,c |
57.08±6.25b,d |
Alterations in bone
histomorphometry
Relatively well-developed and compact trabecular and
cortical bone regions were observed in the femur of the sham
control group rats. Conversely, the OVX control group exhibited a
histological profile that was typical for osteoporosis, including
marked reductions in the trabecular and cortical bone masses, and
increased levels of connective tissue in the periosteum of the
cortical bone due to the resorption of osteoid tissues. Alterations
in the bone histomorphometry were markedly decreased in the
Polycan-treated groups, particularly within the cortical bone, as
compared with the OVX control group (Fig. 1). In the OVX control group, an
increased MAR, as indicated by the distance between the
tetracycline and calcein labeling regions, was observed in the
cortical and trabecular bone regions, as compared with the sham
control group. Conversely, a dose-dependent increase in MAR was
detected in the cortical and trabecular bone regions of the
Polycan-treated groups, as compared with the OVX control group. In
the alendronate-treated group, the MAR in all three bones tested
was markedly decreased compared with the OVX control group
(Fig. 2).
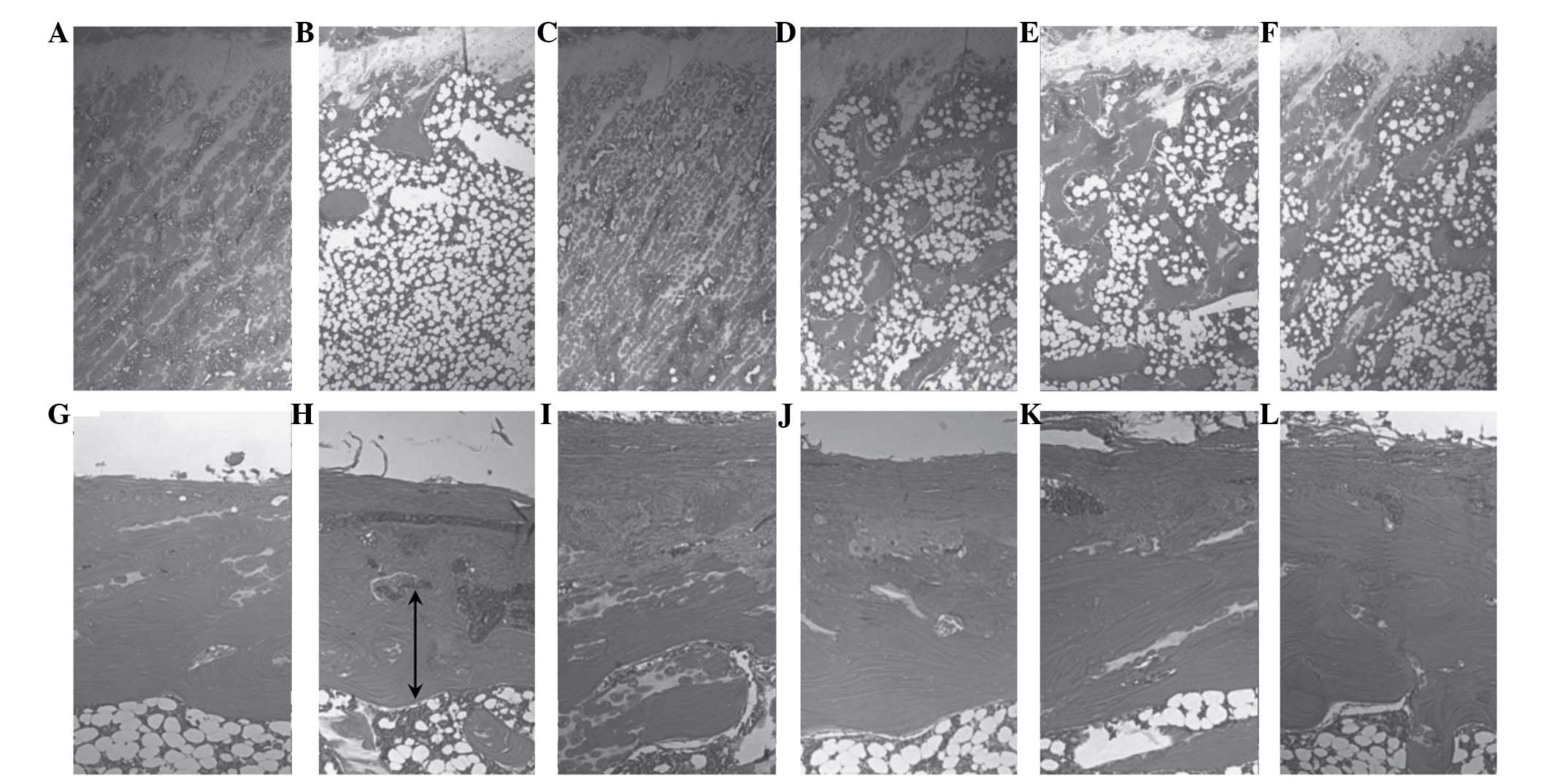 | Figure 1.Histological profiles of the femur of
the (A and B) sham, (C and D) ovariectomized control and (E and F)
alendronate-treated groups, and the (G and H) 31.25, (I and J) 62.5
and (K and L) 125 mg/kg Polycan-treated groups. Polycan inhibited
the ovariectomy-induced reduction in trabecular bone mass and
cortical bone thickness in a dose-dependent manner. Conversely,
alendronate attenuated the ovariectomy-induced reduction in
trabecular bone mass only. A, C, E, G, I and K display epiphyseal
plate-trabecular bone regions (magnification, ×25); B, D, F, H, J,
and L display cortical bone regions (magnification, ×50). Arrows
represent the cortical thickness. All sections were stained with
hematoxylin and eosin. |
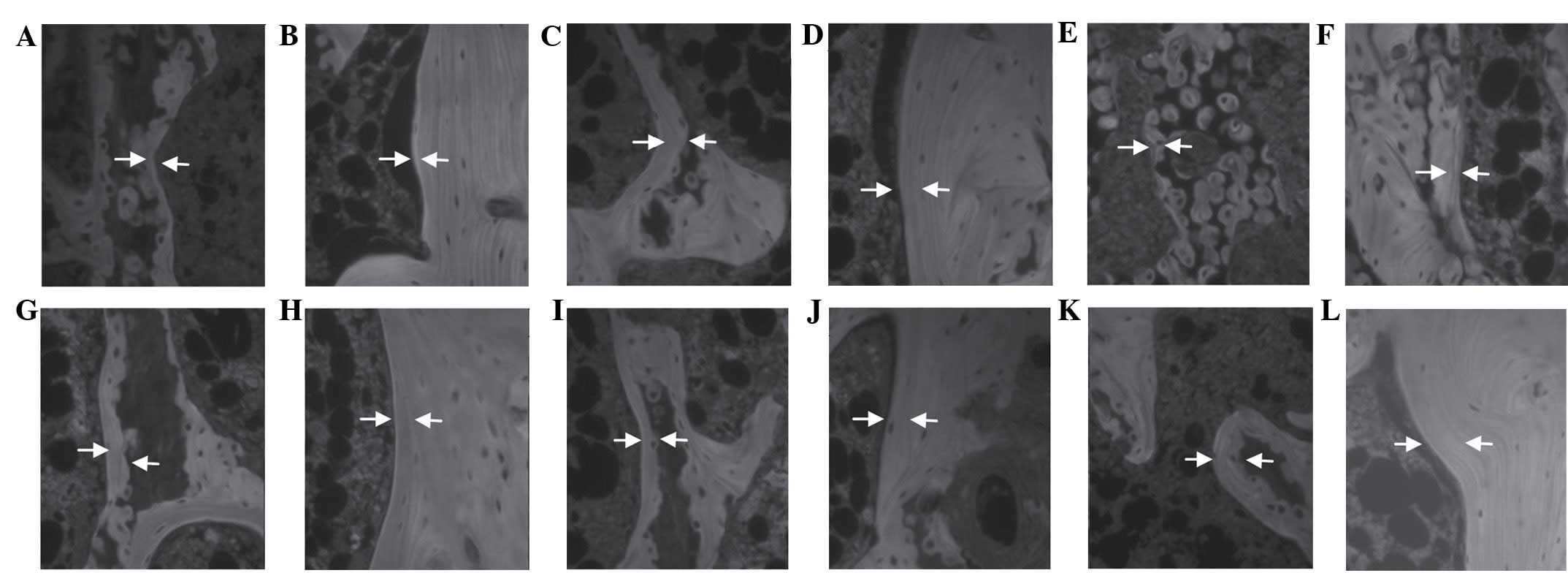 | Figure 2.Fluorescent histological profiles of
the femur of the (A and B) sham, (C and D) ovariectomized (OVX)
control and (E and F) alendronate-treated groups, and the (G and H)
31.25, (I and J) 62.5 and (K and L) 125 mg/kg Polycan-treated
groups. The mineral apposition rate (MAR) was markedly increased in
the trabecular and cortical bone regions of the femur in the
Polycan-treated rats, in a dose-dependent manner, as compared with
the OVX control group. Conversely, the MAR was markedly decreased
in both bone regions of the femur in the alendronate-treated rats,
as compared with the OVX control group. A, C, E, G, I and K display
trabecular bone regions (magnification, ×200); B, D, F, H, J and L
display cortical bone regions (magnification, ×200). Arrows
indicate the tetracycline and calcein labeled lines. The distant
between the arrows represents the MAR over a 10-day period. |
Bone mass and structures
Significant reductions in the Tb.Ar, N.Tb, Tb.Wi,
Tb.Pm and Ct.Wi were detected in the tibia, femur and L5
of the OVX control rats, as compared with the sham control rats
(P<0.01). Conversely, significant (P<0.05) dose-dependent
increases in the histomorphometric indices for bone mass and
structure were detected in all three bones from the Polycan-treated
rats compared with the OVX control, with the exception of the 31.25
mg/kg-treated group, in which no significant increases in these
indices were observed (Tables
VI–VIII).
 | Table VI.Alterations in femur
histomorphometry. |
Table VI.
Alterations in femur
histomorphometry.
|
|
|
|
| Polycan treated
groups |
|---|
|
|
|
|
|
|
|---|
| Indices | Sham control | OVX control | Alendronate | 31.25 mg/kg | 62.5 mg/kg | 125 mg/kg |
|---|
| Bone mass and
structure |
|
|
|
|
|
|
|
Tb.Ar |
42.66±5.11 |
16.48±4.03a |
62.49±4.93a,b |
20.62±3.78a |
23.90±3.28a,b |
25.91±4.99a,b |
|
N.Tb |
20.88±3.36 |
6.38±1.85a |
40.38±8.18a,b |
8.00±1.69a |
10.50±1.51a,b |
11.50±2.00a,b |
|
Tb.Pm |
1,895.31±306.08 |
949.62±152.97a |
1,265.30±196.52a,b |
1,069.78±145.09a |
1,308.83±321.95a,c |
1,326.88±271.85a,b |
|
Tb.Wi |
112.70±10.97 |
75.33±10.21a |
76.35±13.93a |
100.92±25.73c |
103.86±20.33b |
122.20±21.61b |
|
Ct.Wi |
454.86±67.54 |
210.30±26.04a |
200.93±27.21a |
329.64±79.46a,c |
367.91±36.69a,b |
370.46±83.22b |
| Bone
resorption |
|
|
|
|
|
|
|
N.Oc |
13.00±1.41 |
27.00±3.63a |
35.63±10.65a |
27.13±3.52a |
25.25±3.49a |
20.25±2.25a,b |
|
Oc/BS |
4.72±0.79 |
10.96±1.22a |
9.12±1.18a,c |
10.62±1.53a |
10.54±0.90a |
9.13±1.15a,c |
| Trabecular bone
formation |
|
|
|
|
|
|
|
MAR |
0.95±0.13 |
1.54±0.11a |
0.71±0.08a,b |
1.67±0.12a,b |
1.82±0.20a,c |
1.85±0.23a,b |
|
sL.Pm |
7.17±1.22 |
13.21±1.56a |
4.29±0.38a,b |
14.24±1.04a |
15.87±2.36a,c |
16.12±2.44a,c |
|
dL.Pm |
14.39±3.14 |
24.73±3.92a |
7.96±0.97a,b |
27.37±3.14a |
29.41±3.23a,c |
31.25±4.49a,b |
|
Md.Pm |
17.97±3.64 |
31.33±4.53a |
10.11±1.14a,b |
34.50±3.60a |
37.35±4.20a,c |
39.31±5.58a,b |
|
BFR/BS |
0.173±0.054 |
0.484±0.091a |
0.072±0.013a,b |
0.579±0.094a |
0.685±0.138a,c |
0.738±0.208a,b |
| Cortical bone
formation |
|
|
|
|
|
|
|
MAR |
1.17±0.13 |
1.78±0.14a |
0.76±0.09a,b |
1.82±0.16a |
1.98±0.08a,b |
2.06±0.17a,b |
|
sL.Pm |
8.22±1.57 |
15.27±1.70a |
4.21±0.96a,b |
15.00±1.95a |
17.27±1.25a,c |
17.94±1.25a,b |
|
dL.Pm |
15.54±3.01 |
30.71±2.10a |
7.76±1.87a,b |
29.91±2.86a |
34.18±2.72a,c |
35.54±2.94a,b |
|
Md.Pm |
19.65±3.78 |
38.34±2.72a |
9.86±2.32a,b |
37.41±3.82a |
42.82±3.28a,c |
44.51±3.46a,b |
|
BFR/BS |
0.233±0.068 |
0.684±0.093a |
0.076±0.026a,b |
0.685±0.126a |
0.850±0.091a,b |
0.922±0.146a,b |
 | Table VIII.Alterations in L5
histomorphometry. |
Table VIII.
Alterations in L5
histomorphometry.
|
|
|
|
| Polycan treated
groups |
|---|
|
|
|
|
|
|
|---|
| Indices | Sham control | OVX control | Alendronate | 31.25 mg/kg | 62.5 mg/kg | 125 mg/kg |
|---|
| Bone mass and
structure |
|
|
|
|
|
|
|
Tb.Ar |
40.20±6.73 |
20.30±4.07a |
51.66±9.66a,c |
23.78±2.71a,d |
27.18±3.42a,c |
30.31±2.44a,c |
|
N.Tb |
20.38±4.66 |
9.13±1.36a |
28.00±4.34b,c |
10.75±0.89a,d |
11.88±0.64a,c |
12.00±1.69a,c |
|
Tb.Pm |
1,597.39±311.46 |
900.66±158.97a |
1,212.59±194.92b,d |
1,239.49±239.10b,c |
1,409.77±369.62c |
1,538.50±211.31c |
|
Tb.Wi |
130.11±18.75 |
86.26±10.63a |
69.03±5.64a,d |
114.09±13.49c |
124.98±26.79c |
130.69±24.9c |
|
Ct.Wi |
221.60±28.28 |
120.44±34.25a |
123.35±33.39a |
154.38±23.34a,d |
164.45±26.12a,c |
166.47±45.03a,d |
| Bone
resorption |
|
|
|
|
|
|
|
N.Oc |
11.63±1.41 |
23.00±2.14a |
30.75±5.55a,c |
23.63±2.20a |
20.75±1.58a,d |
19.63±2.62a,d |
|
Oc/BS |
3.90±0.68 |
12.09±0.86a |
9.48±1.01a,c |
11.61±1.30a |
10.07±1.23a,c |
9.57±0.92a,c |
| Trabecular bone
formation |
|
|
|
|
|
|
|
MAR |
0.83±0.12 |
1.20±0.09a |
0.77±0.10c |
1.31±0.10a |
1.33±0.15a |
1.43±0.08a,c |
|
sL.Pm |
4.91±1.35 |
9.79±0.92a |
4.07±0.59c |
10.26±1.05a |
11.22±1.34a,d |
11.71±1.34a,c |
|
dL.Pm |
9.23±2.21 |
18.81±2.68a |
7.92±1.09c |
19.75±2.33a |
22.36±2.21a,d |
22.99±2.34a,c |
|
Md.Pm |
11.69±2.86 |
23.71±3.03a |
9.95±1.33c |
24.88±2.66a |
27.97±2.79a,d |
28.85±2.97a,c |
|
BFR/BS |
0.099±0.036 |
0.287±0.055a |
0.078±0.017c |
0.327±0.055a |
0.374±0.074a,d |
0.413±0.063b,c |
| Cortical bone
formation |
|
|
|
|
|
|
|
MAR |
1.01±0.12 |
1.37±0.13a |
0.84±0.13b,c |
1.62±0.20a,c |
1.78±0.14a,c |
1.87±0.10a,c |
|
sL.Pm |
6.96±1.30 |
10.76±0.75a |
5.87±1.52c |
13.04±2.56a,d |
14.62±1.35a,c |
12.79±1.45a,c |
|
dL.Pm |
13.45±2.63 |
22.00±2.32a |
10.57±2.39b,c |
25.83±5.52a |
28.66±2.53a,c |
31.79±2.26a,c |
|
Md.Pm |
16.93±3.25 |
27.39±2.61a |
13.50±3.15c |
32.37±6.79a |
35.97±2.80a,c |
39.68±2.94a,c |
|
BFR/BS |
0.174±0.054 |
0.375±0.051a |
0.117±0.043b,c |
0.536±0.188a,d |
0.640±0.081a,c |
0.743±0.095b,c |
Bone resorption
Significant increases in N.Oc and Oc/BS were
detected in all three bones from the OVX control group, as compared
with the sham control group (P<0.01). However, they appeared to
be significantly decreased by Polycan treatment in a dose-dependent
manner (P<0.05; Tables
VI–VIII).
Bone formation
Significant increases in the MAR, Md.Pm and BFR/BS
were detected in the trabecular and cortical bone regions of all
three bone types in the OVX control group compared with the sham
control group (P<0.01). Conversely, the histomorphometrical
indices for bone formation were significantly increased in the
trabecular and cortical bone regions in the Polycan-treated groups
compared with the OVX control group, in a dose-dependent manner. An
exception was the 31.25 mg/kg Polycan-treated group, in which no
significant increases in the histomorphometrical indices for bone
formation were detected (P>0.05; Tables VI–VIII).
Discussion
The present study investigated the protective
effects of Polycan in a rat model of ovariectomy-induced
osteoporosis by measuring alterations in the BMC, BMD, FL, serum
levels of osteocalcin, bALP, Ca and P, urine Dpd/creatinine ratios
and histomorphometrical indices.
In a previous study, changes in the body weight
(lean and/or fat mass) of OVX rodents was used to predict bone
density (29); however, no
significant ovariectomy-induced alterations in body weight were
detected in the present study.
Osteocalcin is a protein secreted by mature
osteoblasts, levels of which have previously been shown to
correlate with active bone formation and resorption (30). In the present study, the serum levels
of osteocalcin were significantly decreased in OVX mice following
treatment with Polycan for 126 days; thus suggesting that Polycan
may inhibit high bone turnover. bALP levels are generally accepted
as a marker of bone formation (12).
Decreased serum bALP levels by OVX were significantly and
dose-dependently inhibited by Polycan treatment. Increased serum
levels of Ca and P have previously been demonstrated to be a marker
of bone formation in animal models of osteoporosis (31,32). In
the present study, increased serum levels of Ca and P were detected
in the Polycan-treated groups compared with the OVX control group;
thus suggesting that Polycan was able to inhibit the OVX-induced
reduction in serum Ca and P levels. These results suggested that
Polycan may improve bone formation in a dose-dependent manner.
Urine Dpd levels, which are considered to be a
marker of bone resorption, alter in response to alterations in the
levels of creatinine, which is an indicator of kidney state
(33). Therefore, urine
Dpd/creatinine ratios are considered a valuable marker of bone
resorption in osteoporosis (12). In
the present study, Polycan markedly attenuated ovariectomy-induced
increases in the urine Dpd/creatinine ratios; thus suggesting that
Polycan was able to decrease ovariectomy-induced bone resorption.
Similar effects have been observed in OVX rats treated with
alendronate (34).
Patients with osteoporosis typically exhibit a
significantly decreased BMC (35).
Among the various minerals that comprise bone, the levels of Ca and
P have been shown to be the most significantly decreased in
osteoporotic bones, although the Ca/P ratio is not typically
altered (36). In the present study,
the concentrations of Ca and P in the tibia, femur and
L4 bones of the Polycan-treated rats were significantly
increased compared with the OVX control group, and this was
associated with increases in bone strength and quantity, as
determined by BMD.
BMD is considered to be a valuable clinical
indicator of alterations in bone quality, and has been shown to be
significantly decreased in animals with osteoporosis, regardless of
the underlying cause. Furthermore, BMD has previously been used to
predict the efficacy of anti-osteoporosis agents (37), and has provided diagnostic profiles
of bone quality in human clinical research (38). In the present study, Polycan was able
to inhibit ovariectomy-induced decreases in BMD; thus suggesting
that Polycan may be used to prevent and/or treat
osteoporosis-associated fractures.
FL is an key index for predicting the efficacy of
anti-osteoporosis agents (39),
since it has been directly correlated with cortical bone strength.
In the present study, Polycan was able to improve the FL; thus
suggesting that Polycan may directly inhibit ovariectomy-induced
reductions in bone strength.
Bones may be observed under the microscope in order
to analyze alterations in bone morphology (40). The histomorphometrical indices of
bone mass and structure, and bone resorption, have been shown to be
markedly decreased in patients with osteoporosis; thus they may be
considered reliable markers for predicting the efficacy of
anti-osteoporosis agents (41). In
the present study, the histomorphometric indices for bone mass and
structure, and bone resorption, were markedly reduced in the
Polycan-treated rats. In a previous study, the histomorphometric
indices for bone formation were increased in OVX animals due to an
increase in bone turnover (42). In
a previous study, dynamic histomorphometric indices have been shown
to be a reliable indicator of whether a test compound is able to
promote bone-formation (43).
Therefore, the increase in the histomorphometric indices of bone
formation in the Polycan-treated rats in the present study
suggested that Polycan may exert bone anabolic effects. Increases
in the histomorphometrical indices of bone formation have
previously been demonstrated for other bone anabolic agents,
including the parathyroid hormone analogs (44). However, they were not detected
following treatment with selective estrogen receptor modulators
(12) and bisphosphonate (45). Furthermore, alendronate has been
shown to stabilize the indices for bone mass and structure and bone
resorption; however, dynamic histomorphometry demonstrated that the
indices for bone formation were markedly decreased following
treatment with alendronate (45,46).
The results of the present study suggested that
Polycan was able to effectively inhibit ovariectomy-induced changes
associated with osteoporosis through the promotion of osteoblast
differentiation and bone formation, and the inhibition of bone
resorption and osteoclast activity, as has been previously
demonstrated in mouse and in vitro studies (20,21). The
present study demonstrated that Polycan was able to preserve bone
mass and strength, and to increase the rate of bone formation in
the trabecular and cortical bone regions in OVX rats; thus
suggesting that Polycan may be considered a potentially effective
anti-osteoporosis agent. Further studies are required in order to
determined how Polycan is able to stimulate the biomarkers of bone
formation and absorption.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea, and funded by the Korea government
(grant no. 2011-0030124) and the Korea Healthcare Technology
R&D Project, Ministry for Health, Welfare and Family Affairs,
Korea (grant no. A091049-1012-0000400).
References
|
1
|
Sakai A, Nishida S, Okimoto N, Okazaki Y,
Hirano T, Norimura T, Suda T and Nakamura T: Bone marrow cell
development and trabecular bone dynamics after ovariectomy in ddy
mice. Bone. 23:443–451. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madureira MM, Ciconelli RM and Pereira RM:
Quality of life measurements in patients with osteoporosis and
fractures. Clinics (Sao Paulo). 6:1315–1320. 2012. View Article : Google Scholar
|
|
3
|
Melton LJ III, Chrischilles EA, Cooper C,
Lane AW and Riggs BL: How many women have osteoporosis? JBMR
Anniversary Classic. JMBR, Volume 7, Number 9, 1992. J Bone Miner
Res. 20:886–892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen JS and Sambrook PN: Antiresorptive
therapies for osteoporosis: A clinical overview. Nat Rev
Endocrinol. 8:81–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gowen M, Emery JG and Kumar S: Emerging
therapies for osteoporosis. Expert Opin Emerg Drugs. 5:1–43.
2000.
|
|
7
|
Girotra M, Rubin MR and Bilezikian JP:
Anabolic agents for osteoporosis: What is their likely place in
therapy? Treat Endcrinol. 5:347–358. 2006. View Article : Google Scholar
|
|
8
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frost HM and Jee WS: On the rat model of
human osteopenias and osteoporoses. Bone Miner. 18:227–236. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wronski TJ, Yen CF and Scott KS: Estrogen
and diphosphonate treatment provide long-term protection against
osteopenia in ovariectomized rats. J Bone Miner Res. 6:387–394.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimmel DB, Recker RR, Gallagher JC,
Vaswani AS and Aloia JF: A comparison of iliac bone
histomorphometric data in post-menopausal osteoporotic and normal
subjects. Bone Miner. 11:217–235. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ke HZ, Foley GL, Simmons HA, Shen V and
Thompson DD: Long-term treatment of lasofoxifene preserves bone
mass and bone strength and dose not adversely affect the uterus in
ovariectomized rats. Endocrinology. 145:1996–2005. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manolagas SC, Kousteni S and Jilka RL: Sex
steroids and bone. Recent Prog Horm Res. 57:385–409. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bone HG, Hosking D, Devogelaer JP, Tucci
JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J,
Santora AC and Liberman UA: Alendronate Phase III Osteoporosis
Treatment Study Group: Ten years' experience with alendronate for
osteoporosis in postmenopausal women. N Engl J Med. 350:1189–1199.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strewler GJ: Decimal point-osteoporosis
therapy at the 10-year mark. N Engl J Med. 350:1172–1174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lauritzen DB, Balena R, Shea M, Seedor JG,
Markatos A, Le HM, Toolan BC, Myers ER, Rodan GA and Hayes WC:
Effects of combined prostaglandin and alendronate treatment on the
histomorphometry and biomechanical properties of bone in
ovariectomized rats. J Bone Miner Res. 8:871–879. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McClung MR, Wasnich RD, Hosking DJ,
Christiansen C, Ravn P, Wu M, Mantz AM, Yates J, Ross PD and
Santora AC II: Early Postmenopausal Intervention Cohort Study:
Prevention of postmenopausal bone loss: Six-year results from the
early postmenopausal intervention cohort study. J Clin Endocrinol
Metab. 89:4879–4885. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bell NH: Advances in the treatment of
osteoporosis. Curr Drug Targets Immune Endocr Metabol Disord.
1:93–102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boivin G and Meunier PJ: The
mineralization of bone tissue: A forgotten dimension in
osteoporosis research. Osteoporos Int. 14(Suppl 3): S19–S24.
2003.PubMed/NCBI
|
|
20
|
Shin HD, Yang KJ, Park BR, Son CW, Jang HJ
and Ku SK: Antiosteoporotic effect of Polycan, beta-glucan from
Aureobasidium, in ovariectomized osteoporotic mice.
Nutrition. 23:853–860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song HB, Park DC, Do GM, Hwang SL, Lee WK,
Kang HS, Park BR, Jang HJ, Son CW, Park EK, et al: Effect of
exopolymers of Aureobasidium pullulans on improving
osteoporosis induced in ovariectomized mice. J Microbiol
Biotechnol. 16:37–45. 2006.
|
|
22
|
Park SO and Kim JM: Functional food for
immune regulation - beta-glucan. Food Science and Industry.
45:39–47. 2012.
|
|
23
|
Estrada A, Yun CH, Van Kessel A, Li B,
Hauta S and Laarveld B: Immunomodulatory activities of oat
beta-glucan in vitro and in vivo. Microbiol Immunol. 41:991–998.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bell S, Goldman VM, Bistrian BR, Arnold
AH, Ostroff G and Forse RA: Effect of beta-glucan from oats and
yeast on serum lipids. Crit Rev Food Sci Nutr. 39:189–202. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seo HP, Kim JM, Shin HD, Kim TK, Chang HJ,
Park BR and Lee JW: Production of β-1,3/1,6-glucan by
Aureobasidium pullulans SM-2001. Korean J Bitechnol Bioeng.
17:376–380. 2002.
|
|
26
|
Lee HS, Cho HR, Moon SB, Shin HD, Yang KJ,
Park BR, Jang HJ, Kim LS and Ku SK: Effect of β-glucan from
Aureobasidium pullulans on rat rib fracture healing. Lab
Anim Res. 24:39–44. 2008.
|
|
27
|
Han SY, Lee JR, Kwon YK, Jo MJ, Park SJ,
Kim SC, Lee HS and Ku SK: Ostreae testa prevent ovariectomy-induced
bone loss in mice by osteoblast activations. J Ethnopharmacol.
114:400–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parfitt AM, Drezner MK, Glorieux FH, Kanis
JA, Malluche H, Meunier PJ, Ott SM and Recker RR: Bone
histomorphometry: Standardization of nomenclature, symbols, and
units. Report of the ASBMR histomorphometry nomenclature committee.
J Bone Miner Res. 2:595–610. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lorden JF and Caudle A: Behavioral and
endocrinological effects of single injections of monosodium
glutamate in the mouse. Neurobehav Toxicol Teratol. 8:509–519.
1986.PubMed/NCBI
|
|
30
|
Li B and Yu S: Genistein prevents bone
resorption diseases by inhibiting bone resorption and stimulating
bone formation. Biol Pharm Bull. 26:780–786. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ladizesky MG, Cutrera RA, Boggio V, Somoza
J, Centrella JM, Mautalen C and Cardinali DP: Effect of melatonin
on bone metabolism in ovariectomized rats. Life Sci. 70:557–565.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shim JG, Yeom SH, Kim HJ, Choi YW, Lee DI,
Song KY, Kwon SH and Lee MW: Bone loss preventing effect of
Sophorae Fructus on ovariectomized rats. Arch Pharm Res.
28:106–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu M, Dick IM, Day R, Randall D and Prince
RL: Effects of a herbal extract on the bone density, strength and
markers of bone turnover of mature ovariectomized rats. Am J Chin
Med. 31:87–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwamoto J, Seki A, Takeda T, Sato Y,
Yamada H and Yeh JK: Comparative therapeutic effects of alendronate
and alfacalcidol on cancellous and cortical bone mass and
mechanical properties in ovariectomized osteopenic rats. J Nutr Sci
Vitaminol (Tokyo). 52:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pavlik M: Measuring bone mineral content.
Orthop Nurs. 10:39–43. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanaka S, Shimizu M, Debari K, Furuya R,
Kawawa T and Sasaki T: Acute effects of ovariectomy on wound
healing of alveolar bone after maxillary molar extraction in aged
rats. Anat Rec. 262:203–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Syed Z and Khan A: Bone densitometry:
Applications and limitations. J Obstet Gynaecol Can. 24:476–484.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Diez F: Guidelines for the diagnosis of
osteoporosis by densitometric methods. J Manipulative Physiol Ther.
25:403–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bilston LE, Little DG, Smith NC, Williams
P and Briody J: Zoledronic acid improves the mechanical properties
of normal and healing bone. Clin Biomech (Bristol, Avon).
17:716–718. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heikkinen T, Puoliväli J and Tanila H:
Effects of long-term ovariectomy and estrogen treatment on maze
learning in aged mice. Exp Gerontol. 39:1277–1283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weinreb M, Patael H, Preisler O and
Ben-Shemen S: Short-term healing kinetics of cortical and
cancellous bone osteopenia induced by unloading during the
reloading period in young rats. Virchows Arch. 431:449–452. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Verhaeghe J, Oloumi G, van Herck E, van
Bree R, Dequeker J, Einhorn TA and Bouillon R: Effects of long-term
diabetes and/or high-dose 17 beta-estradiol on bone formation, bone
mineral density, and strength in ovariectomized rats. Bone.
20:421–428. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gal-Moscovici A, Gal M and Popovtzer MM:
Treatment of osteoporotic ovariectomized rats with 24,25(OH)2D3.
Eur J Clin Invest. 35:375–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andersson N, Lindberg MK, Ohlsson C,
Andersson K and Ryberg B: Repeated in vivo determinations of bone
mineral density during parathyroid hormone treatment in
ovariectomized mice. J Endocrinol. 170:529–537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
da Paz LH, de Falco V, Teng NC, dos Reis
LM, Pereira RM and Jorgetti V: Effect of 17beta-estradiol or
alendronate on the bone densitometry, bone histomorphometry and
bone metabolism of ovariectomized rats. Braz J Med Biol Res.
34:1015–1022. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Broulik PD, Rosenkrancová J, Růzicka P and
Sedlácek R: Effect of alendronate administration on bone mineral
density and bone strength in castrated rats. Horm Metab Res.
37:414–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
















