Introduction
The guided bone regeneration (GBR) technique is used
for bone tissue reparation in dentistry. The basic concept in GBR
is the placement of a barrier to preserve the blood that is formed
and create a closed area around the bone defect, hence facilitating
the activation of osteoblasts. In general, it has been accepted
that this kind of barrier must be pervious to enable the spreading
of nutrients to the regenerated bone (1–3).
In bone tissue reparation, the use of autogenous
bone grafts is the gold standard. Autogeneous bone grafts have
osteoinductive and osteoconductive properties. Additionally,
autogeneous grafts contain stem cells and growth factors and do not
create any immunological reaction (3). However, the requirement for a second
surgical area, restricted amount of bone graft and graft resorption
have led to the development of various graft materials and
treatment methods for bone augmentation (3,4).
Human-derived bone grafts (allografts) are less osteogenic, more
immunogenic and have a greater rate of resorption than autogeneous
bone grafts, with a potential risk of disease transmission (e.g.,
hepatitis and HIV-AIDS). As a result of these limitations,
synthetic bone grafts (alloplasts) have been developed. Ideally,
alloplastic graft materials should be biocompatible with host
tissues, non-antigenic and non-inflammatory. Calcium phosphate
ceramics, such as β-tricalcium phosphate (β-TCP), have been shown
to induce bone regeneration in experimental animal models, and are
suggested to have high stabiility and osteogenic potential compared
with autologous bone grafts (3,5). Due to
their composition and structure, bioceramic synthetic bone grafts
degrade and are progressively replaced by bone (5).
GBR with a rigid titanium barrier has been used
successfully for enhancing bone tissue in certain in vivo
studies (3,6–9). In this
method, a rigid titanium barrier is installed under the periosteum
to mineralize the underlying blood clot for increase bone height.
There have been a few studies concerning bone augmentation using
autogeneous blood under titanium rigid barriers. In these studies
the researchers aimed to increase bone formation beneath the rigid
titanium barrier. They reported that cortical bone perforation
allowed the movement of angiogenic and osteoblastic cells into the
closed space (3,6–9).
Biphosphonates (BPs) are used to prevent and treat
increased bone resorption in skeletal diseases (10,11). The
influence of BPs on bone healing and the interaction between bone
and implant have been investigated (12,13).
Throughout the bone repair process, BPs have been shown to induce
an anti-osteoclastic effect and, thus, a relatively osteoblastic
effect (12,14).
BPs have some side effects when used systematically.
An initial influenza-like illness, renal failure and osteonecrosis
have been documented in the literature when used systematically
(12,14).
Zoledronic acid (ZA) is the strongest of the BPs in
clinical use. A single dose of ZA, administered intraoperatively,
has been shown to have positive effects on various models of bone
repair and healing (15,16). In the present study the aim was to
evaluate the effect of locally administered ZA with autogeneous
blood on new bone regeneration with or without a β-TCP graft under
a rigid titanium barrier in rabbit calvarium.
Materials and methods
Animal care and ethics
Experimental applications in this study were
authorized by the Animal Experimental Ethics Committee of Firat
University (Elazığ, Turkey). The rabbits were kept and treated
according to advice of the Declaration of Helsinki. Rabbits were
kept in standard cages during the experimental period (21–23°C,
50–65% humidity, 1 atm and a 12-h light-12-h dark cycle). Rabbits
received a balanced standard ration diet and drinking water ad
libitum during the experimental period.
Experimental protocols and surgical
procedure
Eight New Zealand white male rabbits (weight, 3–3.5
kg; age, 0.5–1 year) were used in the study. The rabbits were
obtained from the Experimental Research Center of Firat University.
The 8 rabbits were divided four groups, each containing two
animals: Autogeneous blood (AB) group; AB+ZA group; AB+β-TCP-bone
graft group; and AB+β-TCP-bone graft+ZA group. Prior to all
surgical procedures, rigid dome-shaped pure titanium barriers were
constructed (Elektron Medikal, Ankara, Turkey). These rigid
titanium barriers had a hole in the top for which a Teflon cap was
made. The rigid barriers were cleaned and then sterilized prior to
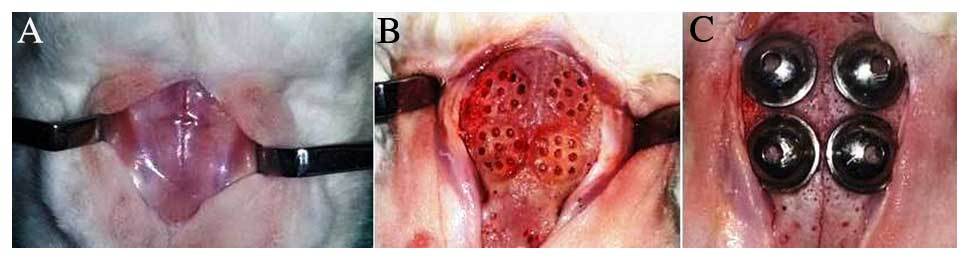
use (Fig. 1).
Surgical operations were conducted in sterile
conditions. General anesthesia was established using 10 mg/kg
xylazine (Rompun®) and 40 mg/kg ketamine. Following
general anesthesia and prior to surgery the skull skin was shaved.
An incision in the skin of the skull was made over the linea media.
A periosteal elevator was used to lift the flap and periosteum in
order to access the parietal and frontal bones of the skull. Nine
holes were drilled using a burr of ~1.5 mm in diameter with saline
irrigation to trigger bleeding. In every rabbit calvarium four sets
of nine holes were created and four titanium barriers (one for each
set) were used (Fig. 1). The edges
of the barriers were bonded to the bone tissue with
N-butyl-2-cyanoacrylate (Histoacryl®; B.Braun,
Melsungen, Germany). In the AB group, decortication of the cortical
layers was executed on the parietal and frontal bones and
autogeneous blood was taken from the rabbit's ear artery and
injected into the titanium barrier through the holes until the
barrier was fully filled. In the AB+ZA group, blood taken from
rabbit's ear artery was mixed with ZA (2 mg ZA/ml autogeneous
blood) and was injected into the titanium barriers through the
holes in each rabbit calvarium. In the AB+β-TCP group, 0.5
cm3 β-TCP (IngeniOs; Zimmer Dental GmbH, Ottobrunn,
Germany) graft material mixed with 2 ml autogeneous blood from the
rabbit's ear artery was used. In the AB+β-TCP+ZA group, 0.5
cm3 β-TCP graft mixed with 2 ml autogeneous blood from
rabbit's ear artery and ZA (2 mg ZA/ml autogeneous blood) was used.
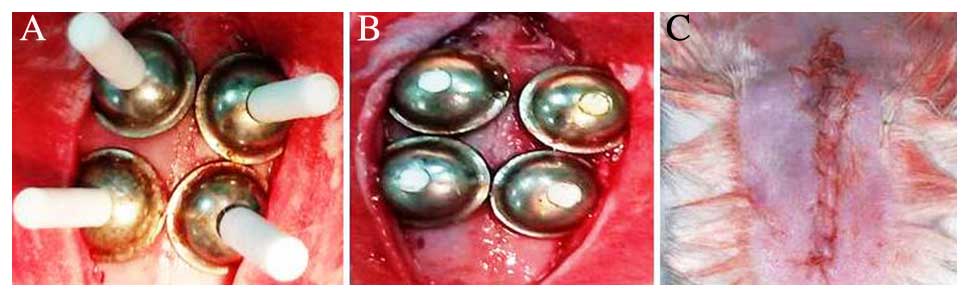
Graft materials were applied under the titanium barriers through a
hole. After this, the holes were closed using the Teflon covers.
The skull skin of the rabbits was sutured with 3/0 polyglactin
resorbable sutures (Ethicon Vicryl; Johnson & Johnson, New
Brunswick, NJ, USA; Fig. 2).
Cefalosporine antibiotic (50 mg/kg) and analgesic (4 mg/kg
acetominophen) were injected intramuscularly in all animals 1 day
before the surgery, and once in a day for 4 days afterwards. All
rabbits were examined for wound cleaning during the healing period
for 2 weeks. After healing for 3 months, the rabbits were
sacrificed with carbon dioxide. Following sacrifice, a surgical
burr attached to an electrical hand motor piece was used to harvest
the bone containing the titanium barriers from the rabbits's
calvarial bone.
Histological and histomorphometric
analysis
The specimens were fixed in 10% formaldehyde for 72
h and demineralised in 10% formic acid; after this, they were
dehydrated, embedded in paraffin wax, and sectioned for
haematoxylin and eosin staining for light microscopic analysis.
Sections 6-µm in thickness, corresponding to the bone area, were
evaluated by light microscopy. The histological sections were
analysed with an Olympus Bx-51 (Olympus Corporation, Tokyo, Japan)
light microscope. The presence of inflammatory cell infiltrate,
connective tissue, bone graft material resorption, new bone
formation, new bone marrow and grafted material were evaluated.
Images of the samples were captured under light
using a photo light microscope with an attached digital camera to
examine mineralized bone formation. Images from each histological
section taken with the attached camera were transferred to a
computer and software with an automatic calibration feature
(Olympus D71 imaging software system; Olympus Corporation, Tokyo,
Japan) was used for histomorphometric analysis of the images. In
the histomorphometric analysis of each specimen, the ratio of the
regenerated new bone areas (µm2) to all areas of the
barriers (new bone area/saggittal surface barrier area) was
calculated with the Dolphin 11.0 Imaging software and an average
value was determined for each sample (3).
Statistical analysis
For the statistical analysis, IBM SPSS Statistics 22
software (IBM SPSS, Armonk, NY, USA) was used. After the 3-month
healing period, mean values and standard deviations were
calculated. The differences among groups were tested using one-way
analysis of variance tests for parameters that showed a normal
distribution and Tukey's honest significant difference test was
used for the identification of specific groups with significant
differences. P<0.05 was considered to indicate a statistically
significant difference.
Results
Histological observations
There were no complications during the surgery.
After the application of the titanium barriers, all animals
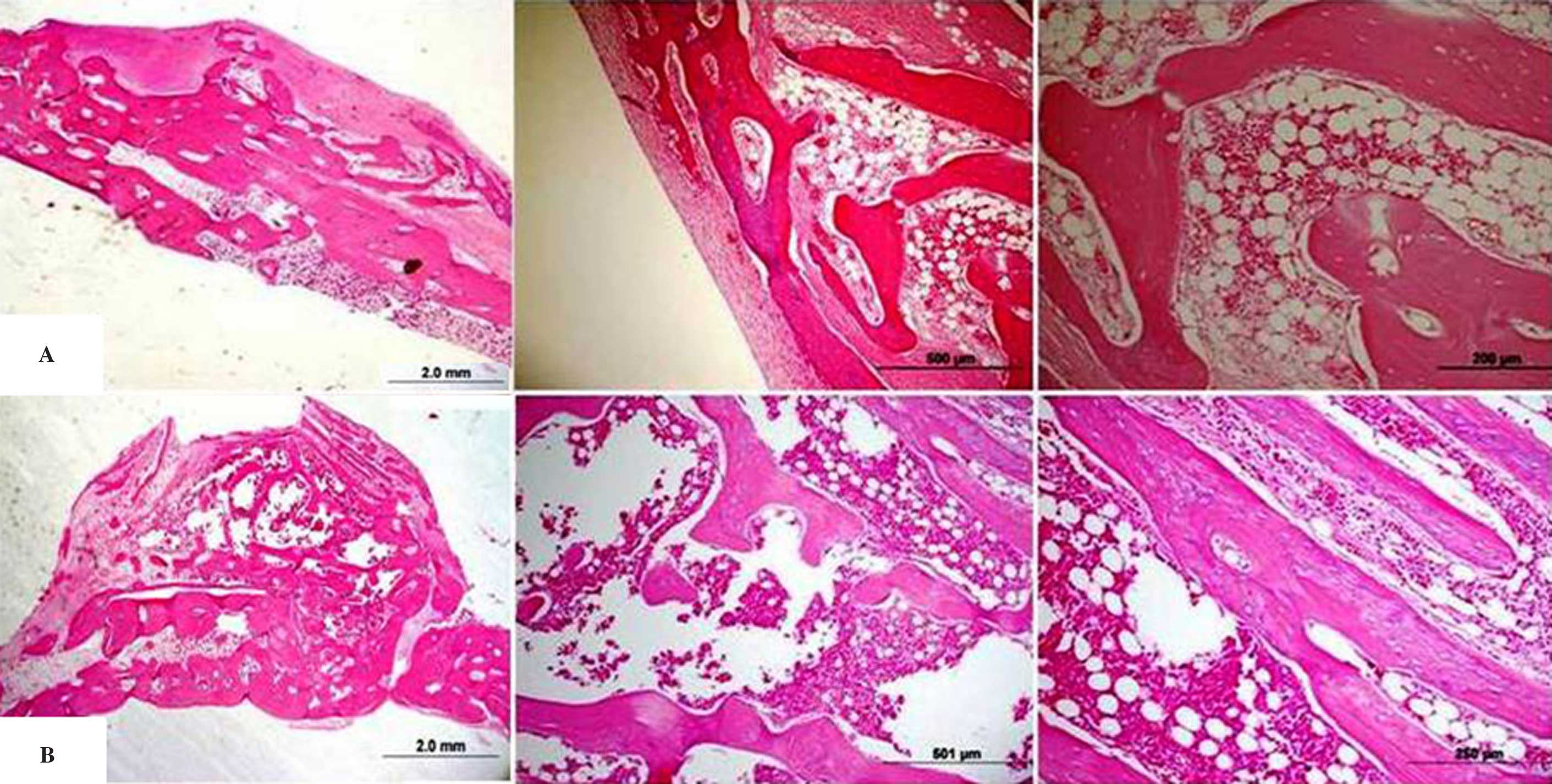
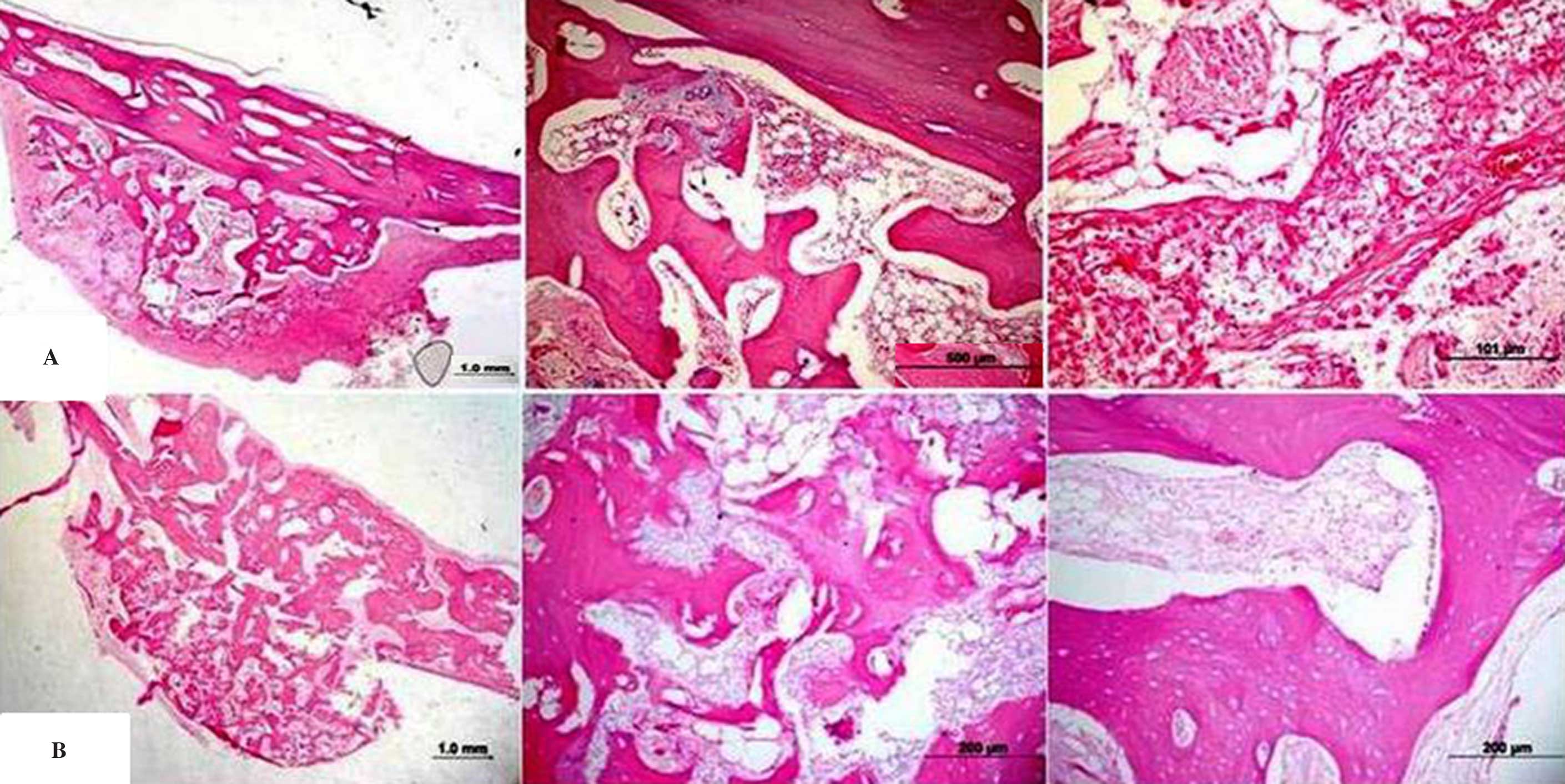
recovered without post-operative signs of infection. In Figs. 3 and 4, histological samples are shown. At
3-months, new bone formation was observed in all groups. A gap was
detected between the border of the titanium rigid barrier and the
bone in some cases. The bone formation rate in the majority of the
histological samples was determined to be much higher in the centre
than at the periphery of the tissue. In all groups a bed of dense
fibrous connective tissue lined the entire periphery of the tissue.
There was active bone formation in all specimens.
Bone formation rates
Bone formation was detected in all groups. There
were statistically significant differences in bone formation rate
among the groups. The bone formation rate in the AB+β-TCP+ZA group
was determined to be statistically significantly higher than that
in the other groups (P<0.05). In group AB+ZA, the bone formation
rate was determined to be significantly higher than that in group
AB (P<0.05). The bone formation rate in group AB+β-TCP was also
determined to be significantly higher than that in group AB
(P<0.05). No statistically significant difference in bone
formation rate was observed between the AB+β-TCP and AB+ZA groups
(Table I).
 | Table I.Bone formation in the four
groups. |
Table I.
Bone formation in the four
groups.
| Groups | New bone formation
(mean ± SD) |
|---|
| AB | 60.52±3.87 |
| AB+ZA |
91.46±4.43a |
| AB+β-TCP |
88.70±1.92a |
| AB+β-TCP+ZA |
96.00±1.65a,c |
Discussion
Advanced periodontitis, resective surgery, trauma
and tumors are considered to be etiologies of alveolar bone defects
(17). Various grafting materials
such as autografts, allografts, xenografts and alloplastic graft
are used in GBR (17–19). During the GBR procedure, graft
materials must be secured in position in the healing period for the
treatment of bone defects. Mechanical stresses may cause
deformation and disruption of the fibrin clot. This causes tissue
regeneration to break down, and fibrous tissue forms. Ensuring the
stability of the matrix during healing enables this to be
controlled. The use of fixation devices, including GBR membranes,
titanium mesh, bone screws or bone tacks can be used to achieve
this (20). The placement of a
subperiosteal titanium barrier is another method for increasing
bone height with the aid of the underlying blood clot for
mineralization (3,9). The use of a titanium barrier on the
rabbit calvarium can markedly increase the proportion of new bone
tissue formation. In earlier studies, the use of a rigid titanium
barrier was observed to stabilize bone grafts and blood clots; the
titanium barrier inhibits resorption of the graft material
(3,17). The results of the present study
confirm earlier reports claiming that it is possible to augment the
skull bone beyond the original form and that the increase is more
substantial if bone grafts are used (6–8,21,22).
Autogenous bone grafts are the gold standard in
dentistry for the treatment of bone defects. However, the available
amount of autogeneous bone graft is often insufficient and the use
of a second surgical area and unpredictable resorption are
disadvantages of autogeneous bone graft procedures. For this
reason, in the treatment and reconstruction of bone defects the use
of synthetic bone graft materials has been considered as a
treatment method. Different bone graft materials have been studied
in the reconstruction of bone defects in medicine (17,23).
β-TCP is a commonly used synthetic bone graft material in the
regeneration of bone defects (17),
and has excellent biocompatibility. In contrast to human- and
animal-derived bone graft materials, the synthetic origin of β-TCP
prevents disease transmission. β-TCP grafts show good biological
suitability and osteoconductive power, as well as a potential
capacity for osteoinductivity (23–25).
β-TCP bone grafts are totally resorbed by host bone tissues
(23).
There have been only a few studies that have
investigated the use of autogenous blood under titanium barriers
for bone augmentation (6,21). In these studies, cortical bone
perforation was created to allow the migration of angiogenic and
osteoblastic cells underneath the membrane to enhance bone
formation (3,26,27). Ito
et al (28) showed that the
use of a titanium barrier membrane can augment the formation of
bone beyond the skeletal cover and into areas where no bone was
formerly present. Maréchal et al (21) reported that the amount of newly
formed bone tissue under the barriers was greater than that
detected using other techniques. The results of the present study
confirm previous reports that it is possible to amplify skull bone
thickness and that new bone formation can occur where no bone was
present initially by the use of a titanium barrier. The current
study demonstrated that ZA with autogeneous blood and bone graft
with ZA plus autogeneous blood exhibits a greater positive effect
on new bone tissue formation with use of a titanium cap compared
with control groups.
Formation of new blood vessels and the triggering of
active osteoblasts are vital factors in bone tissue repair
(26). However, the origin of
osteoblastic cells is controversial. Although several authors
consider stromal fibroblasts of the bone marrow as the
osteoprogenitor cells, others suggest that osteoblasts stem from
the capillary system (3). Thus,
decortication of the cortical layer of the bone may be beneficial
for the accomplishment of tissue regeneration, and may support
bleeding and blood clot formation in the wound area, which has been
hypothesized to be a vital factor in GBR (3,17,26,29).
Lundgren et al (29,30) found that decortication of the
cortical layer of the parietal bone in the rabbit does not result
in additional bone formation beyond the skeletal anatomy after a
3-month healing period when compared with a non-decorticated
cortical bone plate inside a obscured experimental area. In another
study, Min et al (7)
conducted a study using rabbits in order to detect whether or not
calvarial bone decortication size influences bone augmentation
within rigid titanium barriers. The authors detected that
decortication size does not affect augmentation; however, this
result is not in consistent with the findings of other studies
(31–33). For this reason, the same size of
decortication cavity was used for all groups in the present
study.
BP pretreatment can be useful to prevent graft
resorption. In addition, bone cell culture studies have indicated
that the use of very low concentrations of BPs increases
bone-formation parameters (15,34).
Since BPs have a direct action on osteoclasts, it is evident that
they may affect bone formation. Osteoclast function can be changed
by the production of an osteoclast inhibitory factor excreted by
osteoblasts following exposure to BPs. During the bone remodeling
process, cells of osteoblastic lineage control the activity of
osteoclast cells. BPs increase the proliferation and maturation of
osteoblastic cells and reduce apoptosis (15). This information supports the
suggestion that BPs have an anabolic effect on bone tissue cells
and thereafter promote bone tissue formation. Therefore, the target
cells of BPs may include members of the osteoblastic cell family
(15,17). BPs have been shown to increase the
proliferation of osteoblasts and the biosynthesis of collagen and
osteocalcin by bone cells at the cellular level (5,34). In
the present study, the histological analysis indicated that the
newly formed bone area was increased in all groups at the end of
the study. These results appear to confirm similar information in
the literature (5,15–17,34). The
bone formation rate in the AB+ZA group was determined to be
statistically significantly higher than that in the AB group. In
addition, no statistically significant difference was determined
between the AB+ZA group and the AB+β-TCP group. These results
indicate that autologous blood is effective in GBR; in addition,
autologous blood mixed with ZA may provide a greater effect without
bone grafts in bone formation (3).
Bone formation in the AB+β-TCP+ZA group was greater than that in
the other three groups, which may indicate that ZA used with bone
grafts and autogeneous blood is more effective in osteogenesis. In
the present study, we hypothesize that ZA inhibited the resorption
of the bone graft, activated osteoblastic cells and increased
osteogenesis (5,17,34).
Mixing the grafts with BP solution prior to application onto a bone
defect appears to be an innocuous method. By treating bone locally
with BP, the graft can be prevented from undergoing resorption,
without any systemic effects. In an earlier study, it was detected
that the local application of BP solution onto an allograft
protected the graft from resorption (5). In the present study, pre-treatment of
the bone graft with ZA inhibited resorption of the graft material
and enabled bone formation. In addition, the present study revealed
a favourable effect of local pretreatment with BP solution at a
dosage of 2 mg ZA/ml autogeneous blood in the AB+ZA and AB+β-TCP+ZA
groups with regard to new bone formation, consistent with a
previous report using a different BP (5).
In conclusion, the limited results obtained from the
present study suggest that in the GBR procedure executed with a
titanium rigid barrier, the use of local ZA with autogeneous blood
and/or a graft material is more effective than the use of
autogeneous blood or graft alone. It is proposed that this method
may eliminate the use of bone graft materials in the treatment of
bone defects in the future. In addition to this, taking into
account the risks associated with systemic ZA use, further studies
focusing on the local application of ZA with different graft
materials and at different dosages are recommended to improve the
successful development of GBR.
References
|
1
|
Buser D, Brägger U, Lang NP and Nyman S:
Regeneration and enlargement of jaw bone using guided tissue
regeneration. Clin Oral Implants Res. 1:22–32. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwarz F, Herten M, Ferrari D, Wieland M,
Schmitz L, Engelhardt E and Becker J: Guided bone regeneration at
dehiscence-type defects using biphasic hydroxyapatite+beta
tricalcium phosphate (Bone Ceramic) or a collagen-coated natural
bone mineral (BioOss Collagen): An immunohistochemical study in
dogs. Int J Oral Maxillofac Surg. 36:1198–1206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ezirganli S, Polat S, Baris E, Tatar I and
Celik HH: Comparative investigation of the effects of different
materials used with a titanium barrier on new bone formation. Clin
Oral Implants Res. 24:312–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alam S, Ueki K, Nakagawa K, Marukawa K,
Hashiba Y, Yamamoto E, Sakulsak N and Iseki S: Statin-induced bone
morphogenetic protein (BMP) 2 expression during bone regeneration:
An immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 107:22–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toker H, Ozdemir H, Ozer H and Eren K: A
comparative evaluation of the systemic and local alendronate
treatment in synthetic bone graft: A histologic and
histomorphometric study in a rat calvarial defect model. Oral Surg
Oral Med Oral Pathol Oral Radiol. 114(5 Suppl): S146–S152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Steenberghe D, Johansson C, Quirynen
M, Molly L, Albrektsson T and Naert I: Bone augmentation by means
of a stiff occlusive titanium barrier. Clin Oral Implants Res.
14:63–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Min S, Sato S, Saito M, Ebihara H, Arai Y
and Ito K: Micro-computerized tomography analysis: Dynamics of bone
augmentation within a titanium cap in rabbit calvarium. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 106:892–895. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murai M, Sato S, Koshi R, Yokoyama K,
Ikeda K, Narukawa M, Takayama T, Yoshinuma N and Ito K: Effects of
the enamel matrix derivative and beta-tricalcium phosphate on bone
augmentation within a titanium cap in rabbit calvarium. J Oral Sci.
47:209–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molly L, Quirynen M, Michiels K and van
Steenberghe D: Comparison between jaw bone augmentation by means of
a stiff occlusive titanium membrane or an autologous hip graft: A
retrospective clinical assessment. Clin Oral Implants Res.
17:481–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doggrell SA: Clinical efficacy and safety
of zoledronic acid in prostate and breast cancer. Expert Rev
Anticancer Ther. 9:1211–1218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lipton A: The safety of zoledronic acid.
Expert Opin Drug Saf. 6:305–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abtahi J, Tengvall P and Aspenberg P:
Bisphosphonate coating might improve fixation of dental implants in
the maxilla: A pilot study. Int J Oral Maxillofac Surg. 39:673–677.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedl G, Radl R, Stihsen C, Rehak P,
Aigner R and Windhager R: The effect of a single infusion of
zoledronic acid on early implant migration in total hip
arthroplasty. A randomized, double-blind, controlled trial. J Bone
Joint Surg Am. 91:274–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abtahi J, Agholme F, Sandberg O and
Aspenberg P: Effect of local vs. systemic bisphosphonate delivery
on dental implant fixation in a model of osteonecrosis of the jaw.
J Dent Res. 92:279–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ayan M, Dolanmaz D, Mihmanli A, Ayan A and
Kürkcü M: The effect of systemically administrated zoledronic acid
on the osseointegration of dental implants. Oral Dis. 18:802–808.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Back DA, Pauly S, Rommel L, Haas NP,
Schmidmaier G, Wildemann B and Greiner SH: Effect of local
zoledronate on implant osseointegration in a rat model. BMC
Musculoskelet Disord. 13:422012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozdemir H, Ezirganli S, Kara M Isa,
Mihmanli A and Baris E: Effects of platelet rich fibrin alone used
with rigid titanium barrier. Arch Oral Biol. 58:537–544. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greenstein G and Carpentieri JR:
Utilization of d-PTFE barriers for post-extraction bone
regeneration in preparation for dental implants. Compend Contin
Educ Dent. 36:465–473. 2015.PubMed/NCBI
|
|
19
|
Gardin C, Ricci S, Ferroni L, Guazzo R,
Sbricoli L, De Benedictis G, Finotti L, Isola M, Bressan E and
Zavan B: Decellularization and delipidation protocols of bovine
bone and pericardium for bone grafting and guided bone regeneration
procedures. PLoS One. 10:e01323442015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smiler D and Soltan M: The bone-grafting
decision tree: A systematic methodology for achieving new bone.
Implant Dent. 15:122–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maréchal M, Luyten F, Nijs J, Postnov A,
Schepers E and van Steenberghe D: Histomorphometry and
micro-computed tomography of bone augmentation under a titanium
membrane. Clin Oral Implants Res. 16:708–714. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmid J, Hämmerle CH, Olah AJ and Lang
NP: Membrane permeability is unnecessary for guided generation of
new bone. An experimental study in the rabbit. Clin Oral Implants
Res. 5:125–130. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martinez A, Balboa O, Gasamans I,
Otero-Cepeda XL and Guitian F: Deproteinated bovine bone vs.
beta-tricalcium phosphate as bone graft substitutes:
Histomorphometric longitudinal study in the rabbit cranial vault.
Clin Oral Implants Res. 26:623–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daculsi G, Laboux O, Malard O and Weiss P:
Current state of the art of biphasic calcium phosphate bioceramics.
J Mater Sci Mater Med. 14:195–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samavedi S, Whittington AR and Goldstein
AS: Calcium phosphate ceramics in bone tissue engineering: A review
of properties and their influence on cell behavior. Acta Biomater.
9:8037–8045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishimura I, Shimizu Y and Ooya K: Effects
of cortical bone perforation on experimental guided bone
regeneration. Clin Oral Implants Res. 15:293–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buser D, Dula K, Belser UC, Hirt HP and
Berthold H: Localized ridge augmentation using guided bone
regeneration. II. Surgical procedure in the mandible. Int J
Periodontics Restorative Dent. 15:10–29. 1995.PubMed/NCBI
|
|
28
|
Ito K, Minegishi T, Takayama T, Tamura T,
Yamada Y and Sato S: Effects of ipriflavone on augmented bone using
a guided bone regeneration procedure. Clin Oral Implants Res.
18:60–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lundgren AK, Lundgren D, Hämmerle CH,
Nyman S and Sennerby L: Influence of decortication of the donor
bone on guided bone augmentation. An experimental study in the
rabbit skull bone. Clin Oral Implants Res. 11:99–106. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lundgren AK, Lundgren D, Wennerberg A,
Hammerle CH and Nyman S: Influence of surface roughness of barrier
walls on guided bone augmentation: Experimental study in rabbits.
Clin Implant Dent Relat Res. 1:41–48. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Linde A, Thorén C, Dahlin C and Sandberg
E: Creation of new bone by an osteopromotive membrane technique: An
experimental study in rats. J Oral Maxillofac Surg. 51:892–897.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rompen EH, Biewer R, Vanheusden A, Zahedi
S and Nusgens B: The influence of cortical perforations and of
space filling with peripheral blood on the kinetics of guided bone
generation. A comparative histometric study in the rat. Clin Oral
Implants Res. 10:85–94. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmid J, Wallkamm B, Hämmerle CH,
Gogolewski S and Lang NP: The significance of angiogenesis in
guided bone regeneration. A case report of a rabbit experiment.
Clin Oral Implants Res. 8:244–248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toker H, Ozdemir H, Ozer H and Eren K:
Alendronate enhances osseous healing in a rat calvarial defect
model. Arch Oral Biol. 57:1545–1550. 2012. View Article : Google Scholar : PubMed/NCBI
|