Introduction
Immune thrombocytopenic purpura (ITP) is an acquired
immune-mediated disorder characterized by isolated thrombocytopenia
(1). ITP in infants is uncommon and
appears to be predominantly benign. Although the exact cause of ITP
remains unknown, infection is generally considered to serve an
important role in the pathogenesis of ITP. Recently, increasing
evidence has suggested an association between viral infection and
ITP (2,3).
Rotavirus is a double-stranded RNA virus belonging
to the Reoviridae family that was first described in 1973 (4). Human rotavirus is the major etiological
agent of diarrhea in infants and children throughout the world
(5). However, previous studies have
indicated that rotavirus infection is not limited to the
gastrointestinal tract (6). Viremia
and other systemic infections are commonly reported in patients and
animals with rotavirus infections (7). Furthermore, rotavirus infection may be
an etiological agent of autoimmune disease (8).
To date, the existence of an association between
rotavirus infection and ITP remains unclear. The present
retrospective study described a series of cases in children who
suffered from both rotavirus diarrhea and ITP simultaneously in
order to investigate whether rotavirus infection serves a causative
role in ITP.
Patients and methods
Participants and study procedure
Initially, the incidence of ITP in children with or
without simultaneous rotavirus infection was compared. A total of
601 children hospitalized for diarrhea in the Department of
Hematology at Tianjin Children's Hospital (Tianjin, China) between
December 1, 2002 and December 1, 2010 were included in the study.
The rotavirus antigens were investigated in all children. Patients
that were rotavirus-positive were classified as the rotavirus
group, while rotavirus-negative patients served as the controls.
The diagnosis of ITP was established according to the medical
history of the patients, including the results of physical
examination, complete blood count and peripheral blood smear that
eliminated other causes of the thrombocytopenia.
Subsequently, the clinical features in ITP children
with or without rotavirus infection were compared. The clinical
features that we evaluated were as follows: i) Demographic
characteristics; ii) seasonal variation; iii) untreated platelet
count; iv) mean platelet volume (MPV); v) bleeding severity; and
vi) response to therapy.
Examinations
Fecal specimens collected from the patients were
examined on admission for the presence of rotavirus antigen using
Group A Rotavirus Antigen Rapid Test Kit (colloidal gold method;
Gentaur, Kampenhout, Belgium).
Statistical analysis
The STATA statistical software (version 12.2;
StataCorp, College Station, TX, USA) was used to analyze
statistical correlations in the collected data. Student's t-test or
Fisher's exact test were used to calculate statistically
significant differences, which were indicated by P<0.05.
Results
Incidence of ITP in children with or
without rotavirus infection
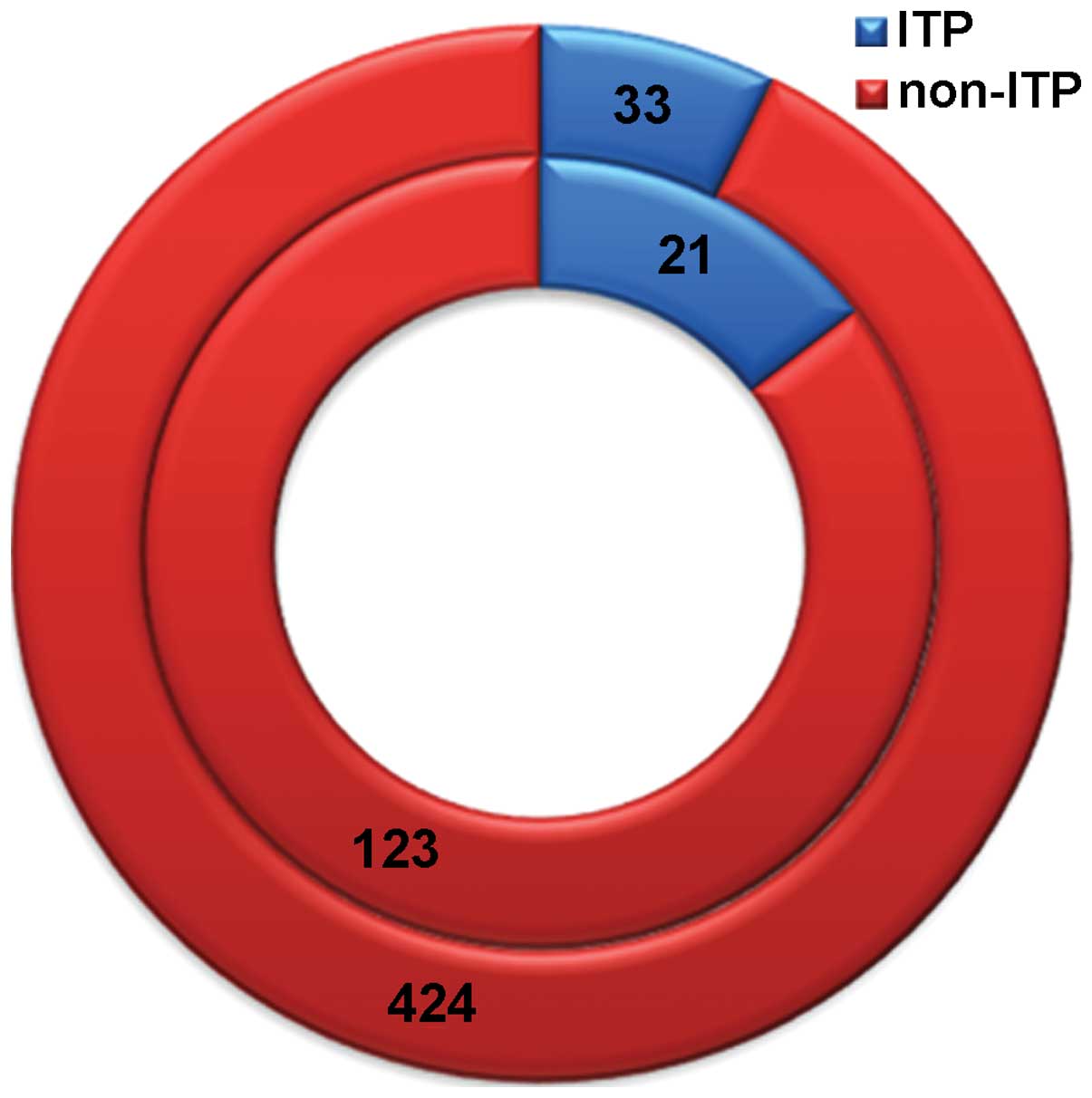
Among the children with rotavirus infection, 21
(14.58%) demonstrated simultaneous ITP. Among the 457 children with
non-rotavirus-associated diarrhea, 33 (7.22%) presented ITP.
As shown in Fig. 1,
the inner circle represents the 144 children with rotavirus
diarrhea, which includes 21 children (14.58%) with simultaneous ITP
(blue color) and 123 children (85.42%) who did not present ITP (red
color). Similarly, the outer circle represents the 457 children
with non-rotavirus diarrhea, including 33 children (7.22%) with ITP
(blue color) and 424 children (92.78%) without ITP (red color).
Demographic characteristics and
clinical features of ITP children with or without rotavirus
infection
Table I lists the
demographic and clinical features of the included patients. The
median age of children with simultaneous ITP and rotavirus
infection was 18.42 months, with a range of 4–49 months. The median
month age of the 601 children presenting ITP without rotavirus
infection was 35.85 months, with a range of 5–98 months. Children
presenting ITP with rotavirus infection were significantly younger
in age (P<0.05) compared with children presenting ITP without
rotavirus infection. In addition, the male:female ratio in children
presenting ITP with rotavirus infection was 2.2:1, while the ratio
was 1.5:1 in children presenting ITP without rotavirus infection.
Thus, the possibility of developing ITP is higher in males with
rotavirus infection.
 | Table I.Clinical features and laboratory
findings in ITP. |
Table I.
Clinical features and laboratory
findings in ITP.
| Parameter | ITP with RV
(n=21) | ITP without RV
(n=33) | P-value |
|---|
| Median age,
months | 18.42 | 35.85 | <0.001 |
| Age range,
months | 4–49 | 5–98 |
|
| Gender ratio
(M/F) | 2.2:1 | 1.5:1 |
|
| Season of onset,
n |
|
|
|
| Spring
(March-May) | 1 | 7 |
|
| Summer
(June-August) | 6 | 10 |
|
| Autumn
(September-November) | 1 | 8 |
|
| Winter
(December-February) | 13 | 8 |
|
| Untreated platelet
count, ×109/l | 23.51 | 22.69 | 0.722 |
| Mean platelet volume,
fl | 7.88 | 9.98 | 0.032 |
| Bleeding grades |
|
|
|
| 0 | 1 | 0 | 0.400 |
| 1 | 12 | 10 | 0.160 |
| 2 | 4 | 2 | 0.191 |
| 3 | 4 | 20 | 0.044 |
| 4 | 0 | 1 | 0.618 |
Seasonal variation
The seasonal variation in children with ITP and
rotavirus infection was as follows: 1 case in spring, 6 cases in
summer, 1 case in autumn, and 13 cases in winter. Therefore, the
majority of ITP cases with rotavirus infection occurred during the
winter months (13/21). The seasonal variation in ITP cases without
rotavirus infection was as follows: 7 cases in spring, 10 in
summer, 8 in autumn and 8 in winter. Thus, the number of ITP cases
in children without rotavirus infection occurred evenly throughout
the seasons, without a significant seasonal variation observed.
Untreated platelet count
The mean platelet count in children presenting ITP
with and without rotavirus infection was 23.51×109 and
22.69×109/l, respectively. No statistically significant
difference was observed between the two groups (Table I; P>0.05).
MPV measurement
The MPV in children presenting ITP with and without
rotavirus infection was 7.88 and 9.21 fl (9.6–13.0 fl),
respectively. A statistically significant decrease in MPV was
observed in ITP children with rotavirus infection when compared
with those without rotavirus infection (Table I; P<0.05).
Bleeding severity
The scoring system described by Buchanan and Adix
(9) was used to assess bleeding. The
following four domains were assessed: i) Overall bleeding tendency;
ii) bleeding from the oral cavity; iii) bleeding from the nose; and
iv) cutaneous hemorrhage. The bleeding severity in each of the four
domains was graded between 0 and 4. Subsequently, the grades from
each domain were added together to obtain the Buchanan score, which
indicated the following: 0, no evidence of bleeding; 1, minor
bleeding; 2, mild bleeding; 3, moderate bleeding; and 4, severe
bleeding.
Children presenting ITP with rotavirus infection
demonstrated a significantly higher frequency of a bleeding score
of 3 when compared with those without rotavirus infection
(P<0.05), as determined using the Fisher's exact test. However,
there was no significant difference in bleeding scores 0–2 or 4
between the ITP children with or without rotavirus infection,
according to the results of Fisher's exact test.
Response to therapy
Children with platelet counts
<50×109/l received pharmacological therapy, which
included intravenous immunoglobulin (IVIG), high-dose steroids and
conventional-dose steroids (10).
They were evaluated for their response to therapy at day 7. By
contrast, children with platelet counts of >50×109/l
did not receive any pharmacological therapy. The response to
therapy was defined as complete (CR) if the platelet count was
>150×109/l following treatment and partial (PR) if
the platelet count was between 50–150×109/l following
treatment. All other children were considered to have no-response
(NR) to treatment.
IVIG therapy was administered to 3 ITP children with
rotavirus infection and to 7 without rotavirus infection. CR was
achieved in 2 ITP children with rotavirus infection and 5 ITP
children without rotavirus infection, while PR was achieved in 1
ITP child with rotavirus infection and 1 ITP child without
rotavirus infection. In addition, 1 child without rotavirus
infection showed NR to treatment.
High-dose steroid therapy (methylprednisolone or
dexamethasone) was administered to 2 ITP children with rotavirus
infection and to 5 without rotavirus infection. The treatment
resulted in CR in 1 ITP child with rotavirus infection and 3 ITP
children without rotavirus infection, as well as in PR in 1 ITP
child with rotavirus infection and 1 ITP children without rotavirus
infection. NR was observed in 1 children without rotavirus
infection.
Furthermore, conventional-dose steroid therapy
(prednisolone or dexamethasone) was administered to 17 ITP children
with rotavirus infection and 16 without rotavirus infection. CR was
achieved in 14 ITP children with rotavirus infection and 12 without
rotavirus infection, while PR was achieved in 2 ITP children with
rotavirus infection and 3 ITP children without rotavirus infection.
NR was observed in 1 ITP child with rotavirus infection and 1 ITP
children without rotavirus infection.
A total of 21 children with ITP received no
treatment, and CR was achieved in all these children.
Discussion
Approximately 66% of children with ITP show a
history of infectious illness a few days or a week prior to the
onset of thrombocytopenia. A viral infection (such as varicella
zoster, rubella, Epstein-Barr, influenza or human immunodeficiency
virus-1) have been identified in a subset of these children, which
indicates an etiological role of a preceding viral infection that
leads to ITP (11). A previous case
study indicated the existence of an association between rotavirus
infection and ITP (12). The data
presented in the current study demonstrated a higher prevalence of
ITP in children presenting diarrhea with rotavirus infection
compared with children without rotavirus infection. This suggests
that an association exists between the clinical manifestation of
diarrhea (9).
The mechanism of rotavirus-associated ITP could be
elucidated by analyzing the kinetics of the immune response during
rotavirus infection. Rotavirus antibodies can be initially detected
in the serum as early as 2 days after diarrhea onset, which
indicates a causative role of rotavirus infection in producing the
diarrhea (13). However, in the
present study, only 2 children developed ITP 2–4 days after
diarrhea onset. Consequently, the kinetics of the immune response
during rotavirus infection may not be the main mechanism of
rotavirus-associated ITP.
In the present study, the majority of ITP cases
(61.9%) in children with rotavirus infection occurred during the
winter season. However, the incidence of ITP in children without
rotavirus infection demonstrated no seasonal variation. In
addition, the majority of subjects included in the current study
dwelled in Tianjin in northern China. Notably, a previous
large-scale epidemic study of rotavirus infection in the
neighboring city of Beijing showed a peak number of cases in winter
(14). By contrast, another study of
new ITP cases from the Beijing Children's Hospital showed no
seasonal variation (15).
In the current study, it is speculated that
thrombocytopenia is not the only bleeding risk factor in ITP
children with rotavirus infection, since the untreated platelet
count showed no significant difference between the two groups.
However, a significant decrease in MPV was observed in ITP children
with rotavirus infection when compared with those without rotavirus
infection. Similarly, Mete et al observed a significant
decrease in MPV in children with acute rotavirus gastroenteritis
(16). Since MPV is a marker of
platelet function, we suggest that rotavirus infection may decrease
platelet function.
However, the present study had a number of inherent
limitations. Firstly, a large prospective cohort study was
precluded due to the relatively small number of ITP children with
rotavirus infection. Secondly, an observation bias is acknowledged
since the study was not blinded. Finally, more sensitive detection
methods for rotavirus have emerged over the past years, other than
colloidal.
In conclusion, the present retrospective study
investigated the association of rotavirus infection with the
incidence of ITP in children. The results suggested that rotavirus
may serve a causative role in ITP. Further prospective studies are
required to determine how rotavirus affects platelets.
Acknowledgements
The authors would like to thank Dr Ronald W. Dudek
(East Carolina University) for the helpful critical reading of the
manuscript and for providing constructive comments.
References
|
1
|
Provan D, Stasi R, Newland AC, Blanchette
VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB,
Godeau B, et al: International consensus report on the
investigation and management of primary immune thrombocytopenia.
Blood. 115:168–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagamine T, Ohtuka T, Takehara K, Arai T,
Takagi H and Mori M: Thrombocytopenia associated with hepatitis C
viral infection. J Hepatol. 24:135–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ratner L: Human immunodeficiency
virus-associated autoimmune thrombocytopenic purpura: A review.
American J Med. 86:194–198. 1989. View Article : Google Scholar
|
|
4
|
Chizhikov V, Wagner M, Ivshina A, Hoshino
Y, Kapikian A and Chumakov K: Detection and genotyping of human
group A rotaviruses by oligonucleotide microarray hybridization. J
Clin Microbiol. 40:2398–2407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harrington M, Butler K and Cafferkey M:
Rotavirus infection in hospitalised children: Incidence and impact
on healthcare resources. Ir J Med Sci. 172:33–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramig RF: Pathogenesis of intestinal and
systemic rotavirus infection. J Virol. 78:10213–10220. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramig RF: Systemic rotavirus infection.
Expert Rev Anti Infect Ther. 5:591–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eichmann M and Peakman M: The role of T
lymphocytes in the pathogenesis of autoimmune type 1 diabetes:
implications for potential virus-mediated pathwaysDiabetes and
Viruses. Taylor K, Hyöty H, Toniolo A and Zuckerman A: Springer;
New York, NY: pp. 271–286. 2013, View Article : Google Scholar
|
|
9
|
Buchanan GR and Adix L: Grading of
hemorrhage in children with idiopathic thrombocytopenic purpura. J
Pediatr. 141:683–688. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Veneri D, Franchini M, Gottardi M, D'Adda
M, Ambrosetti A, Krampera M, Zanetti F and Pizzolo G: Efficacy of
helicobacter pylori eradication in raising platelet count in adult
patients with idiopathic thrombocytopenic purpura. Haematologica.
87:1177–1179. 2002.PubMed/NCBI
|
|
11
|
Rand ML and Wright JF: Virus-associated
idiopathic thrombocytopenic purpura. Transfus sci. 19:253–689.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siddiqui AH and Chitlur MB: Immune
thrombocytopenic purpura in a 5-month-old female with rotavirus
infection. Pediatr Blood Cancer. 54:6332010.PubMed/NCBI
|
|
13
|
Grimwood K, Lund JC, Coulson BS, Hudson
IL, Bishop RF and Barnes GL: Comparison of serum and mucosal
antibody responses following severe acute rotavirus gastroenteritis
in young children. J Clin Microbiol. 26:732–738. 1988.PubMed/NCBI
|
|
14
|
Fang ZY, Yang H, Zhang J, Li YF, Hou AC,
Ma L, Sun LW and Wang CX: Child rotavirus infection in association
with acute gastroenteritis in two chinese sentinel hospitals.
Pediatr Int. 42:401–405. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu RH, Wu MY, Zhang YH, Shi HW, Xie J and
Duan Y: Two hundred and sixty two Children with idiopathic
thrombocytopenic purpura. Shi Yong Er Ke Lin Chuang Za Zhi.
21:1002–1003. 2006.(In Chinese).
|
|
16
|
Mete E, Akelma AZ, Cizmeci MN, Bozkaya D
and Kanburoglu MK: Decreased mean platelet volume in children with
acute rotavirus gastroenteritis. Platelets. 25:51–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|