Introduction
Resveratrol (Res) is a natural polyphenolic compound
present in grapes and red wine. It is a specific agonist of sirtuin
1 (Sirt1), and has many cardiovascular protective effects, such as
anti-inflammatory, anti-oxidative and anti-proliferative effects
(1,2).
Previous studies have shown that Res is able to
relax vascular beds of various types, including conductance
arteries, such as the uterine artery (3), aorta (4–6),
abdominal aorta (7) and thoracic
aorta (8), and resistance arteries,
such as the internal mammary artery (9), mesenteric artery (3,10,11) and
coronary artery (12). The
vasorelaxant effects of Res on conductance arteries and the
underlying mechanism have been well clarified. However, the
vasodilatation and vasodilatory mechanisms in small resistance
arteries are associated with cardiovascular events (13). Although Res possesses the
pharmacological property of vasodilatation in resistance arteries,
several pathways involved in the mechanism of vasodilatation are
unclear.
Therefore, the present study was designed to explore
the mechanism by which Res induces vasodilatation in rat superior
mesenteric arteries. This should further reveal the underlying
mechanisms involved in the vasorelaxant effect of Res on resistance
arteries, and provide a theoretical basis for the development of
cardiovascular drugs.
Materials and methods
Reagents
Phenylephrine hydrochloride (PE), acetylcholine
chloride (ACh), NG-nitro-L-arginine methyl ester
(L-NAME), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ),
indomethacin (Indo), 4-aminopyridine (4-AP), barium chloride
dehydrate (BaCl2), glibenclamide (Gli),
tetraethylammonium chloride (TEA) and Triton X-100 were obtained
from Sigma-Aldrich (St. Louis, MO, USA). Res was obtained from the
College of Life Science, Northwest University (Xi'an, China). ODQ,
TEA, Gli, 4-AP, and Res were dissolved in dimethylsulfoxide. All
other compounds were dissolved in distilled water.
Artery preparation and testing
Thirty male Sprague-Dawley rats (8 weeks old; body
weight, 300–350 g), which were obtained from the Animal Center of
Xi'an Jiaotong University (Xi'an, China), were euthanized with
CO2. The superior mesenteric artery was gently removed
and freed from adhering tissue under a dissecting microscope. The
animal experiments in this study were approved by the Laboratory
Animal Administration Committee of Xi'an Medical University (Xi'an,
China) and performed according to the Guidelines for Animal
Experimentation of Xi'an Medical University and the Guide for the
Care and Use of Laboratory Animals published by the US National
Institutes of Health (NIH Publication No. 85–23, revised 1996).
Triton X-100 is a non-ionic detergent. It directly
dissolves the lipid bilayer in endothelial cell membranes to cause
destruction of endothelial cell surfaces. In this study, the
endothelium was denuded by perfusion of the vessel for 10 sec with
X-100 (0.1%, v/v) followed by another 10 sec with a physiological
buffer solution (PSS; NaCl 119 mM, KCl 4.6 mM, NaHCO3 15
mM, NaH2PO4 1.2 mM, MgCl2 1.2 mM,
CaCl2 1.5 mM and glucose 5.5 mM). The vessels were then
cut into 1–3-mm long cylindrical segments.
The segments, with and without endothelium, were
immersed in individual temperature-controlled (37°C) myograph baths
(Organ Bath Model 700MO; J.P. Trading, Aarhus, Denmark) containing
PSS (5 ml). The solution was continuously aerated with gas
comprising 5% CO2 and 95% O2, resulting in a
pH of 7.4. The arterial segments were mounted for continuous
recording of isometric tension using LabChart 7 Pro software
(ADInstruments, Hastings, UK). A resting tone of 2 mN was applied
to each segment, and the segments were allowed to stabilize at this
tension for at ≥1.5 h prior to exposure to K+-rich (60
mM) buffer solution with the same composition as the standard
solution, with the exception that NaCl was replaced by an equimolar
concentration of KCl (KPSS). The potassium-induced contraction was
used as a reference for contractile capacity, and the segments were
used only if potassium elicited reproducible responses >1.0 mN.
Following equilibration, PE (10 µM) or KPSS (containing 60 mM
K+) was added to the bath. When a sustained tension was
obtained, Res (5×10−7-5×10−4 M) was added
cumulatively to the baths and concentration-response curves to Res
were constructed. After the experiment, the bath was washed with
PSS three times. PE (10 µM) or KPSS was added to the bath again
following equilibration. The difference in contractile capacity
between before and after the experiment was used as a reference for
the toxicity of Res.
With regard to the endothelium, the completeness of
endothelium denudation was tested with ACh (10 µM) following
pre-contraction with KPSS. No relaxation in response to ACh in the
denuded preparation indicated an effective functional removal of
the endothelium. Endothelium-intact rings that produced <30%
relaxation in response to ACh were discarded (14).
In vitro pharmacology
To evaluate the effects of Res on the contraction
induced by PE or KCl, superior mesenteric artery rings were
pre-contracted with PE (10 µM) or KCl (60 mM), and once a plateau
was attained, concentration-response curves were obtained by adding
cumulative doses of Res to the bath.
To identify the endothelial mediator (s) associated
with the vasodilatory effect of Res, an endothelial nitric oxide
synthase (eNOS) inhibitor [L-NAME (100 µM)], a guanylate cyclase
inhibitor [ODQ (10 µM)] and a cyclooxygenase inhibitor [Indo (5
µM)] were used. The endothelium-intact artery rings were
pre-incubated with each of these inhibitors for 20 min before KCl
(60 mM) was added to the bath, and then Res was added
cumulatively.
In order to demonstrate the role of K+
channels in Res-induced relaxation, artery rings without
endothelium were pre-incubated with the K+ channel
blockers 4-AP (100 µM), BaCl2 (10 µM), Gli (10 µM) and
TEA (1 mM), independently, for 20 min before KCl (60 mM) was added,
and then Res was added cumulatively.
To clarify whether the relaxation induced by Res was
associated with intracellular Ca2+ release, experiments
were carried out in Ca2+-free PSS (100 µM). Rings
without endothelium were washed with Ca2+-free PSS.
Following incubation with or without Res (500 µM) for 20 min, PE
(10 µM) was added to stimulate the release of intracellular
Ca2+ and the contraction was recorded (15).
Finally, to determine whether the inhibition of
extracellular Ca2+ influx was involved in the relaxation
induced by Res, experiments were carried out in
Ca2+-free PSS (100 µM). Artery rings without endothelium
were washed with Ca2+-free PSS containing ethylene
glycol tetraacetic acid (EGTA; 100 µM) and then rinsed with
Ca2+-free PSS (without EGTA) containing KCl (60 mM
K+). Following incubation with or without Res (500 µM)
for 20 min, CaCl2 (2 mM) was added to contract the
artery rings (15).
Statistical analysis
Data are expressed as mean ± standard error of the
mean. The effects of Res are expressed as percentage of relaxation
from the pre-contraction. The negative logarithm of the dilator
concentration that caused 50% of the maximum response
(pD2) and the maximum relaxation (Emax%) were
calculated. Statistical analysis was performed with unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant result. The analysis was performed using
SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
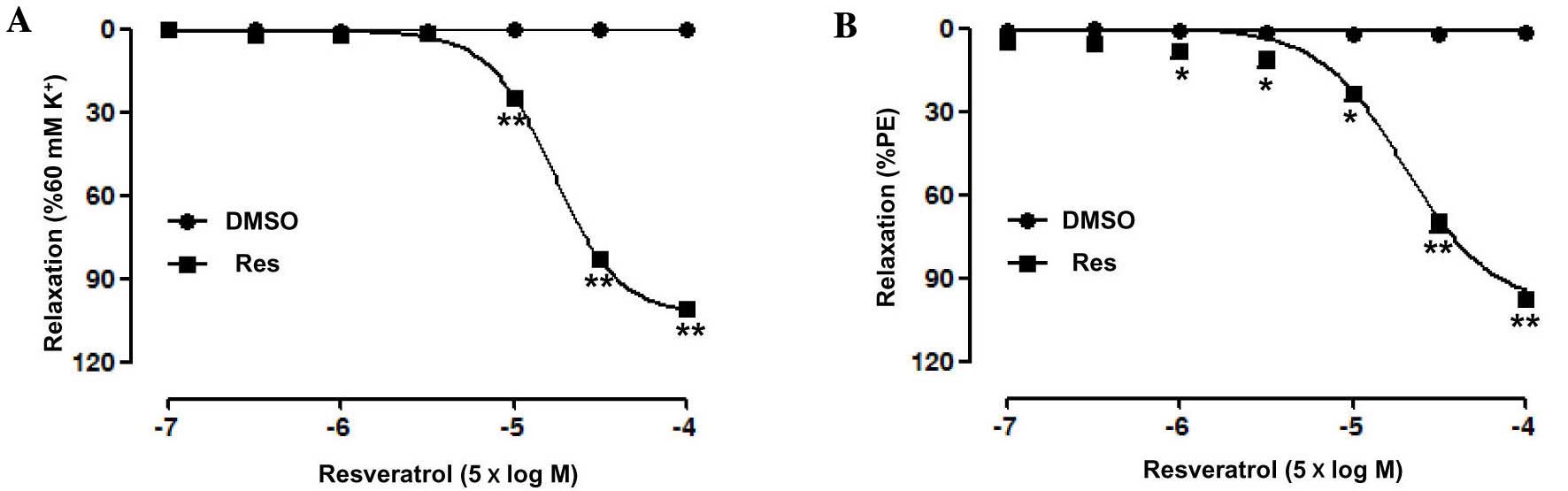
Effect of Res on rat superior
mesenteric artery pre-constricted by PE or KCl
Res (0.5–500 µM) concentration-dependently relaxed
the endothelium-intact superior mesenteric artery rings
pre-contracted by PE (Emax, 97.66±0.79%; pD2,
4.30±0.14) or KCl (Emax, 101.3±0.6%; pD2,
4.12±0.03) (Fig. 1). In addition,
there was no significant change in the contractile capacity of the
superior mesenteric artery induced by PE or KPSS between before and
after the experiment, suggesting that Res has no toxicity.
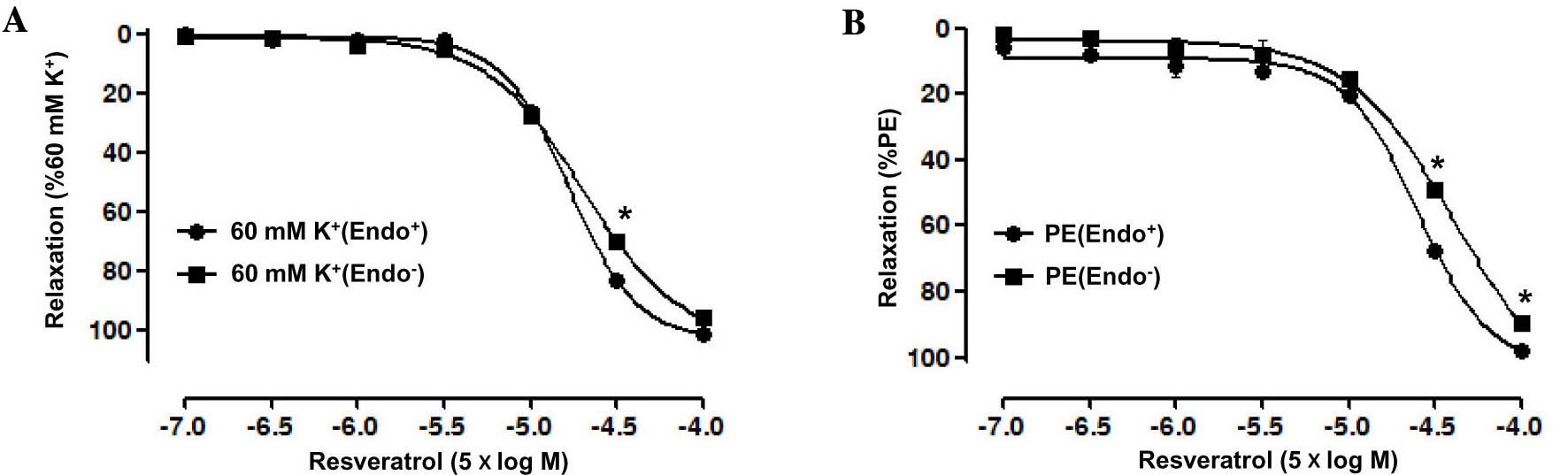
Role of the endothelium in Res-induce
relaxation of rat superior mesenteric artery pre-constricted by
KCl
The vasorelaxant effect of Res on endothelium-intact
superior mesenteric artery rings pre-contracted by PE (10 µM) was
significantly stronger than that on artery rings without
endothelium, with an Emax of 97.69±0.82 vs.
89.72±0.1.89% for the artery rings without endothelium group, and a
pD2 of 4.31±0.14 vs. 3.86±0.04 for the artery rings
without endothelium group. Moreover, the vasorelaxation induced by
Res in endothelium-intact artery rings pre-contracted by KCl (60
mM) also was significantly stronger than that in artery rings
without endothelium, with an Emax of 100.94±0.59 vs.
95.63±0.63% for the artery rings without endothelium group and a
pD2 of 4.13±0.03 vs. 4.09±0.01 for the artery rings
without endothelium group (P<0.05; Fig. 2).
The endothelial mediator(s) associated with the
vasodilatory effect of Res were investigated by pre-incubation with
the eNOS inhibitor L-NAME, guanylate cyclase inhibitor ODQ and
cyclooxygenase inhibitor Indo, independently, prior to treatment
with KCl or Res. The results showed that L-NAME significantly
inhibited the relaxation induced by Res in the artery rings with
endothelium, with an Emax of 89.93±0.17 vs. 100.96±0.76%
in the control group, and a pD2 of 3.91±0.03 vs.
4.15±0.02 in the control group (P<0.05; Fig. 3A). However, ODQ and Indo each did not
significantly affect the relaxation induced by Res in the artery
rings with endothelium (Fig. 3B and
C).
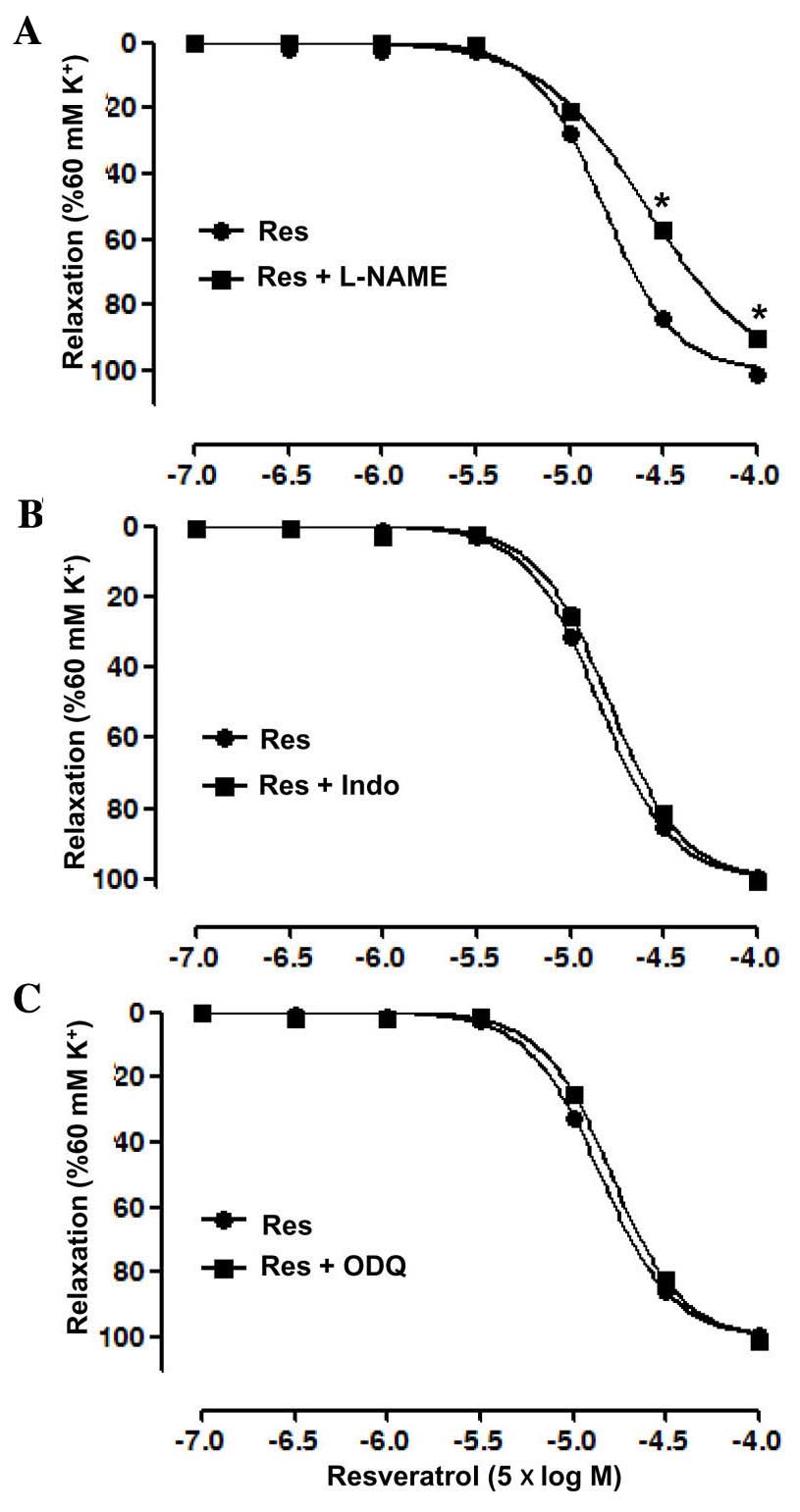 | Figure 3.Vasodilatation effects of resveratrol
(Res) on endothelium-intact superior mesenteric arterial rings
pre-contracted with KCl (60 mM) in the presence of (A) the
endothelial nitric oxide synthase inhibitor (L-NAME, 100 µM), (B)
the cyclooxygenase inhibitor (Indo, 5 µM) and (C) the guanylate
cyclase inhibitor (ODQ, 10 µM). Data are presented as mean ±
standard error of the mean (n=6–8). *P<0.05 vs. Res. L-NAME,
NG-nitro-L-arginine methyl ester; Indo, indomethacin;
ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. |
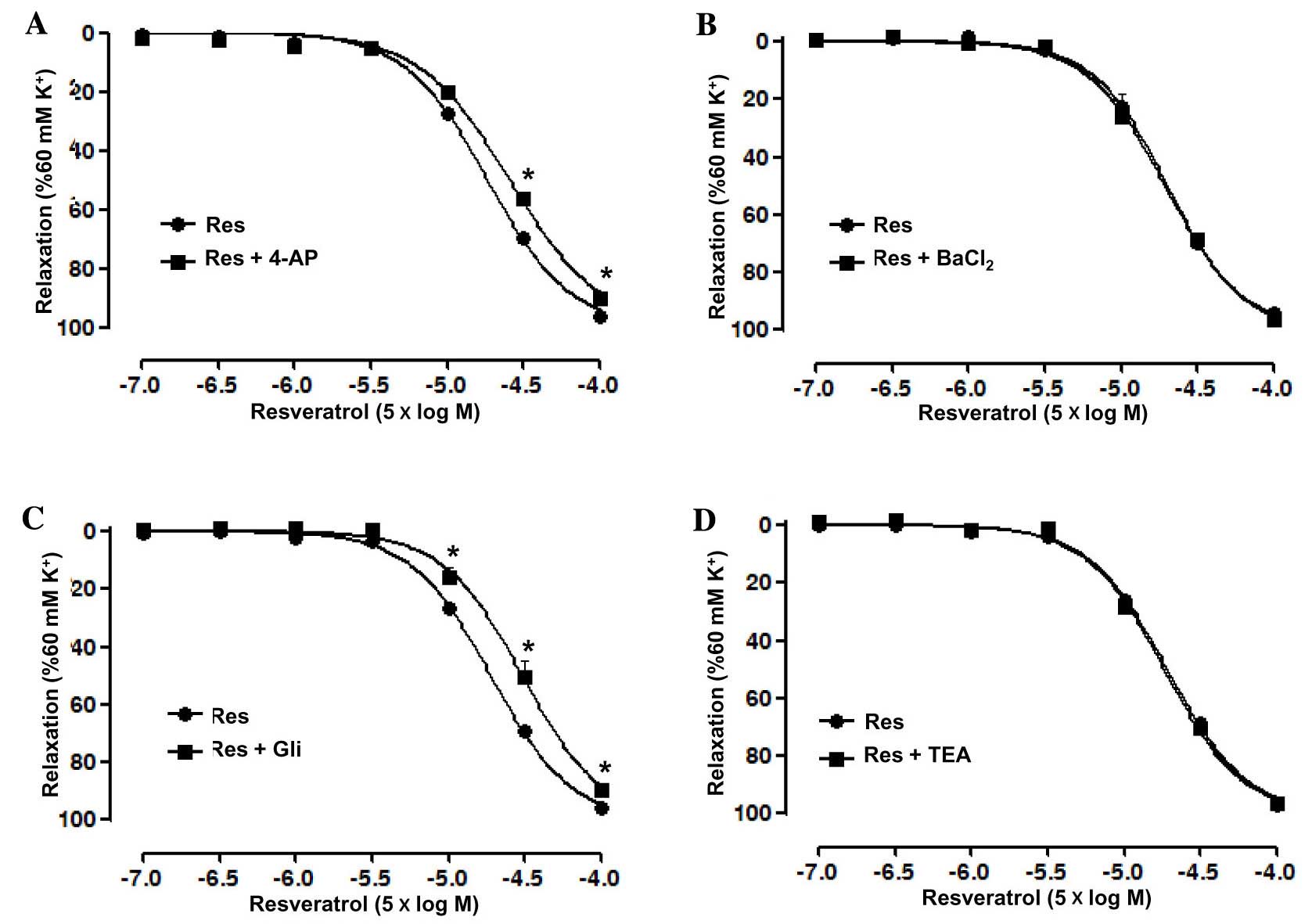
Role of K+ channels in the
Res-induced relaxation of rat superior mesenteric artery
pre-constricted by KCl
Artery rings without endothelium were pre-incubated
with the K+ channel blockers 4-AP, BaCl2, Gli
and TEA, independently, prior to treatment with KCl and Res in
order to investigate the role of K+ channels in the
Res-induced relaxation. The results showed that 4-AP significantly
reduced the relaxation induced by Res in the artery rings without
endothelium, with an Emax of 90.15±1.6 vs. 96.38±0.44%
in the control group and pD2 of 3.94±0.03 vs. 4.1±0.02
in the control group (P<0.05; Fig.
4A). However, BaCl2 did not significantly affect the
relaxation induced by Res in the artery rings without endothelium
(Fig. 4B). In addition, Gli also
significantly reduced the relaxation induced by Res in the artery
rings without endothelium, with an Emax of 89.75±1.24
vs. 95.82±0.49% in the control group and pD2 of
3.86±0.05 vs. 4.07±0.02 in the control group (P<0.05; Fig. 4C), whilst TEA, similar to
BaCl2, did not significantly affect the relaxation
induced by Res in the artery rings without endothelium (Fig. 4D).
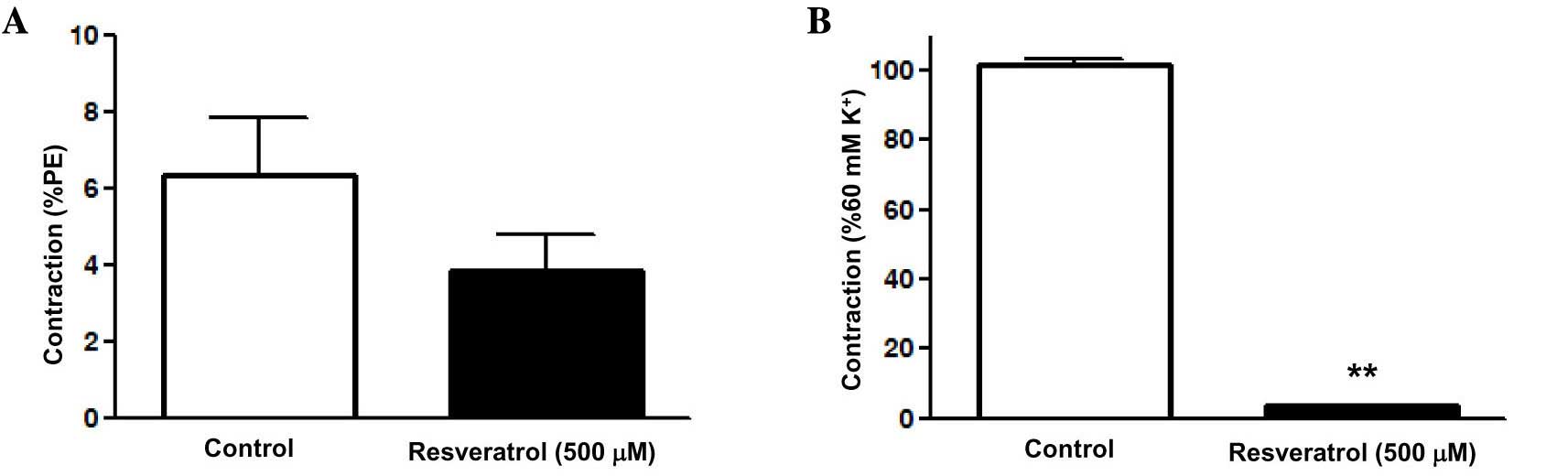
Effect of Res on calcium release by
the sarcoplasmic reticulum in rat superior mesenteric artery
pre-constricted by PE
Experiments were carried out in Ca2+-free
buffer to clarify whether the relaxation induced by Res was
associated with intracellular Ca2+ release. The results
showed that PE induced a transient contraction due to the release
of intracellular Ca2+ in the Ca2+-free
solution, with an Emax of 6.35±1.5%. Res attenuated the
contraction induced by PE, with an Emax of 3.85±0.95%;
however, the difference was not significant (Fig. 5A).
Effect of Res on extracellular
Ca2+-induced contraction in rat superior mesenteric
artery pre-constricted by KCl
Experiments were carried out in Ca2+-free
buffer to determine whether the inhibition of extracellular
Ca2+ influx is involved in the Res-induced relaxation of
artery rings without endothelium. The results showed that Res
significantly attenuated CaCl2-induced contraction in
the Ca2+-free PSS containing KCl (60 mM K+),
with an Emax of 3.6±0.31 vs. 101.4±1.79% in the control
group (P<0.01; Fig. 5B). This
suggests that Ca2+ influx was inhibited by Res in the
superior mesenteric artery.
Discussion
The present study found that Res
concentration-dependently relaxed superior mesenteric artery rings
with or without endothelium that had been pre-contracted using PE
or KCl. This suggests that Res induced vasorelaxation via
endothelium-dependent and-independent pathways. Moreover, the
vasorelaxation induced by Res was inhibited by L-NAME, and not
affected by ODQ or Indo in artery rings with endothelium. In
addition, the vasorelaxation induced by Res was inhibited by 4-AP
and Gli, and not affected by BaCl2 and TEA in artery
rings without endothelium. Finally, it was also found that the
vasorelaxation induced by Res was mediated through blockade of
Ca2+ influx from extracellular medium.
Vascular endothelium, occupying a location between
circulating blood and vascular smooth muscle, is considered to be
important in the regulation of vascular tone. The vasorelaxation is
mediated by relaxing substances synthesized in and released by the
endothelium (16). In the present
study, the relaxant effect induced by Res was attenuated in the
superior mesenteric artery rings without endothelium, suggesting
that Res relaxed the artery through an endothelium-dependent
pathway. The data showed that L-NAME (an eNOS inhibitor)
significantly reduced the vasorelaxation induced by Res. However,
Indo (a cyclooxygenase inhibitor) and ODQ (a guanylate cyclase
inhibitor) did not affect the action of Res. This suggests that
nitric oxide (NO) is involved in the relaxation of Res in the
superior mesenteric artery with endothelium, whereas the cGMP
pathway and prostanoids are not associated with this effect. This
finding is consistent with previous studies; in the abdominal
aorta, thoracic aorta and coronary artery, the efficacy of Res has
been found to be closely associated with the NO system in
endothelial cells (4,7,8,12).
In the present study, it was found that Res also
induced a relaxant effect in superior mesenteric artery without
endothelium, suggesting that Res has a direct effect on vascular
smooth muscle cells (VSMCs). The opening of K+ channels
in vsMCs causes membrane potential hyperpolarization, decreases
Ca2+ entry through voltage-operated Ca2+
channels, and induces vasorelaxation (17,18).
Several types of K+ channels have been identified in
vascular smooth muscle. The most abundant types include large
conductance Ca2+-activated K+ channels,
voltage sensitive K+ channels, ATP-sensitive
K+ channels and inward rectifying potassium channels
(19). In order to detect the
contribution of different types of K+ channels to the
endothelium-independent relaxation induced by Res in superior
mesenteric artery rings, agents that are known to possess
K+ channel-blocking activity, namely 4-AP (a
voltage-dependent K+ channel blocker), BaCl2
(an inward rectifying potassium channel blocker), Gli (an
ATP-sensitive K+ channel blocker) and TEA (a
Ca2+-activated K+ channel blocker) (20,21) were
used.
Previous studies have found that Res relaxes many
types of vascular beds without endothelium through the activation
of different types of K+ channels. The voltage-dependent
K+ channel plays an important role in the vasodilatation
induced by Res in the aorta (6) and
internal mammary artery (9),
whereas, the voltage-dependent K+ channel is involved in
the vasodilatation induced by Res in the thoracic aorta (6). In addition, Res has been shown to
induce relaxation of the abdominal aorta through activation of
ATP-sensitive K+ channels and Ca2+-activated
K+ channels (7). However,
Gojkovic-Bukarica et al found that K+
channel-independent mechanisms are involved in its vasorelaxant
effect in mesenteric arteries (10).
In the present study, both 4-AP and Gli significantly inhibited the
relaxant effect of Res, indicating that voltage-dependent
K+ channels and ATP-sensitive K+ channels are
involved in the relaxation of the superior mesenteric artery
induced by Res. However, neither BaCl2 nor TEA affected
the concentration-response curves of Res, suggesting that inward
rectifying K+ channels and Ca2+-activated
K+ channels are not involved in the Res-induced
relaxation.
Accumulation of intracellular calcium is involved in
vascular smooth muscle contraction. Moreover, both extracellular
Ca2+ influx, through voltage-dependent calcium channels
(VDCCs) or receptor-operated calcium channel (ROCCs), and
intracellular Ca2+ release result in an increase of the
intracellular calcium level (22).
Contractions of vsMCs induced by KCl rely almost exclusively on
Ca2+ influx induced by the activation of
voltage-sensitive channels (23),
whereas contractions induced by PE are mediated by an increase in
Ca2+ influx through both receptor-operated channels
(24) and voltage-sensitive channels
(25). The results of the present
study show that Res is able to inhibit the contractile effects
induced by PE or KCl on the superior mesenteric artery without
endothelium. This suggests that Res may exert effects on both VDCCs
and ROCCs. The release of intracellular stored Ca2+ is
mainly regulated by the inositol trisphosphate (IP3) receptor
system and the ryanodine receptor system (26). Contractions induced by PE in
Ca2+-free medium occur due to intracellular
Ca2+ release through Ca2+ channels in the
sarcoplasmic reticulum activated by IP3 (27). Previous studies have shown that Res
attenuates extracellular calcium influx and intracellular calcium
release, which results in vasodilatation in the abdominal aorta
(7) or thoracic aorta (8). However, Res has Ca2+
antagonistic properties and inhibits extracellular Ca2+
influx through VDCCs in coronary arteries (12). In the present study, it was found
that Res significantly inhibited CaCl2-induced
contraction in the superior mesenteric artery rings without
endothelium in Ca2+-free PSS containing KCl (60 mM),
indicating that Res exhibits Ca2+ entry blocking
activity. However, Res did not inhibit the contraction triggered by
PE in Ca2+-free PSS, suggesting that Res does not
inhibit Ca2+ mobilization from intracellular stores. In
the superior mesenteric artery, it appears that Res induced
vasorelaxation via the inhibition of extracellular calcium in
vsMCs.
In conclusion, the results of the present study
suggest that Res-induced relaxation of the rat superior mesenteric
artery occurs via an endothelium-dependent pathway involving NO
release, as well as an endothelium-independent pathway, with
opening of voltage-dependent K+ channels and
ATP-sensitive K+ channels, and blockade of extracellular
Ca2+ influx.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81500350) and China
Postdoctoral Science Foundation (grant no. 2015M582607).
Glossary
Abbreviations
Abbreviations:
|
PE
|
phenylephrine hydrochloride
|
|
ACh
|
acetylcholine chloride
|
|
4-AP
|
4-aminopyridine
|
|
BaCl2
|
barium chloride dehydrate
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
Gli
|
glibenclamide
|
|
Indo
|
indomethacin
|
|
L-NAME
|
NG-nitro-L-arginine methyl ester
|
|
NO
|
nitric oxide
|
|
ODQ
|
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
|
|
ROCC
|
receptor-operated calcium channel
|
|
Res
|
resveratrol
|
|
TEA
|
tetraethylammonium chloride
|
|
VDCC
|
voltage-dependent calcium channel
|
|
vsMCs
|
vascular smooth muscle cells
|
References
|
1
|
Koo SH and Montminy M: In vino veritas: A
tale of two sirt1s? Cell. 127:1091–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Yang YJ, Qian HY, Zhang Q, Xu H
and Li JJ: Resveratrol in cardiovascular disease: What is known
from current research? Heart Fail Rev. 17:437–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naderali EK, Doyle PJ and Williams G:
Resveratrol induces vasorelaxation of mesenteric and uterine
arteries from female guinea-pigs. Clin Sci (Lond). 98:537–543.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soylemez S, Gurdal H, Sepici A and Akar F:
The effect of long-term resveratrol treatment on relaxation to
estrogen in aortae from male and female rats: Role of nitric oxide
and superoxide. Vascul Pharmacol. 49:97–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taguchi K, Hida M, Matsumoto T and
Kobayashi T: Resveratrol ameliorates clonidine-induced
endothelium-dependent relaxation involving Akt and endothelial
nitric oxide synthase regulation in type 2 diabetic mice. Biol
Pharm Bull. 38:1864–1872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Novakovic A, Bukarica LG, Kanjuh V and
Heinle H: Potassium channels-mediated vasorelaxation of rat aorta
induced by resveratrol. Basic Clin Pharmacol Toxicol. 99:360–364.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen M, Zhao L, Wu RX, Yue SQ and Pei JM:
The vasorelaxing effect of resveratrol on abdominal aorta from rats
and its underlying mechanisms. Vascul Pharmacol. 58:64–70. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang HY, Xu CQ, Li HZ, Li BX, Zhang YQ
and Zhang YN: Effects of resveratrol on isolated thoracic aorta
rings of rats. Zhongguo Zhong Yao Za Zhi. 30:1283–1286. 2005.(In
Chinese). PubMed/NCBI
|
|
9
|
Novakovic A, Gojkovic-Bukarica L, Peric M,
Nezic D, Djukanovic B, Markovic-Lipkovski J and Heinle H: The
mechanism of endothelium-independent relaxation induced by the wine
polyphenol resveratrol in human internal mammary artery. J
Pharmacol Sci. 101:85–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gojkovic-Bukarica L, Novakovic A, Kanjuh
V, Bumbasirevic M, Lesic A and Heinle H: A role of ion channels in
the endothelium-independent relaxation of rat mesenteric artery
induced by resveratrol. J Pharmacol Sci. 108:124–130. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naderali EK, Smith SL, Doyle PJ and
Williams G: The mechanism of resveratrol-induced vasorelaxation
differs in the mesenteric resistance arteries of lean andobese
rats. Clin Sci (Lond). 100:55–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li HF, Tian ZF, Qiu XQ, Wu JX, Zhang P and
Jia ZJ: A study of mechanisms involved in vasodilatation induced by
resveratrol in isolated porcine coronary artery. Physiol Res.
55:365–372. 2006.PubMed/NCBI
|
|
13
|
Mathiassen ON, Buus NH, Sihm I, Thybo NK,
Mørn B, Schroeder AP, Thygesen K, Aalkjaer C, Lederballe O, Mulvany
MJ and Christensen KL: Small artery structure is an independent
predictor of cardiovascular events in essential hypertension. J
Hypertens. 25:1021–1026. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao YX, Zhang W, He JY, He LC and Xu CB:
Ligustilide induces vasodilatation via inhibiting voltage dependent
calcium channel and receptor-mediated Ca2+influx and
release. Vascul Pharmacol. 45:171–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu XM, Fang LH, Li YJ and Du GH:
Endothelium-dependent and -independent relaxation induced by
pinocembrin in rat aortic rings. Vascul Pharmacol. 46:160–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubanyi GM: The role of endothelium in
cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol.
22:(Suppl 4). S1–S14. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nelson MT and Quayle JM: Physiological
roles and properties of potassium channels in arterial smooth
muscle. Am J Physiol. 268:C799–C822. 1995.PubMed/NCBI
|
|
18
|
Bolotina VM, Najibi S, Palacino JJ, Pagano
PJ and Cohen RA: Nitric oxide directly activates calcium-dependent
potassium channels in vascular smooth muscle. Nature. 368:850–853.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jackson WF: Potassium channelsin the
peripheral microcirculation. Microcirculation. 12:113–127. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brayden JE: Potassium channels in vascular
smooth muscle. Clin Exp Pharmacol Physiol. 23:1069–1076. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vergara C, Latorre R, Marrion NV and
Adelman JP: Calcium-activated potassium channels. Curr Opin
Neurobiol. 8:321–329. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horowitz A, Menice CB, Laporte R and
Morgan KG: Mechanisms of smooth muscle contraction. Physiol Rev.
76:967–1003. 1996.PubMed/NCBI
|
|
23
|
Hirata S, Enoki T, Kitamura R, Vinh VH,
Nakamura K and Mori K: Effects of isoflurane on receptor-operated
Ca2+ channels in rat aortic smooth muscle. Br J Anaesth.
81:578–583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee CH, Poburko D, Sahota P, Sandhu J,
Ruehlmann DO and van Breemen C: The mechanism of
phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic
contraction in the rabbit inferior vena cava. J Physiol.
534:641–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CN, Wong KL, Liu JC, Chen YJ, Cheng JT
and Chan P: Inhibitory effect of stevioside on calcium influx to
produce antihypertension. Planta Med. 67:796–799. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka Y and Tashjian AH Jr: Thimerosal
potentiates Ca2+ release mediated by both the inositol
1,4,5-trisphosphate and the ryanodine receptors in sea urchin eggs.
Implications for mechanistic studies on Ca2+ signaling.
J Biol Chem. 269:11247–11253. 1994.PubMed/NCBI
|
|
27
|
Eckert RE, Karsten AJ, Utz J and Ziegler
M: Regulation of renal artery smooth muscle tone by
alpha1-adrenoceptors: Role of voltage-gated calcium channels and
intracellular calcium stores. Urol Res. 28:122–127. 2000.
View Article : Google Scholar : PubMed/NCBI
|