Introduction
The application of mesenchymal stem cells (MSCs) as
a cell therapy for various diseases has been extensively
investigated. MSCs possess high proliferative activity and are able
to differentiate into various cell types (1,2). The
main advantage of MSCs for clinical application is the potential
for using a patient's own cells (autologic MSCs) for
transplantation (1,2). This reduces the risks of rejection and
undesirable immunological reactions. For a long time, bone marrow
has been the main source of MSCs. To date, MSCs have been
established from adipose tissue (3),
umbilical cord blood (4), amniotic
fluid (5) and endometrium (6,7).
However, the methods used to harvest cellular material from bone
marrow and adipose tissue are painful and can be dangerous for
donors. In addition, cells from the umbilical cord can only be
obtained from newborns.
Human endometrium, which is composed of endometrial
glands lined with stromal cells, is a dynamic tissue that undergoes
~400 cycles of regeneration, differentiation and shedding (8). It has previously been demonstrated that
endometrium fragments in menstrual blood are a source of
endometrial MSCs (eMSC); thus menstrual blood represents a
noninvasive and readily available source of MSCs (9,10). Their
high proliferative ability during long-term cultivation, genetic
stability (11), lack of
tumorigenicity and low immunogenicity (12) makes the eMSCs from menstrual blood a
promising source of stem cells for future clinical
applications.
During pregnancy, specific morphological and
biochemical changes, known as decidual reactions, occur in the
endometrium. Decidualization of the endometrium is an essential
process for embryo implantation, placenta formation and maintenance
of pregnancy. Therefore, during all terms of pregnancy, loss of the
decidual reactions of endometrial cells may cause miscarriages and
fetal growth delay (13,14). Insufficient decidualization of the
endometrium may lead to infertility and pathologies such as
Asherman's syndrome and endometrium atrophy (15,16).
Currently, Asherman's syndrome is treated by surgery, followed by
cyclic hormonal therapies over the subsequent 3–6 months. Previous
studies have investigated the possibility of using stem cell
therapy to correct endometrium failure (17,18). In
these efforts, stem cells derived from bone marrow were used. In
our previous study, it was demonstrated that eMSCs transplanted
into pseudopregnant rats facilitated the development of all
elements of decidual tissue (19).
Cell therapy typically requires substantial cell
biomass. The initial content of MSCs in tissues is usually very
low; thus, in order to obtain sufficient biomass, MSCs must be
expanded in culture. However, cultivation of somatic cells may be
accompanied by significant changes to their properties, including
malignant transformation. Therefore, the cells used in clinical
applications should be carefully evaluated for their safety
(1).
The majority of researchers consider that MSCs do
not undergo spontaneous transformation during cultivation (1,11).
However, the results of previous studies have been controversial.
Mouse MSCs were easily immortalized and transformed during
long-term cultivation (20,21), whereas the long-term growth of human
bone marrow MSCs was not accompanied by transformation (22). Previous reports on the spontaneous
transformation of human stem cells from adipose tissue (23), bone marrow (24) and neuronal stem cells (25) are questionable. The first two papers
were retracted as, in both cases, stem cell cultures had been cross
contaminated with cells derived from immortal cell lines (26). Therefore, it was more likely that
cross-contamination with HeLa cells, and not spontaneous
transformation, had taken place. Although the spontaneous
transformation of human stem cells has not been verified, these
papers continue to be cited (1).
The use of MSCs with irreversibly arrested
proliferation may reduce the oncogenic risks of transplanted cells.
The present study aimed to investigate the effect of human eMSCs
with arrested proliferation on the decidual differentiation of the
endometrium in a rat model of pseudopregnancy. The proliferation of
eMSCs was blocked using mitomycin C exposure or ionizing radiation
(IR), both of which are well known inhibitors of the cell cycle
(27–30).
Materials and methods
eMSC derivation and cultivation
eMSCs were isolated, as described previously
(31). Briefly, menstrual blood
containing endometrium fragments was obtained from three female
volunteers aged 27 years. Written informed consent was obtained
from all donors. A total of 2 ml menstrual blood was collected on
the second day of the menstrual cycle and centrifuged at 400 × g
for 5 min at room temperature. The resulting pellet containing the
endometrium fragments was resuspended in phosphate-buffered saline
(PBS) supplemented with 10% antibiotic-antimycotic mixture,
incubated for 1 h at 37°C and centrifuged at 400 × g for 5 min at
room temperature. Subsequently, the cell pellet was resuspended in
Dulbecco's modified Eagle's medium/F12 medium (DMEM) supplemented
with 10% fetal calf serum (FCS; GE Healthcare Life Sciences, Logan,
UT, USA), 1% antibiotic-antimycotic mixture and 1% glutamax, and
seeded into 6-cm Petri dishes (Corning Incorporated, Corning, NY,
USA) at a cell density of 2×104 cells/cm2.
Cells were cultivated for 3–7 days at 37°C, during which the medium
was exchanged several times to ensure that only adhesive cells
formed the culture.
Immunophenotyping
Immunophenotyping of eMSCs [cluster of
differentiation (CD) marker expression] was performed using an
Epics XL flow cytometer (Beckman Coulter, Inc., Brea, CA, USA). A
single cell suspension was obtained using 0.05% trypsin/EDTA. Cells
(1×106) were suspended in 1 ml PBS containing 5% FCS.
Subsequently, according to the manufacturer's instructions, the
cells were incubated at room temperature for 30 min with the
following mouse anti-human fluorescein isothiocyanate-conjugated
monoclonal antibodies: anti-CD34 (555821; 1:250), CD45 (555482;
1:250) and CD90 (555595; 1:250), and phycoerythrin-conjugated
monoclonal antibodies: Anti-CD19 (557835; 1:250), CD73 (561014;
1:200), CD105 (560839; 1:250), CD146 (561013; 1:200) and human
leukocyte antigen (HLA)-DR (555812; 1:200; all BD Pharmingen, San
Diego, CA, USA). All antibodies were pre-diluted for use according
to the recommended volume per test. For each test, 1×106
cells in 100 µl PBS were used. For CD73, CD146 and (HLA)-DR the
volume per test was 20 µl. Cells were analyzed by flow
cytometry.
Immunocytochemistry
Immunofluorescent staining for nestin was performed
according to a standard protocol (31). Briefly, the cells were incubated with
rabbit anti-nestin polyclonal antibody (1:100; AB5922; EMD
Millipore, Billerica, MA, USA), followed by incubation with cyanine
2-conjugated goat anti-rabbit (1:300; 111-225-144) or DyLight
488-conjugated goat anti-rabbit (1:400; 111-545-003) antibodies
(both Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA). Cells were then observed under a fluorescent microscope.
Adipogenic differentiation
Cells (2×104/cm2) were seeded
into Petri dishes coated with 0.1% gelatin (Sigma-Aldrich, St.
Louis, MO, USA). When the cells reached ~80% confluence, 1 mM
dexamethasone (Sigma-Aldrich), 0.5 mM isobutylmethylxanthine
(Sigma-Aldrich), 10 µg/ml human recombinant insulin (Sigma-Aldrich)
and 100 mM indometacin were added to the cells. Cells were
cultivated at 37°C in the differentiation medium for 5 days, with
half of the medium replaced every other day. Under these
conditions, the cells had been differentiated for 3–5 weeks. Lipid
drops were visualized with Oil Red staining (Sigma-Aldrich),
according to the manufacturer's protocol.
Osteogenic differentiation
Cells (2×104 cells/cm2) were
seeded into Petri dishes coated with 0.1% gelatin. After the cells
had reached 100% confluence, 100 nM dexamethasone, 10 mM β-glycerol
phosphate and 0.2 mM ascorbate 2-phosphate were added to the cells.
In this medium, the cells were differentiated at 37°C for 3–5
weeks, with half of the medium changed every 2–3 days.
Subsequently, the cells were fixed with 70% cold ethanol for 1 h,
stained with Alizarin Red (pH 4.1; Sigma-Aldrich) and observed
under a light microscope.
Mitomycin C treatment of eMSCs
eMSCs between the third and fourth passages were
treated with 10 µg/ml mitomycin C (Sigma-Aldrich) for 1.5 h.
Treated cells were then washed with PBS three times, resuspended in
DMEM supplemented with 10% FCS, 1% antibiotic antimycotic mixture
and 1% glutamax, and seeded (1×105 cells) into 35-mm
Petri dishes.
Irradiation of eMSCs
eMSCs (105) between the third and fourth
passages were seeded into 35-mm Petri dishes. Subsequently, the
cells were exposed to 3, 6 and 10 Gy doses of IR using a stationary
X-ray device (0,49 Gy/min).
Cell growth kinetics
Cell growth properties were assessed by generating
growth curves. eMSCs (105) were seeded into 35-mm Petri
dishes, and the number of cells were counted daily using a
Goryaev's chamber for 4 days. Two plates were used for each
measurement and assays were performed in triplicate.
Cell cycle distribution analysis
Adherent cells were rinsed with PBS, harvested using
trypsin-EDTA solution and resuspended in PBS at 1×108
cells/ml. Subsequently, 200 µg/ml saponin, 250 µg/ml RNase A and 50
µg/ml propidium iodide (PI; all Sigma-Aldrich) were added to each
sample tube. Following incubation for 60 min at room temperature,
the samples were analyzed using an Epics XL flow cytometer. Cell
cycle distribution analysis was performed using WinMDI 2.8
(http://winmdi.software.informer.com/2.8/) and ModFit
LT 4.1 software (http://www.vsh.com/products/mflt/index.asp).
Assessment of cell viability
Cell viability was evaluated by flow cytometry using
PI staining. Briefly, 50 µg/ml PI was added to each sample and
mixed gently. Samples were analyzed using the Epics XL flow
cytometer.
Animal model
A total of 48 adult female albino rats weighing
200–250 g were purchased from Rappolovo Animal Farm (St.
Petersburg, Russia) and maintained at the Institute of Cytology
(Russian Academy of Sciences, St. Petersburg, Russia) animal care
facility at 24°C with free access to food and water and a 14/10-h
dark/light cycle, according to the institutional guidelines for the
care and use of laboratory animals. Vaginal cytological analyses
were performed to assess the estrous cycle. Briefly, a sterile swab
was moistened with saline and rotated against the vaginal wall to
obtain rat vaginal cells. Vaginal smears were visualized under a
light microscope. Pseudopregnancy and an artificial decidual
response was induced by electrical stimulation of the cervix during
estrus. On the fifth day following stimulation, the rats were
anesthetized via an intramuscular injection of Zoletil 100 (5 mg/kg
body weight; Virbac, Carros, France) and surgical procedures were
performed under aseptic conditions. Briefly, the rats were placed
in the dorsal position and double 1.5-cm incisions into the skin
and muscles, lateral to the vertebrae, were made. Subsequently, the
uterine horns were carefully removed to avoid any trauma.
Rats were divided into four groups (n=12). In the
first group, a normal eMSC (5×105) single cell
suspension in 20 µl PBS and 20 µl PBS without cells was injected
into the experimental uterine horn and the contralateral control
horn, respectively. In the second group, eMSCs were fixed with 95%
ethanol at −20°C for 20 min and washed three times with PBS
solution. Subsequently, 5×105 single cell suspensions of
ethanol-fixed eMSCs in 20 µl PBS and 20 µl PBS without cells were
injected into the experimental uterine horn and the contralateral
control horn, respectively. In the second and third groups, a
single cell suspension of eMSC (5×105) previously
treated with mitomycin C (group 3) and 6 Gy irradiated (group 4) in
20 µl PBS and 20 μl PBS without cells were injected into the
experimental uterine horn and the contralateral control horn,
respectively.
Rats were sacrificed via cervical dislocation
following administration of diethyl ether (80 mg/kg body weight;
Ural Profchem Co., Nizhniy Tagil, Russia) on day 11 of
pseudopregnancy. To estimate deciduas development in the
experimental and control uterine horns, decidual tissue was
collected and weighed.
Histological analysis
Frozen 10-µm sections of formed deciduas were
generated and mounted onto slides. Slides were fixed in an
ethanol/methanol mixture for 2 min at −20°C, followed by staining
with hematoxylin and eosin. The differentiation extent of the
decidua, structural alterations in the decidual tissue, and the
presence of inflammation and necrosis were assessed by light
microscopy.
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± standard deviation, when
indicated. Statistical significance was evaluated by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
eMSC properties
In our previous study, endometrial MSCs were
established from desquamated endometrium in menstrual blood
(31). The present study established
a novel MSC line from endometrium fragments in menstrual blood, and
investigated its potential application for the treatment of
infertility.
eMSCs obtained from menstrual blood in the present
study met the criteria for human multipotent stromal MSCs suggested
by the International Society for Cellular Therapy. eMSCs were
adhesive to plastic under standard culture conditions, had a
fibroblast-like shape and formed a monolayer with a typical round
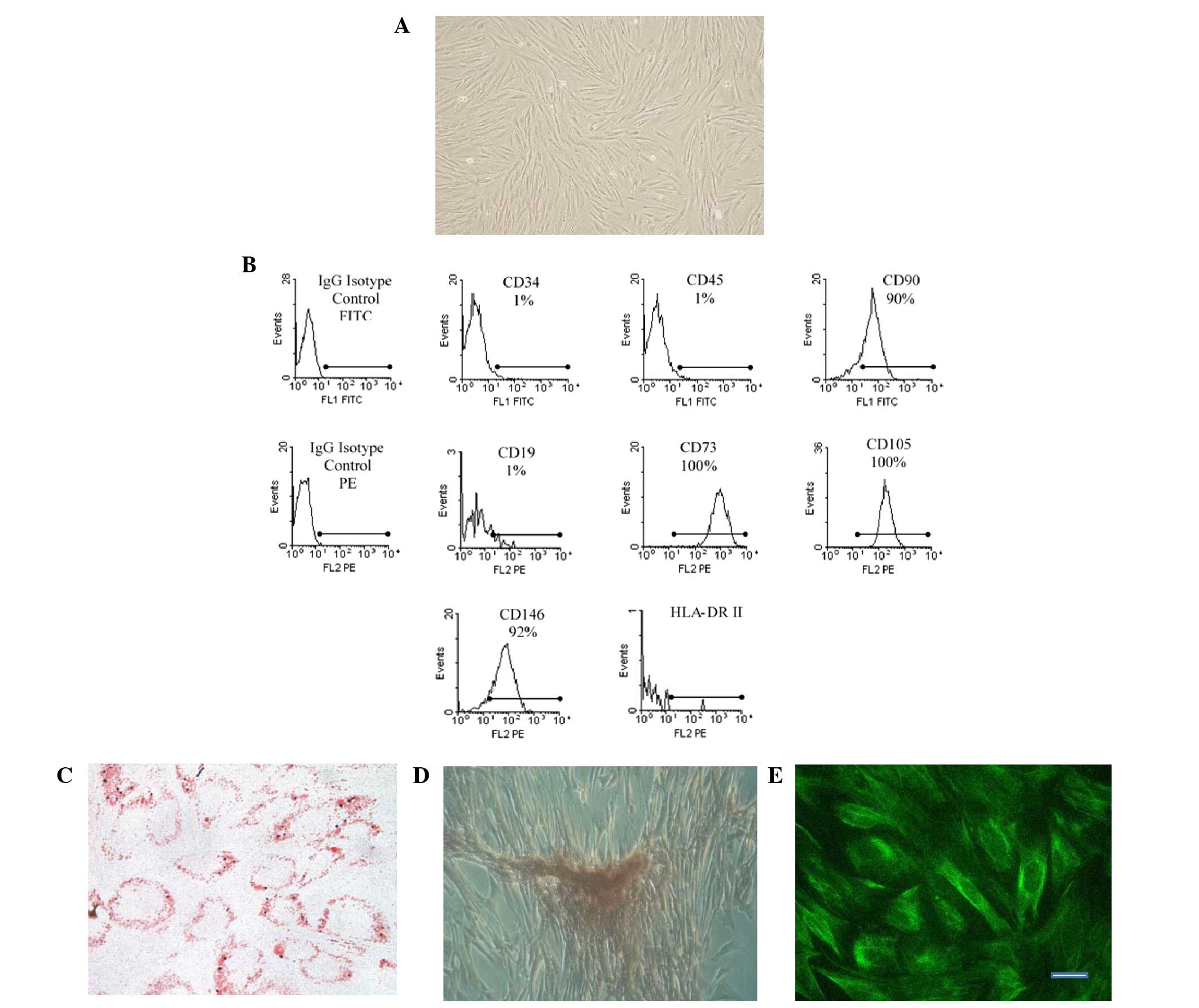
swirling pattern (Fig. 1A). In
addition, the eMSCs were positive for CD146, CD105, CD73 and CD90
expression, but negative for CD19, CD34, CD45 and HLA-DR (class II)
expression (Fig. 1B). Furthermore,
the cells were multipotent and able to differentiate into mesoderm
lineages, including osteoblasts and adipocytes (Figs. 1C and D). Immunostaining for nestin,
a neural cell marker, was positive (Fig.
1E).
eMSCs have a high level of proliferation activity,
with a doubling time of 22–23 h. Cells undergo >45 population
doublings during culture before this phase is terminated in favor
of dividing and entering into replicative senescence. Replicative
senescence is a common feature of normal human MSCs cultivated
in vitro. As a noninvasive, easily accessible cell source
with a high proliferation activity (higher than bone marrow and
umbilical cord blood MSCs), multipotency and ability to undergo
long-term cultivation without developing karyotypic abnormalities,
MSCs derived from endometrium may be considered to be a promising
stem cell source for cell therapy.
IR and mitomycin C treatment induce
the irreversible cell cycle arrest of eMSCs
Mitomycin С belongs to a class of compounds that
cause Gl and G2 cell cycle arrest by activating a DNA-damage
checkpoint in the cell cycle, resulting in the inhibition of
cell-cycle progression and activation of DNA-repair machinery
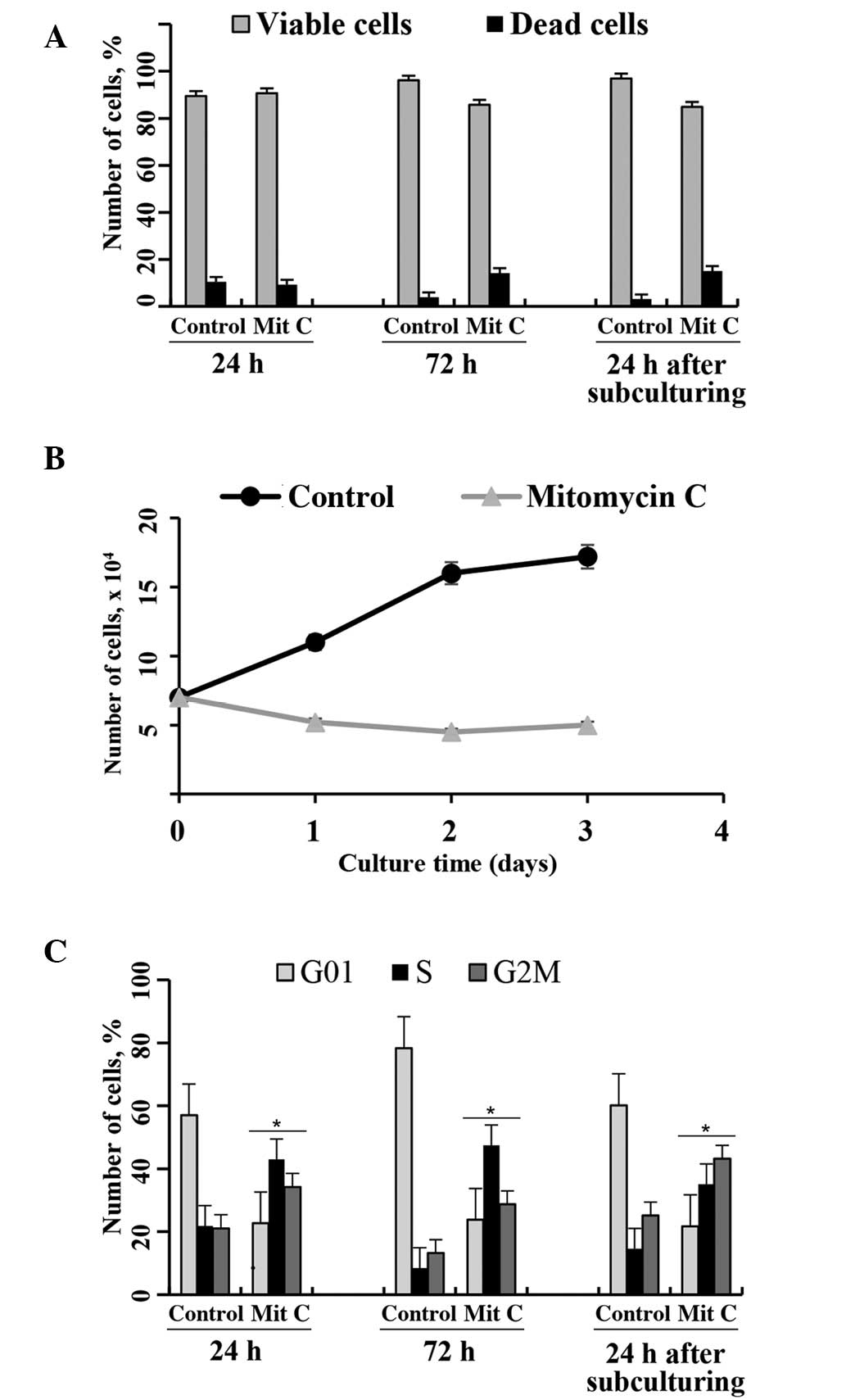
(29,30). In the present study, the
proliferation of eMSCs was inhibited by treatment with 10 µg/ml
mitomycin С for 1.5 h (Fig. 2).
Mitomycin С-treated eMSCs maintained a normal adherent cell
morphology (data not shown). The cell viability of mitomycin
C-treated eMSCs, as assessed by flow cytometry using PI-staining
(Fig. 2A), was only slightly
altered, as compared with the untreated cells. However, the growth
of eMSCs was entirely suppressed following treatment with mitomycin
C. Fig. 2B shows growth curves for
normal and mitomycin C-treated cells. The number of eMSCs following
treatment with mitomycin C slightly declined over time.
Irreversible cell cycle arrest was confirmed by cell cycle
distribution analyses via flow cytometry. Fig. 2C shows that mytomycin C-treated
cells, unlike normal eMSCs, accumulated in the S and G2/M phases of
the cell cycle, and that cell cycle arrest was not overcome
following subculturing of the cells.
IR was also used to induce cell cycle arrest. The
present study aimed to identify the minimal IR dose that was able
to induce eMSC proliferation arrest without altering the viability
of the cells. Previous studies have demonstrated that MSCs from
different tissue sources are relatively resistant to IR exposure
(27,28,32,33). In
addition, no significant apoptosis induction was observed in MSCs
exposed to IR doses up to 10 Gy (27).
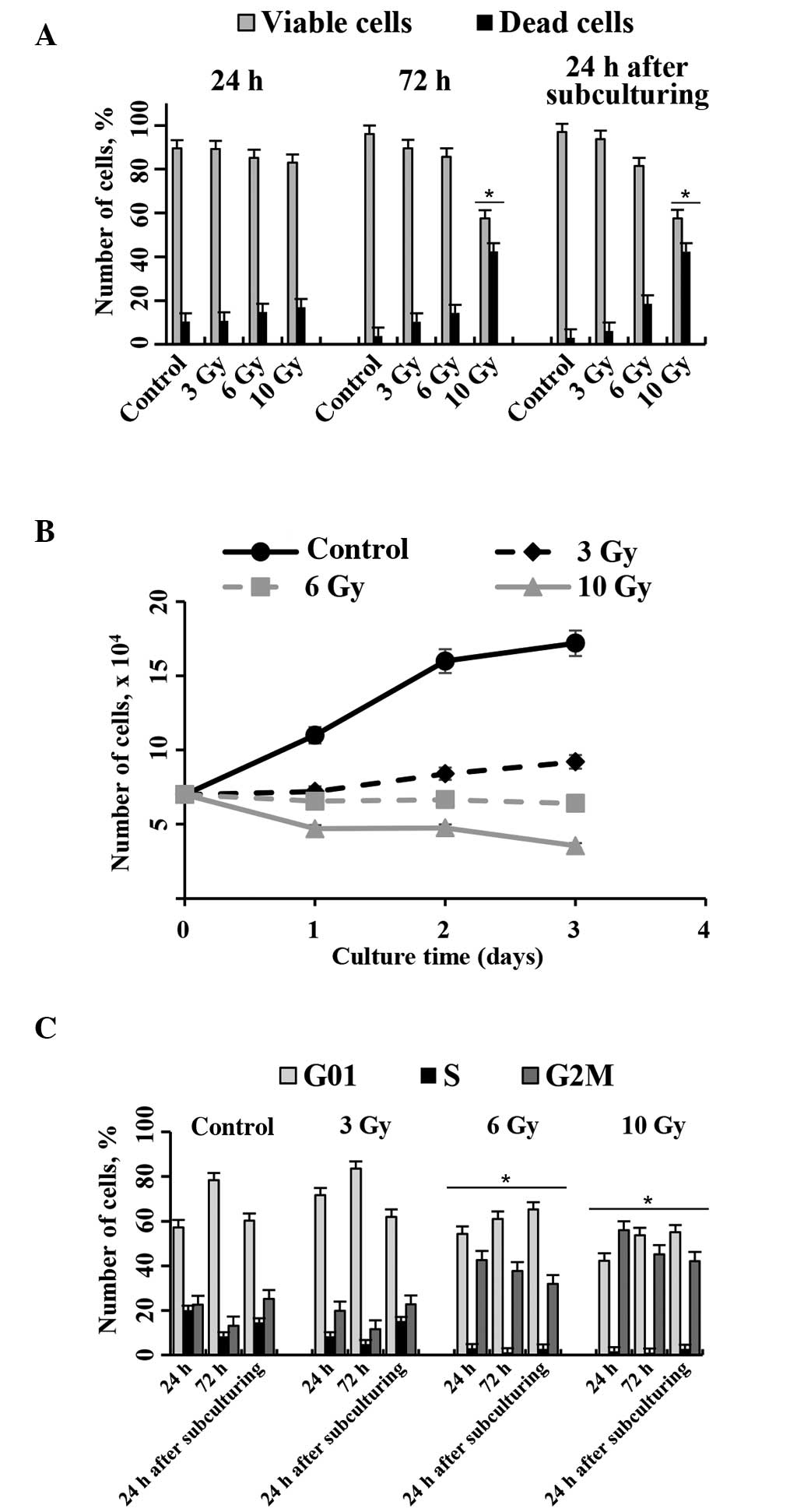
On the basis of existing literature, the present
study selected three IR doses (3, 6 and 10 Gy) to assay the cell
cycle distribution and cell viability at 24 and 72 h following
irradiation, and 24 h after subculture of the MSCs. Fig. 3 shows the viability and proliferation
status of eMSCs exposed to 3, 6 and 10 Gy IR for various durations.
The viability of eMSCs irradiated with 3 and 6 Gy doses was not
significantly different, as compared with normal eMSCs. Conversely,
irradiation with 10 Gy markedly reduced the number of viable cells
(Fig. 3A).
Cell growth curves (Fig.
3B) show that the eMSCs irradiated with 3 and 6 Gy exhibited
cell division arrest, and the number of eMSCs gradually reduced
following treatment with 10 Gy. The growth properties of irradiated
eMSCs were also assessed by cell cycle distribution analyses via
flow cytometry. In all groups, >50% of cells were in the peak
corresponding to the G1/G0 phases at 24 h following IR exposure.
Percentage of irradiated eMSCs located in the S phase varied from
1.06 to 7.75%, which was markedly lower than in normal eMSCs
(21.15%). The main difference between the groups was the number of
cells in the G2/M phase. At 24 h following eMSC exposure to 3 Gy,
the percentage of cells in the G2/M phase was not significantly
different, as compared with the normal eMSCs. Conversely, the cells
irradiated with 6 and 10 Gy were preferentially accumulated in the
G2/M phase (45.2 and 55.4%, respectively).
At 72 h, the number of normal and 3 Gy-irradiated
eMSCs in the S and G2/M phases were decreased, and those in the
G0/G1 phase were increased. During this period, the normal eMSCs
had reached confluency and had ceased to proliferate. Conversely,
eMSCs irradiated with 6 and 10 Gy exhibited unaltered phase ratios
between 24 and 72 h.
On day 4 following irradiation, normal and
irradiated cells were subcultured to assess their capacity for
proliferation. At 24 h following subculturing, normal and 3
Gy-irradiated eMSCs exhibited increased numbers of cells in the S
and G2/M phases (Fig. 3C). These
results suggested that eMSCs exposed to 3 Gy had recovered
following irradiation-induced stress. Conversely, no changes in the
cell cycle distribution were observed in cells exposed to 6 and 10
Gy. No proliferation for these cells was observed at 24 h after
passaging. These results suggest that irradiation of eMSCs with 6
Gy to induce irreversible cell cycle arrest while maintaining cell
viability is the optimum approach for experiments on
transplantation.
Malignant transformation is a potential risk of cell
therapy (34). Although it has been
widely accepted that MSCs cultured in vitro do not undergo
malignant transformation, it cannot be concluded that they will not
undergo malignant transformation within humans (1,34). In
vivo conditions may alter the regulation of proliferation in
transplanted cells. Therefore, the transplantation of MSCs that
remain viable but have lost their ability to divide may
significantly reduce their oncogenic potential.
Various preconditioning (pretreatment) strategies
have been tested on various stem cells and progenitor cells to
enhance transplanted cell viability and function (35). Stem cells and progenitors
preconditioned with growth factors, including transforming growth
factor-α, insulin-like growth factor-1 and fibroblast growth
factor-2, pharmacological agents or ischemia/hypoxia have exhibited
improved survival, increased neuronal differentiation, enhanced
paracrine effects that lead to increased trophic support, and
improved homing to the lesion site (36). The present study focused on cell
preconditioning that decreased the oncogenic risk of transplanted
cells.
eMSC transplantation into
pseudopregnant rats
The present study investigated the capacity of eMSCs
with arrested proliferation to stimulate decidual tissue
development in a rat model of pseudopregnancy. In our previous
study, the effect of intact human eMSCs on decidualization
processes was analyzed using pseudopregnant rats (19). It was demonstrated that inoculation
of human eMSC suspension into the uterus facilitated the
development of decidual tissue in pseudopregnant rats, as compared
with control PBS injection. Transplantation of rat bone marrow
cells into the same model gave similar results, which suggested
that the effect of transplanted human eMSCs was not xenogeneic.
The present study compared the development of
decidual tissue in rats transplanted with normal eMSCs and those
transplanted with eMSCs with irreversibly arrested proliferation.
eMSC proliferation was blocked by treatment with mitomycin C or IR.
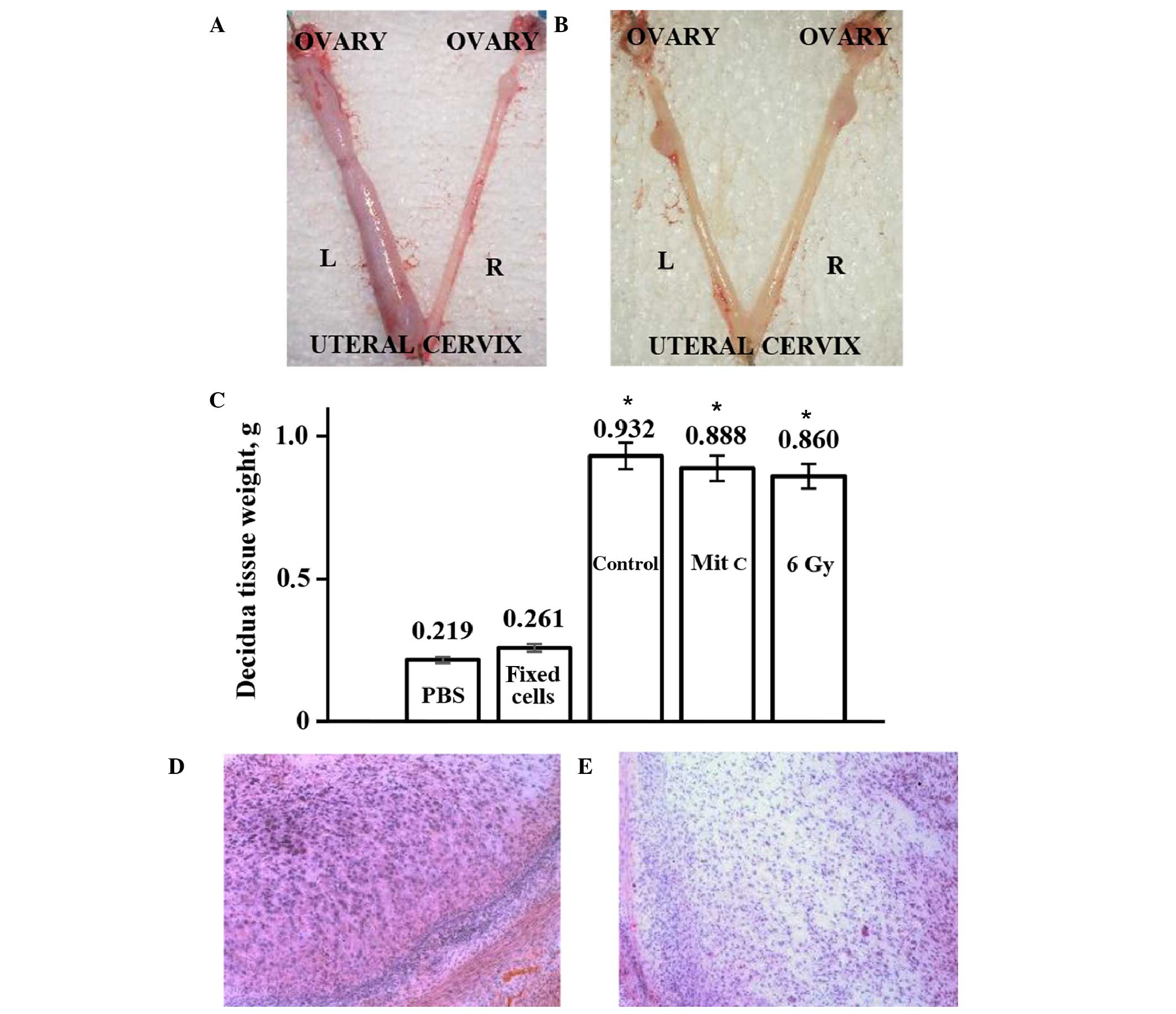
Decidua development on day 11 of pseudopregnancy was more visible
in the uterine horns transplanted with the human eMSCs with
arrested proliferation, as compared with the horns injected with
PBS control (Fig. 4A). In order to
verify that only viable cells are able to promote decidualization,
eMSCs killed with 95% ethanol were transplanted into pseudopregnant
rats. Fig. 4B shows that, unlike
viable eMSCs, there was no difference in size between the decidual
tissues derived from the experimental and control horns of rats
transplanted with non-viable eMSCs or injected with PBS. Visible
differences in the sizes of the experimental and control horns were
quantitated by weighing the isolated decidual tissue (Fig. 4C). The weight of decidual tissue from
the experimental horns was significantly increased, as compared
with the tissue from the control horns. These results suggested
that transplantation of rats with eMSCs with arrested proliferation
stimulated decidualization to the same extent as normal cells.
Histological analysis of decidua tissue did not detect any changes
in cell differentiation or tissue structure following
transplantation of normal or treated human eMSCs into the uterus of
pseudopregnant rats (Fig. 4D and E).
Rodent decidual tissue is formed by large decidual cells (LDCs),
small decidual cells and endometrial granulated cells (13). Huge polygonal LDCs of decidua are
shown in Fig. 4D, and Fig. 4E shows part of the decidua composed
of small decidual cells. The percentage of the total decidua that
consisted of LDCs only was 30–40%. Following transplantation, the
LDC zone ratio in the decidua section was not altered. These
results suggested that transplantation does not modify the tissue
structure; the increase in the decidua size resulted from intensive
development of all elements of decidual tissue. Furthermore, no
leukocyte infiltration into sites of transplanted cells was
observed (Fig. 4D and E).
The loss of decidual reactions in endometrial cells
is one of the reasons for miscarriages and fetal growth delay at
all terms of pregnancy (15). The
insufficient decidualization of the endometrium leads to
infertility in such pathologies as Asherman's syndrome and
endometrium atrophy. The incidence of Asherman's syndrome in women
who have undergone a hysteroscopy is 1.55%, and 39% in women who
have had recurrent miscarriage (16). At present, Asherman's syndrome is
treated by surgery, followed by cyclic hormonal therapies during
the subsequent 3–6 months. However, it remains a challenging
disease to treat. A few previous studies, including several animal
models of Asherman's syndrome (37–39),
have investigated the application of cell therapy to the treatment
of endometrium-determined infertility. In addition, reports on the
successful application of cell therapy to Asherman's syndrome
treatment have been published (17,18). The
authors described a clinical case of Asherman's syndrome in which
intrauterine injection of autologous bone marrow cells led to an
increase in the thickness of the patient's endometrium (18). However, to the best of our knowledge,
no previous study has investigated the effect of using endometrial
cells for the recovery of endometrial disorders, either in animal
models or clinical trials.
In conclusion, the present study demonstrated that
transplantation of human eMSCs with arrested proliferation into
pseudopregnant rats facilitated the development of all elements of
decidual tissue. Rats were used to model the endometrial
transformation into decidual tissue during the normal pregnancy
process. In addition, it was demonstrated that human eMSCs exposed
to mitomycin C (10 µg/ml) or IR (6 Gy) remained viable but
irreversibly lost their proliferation capacity, as assessed by
growth curves and flow cytometry. These results suggested that
preconditioning eMSCs with division blocking agents may diminish
their oncogenic potential in vivo. Transplantation of eMSCs
killed by ethanol treatment did not promote the decidualization
process, thus suggesting that viable MSCs may be required. The
results of the present study supported the application of eMSCs to
the cell therapy of infertility associated with decidualization
insufficiency.
Acknowledgements
The authors would like to thank the Russian Science
Foundation (grant no. 14-50-00068) and Russian Federal Agency of
Scientific Organizations for their financial support. Professor
Irina Fridlyanskaya and Dr Polina Novikova are grateful to the RAS
Presidium program of ‘Fundamental Sciences for Medicine’ for the
support.
References
|
1
|
Wang S, Qu X and Zhao RC: Clinical
applications of mesenchymal stem cells. J Hematol Oncol. 5:19–20.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Husein KS and Thiemermann C: Mesenchymal
stromal cells: current understanding and clinical status. Stem
Cells. 28:585–596. 2010.PubMed/NCBI
|
|
3
|
Parker AM and Katz AJ: Adipose derived
stem cells for the regeneration of damaged tissues. Expert Opin Bio
Ther. 6:567–578. 2006. View Article : Google Scholar
|
|
4
|
Harris DT, Badowski M, Ahmad N and Gaballa
MA: The potential of cord blood stem cells for use in regenerative
medicine. Exper Opin Biol Ther. 7:1311–1322. 2007. View Article : Google Scholar
|
|
5
|
Coppi P, De Bartsch G Jr, Siddiqui MM, Xu
T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ,
et al: Isolation of amniotic stem cell lines with potential for
therapy. Nat Biotechnol. 25:100–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho NH, Park YK, Kim YT, Yang H and Kim
SK: Lifetime expression of stem cell markers in the uterine
endometrium. Fertil Steril. 81:403–407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gargett CE: Identification and
characterization of human endometrial stem/progenitor cells. Aust
Nz J Obstet Gynaecol. 46:250–253. 2006. View Article : Google Scholar
|
|
8
|
Gargett CE and Masuda H: Adult stem cells
in the endometrium. Mol Hum Reprod. 16:818–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng X, Ichim TE, Zhong J, Rogers A, Yin
Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, et al: Endometrial
regenerative cells: A novel stem cell population. J Transl Med.
5:57–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel AN, Park E, Kuzman M, Benetti F,
Silva FJ and Allickson JG: Multipotent menstrual blood stromal stem
cells: isolation, characterization, and differentiation. Cell
Transplant. 17:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dominina AP, Fridliandskaia II, Zemel'ko
VI, Pugovkina NA, Kovaleva ZV, Zenin VV, Grinchuk TM and Nikol'skiĭ
NN: Mesenchymal stem cells from human endometrium do not undergo
spontaneous transformation during long-term cultivation. Cell
tissue biol. 7:221–226. 2013. View Article : Google Scholar
|
|
12
|
Murphy MP, Wang H, Patel AN, Kambhampati
S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE and
Riordan NH: Allogeneic endometrial regenerative cells: An ‘off the
shelf solution’ for critical limb ischemia? J Transl Med. 6:452008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mikhailov VM: Life cycle of decidual
cells. Int Rev Cytol. 227:1–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JJ, Taylor HS, Lu Z, Ladhani O,
Hastings JM, Jackson KS, Wu Y, Guo SW and Fazleabas AT: Altered
expression of HOXA10 in endometriosis: Potential role in
decidualization. Mol Hum Reprod. 13:323–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Csemiczky G, Wramsby H, Johannisson E and
Landgren BM: Importance of endometrial quality in women with tubal
infertility during a natural menstrual cycle for the outcome of IVF
treatment. J Assist Reprod Genet. 15:55–61. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dmowski WP and Greenblatt RB: Asherman';s
syndrome and risk of placenta accrete. Obstet Gynecol. 34:288–299.
1969.PubMed/NCBI
|
|
17
|
Zhao Y, Wang A, Tang X, Li M, Yan L, Shang
W and Gao M: Intrauterine transplantation of autologous bone marrow
derived mesenchymal stem cells followed by conception in a patient
of severe intrauterine adhesions. Open J Obstet Gynecol. 3:377–380.
2013. View Article : Google Scholar
|
|
18
|
Nagori CB, Panchal SY and Patel H:
Endometrial regeneration using autologous adult stem cells followed
by conception by in vitro fertilization in a patient of severe
Asherman';s syndrome. J Hum Reprod Sci. 4:43–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Domnina AP, Zemelko VI, Mikhailov VM and
Nikolsky NN: Stimulation of decidua development by transplantation
of endometrial stem cells. J Biomed Sci Eng. 6:59–65. 2013.
View Article : Google Scholar
|
|
20
|
Grinchuk TM, Ivantsov KM, Alekseenko LL,
Kozhukharova IV, Zaĭchik AM, Petrov NS, Mikhaĭlov VM and Popov BV:
Characterization of cultured murine mesenchymal stem cell line
expressing GFP. Tsitologiia. 50:1030–1035. 2008.(In Russian).
PubMed/NCBI
|
|
21
|
Popov BV, Petrov NS, Mikhaĭlov VM, Tomilin
AN, Alekseenko LL, Grinchuk TM and Zaĭchik AM: Spontaneous
transformation and immortalization of mesenchymal stem cells in
vitro. Tsitologiia. 51:91–102. 2009.(In Russian). PubMed/NCBI
|
|
22
|
Bernardo ME, Zaffaroni N, Novara F, Cometa
AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R,
Daidone MG, et al: Human bone marrow derived mesenchymal stem cells
do not undergo transformation after long-term in vitro culture and
do not exhibit telomere maintenance mechanisms. Cancer Res.
67:9142–9149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rubio D, Castro J Garcia, Martin MC, de la
Fuente R, Cigudosa JC, Lloyd AC and Bernad A: Spontaneous human
adult stem cell transformation. Cancer Res. 65:3035–3039.
2005.PubMed/NCBI
|
|
24
|
Røsland GV, Svendsen A, Torsvik A, Sobala
E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R,
Lønning PE, et al: Long-term cultures of bone marrow derived human
mesenchymal stem cells frequently undergo spontaneous malignant
transformation. Cancer Res. 69:5331–5339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu W, He Q, Li X, Zhang X, Lu A, Ge R,
Zhen H, Chang AE, Li Q and Shen L: Long-term cultured human neural
stem cells undergo spontaneous transformation to tumor-initiating
cells. Int J Biol Sci. 7:892–901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Torsvik A, Rosland GV and Bjerkvig R:
Spontaneous transformation of stem cells in vitro and the issue of
cross contamination. Int J Biol Sci. 8:1051–1052. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sokolov MV and Neumann RD: Lessons learned
about human stem cell responses to ionizing radiation exposures: A
long road still ahead of us. Int J Mol Sci. 14:15695–15723. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sokolov MV and Neumann RD:
Radiation-induced bystander effects in cultured human stem cells.
PLoS One. 5:e141952010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nieto A, Cabrera CM, Catalina P, Cobo F,
Barnie A, Cortés JL, del Jesus A Barroso, Montes R and Concha A:
Effect of mitomycin C on human foreskin fibroblasts used as feeders
in human embryonic stem cells: Immunocytochemistry MIB1 score and
DNA ploidy and apoptosis evaluated by flow cytometry. Cell Biol
Int. 31:269–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hung DT, Jamison TF and Schreiber SL:
Understanding and controlling the cell cycle with natural products.
Chem Biol. 3:623–639. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zemel'ko VI, Grinchuk TM, Domnina AP,
Artsybasheva IV, Zenin VV, Kirsanov AA, Bichevaia NK, Korsak VS and
Nikol'skiĭ NN: Multipotent mesenchymal stem cells of desquamated
endometrium: Isolation, characterization and application as a
feeder layer for maintenance of human embryonic stem cells. Cell
Tiss Biol. 6.1:1–11. 2011.(In Russian).
|
|
32
|
Cmielova J, Havelek R, Soukup T, Jiroutová
A, Visek B, Suchánek J, Vavrova J, Mokry J, Muthna D, Bruckova L,
et al: Gamma radiation induces senescence in human adult
mesenchymal stem cells from bone marrow and periodontal ligaments.
Int J Radiat Biol. 88:393–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ko E, Lee KY and Hwang DS: Human umbilical
cord blood-derived mesenchymal stem cells undergo cellular
senescence in response to oxidative stress. Stem Cells Dev.
21:1877–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prockop DJ, Brenner M, Fibbe WE, Horwitz
E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L and Keating A:
Defining the risks of mesenchymal stromal cell therapy.
Cytotherapy. 12:576–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu SP, Wei Z and Wei L: Preconditioning
strategy in stem cell transplantation therapy. Transl Stroke Res.
4:76–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu P, Wei Z and Wei L: Preconditioning
strategy in stem cell transplantation therapy. Transl Stroke Res.
4:76–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alawadhi F, Du H, Cakmak H and Taylor HS:
Bone marrow-derived stem cell (bmdsc) transplantation improves
fertility in a murine model of Asherman's syndrome. PLoS One.
9:e966622014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kilic S, Yuksel B, Pinarli F, Albayrak A,
Boztok B and Delibasi T: Effect of stem cell application on
Asherman syndrome, an experimental rat model. J Assist Reprod
Genet. 31:975–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jing Z, Hong G and Li Y: Development of an
animal model for thin endometrium using 95% ethanol. J Fert In
Vitro. 2:42012.
|