Introduction
Osteoporosis is a considered to be one of the most
common diseases and public health problems (1,2). Spinal
compression fractures and hip fractures are easily caused in
individuals with osteoporosis, thus severely affecting the health
of older individuals. Along with the gradual increase in the aging
population, the medical community is increasingly concerned about
this widely existing systemic bone disease (3,4). The
early diagnosis and prevention of osteoporosis is of great
importance, and detecting the bone mineral density (BMD) is the
most commonly used clinical method of diagnosis (5,6).
Currently, dual-energy X-ray absorptiometry (DXA) is recognized as
the gold standard for measuring BMD (7,8);
however, the results of BMD provide the sum of minerals in the
cortical and cancellous bones, and thus early detection of
osteoporosis originating from the vertebral cancellous bone is
difficult (9,10). Conventional computed tomography (CT)
examination of osteoporosis typically depends on the clinicians
experience in detecting trabecular bone rarefraction, vertebral
double-concave deformation and density decreases, which cannot
accurately reflect the alteration in BMD; therefore, dual-energy
quantitative CT may theoretically be the best technique for
measuring BMD (11). It has
previously been demonstrated that the Ca2+-water and
water-Ca2+ densities can be used for diagnosing
osteoporosis and calculating the BMD (12). Gemstone spectral CT (GSCT) uses
single-energy transient kVp switching technology to capture images
of material densities, thus obtaining Ca2+-based and
water-based images that reflect the bone mineral content and
changes in the content via the Ca2+-water and
water-Ca2+ densities (12,13).
The present study used Ca2+-based
gemstone spectral imaging (GSI) to measure the L3 vertebral
Ca2+-water densities and water-based imaging to measure
the L3 vertebral water-Ca2+ densities in 235 subjects.
The study aimed to compare the association of gender and age with
the GSI-measured Ca2+-water and water-Ca2+
densities, and to examine the feasibility of GSI technology in
measuring the BMD.
Materials and methods
General information
A total of 235 patients with osteoporosis who
underwent abdominal or lumbar GSI at the Affiliated Hospital of
Weifang Medical University (Weifang, China) between May 2012 and
May 2013 were enrolled into the present study, including 113 males
and 122 females. The study was conducted in accordance with the
Declaration of Helsinki, and was confirmed and approved by the
hospital's Ethical Committee. Signed informed consent was obtained
from all patients.
The selected patients had no history of cancer,
spinal trauma, surgery and other diseases that may affect the BMD,
including hyperthyroidism, hyperparathyroidism, diabetes,
congenital osteoarthrosis, sequelae of cerebrovascular disease,
immune dysfunction, long-term administration of corticosteroids,
and liver, kidney or rheumatic diseases. The patients were aged
between 20 and 87 years, with a mean age of 50.6±8.2 years. The
patients were divided into seven age groups of 10 years each, as
follows: Group 1, 20–29 years; group 2, 30–39 years; group 3, 40–49
years; group 4, 50–59 years; group 5, 60–69 years; group 6, 70–79
years; and group 7, ≥80 years.
CT imaging technique
A Discovery CT750 HD spectral CT scanner (GE
Healthcare, Waukesha, WI, USA) was used in the present study. The
L3 vertebral bodies of all patients were subjected to GSI with the
following scanning conditions: Instantaneous switching, 80–140 kVp;
automatic mAs; slice thickness, 5 mm; spacing, 5 mm; pitch, 0.984;
speed, 39.37 mm/cycle; and rotation time, 0.8 sec/rotation.
Image post-processing and density
measurement
The single-energy image, with a slice thickness of
0.625 mm, was reconstructed and transmitted to the Advantage
Workstation version 4.4 (GE Healthcare) for processing with GSI
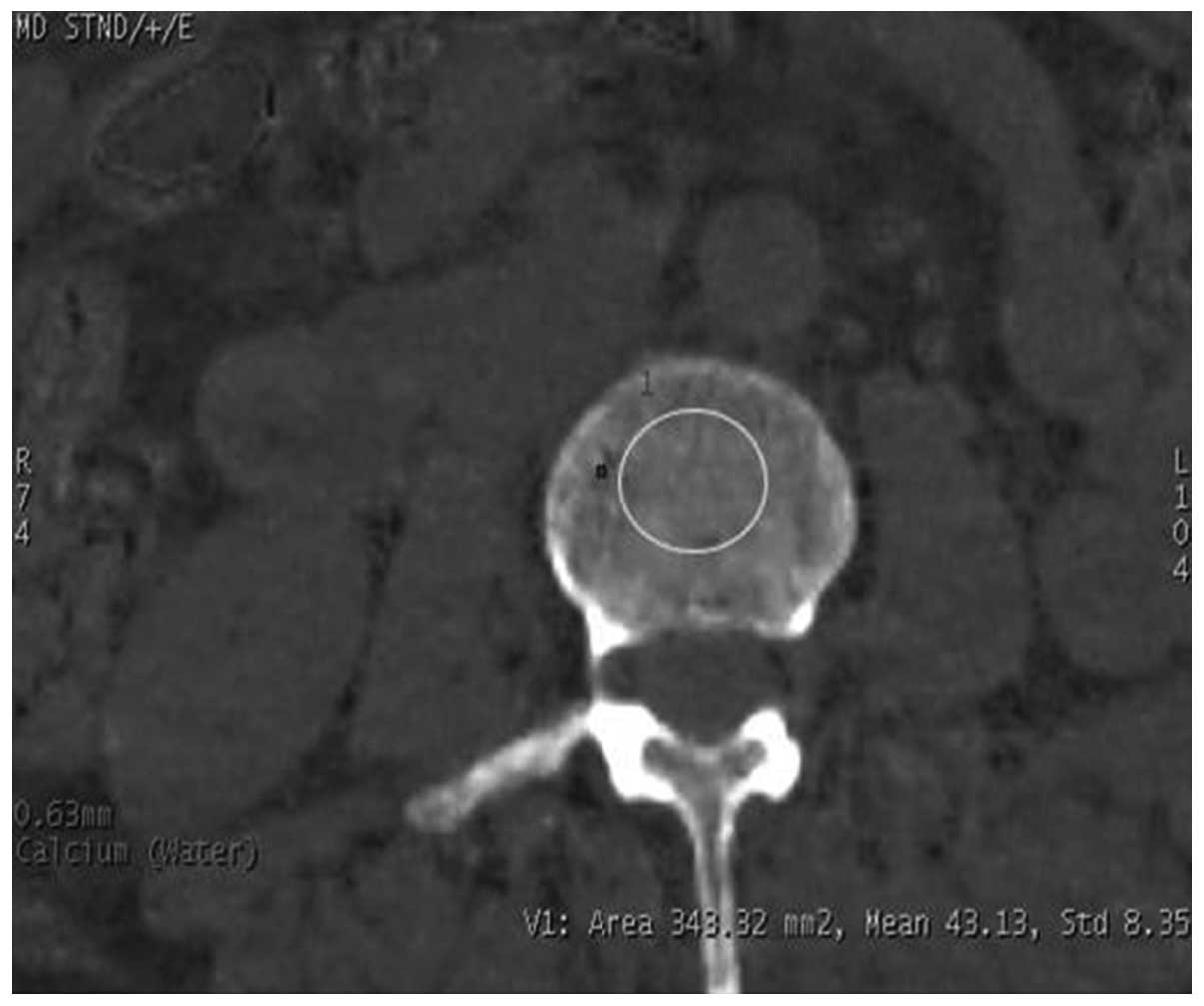
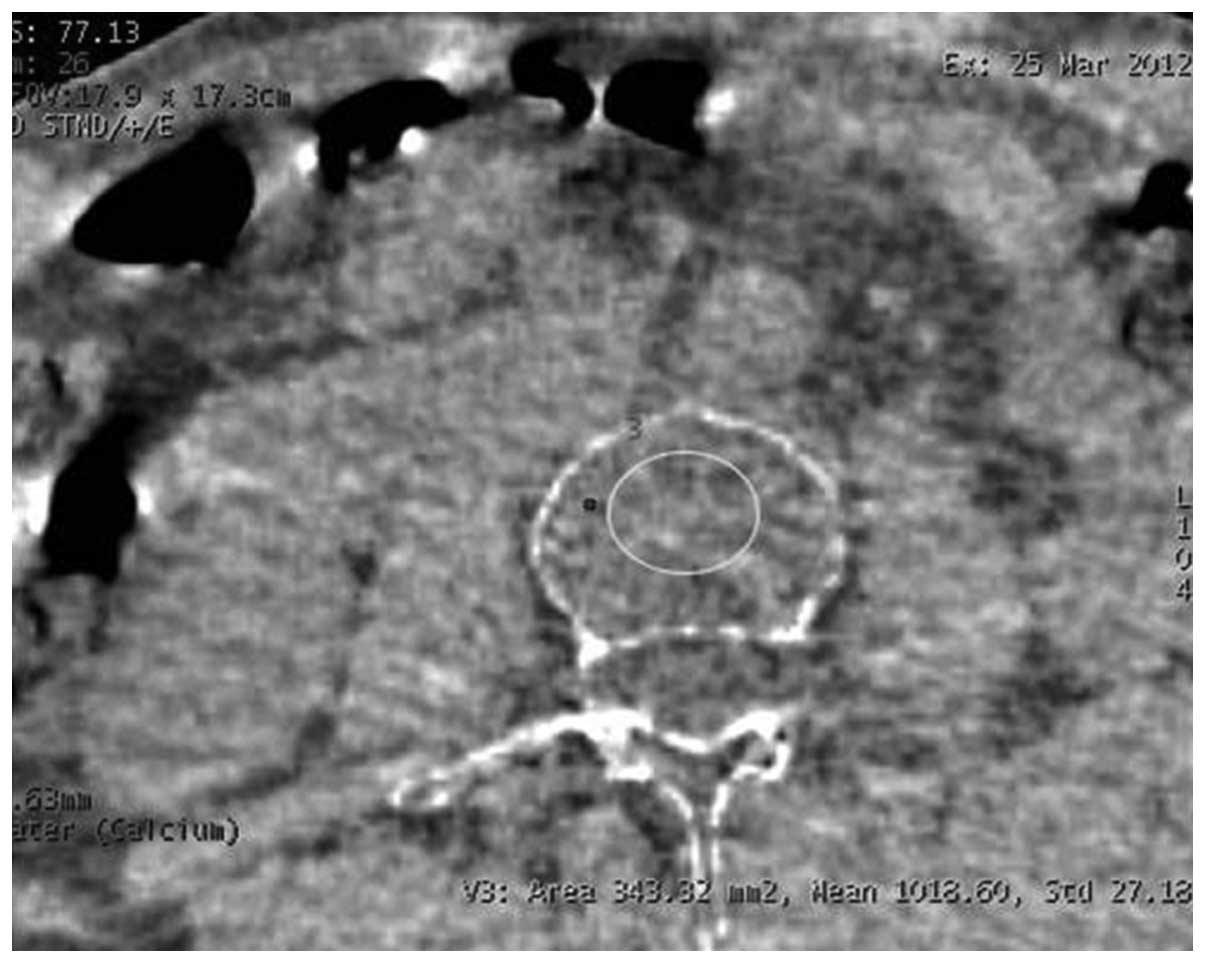
Viewer (GE Healthcare). The Ca2+-water-based (Fig. 1) and water-Ca2+-based
(Fig. 2) material images were thus
obtained, and the Ca2+-water and water-Ca2+
densities of the region of interest (ROI) were measured on the L3
vertebral cancellous bone level, and the mean value was then
obtained. When selecting the ROI, the entire cancellous bone was
maximally covered, and the region was ~5 mm to the cortical edge in
order to avoid any bone islands and venous plexuses. In all cases,
the size of the ROI was ~300 mm2.
Statistical analysis
SPSS version 13.0 software package (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. One-way
analysis of variance was performed for the statistical processing
of measurement data. The association of age with the vertebral
GSI-obtained Ca2+-water and water-Ca2+
densities was investigated with Pearson correlation analysis. In
addition, intergroup differences between the male and female groups
were investigated by Student's t-test. P<0.05 was considered to
indicate a statistical significant difference.
Results
Specific values of L3 vertebral
GSI-determined Ca2+-water and water-Ca2+
densities of all age groups
The highest vertebral Ca2+-water and
water-Ca2+ density determined by GSI was observed in the
20–29 year-old group in males and in the 30–39 year-old group in
females (Tables I and II). The vertebral Ca2+-water
density determined by GSI in males aged 20–29 years old was
79.4012±7.8896 mg/cm3, which was the highest among all
groups; the vertebral Ca2+-water density in females aged
30–39 years old was 80.6257±8.6175 mg/cm3, which was the
highest among all groups (Table I).
The vertebral water-Ca2+ density in males aged 20–29
years old was 1,024.1680±7.5345 mg/cm3, which was the
highest among all the groups. The vertebral water-Ca2+
density in females aged 30–39 years old was 1,028.3190±10.3976
mg/cm3, which was the highest among all the groups
(Table II).
 | Table I.Ca2+-water densities of L3
vertebrae, as determined by gemstone spectral imaging, and
statistical analysis in the various age groups. |
Table I.
Ca2+-water densities of L3
vertebrae, as determined by gemstone spectral imaging, and
statistical analysis in the various age groups.
|
|
|
| Ca2+-water
density (mg/cm3) | t-test |
|---|
|
|
|
|
|
|
|---|
| Age group
(years) | No. of males | No. of females | Males (n=113) | Females
(n=122) | t-value | P-value |
|---|
| 20–29 | 17 | 15 |
79.4012±7.8896 |
80.3740±9.8189 | −0.283 |
0.780 |
| 30–39 | 15 | 18 |
75.4280±8.5935 |
80.6257±8.6175 | −1.625 |
0.116 |
| 40–49 | 19 | 22 |
67.9171±8.2366 |
63.2672±8.9045 |
1.685 |
0.100 |
| 50–59 | 23 | 25 |
63.2400±4.6890 |
51.7106±8.1675 |
4.512 | <0.001 |
| 60–69 | 15 | 15 |
51.7440±4.4829 |
35.8400±3.6696 | 10.632 | <0.001 |
| 70–79 | 13 | 14 |
43.8656±4.4570 |
29.2200±6.9053 |
5.346 | <0.001 |
| ≥80 | 11 | 13 |
33.9017±6.7504 |
25.8386±4.8065 |
2.511 |
0.029 |
 | Table II.Water-Ca2+ densities of L3
vertebrae, as determined by gemstone spectral imaging, and
statistical analysis in the various age groups. |
Table II.
Water-Ca2+ densities of L3
vertebrae, as determined by gemstone spectral imaging, and
statistical analysis in the various age groups.
|
|
|
|
Water-Ca2+ density
(mg/cm3) | t-test |
|---|
|
|
|
|
|
|
|---|
| Age group
(years) | No. of males | No. of females | Males (n=113) | Females
(n=122) | t-value | P-value |
|---|
| 20–29 | 17 | 15 |
1,024.1680±7.5345 |
1,021.4307±16.9910 | −1.599 | 0.122 |
| 30–39 | 15 | 18 |
1,019.9606±14.4219 |
1,028.3190±10.3976 |
0.567 | 0.575 |
| 40–49 | 19 | 22 |
1,009.3271±8.7338 |
1,014.9172±9.7994 | −1.866 | 0.070 |
| 50–59 | 23 | 25 |
1,003.2423±17.4680 |
999.9331±14.3856 |
0.560 | 0.580 |
| 60–69 | 15 | 15 |
1,001.4167±13.7474 |
989.4767±9.4838 |
1.769 | 0.135 |
| 70–79 | 13 | 14 |
987.4067±14.3953 |
980.0378±11.7370 |
1.190 | 0.251 |
| ≥80 | 11 | 13 |
984.0467±6.3942 |
982.7571±6.4488 |
0.361 | 0.725 |
Comparison of Ca2+-water
and Ca2+-water densities in males and females
The vertebral Ca2+-water densities
determined by GSI in age groups 1–3 showed no statistically
significant differences between males and females, with t values of
−0.283, −1.625 and 1.685, respectively (all P>0.05). By
contrast, the Ca2+-water densities of individuals in age
groups 4–7 demonstrated statistically significant differences
between males and females (group 4: t=4.512, P<0.001; group 5:
t=10.632, P<0.001; group 6: t=5.346, P<0.001; group 7:
t=2.511, P=0.029) (Table I).
Furthermore, the vertebral GSI-determined water-Ca2+
densities in all age groups were not found to be significantly
different between males and females (Table II).
Correlation of age and gender with
Ca2+-water and water-Ca2+ densities
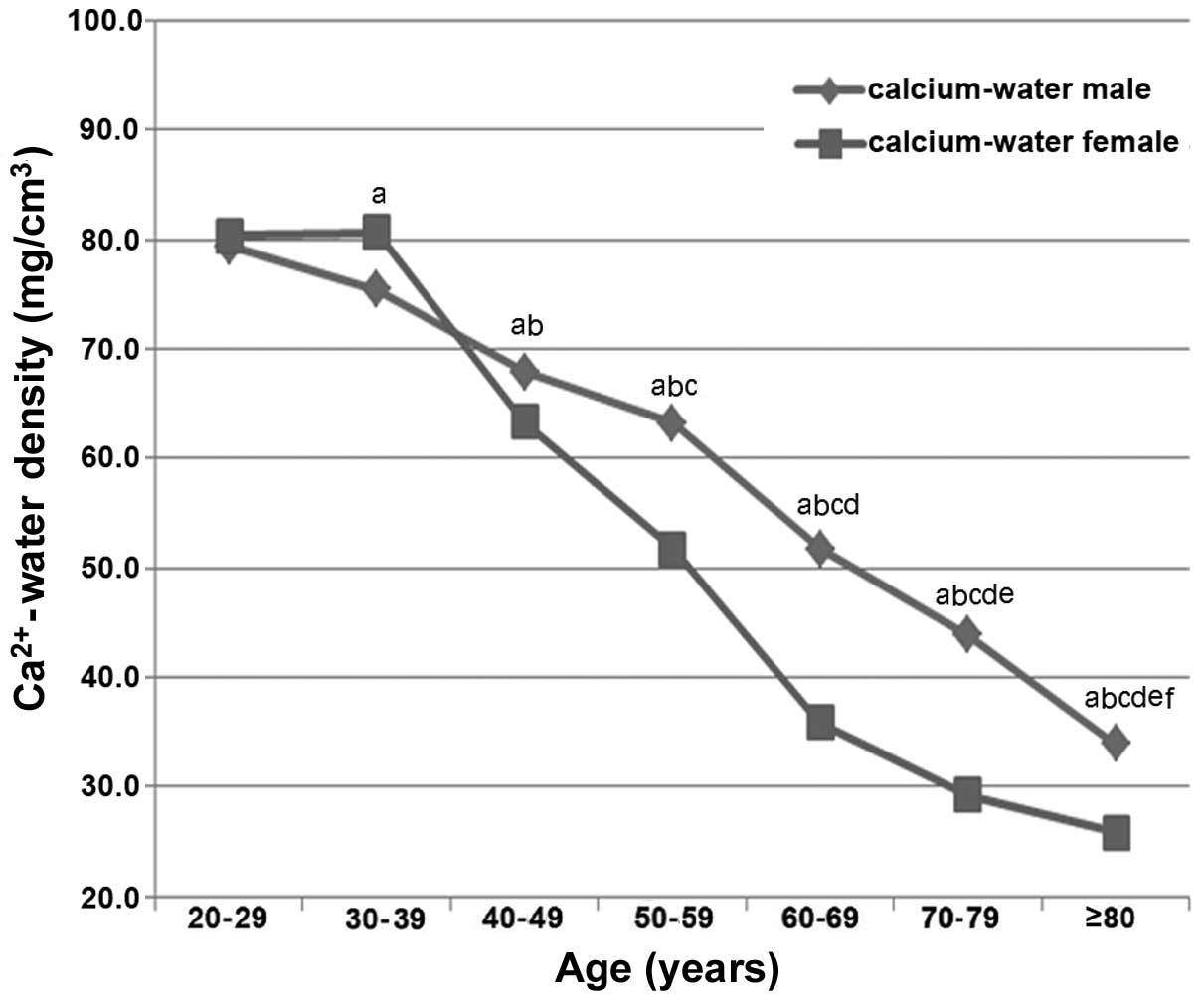
As shown in Fig. 3,
the male participants exhibited a decreasing trend in vertebral
GSI-determined Ca2+-water density with increasing age
(r=−0.6815; P<0.01); whereas females aged ≥40 years presented a
decreasing trend in Ca2+-water density with increasing
age (r=−0.7961; P<0.01). Females age ≤39 years exhibited no
significant correlation between age and Ca2+-water
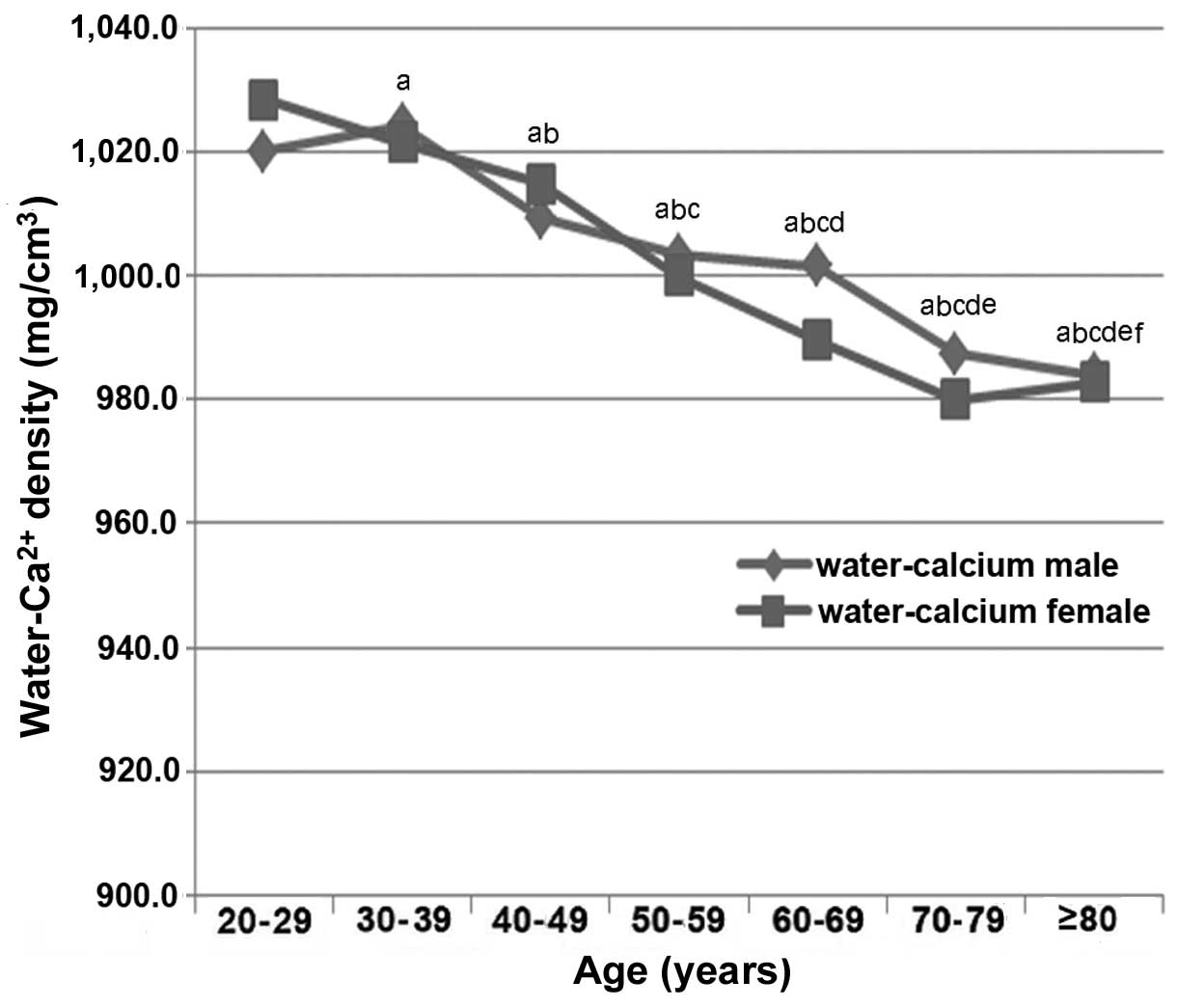
density (r=0.1901; P=0.3735). As shown in Fig. 4, males aged ≥40 years exhibited a
decreasing trend in water-Ca2+ density with increasing
age (r=−0.9065; P<0.01); whereas females aged ≤70 years
demonstrated a decreasing trend in water-Ca2+ density
with increasing age (r=−0.8877; P<0.01). Males aged ≤39 years
(r=−0.0233; P=0.9140) and females aged ≥71 years (r=−0.0432;
P=0.5632) exhibited no significant correlation between their age
and water-Ca2+ density.
Discussion
Osteoporosis, which refers to the reduction of bone
mass per unit volume, is a disease that is closely associated with
age. BMD detection is an important means for the early diagnosis
and evaluation of osteoporosis. Since the lumbar spine is the most
common site involved in the early stages of osteoporosis, BMD
measurement is typically performed at the lumbar spine region
(14). Currently, lumbar spinal BMD
detection using the DXA and quantitative CT methods remains the
best technique to diagnose and evaluate osteoporosis (14,15). In
particular, use of the DXA method has been recommended by the World
Health Organization as part of the diagnostic criteria for
osteoporosis (14,16); however, this method has certain
limitations, since it is unable to distinguish between the
cancellous and the cortical bones. Due to the different conversion
rates of cortical and cancellous bones, alterations in the bone
during early osteoporosis initially occur in the cancellous bone.
This is due to the large surface area and high metabolic conversion
rate of the cancellous bone, which were greater than those in the
cortical bone. Thus, the DXA method may not detect early
osteoporosis originating from the vertebral cancellous bone, and
may reduce the sensitivities of osteoporosis towards treatment
(17). In addition, detection from
the posteroanterior lumbar may only obtain the area density within
that region, which also includes images of surrounding structures
such as accessories, paraspinal ligaments and abdominal aorta other
than the lumbar vertebrae; therefore, this detection technique
would be greatly affected by the osteosclerotic images of
hyperostosis, which may lead to the distortion of measurement data
to a certain extent (18).
Quantitative CT can be used to measure the actual
volumetric BMD, completely ruling out the effects of bone size,
while it can also measure the densities of the cortical and
cancellous bones, as well as more accurately determine the
morphological parameters. Therefore, measurements by quantitative
CT may be able to reflect the early changes in vertebral bone mass.
However, a limitation of this method is that it requires a
dedicated phantom and specialized software for bone density
measurement and analysis (19,20).
Since each manufacturer has different operating and preparing
standards for the phantom, the measurement results of operating
phantoms may present various uncertainties, while the method is
more complex and requires a higher dose of radiation compared with
other techniques; therefore, the widespread clinical applications
of quantitative CT are limited (21).
Theoretically, the ideal technique for BMD
measurement would be dual-energy CT scanning. However, this method
requires duplicated scanning, and is thus limited by image
mismatching and high radiation doses; in addition, it is a
complicated technique with no corresponding software available,
therefore limiting its clinical applications (22). GSCT is another technique that can
achieve spectral imaging by instant switching of dual energy
through a single bulb, using unique technologies of physical
separation and quantitative determination of the material density,
measuring the Ca2+-water and water-Ca2+
densities of the vertebral cancellous bone.
The absorption of objects results in changes in
X-ray energy, and during the application of two X-ray energy
spectra, the X-ray attenuation is altered. The base materials in
any tissue have different absorption proportions towards X-ray, and
their combinations represent the X-ray attenuation (23).
The base materials may not necessarily be the actual
substances contained in the tissue, as they simply represent the
X-ray attenuation of this tissue; the measurement of matched base
materials may reflect the relative contents of these substances in
the tissue (24). The main
components of vertebral cancellous bone are bone minerals (such as
Ca2+ salt), water and collagen; therefore,
water-Ca2+ or Ca2+-water-based substances may
pair up and indirectly reflect the Ca2+ content and
changes in the cancellous bone.
In the present study, GSCT was used to measure the
Ca2+-water densities of L3 vertebral cancellous bone
among different age groups. The results demonstrated that the
highest GSI-determined Ca2+-water density was observed
in males with ages of 20–29 years and females with ages of 30–39
years, suggesting that the BMD peak in male and female vertebral
cancellous bones was reached at different ages. In addition, the
results indicated that the age of male participants was negatively
correlated with vertebral GSI-determined Ca2+-water
densities, suggesting that the male vertebral Ca2+
contents decreased with increasing age. In females ≤39-years-old,
the GSI-determined Ca2+-water density of vertebral
cancellous bone was slightly elevated with increasing age, but
there was not statistically significant correlation between the age
and vertebral Ca2+-water densities. By contrast, females
≥40-years-old presented a negative correlation between age and
vertebral GSI-determined Ca2+-water densities,
suggesting a gradual decrease in vertebral Ca2+ content
from 40 years of age. Furthermore, the results of the current study
revealed that the Ca2+ content began to decrease at a
later age in females compared with males, and the reason behind
this trend requires further investigation. Regarding the slight
difference in the ages at which the peak BMD of vertebral
cancellous bone appeared in males and females, which was also
reported in a previous study (25),
we hypothesize that it may be associated with the separative
statistics of males and females, and the numbers of selected cases.
Before 40 years of age, the GSI-determined Ca2+-water
density of the vertebral cancellous bone in females was
significantly higher compared with that in males within the same
age period; however, after 50 years of age, this density was
significantly reduced in females compared with that in males, and
the difference was statistically significant (P<0.01), which is
consistent with numerous previous studies (26,27).
This trend may be caused by the decline of estrogen in females
>50-years-old, resulting in reduced vertebral Ca2+
content (27). Furthermore,
lactation, menstruation and diet may also affect the vertebral
Ca2+ content in these individuals.
The present study also measured the
water-Ca2+ densities of vertebral cancellous bone in
different age groups using GSI. The results revealed a correlation
between their peak water-Ca2+ density values and age,
but no statistically significant differences were detected among
the different age groups and genders; therefore, the significance
remains of water-Ca2+ density remains to be further
examined.
GSCT is a newly-developed method for measuring the
BMD, which may also be used as the means of monitoring the BMD
prior to and following treatment. When performing chest and
abdominal CT examination, the GSI Ca2+-water and
water-Ca2+ densities of the vertebral cancellous bone
could be measured at the same time, without the need to
re-irradiate the vertebral body, which reduces the radiation doses
and additional costs (28).
The number of patients in the present study was
limited and large sample analysis could not be performed, therefore
reference values of GSI-determined Ca2+-water and
water-Ca2+ densities of normal vertebral cancellous bone
could not be obtained. In addition, the water and fat contents
inside the vertebral body may affect the results. Although the
water content is low inside the vertebral body, it may have a
greater impact on the results when combined with vertebral
fractures and bone marrow edema. Given the aforementioned
limitations of the current technique compared with other methods,
further investigation is required. In conclusion, GSI may be used
as a novel method of measuring the vertebral adult bone mineral
density.
Acknowledgements
The present study was supported by grants from the
Science and Technology Development Project of Weifang (no.
201301068) and the Science and Technology Project of Weifang
Municipal Health Bureau (no. 2013089).
References
|
1
|
Charles W, Slemenda C and Johnston CJ:
Epidemiology of osteoporosisTreatment of the Postmenopausal Woman:
Basic and Clinical Aspects. Lobo RA: Lippincott Williams &
Wilkins; Baltimore: pp. 279–285. 1999
|
|
2
|
Riggs BL: The problem of osteoporosis-its
prevention and treatmentPrevention and Treatment of Osteoporosis.
Riggs BL: Hogrefe & Huber; Lewiston, N.Y.: pp. 9–11. 1992
|
|
3
|
Li Y, Lin J, Wang P, Yao X, Yu H, Zhuang
H, Zhang L and Zeng Y: Effect of time factors on the mortality in
brittle hip fracture. J Orthop Surg Res. 9:372014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnell O and Kanis JA: An estimate of the
worldwide prevalence and disability associated with osteoporotic
fractures. Osteoporos Int. 17:1726–1733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lenchik L, Kiebzak GM and Blunt BA:
International Society for Clinical Densitometry Position
Development Panel and Scientific Advisory Committee: What is the
role of serial bone mineral density measurements in patient
management? J Clin Densitom. 5(Suppl): S29–S38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crandall C: The role of serial bone
mineral density testing for osteoporosis. J Womens Health Gend
Based Med. 10:887–895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lewiecki EM: Bone density measurement and
assessment of fracture risk. Clin Obstet Gynecol. 56:667–676. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shuler FD, Conjeski J, Kendall D and
Salava J: Understanding the burden of osteoporosis and use of the
World Health Organization FRAX. Orthopedics. 35:798–805. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanterewicz E, Puigoriol E,
García-Barrionuevo J, del Rio L, Casellas M and Peris P: Frodos
Research Group: Prevalence of vertebral fractures and minor
vertebral deformities evaluated by DXA-assisted vertebral fracture
assessment (VFA) in a population-based study of postmenopausal
women: The FRODOS study. Osteoporos Int. 25:1455–1464.
2014.PubMed/NCBI
|
|
10
|
Bazzocchi A, Spinnato P, Fuzzi F, Diano D,
Morselli-Labate AM, Sassi C, Salizzoni E, Battista G and Guglielmi
G: Vertebral fracture assessment by new dual-energy X-ray
absorptiometry. Bone. 50:836–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nickoloff EL, Feldman F and Atherton JV:
Bone mineral assessment: New dual-energy CT approach. Radiology.
168:223–228. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng S, Dong Y, Miao Y, Liu A, Zhang X,
Wang B, Ge Y, Liu Y and Wang S: Differentiation of osteolytic
metastases and Schmorl's nodes in cancer patients using dual-energy
CT: Advantage of spectral CT imaging. Eur J Radiol. 83:1216–1221.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duan X, Wang J, Yu L, Leng S and
McCollough CH: CT scanner X-ray spectrum estimation from
transmission measurements. Med Phys. 38:993–997. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Writing Group for the ISCD Position
Development Conference: Technical standardization for dual-energy
X-ray absorptiometry. J Clin Densitom. 7:27–36. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gnudi S, Sitta E and Fiumi N: Bone density
and geometry in assessing hip fracture risk in post-menopausal
women. Br J Radiol. 80:893–897. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prevention and management of osteoporosis.
World Health Organ Tech Rep Ser. 921:1–164. 2003.PubMed/NCBI
|
|
17
|
Yu EW, Bouxsein ML, Roy AE, Baldwin C,
Cange A, Neer RM, Kaplan LM and Finkelstein JS: Bone loss after
bariatric surgery: Discordant results between DXA and QCT bone
density. J Bone Miner Res. 29:542–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klingberg E, Lorentzon M, Göthlin J,
Mellström D, Geijer M, Ohlsson C, Atkinson EJ, Khosla S, Carlsten H
and Forsblad-d'Elia H: Bone microarchitecture in ankylosing
spondylitis and the association with bone mineral density,
fractures and syndesmophytes. Arthritis Res Ther. 15:R1792013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bauer JS, Henning TD, Müeller D, Lu Y,
Majumdar S and Link TM: Volumetric quantitative CT of the spine and
hip derived from contrast-enhanced MDCT: conversion factors. AJR Am
J Roentgenol. 188:1294–1301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Genant HK, Lang T, Fuerst T, Pinette KV,
Zhou C, Thiebaud D and Diez-Perez A: Treatment with raloxifene for
2 years increases vertebral bone mineral density as measured by
volumetric quantitative computed tomography. Bone. 35:1164–1168.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cann CE: Quantitative CT for determination
of bone mineral density: A review. Radiology. 166:509–522. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Graser A, Johnson TR, Hecht EM, Becker CR,
Leidecker C, Staehler M, Stief CG, Hildebrandt H, Godoy MC, Finn
ME, et al: Dual-energy CT in patients suspected of having renal
masses: Can virtual nonenhanced images replace true nonenhanced
images? Radiology. 252:433–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ascenti G, Siragusa C, Racchiusa S, Ielo
I, Privitera G, Midili F and Mazziotti S: Stone-targeted
dual-energy CT: A new diagnostic approach to urinary calculosis.
AJR Am J Roentgenol. 195:953–958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang D, Li X and Liu B: Objective
characterization of GE discovery CT750 HD scanner: Gemstone
spectral imaging mode. Med Phys. 38:1178–1188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sai H, Iguchi G, Tobimatsu T, Takahashi K,
Otani T, Horii K, Mano I, Nagai I, Iio H, Fujita T, et al: Novel
ultrasonic bone densitometry based on two longitudinal waves:
Significant correlation with pQCT measurement values and
age-related changes in trabecular bone density, cortical thickness
and elastic modulus of trabecular bone in a normal Japanese
population. Osteoporos Int. 21:1781–1790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nindl BC, Pierce JR, Durkot MJ, Tuckow AP,
Kennett MJ, Nieves JW, Cosman F, Alemany JA and Hymer WC:
Relationship between growth hormone in vivo bioactivity, the
insulin-like growth factor-I system and bone mineral density in
young, physically fit men and women. Growth Horm IGF Res.
18:439–445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krum SA and Brown M: Unraveling estrogen
action in osteoporosis. Cell Cycle. 7:1348–1352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Dong Y, Ge Y and Liu YJ:
Feasibility of bone density measurement based on CT gemstone
spectral imaging. Zhong Guo Yi Xue Ying Xiang Ji Shuy. 29:133–137.
2013.(In Chinese).
|