Introduction
Orthodontic treatment on adults usually lasts 2–3
years (1–3). However, patients under exodontia
require a longer period of time (4).
The longer the treatment course is, the greater the risks of
gingivitis, enamel demineralization, decayed tooth, and even root
resorption (5–6). At present, the therapies that expedite
the movement of orthodontic tooth mainly include drugs,
physiotherapy and surgical operations.
As piezosurgery becomes widely applied in tumor
resection, fracture healing promotion, bone non-union treatment,
maturity and remodeling acceleration after distraction
osteogenesis, it is gradually introduced into orthodontic alveolar
bone remodeling to promote the movement effect of tooth (7). Bone morphogenetic protein-2 (BMP-2) is
a multifunctional growth factor (8).
As a type of signal protein, BMP-2 promotes the proliferation,
differentiation and apoptosis of many cells, and is involved in the
regeneration and repair of tissues (8). Specifically, this protein plays a
significant role in the remodeling of bone tissues (8).
The aim of the present study was to establish an
animal model to investigate the effects of piezosurgery in
accelerating the movement of orthodontic alveolar bone tooth of
rats and the expression mechanism of BMP-2.
Materials and methods
Animals
Thirty healthy adult male Wistar rats provided by
the Experimental Animal Center of Wuhan University were included in
the present study. The age range of the rats was 14–15 weeks, with
an average weight of 250±16 g. The rats were kept in cages at a
temperature of 22.5±0.5°C, with a 12-h light/dark cycle, were fed a
normal diet and had access to water. The present study was approved
by the ethics committee of Wuhan University.
Reagents
The reagents used in the study included, isoflurane
(SurgiVet, Inc., Waukesha, WI, USA), superfine diamond bar
(Dentsply Maillefer, Tulsa, OK, USA), ligature wire (Changsha
Tianmei Co., Ltd., Changsha, China), NiTi coil spring (3M Unitek,
St. Paul, MN, USA), orthodontic stress and tension gauge (Hangzhou
Tianmei Co., Ltd., Xiaoshan, China), ultrasonic coupling agent
(Aquasonic; Parker Laboratories, Inc., Fairfield, NJ, USA),
silicone impression material (Heraeus, Hanau, German), goat serum
(Invitrogen Life-Technologies, Carlsbad, CA, USA), BMP-2 antibody
(Abcam, Cambridge, MA, USA), horseradish peroxidase-labeled sheep
anti-rabbit IgG (Sigma, St. Louis, MO, USA), methanol (Merck,
Darmstadt, Germany), streptavidin-biotin complex (SABC)
immunohistochemistry kit (Dako, Glostrup, Denmark),
3,3′-diaminobenzidine (DAB) colour-producing reagent kit (Boster
Inc., Wuhan, China), hematoxylin (Biosharp, Hefei, China), BCA
protein quantitative detection kit (Boster Inc.), polyvinylidene
fluoride membrane (Millipore Corp., Billerica, MA, USA), enhanced
chemiluminescence (ECL) kit (Pierce Biotechnology, Inc., Rockford,
IL, USA), β-actin antibody (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA), primer (R&F; Sangon Biotech Co., Ltd., Shanghai,
China), total RNA extraction kit (Omega Bio-Tek, Inc., Norcross,
GA, USA), quantitative polymerase chain reacyion (qPCR) kit (Takara
Bio, Inc., Otsu, Japan), reverse transcription kit (Takara Bio,
Inc.), and TRIzol reagent (Invitrogen Life-Technologies, Carlsbad,
CA, USA).
Instruments
The instruments used in the study were Piezosurgery
(Exploiter TM UOSS-II; Beijing Boda Hi-Tech Co., Ltd., Beijing,
China), thermometer (Shanghai Medical University Instrument
Factory, Shanghai, China), turbine mixer (Shanghai Medical
University Instrument Factory), water bath (Shanghai Pudong
Physical Optical Instrument Factory, Shanghai, China), refrigerator
(Haier Co., Ltd., Qingdao, China), inverted microscope (Olympus
Corp., Tokyo, Japan), and qPCR instrument (Applied Biosystems Life
Technologies, Foster City, CA, USA).
Establishment of animal model
After anesthesia by xylazine (5 mg/kg, i.m) and
ketamine (100 mg/kg, i.m), the superfine diamond bar of the turbine
motor was used to form a small groove of 0.15–0.25 mm in the
cervical margin parallel to gingival margin on the flip side of the
two central incisors in the rat's maxillary, and in the centrifugal
axial surface corner of the neck of the first molar parallel to the
gingival margin to fix the ligature wire. Then, 0.25 mm orthodontic
stainless steel ligation wire was used to bind the NiTi coil spring
with a diameter of 0.030′' between the first molar and the central
incisor on the left side and right side, respectively. The force
value was set to 0.1 N, and the first molars were dragged on the
two sides to move towards the mesial position, and thus the
direction of coil spring stress segmentation was guaranteed to make
the first molar move towards the mesial position horizontally. Any
damages or detachment was closely monitored and repaired. If the
retention was unfavorable or the stress value was changed due to
the growth of the rats' central incisors, a new retention groove
was made on the centrifugal cervical margin of the central
incisors, and the retention equipment was relocated.
Experimental grouping
The animals were randomly divided them into 5
groups, with control and observation groups. Rats in the control
group were injected with 25-dihydroxyvitamin
[1,25-dihydroxycholecalciferol (DHCC)] into their dental ligament.
Rats in the observation group were placed with an orthodontic
device between the first molar and central incisor in the
maxillary. On the first day after the model was successfully
established, piezosurgery stimulation was performed on the first
molar in maxillary. The animals were anesthetized with 3%
isoflurane, and the first molar regions were defeathered in
bilateral maxillary when the activity of rats was significantly
decreased. Low-intensity pulse piezosurgery stimulation was carried
out on the first molar region in the maxillary of the experimental
side. The stimulation intensity was 30 mW/cm2, frequency
was 1.5 MHz, pulse width was 200 µsec, repeat frequency was 1 kHz,
once a day, each time for 20 min.
Observation index and detection
methods
Changes of the movement distance of the first molar
and gum surface temperature were compared on days 1, 3, 5, 7 and
14. Immunohistochemical staining was used to detect the expression
of BMP-2 of periodontal tissue in the tension side of the first
molar. RT-PCR and western blot analysis was performed to detect the
BMP-2 mRNA and protein expression. The distance between the central
fossas of the first molar and the second molar in the maxillary was
measured using a vernier caliper, and a temperature laser infrared
thermometer was used to measure temperature changes on the gum
surface.
Tissue preparation
The animals were sacrificed using 5% isoflurane
followed by cervical dislocation. The thoracic cavity was opened to
expose the heart and the aorta was separated. A thread was used to
prepare for the ligation of the perfusion needle. A small mouth on
the left ventricle was opened to insert the perfusion needle into
the left ventricle until it was inside the ascending aorta. The
perfusion needle was fixed using silk thread, then perfused with
normal saline, at a flow rate of ~20 ml/min. The auricula dextra
was then opened to allow the blood to flow out, followed by
suspended perfusion until the liver gradually turned white, as the
blood color of the excurrent liquid became thickened and then
clear.
The procedure used was as follows: Internal fixation
(perfusion of 4% paraformaldehyde for approximately 2 h), external
fixation (the tissue blocks containing the first molar and the
surrounding alveolar bone were extracted. These blocks were placed
in 4% paraformaldehyde fixation liquid at 4°C), decalcification
(fixed for 48 h, decalcified with 5% EDTA at 4°C for 10 days),
dehydration (dehydration of tissues using 75% alcohol, 85% alcohol,
95% alcohol, 95% alcohol, 100% alcohol, and 100% alcohol, for 12 h
each), transparentizing (immersion of the tissue blocks into
dimethylbenzene until the tissue blocks became transparent), waxdip
(at 60°C, the transparent tissue blocks were dipped into the
transparent agent and paraffin was melted for 20 min, after which
the blocks were dipped into the melted pure paraffin wax for 1 h),
embedding (the wax was melted to liquid, poured into the embedding
frame, slightly heated tweezers were used to clip the tissue blocks
into the embedding frame, the surface was placed in such a position
as to be cut downward, the embedding frame was then moved until the
paraffin wax was completely coagulated), and section (serial
section was produced alongside the long axis of the molar from
near, far to middle, with each section at 5 µm).
Immunohistochemical staining
Paraffin sections (3 µm) mounted on
poly-L-lysine-coated slides were deparaffinized and rehydrated.
Using 0.6% H2O2 in methanol, peroxidase
activity was removed. Then, the sections were washed in tap water
and in PBS (pH 7.4) for 10 min, treated with normal 1.5% goat serum
for 20 min at room temperature and incubated with the first
antibody: BMP-2 (diluted 1:250) overnight at 4°C. Subsequently, the
sections were washed in PBS and incubated with the secondary
antibody (sheep anti rabbit IgG) for 1 h at room temperature.
Peroxidatic activity was detected with a DAB colour-producing
reagent kit. The sections were then counterstained with
hematoxylin.
RNA extraction
For RNA extraction, 2 mm alveolar bone was collected
and frozen in liquid nitrogen at −80°C. Tissue (100 mg) was
immersed in 1 ml TRIzol and placed at room temperature for 2–5 min.
Subsequently, 200 µl chloroform was added to each tube and placed
at room temperature for 5 min. The contents were centrifuged at
10,000 × g for 15 min at 4°C, the supernatant was collected in the
tube, and pre-cooled isopropanol of the same volume was added to
the tube. The solution was placed at room temperature for 10 min
and the contents were centrifuged at 10,000 × g for 10 min. Then, 1
ml of 75% alcohol was used to wash the precipitate. RNA extracted
in the form of pellets was dried at room temperature for 5 min
until it became transparent. This was followed by the addition of
20 µl RNase-free water to dissolve the pellet. The RNA
concentration was quantified using UV spectrophotometry (Thermo
Fisher Scientific Inc., Orlando, FL, USA).
Preparation of cDNA
In detail, 1 µl Oligo dT and 2.0 µg total RNA were
mixed in a PCR tube, and DEPC-treated water was added until 9 µl
was reached. The contents were centrifuged after blending, washed
in 70°C warm water for 5 min and then washed in ice. The reaction
mixture was prepared on ice with 4 µl 5X RT buffer, 2 µl 10 mM
dNTPs, 0.5 µl RNase inhibitor, 1 µl M-MLV-RTase, 3.5 µl DEPC
H2O, vortexed uniformly, and centrifuged. The reaction
mixture was reacted at 42°C for 1 h, followed by 70°C for 10 min.
The cDNA was then preserved at −80°C for subsequent use.
qPCR
For qPCR, 1.0 µl cDNA, 10 µl SYBR Premix Ex Taq II
(2X), 0.5 µl upstream primer (5 µM), 0.5 µl downstream primer (5
µM), and 8.0 µl ddH2O were mixed on ice, vortexed and
centrifuged. Table I shows the
primers used. PCR conditions included pre-denaturation at 95°C for
5 sec, annealing and extension at 60°C for 30 sec for a total of 45
cycles, denaturation at 95°C for 1 min, then cooled down to 55°C.
Starting from 55°C, the temperature was raised by 0.5°C in each
stage until 95°C for 30 sec.
 | Table I.Primers for quantitative polymerase
chain reaction. |
Table I.
Primers for quantitative polymerase
chain reaction.
| Gene | Sequence |
|---|
| BMP-2 |
5′-CTACATGCTAGACCTGTATCGC-3′ |
|
|
5′-CCCACTCGTTTCTGGTAGTTC-3′ |
| β-actin
(human) |
5′-CACCACACCTTCTACAATGAG-3′ |
|
|
5′-GCATACCCCTCGTAGATGGGC-3′ |
Western blotting
Western blot analysis included protein extraction,
electrophoresis, membrane transfer, and immune coloration. Total
protein (30 µg) was loaded to SDS-PAGE gel and then transferred to
the nitrocellulose membrane. The membrane was incubated at room
temperature with TBST containing 50 g/l milk. The membrane was
incubated with primary rabbit polyclonal BMP-2 antibody (Abcam,
Cambridge, MA, USA; catalog no.: ab14933) overnight at 4°C. The
membrane was washed repeatedly with TBST and incubated with
corresponding secondary antibody (1:5,000). The antibody was
exposed with ECL solution and the gray value was calculated. The
assay was performed in triplicate.
Statistical analysis
The SPSS 20.0 software (IBM SPSS Armonk, NY, USA),
was used for statistical analysis. The measurement data were
presented as means ± standard deviation. An independent sample
t-test was used to compare between groups. Countable data were
presented as case or percentage, and the χ2 test was
applied for intra-group comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
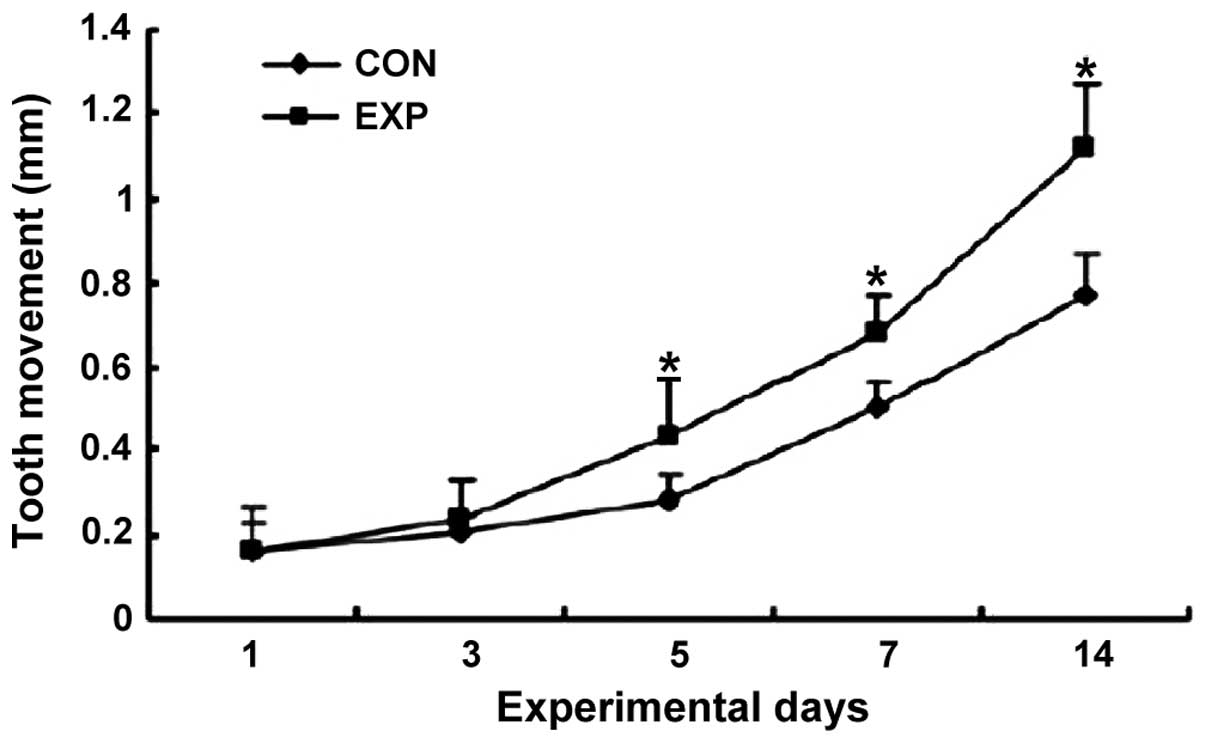
Tooth movement distance
Tooth movement distance in the observation group on
days 5, 7 and 14 was significantly longer than that in the control
group (p<0.05) (Fig. 1).
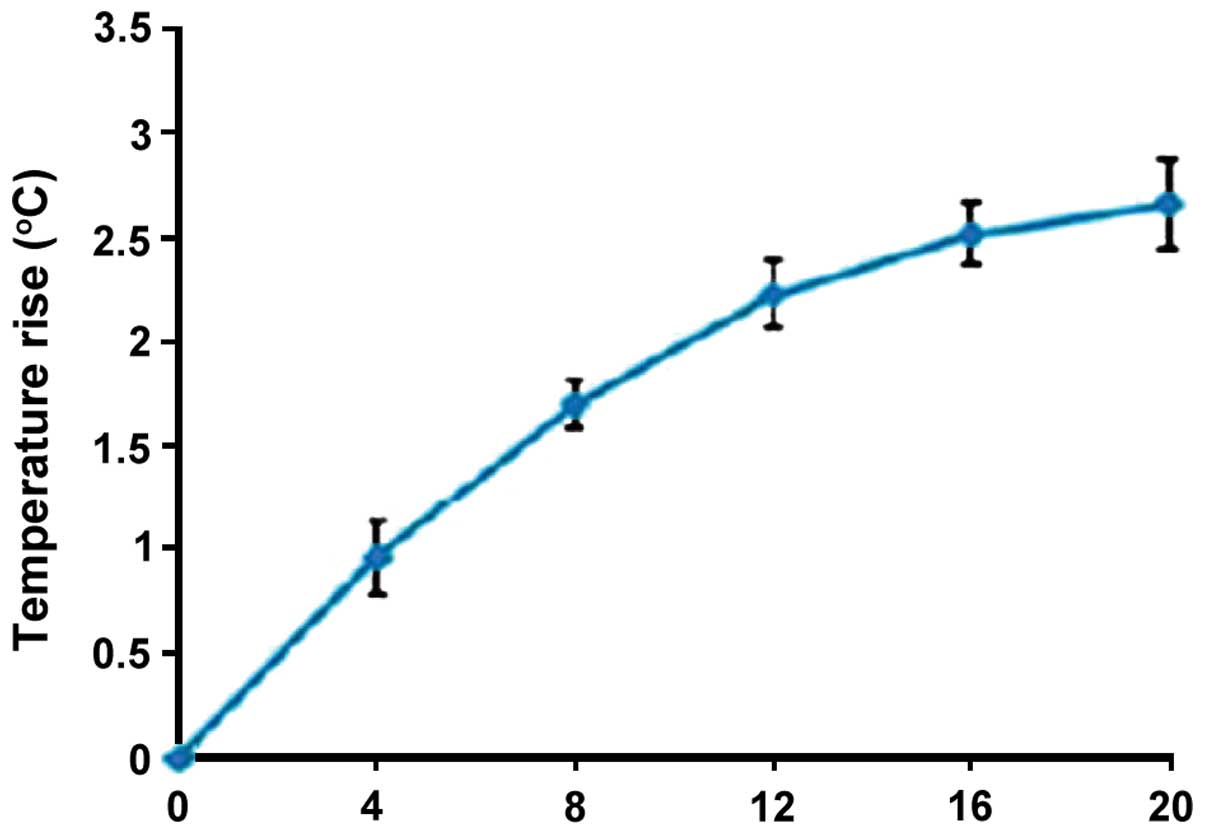
Gum surface temperature of observation
group
The gum surface temperature of the observation group
was elevated to some extent, and peaked after 20 min (Fig. 2).
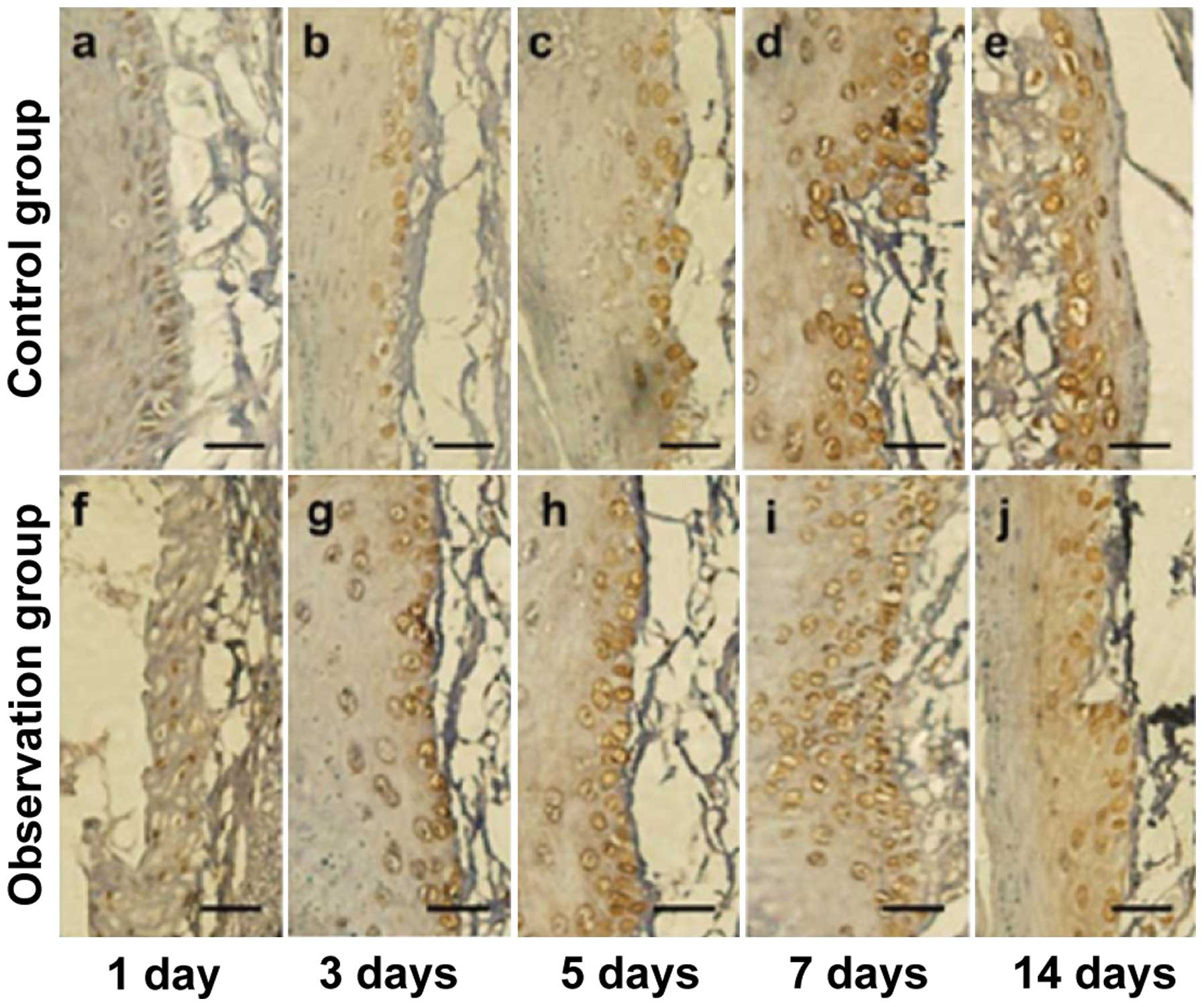
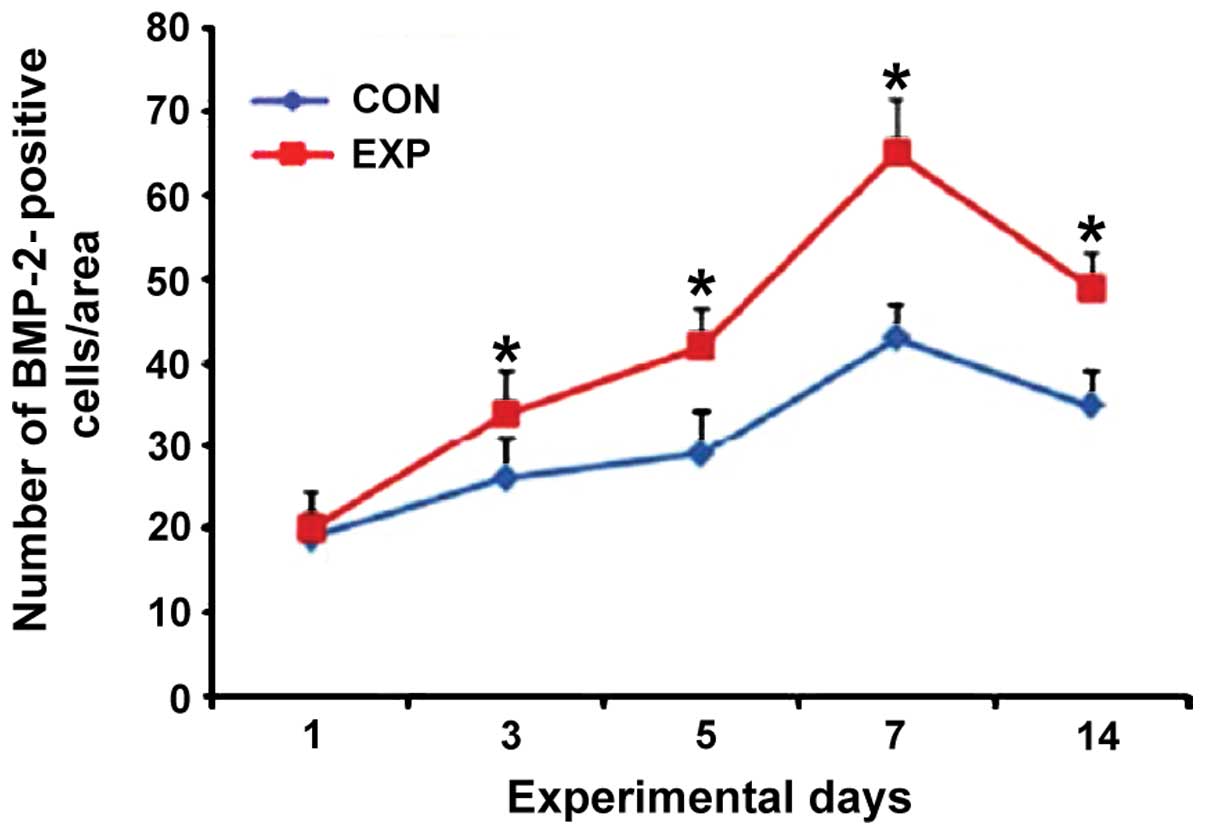
BMP-2 immunohistochemical
staining
The number of positive cells in the observation
group was significantly higher than that in the control group ats
day 3, 5, 7 and 14, and the difference was statistically
significant (p<0.05) (Figs. 3 and
4).
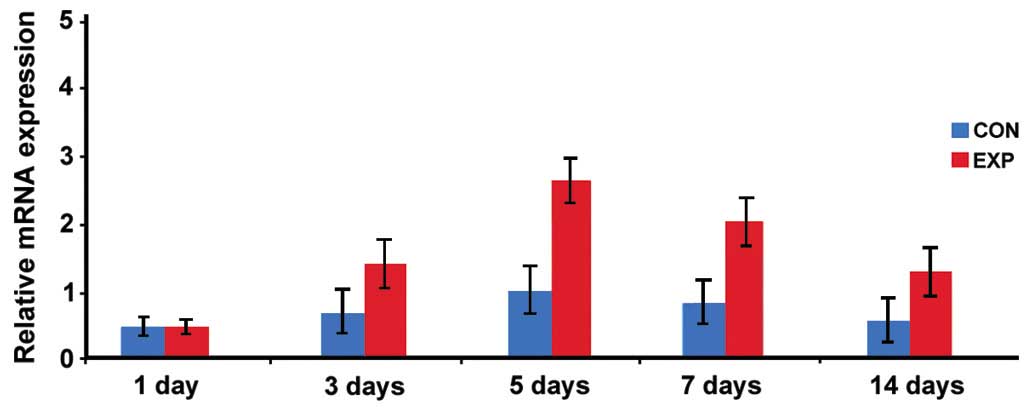
Expression of BMP-2 mRNA
The expression level of BMP-2 mRNA in the
observation group was significantly higher than that in the control
group at days 3, 5, 7 and 14, and the difference was statistically
significant (p<0.05; Fig. 5).
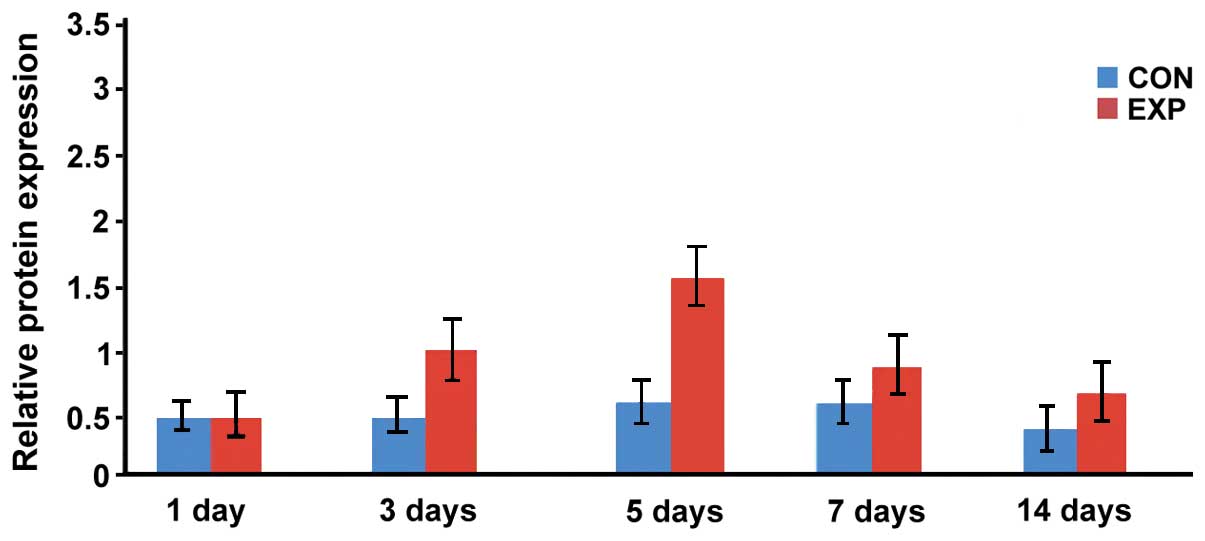
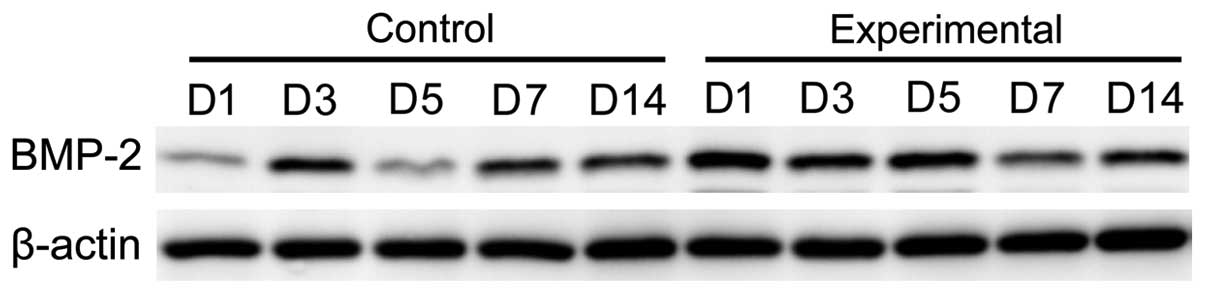
Expression level of BMP-2 protein
The expression levels of BMP-2 protein in the
observation group at days 3, 5, 7 and 14 were significantly higher
than that in the control group, and the difference was
statistically significant (p<0.05; Figs. 6 and 7).
Discussion
Tooth movement is closely associated with the
application of orthodontic force. Orthodontic force is the key
factor of alveolar bone remodeling (10). The periodontal ligament is a thin
layer of dense connective tissue between the alveolar bone and
cementum with the capacity for self-renewal and self-restoration.
Orthodontic force is important in keeping the dynamic balance of
periodontal tissues (11). In the
process of orthodontic treatment on tooth movement, a tension zone
and pressure zone may appear in the parodontium area of the tooth
under pressure. The periodontal ligament in the tension area may be
in tensional state, the expression of genes related to bone
formation, such as osteocalcin and bone sialoprotein, were
upregulated, and thus promoted the formation of new bones in the
tension area. The periodontal ligament in the tension area has a
compression force, which may activate the osteoclast, promote the
absorption of alveolar bone in the tension area, and eventually
promote the balance between bone formation and bone absorption of
alveolar bone surrounding the stressed tooth (12).
1,25-DHCC is the most active metabolite of vitamin
D, which can promote bone deposition and inhibit the release of
parathyroid hormone. A physiological dose of 1,25-DHCC would not
stimulate bone resorption while a low dose of 1,25-DHCC promotes
the differentiation of osteoclasts by upregulating the expression
of receptor activator of nuclear factor-κB ligand (13). Collins et al injected
1,25-DHCC into the dental ligament of cats' cuspid teeth and found
that 3 weeks later, the movement speed of the cuspid teeth of the
observation group was 60% faster than that of the control group
(14). The main effects of the
ultrasonic cavitation effect on the human body mainly included
cavitation, mechanical, thermal, thixotropic, dispersion,
fragmentation and hemostatic effect. As a type of high frequency
soundwave, ultrasonic was able to transmit the wave into the organs
via the form of mechanical energy. Ultrasonic has been widely
applied in the medical field. Low intensity-pulsed ultrasound
treatment is the best known parameter for promoting fracture
healing. A large number of animal experiments and clinical studies
have demonstrated that low intensity-pulsed ultrasound can reduce
the healing time of fracture, and effectively treat the delay
fracture healing and bone non-union. Compared with other
treatments, it is safer and more invasive (15).
In the process of proliferation and differentiation,
osteoblasts may undergo four stages: cell proliferation, matrix
secretion, matrix maturation and mineralization formation. This
process is regulated by a series of cytokines, signaling molecules,
and the surrounding environment, such as BMPs, transforming growth
factor-β. BMPs play an important role in the metabolism of bone.
BMP-2 is important in controlling the proliferation,
differentiation and bone matrix secretion of osteoblasts by
stimulating specific transcription procedures in the period of
embryonic skeletal development and bone remodeling after birth
(16,17).
In the present study, we found that tooth movement
distance in the observation group at days 5, 7 and 14 was
significantly longer than that in the control group. The number of
positive cells in the observation group was significantly more than
that in the control group at days 3, 5, 7 and 14. The levels of
BMP-2 mRNA and protein expression in the observation group were
significantly higher than those in the control group at days 3, 5,
7 and 14. The data show that piezosurgery may significantly
accelerate the movement of rat orthodontic alveolar bone tooth. It
may be associated with an increasing BMP-2 expression.
Acknowledgements
The present study was funded by the Shandong
Province Natural Science fund project (no. ZR2014HL052) and
Shandong province Medical Science and Technology Development plan
project (no. 2014 ws0048).
References
|
1
|
Yang EY and Kiyak HA: Orthodontic
treatment timing: A survey of orthodontists. Am J Orthod
Dentofacial Orthop. 113:96–103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grippaudo C, Pantanali F, Paolantonio EG,
Saulle R, Latorre G and Deli R: Orthodontic treatment timing in
growing patients. Eur J Paediatr Dent. 14:231–236. 2013.PubMed/NCBI
|
|
3
|
Viazis AD: Efficient orthodontic treatment
timing. Am J Orthod Dentofacial Orthop. 108:560–561. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Melo AC, Carneiro LO, Pontes LF, Cecim RL,
de Mattos JN and Normando D: Factors related to orthodontic
treatment time in adult patients. Dental Press J Orthod. 18:59–63.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Bialy T, Hassan A, Albaghdadi T, Fouad
HA and Maimani AR: Growth modification of the mandible with
ultrasound in baboons: a preliminary report. Am J Orthod
Dentofacial Orthop. 130:435e7–14. 2006. View Article : Google Scholar
|
|
6
|
Ge MK, He WL, Chen J, Wen C, Yin X, Hu ZA,
Liu ZP and Zou SJ: Efficacy of low-level laser therapy for
accelerating tooth movement during orthodontic treatment: a
systematic review and meta-analysis. Lasers Med Sci. 30:1609–1618.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Charavet C, Lecloux G, Bruwier A, Rompen
E, Maes N, Limme M and Lambert F: Localized piezoelectric alveolar
decortication for orthodontic treatment in adults: A randomized
controlled trial. J Dent Res. 95:1003–1009. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aldinger G, Herr G, Kusswetter W, Reis HJ,
Thielemann FW and Holz U: Bone morphogenetic protein: A review. Int
Orthop. 15:169–177. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He X, Dziak R, Yuan X, Mao K, Genco R,
Swihart M, Sarkar D, Li C, Wang C, Lu L, Andreadis S and Yang S:
BMP2 genetically engineered MSCs and EPCs promote vascularized bone
regeneration in rat critical-sized calvarial bone defects. PLoS
One. 8:e604732013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olson C, Uribe F, Kalajzic Z, Utreja A,
Nanda R, Rowe D and Wadhwa S: Orthodontic tooth movement causes
decreased promoter expression of collagen type 1, bone sialoprotein
and alpha-smooth muscle actin in the periodontal ligament. Orthod
Craniofac Res. 15:52–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Proff P and Römer P: The molecular
mechanism behind bone remodelling: a review. Clin Oral Investig.
13:355–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sirisoontorn I, Hotokezaka H, Hashimoto M,
Gonzales C, Luppanapornlarp S, Darendeliler MA and Yoshida N: Tooth
movement and root resorption; the effect of ovariectomy on
orthodontic force application in rats. Angle Orthod. 81:570–577.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SK, Kalinowski J, Jastrzebski S and
Lorenzo JA: 1,25(OH)2 vitamin D3-stimulated osteoclast formation in
spleen-osteoblast cocultures is mediated in part by enhanced IL-1
alpha and receptor activator of NF-kappa B ligand production in
osteoblasts. J Immunol. 169:2374–2380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collins MK and Sinclair PM: The local use
of vitamin D to increase the rate of orthodontic tooth movement. Am
J Orthod Dentofacial Orthop. 94:278–284. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M, Hayashi M, Fujita S, Yoshida
T, Utsunomiya T, Yamamoto H and Kasai K: Low-energy laser
irradiation facilitates the velocity of tooth movement and the
expressions of matrix metalloproteinase-9, cathepsin K, and
alpha(v) beta(3) integrin in rats. Eur J Orthod. 32:131–139. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li P, Bai Y, Yin G, Pu X, Huang Z, Liao X,
Chen X and Yao Y: Synergistic and sequential effects of BMP-2, bFGF
and VEGF on osteogenic differentiation of rat osteoblasts. J Bone
Miner Metab. 32:627–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu X, Zhao Q, Yang S, Fu G and Chen Y: A
new approach to accelerate orthodontic tooth movement in women:
Orthodontic force application after ovulation. Med Hypotheses.
75:405–407. 2010. View Article : Google Scholar : PubMed/NCBI
|