Introduction
Osteoarthritis (OA) is a complex degenerative joint
disease that characterized by the progressive loss of articular
cartilage that leads to chronic pain and functional limitations.
The prevalence of OA has markedly increased in the past two decades
due to an ageing population and increasing obesity, and the public
health consequences of OA and OA-associated disability are expected
to increase as a result of the increasing incidence of obesity and
the aging of the population (1–3).
However, the molecular mechanisms underlying OA remain to be fully
elucidated. Several biochemical and biomechanical factors are
thought to underlie OA pathogenesis.
OPN (osteopontin) is a 44–75 KD multifunctional
phosphoprotein secreted by numerous cell types, including
osteoclasts, macrophages, lymphocytes, epithelial cells and
vascular smooth muscle cells (SMC) (4–5). This
protein, also known as early T cell activation gene-1 is abundant
in bone, where it mediates important cell-matrix and cell-cell
interactions (5). During the past
two decades, OPN has become the subject of increased research in OA
pathogenesis. Previous studies have demonstrated a close
association between OPN and OA (6–17), which
suggests OPN may serve as a biochemical marker of disease severity
in knee OA. In addition, the functions of OPN are tightly regulated
by its phosphorylation status in normal and pathological states
(4). OPN phosphorylation has been
demonstrated to regulate cell adhesion and migration (18–21).
Furthermore, the different types and the extent of OPN
phosphorylation contribute to the greater complexity of
OPN-receptor binding and downstream signaling pathways (18). Xu et al (22) revealed that OA cartilage had higher
phosphorylation levels of OPN compared with normal cartilage, OPN
increases matrix metalloproteinase (MMP)-13 expression, and the
upregulation of MMP-13 expression induced by phosphorylated (p)-OPN
was more marked than that induced by non-phosphorylated OPN.
Further studies are required in order to elucidate the detailed
molecular mechanisms underlying the effect of p-OPN on cartilage
degeneration.
Apoptosis, or genetically programmed cell death, has
been associated with OA (23,24).
Since chondrocytes are the only resident cells located in articular
cartilage and are responsible for both the synthesis and the
breakdown of the extracellular matrix (ECM) and tissue function
(25), research to elucidate the
detailed mechanism underlying apoptosis in cartilage is of great
significance for understanding OA pathogenesis (26). Dalal et al (27) reported that OPN stimulates apoptosis
in adult cardiac myocytes via the involvement of CD44 receptors.
However, to date no investigation into the roles of p-OPN in
apoptosis and pro-inflammatory cytokines in human chondrocytes have
been reported.
The aim of the present study was to investigate the
effects of p-OPN on apoptosis and pro-inflammatory cytokine
expression of human knee OA chondrocytes, which may serve as a
useful tool to mark the OA disease process and to further elucidate
the molecular changes and signaling pathways underlying OA.
Materials and methods
OA cartilage acquisition
OA cartilage samples were obtained from the knees of
patients (n=16; 6 males and 10 females; mean age, 63.5±10.3 years)
during total knee arthroplasty at Xiangya Hospital (Changsha,
China) between January 2014 and June 2014. Clinical data were
carefully reviewed to exclude any secondary forms of OA, rheumatoid
arthritis or other arthritis forms. The cartilage samples were
macroscopically altered and histological analysis of representative
samples showed typical OA changes, such as focal cell loss,
chondrocyte cluster formation and fibrillation. The present study
was approved by the ethics committee of the Xiangya Hospital. All
patients willing to donate knee tissue samples provided
written-informed consent.
p-OPN preparation
Phosphorylation of recombinant osteopontin increases
its ability to support osteoclast adhesion and cell attachment,
which is dependent on an RGD sequence to the same extent as the
native phosphorylated osteopontin (28). Therefore, mitogen-activated protein
kinase (MAPK) was used to obtain phosphorylated exogenous OPN.
rhOPN (R&D Systems, Inc., Minneapolis, MN, USA) was diluted
with phosphate-buffered saline (PBS) to 0.1 µg/ml. The diluted OPN
(5 µl) was phosphorylated in a total volume of 50 µl containing 10
mM ATP (10 µl), 1 µl MAPK (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) 10X kinase buffer [25 mM Tris-HCl (pH 7.5), 5 mM
β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM
Na3VO4, 10 mM MgCl2] at 30°C for
30 min. The phosphorylation was terminated by adding 10 µl of stop
mixture (1% SDS and 100 mM EDTA). These reagents were purchased
from Cell Signaling Technology Inc. (Danvers, MA, USA).
Cell isolation and culture
conditions
OA cartilage samples were cut from the subchondral
bone and homogenized to form pieces <1 mm3, prior to
being treated with 2% penicillin/streptomycin and 0.2% amphotericin
B (Gibco; Thermo Fisher Scientific, Inc.) in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.). OA
chondrocytes were isolated from the OA articular cartilage using a
sequential enzymatic digestion with 0.1% hyaluronidase for 30 min,
then 0.5% pronase for 1 h, and 0.2% collagenase (Gibco; Thermo
Fisher Scientific, Inc.) for 1 h at 37°C carried out in the washing
solution (DMEM, penicillin/streptomycin and amphotericin B). The
suspension was then filtered twice through a 70 µm nylon mesh,
washed twice with 4°C PBS, and centrifuged at 450 × g for 10 min at
4°C. A trypan blue viability test was conducted and demonstrated
that 93% of the recovered cells were alive. The primary cultures of
chondrocytes were kept at 37°C in an atmosphere containing 5%
CO2 for 2 weeks.
Treatments
Human OA chondrocytes at first passage were seeded
on a 24-well plate at a starting density of 1×104
cells/well with two medium changes (DMEM) per week until they
became confluent. The cells were then divided into three groups:
(1) The p-OPN group, treated with 4
µg/ml p-OPN for 48 h as previously described (23); (2) the
OPN group, treated with 4 µg/ml OPN (R&D Systems, Inc.) for 48
h as previously described (15,16,23); and
(3) the control group, stimulated
with 4 µg/ml buffer (Cell Signaling Technology, Inc.) for 48 h.
Apoptosis assay
The frequency of chondrocyte apoptosis was measured
by flow cytometry with Annexin V-fluorescein isothiocyanate (FITC)
and propidium iodide (PI; both from Roche Diagnostics GmbH,
Mannheim, Germany). A total of 1×104 treated
chondrocytes were collected from each group, washed in cold PBS and
incubated with Annexin V-FITC and PI at room temperature for 15 min
in the dark on ice. These samples were then analyzed using a
fluorescence-activated cell sorter (BD Biosciences, San Jose, CA,
USA). Cell Quest software (version 7.5.3; BD Biosciences) was used
to analyze the percentage of apoptosis. All tests were repeated in
triplicate.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from OA chondrocytes
following treatments using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Total RNA (1 µg) was quantified by spectrophotometry
and reverse transcribed to cDNA using an AllinOne™ First Strand
cDNA Synthesis kit (GeneCopoeia, Inc., Rockville, MD, USA). RT-qPCR
was performed using a SYBR Green qPCR SuperMix (GeneCopoeia, Inc.)
and ABI 7900 Sequence Detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The qPCR thermal cycling conditions were
as follows: Initial denaturation at 95°C for 10 min, followed by 40
cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 30
sec and extension at 72°C for 30 sec. Melting curve analysis was
performed following the final amplification period via a
temperature gradient of 95°C for 15 sec, 60°C for 15 sec and 95°C
for 15 sec. The specific primers used for the various human mRNAs
(GenScript, Nanjing, China) were: Interleukin (IL)-1β forward,
5′-CGTTCCCATTAGACAACTGCA-3′, and reverse,
5′-GGTATAGATTCTTTCCTTTGAGGC-3′; tumor necrosis factor (TNF)-α
forward, 5′-AAAGCATGATCCGAGATGTGTGGAA-3′, and reverse,
5′-AGTAGACAGAAGAGCGTGGTGGC-3′; IL-6 forward,
5′-CCAGTTGCCTTCTTGGGACT-3′, and reverse,
5′-GTCTGTTGTGGGTGGTATCCTCTGT-3′; nuclear factor (NF)-κB forward,
5′-CCCATCGGGTTCCCATAAAG-3′, and reverse,
5′-GCCTGAAGCAAATGTTGGCGTA-3′; and β-actin forward,
5′-CATCCTGCGTCTGGACCTGG-3′, and reverse,
5′-TAATGTCACGCACGATTTCC-3′. The data were given as a quantitative
cycle (Cq). IL-1β, TNF-α, IL-6 mRNA and NF-κB mRNA expression
levels were normalized to β-actin mRNA controls using the
comparative 2−ΔΔCq method (29).
Western blot analysis
Total proteins were extracted from the cells using
whole-cell ice-cold lysis buffer containing 50 mM Tris-HCl, pH 7.4,
1% NP-40, 150 mM NaCl and 0.1% SDS supplemented with proteinase
inhibitor (one tablet/10 ml; Roche Diagnostics, Indianapolis, IN,
USA). For the western blot analysis, 40 µg of protein extracts were
size-fractionated by 4–20% SDS-PAGE, and transferred onto
nitrocellulose membranes (Invitrogen; Thermo Fisher Scientific,
Inc.). The membrane was blocked with 5% bovine serum albumin in
Tris-buffered saline with Tween 20 (TBST) for 1 h. The membranes
were then incubated with the following primary antibodies for 24 h
at 4°C: Anti-IL-1β (1:1,000; cat. no. 12242; Cell Signaling
Technology, Inc.), TNF-α (1:1,000; cat. no. 37078; Cell Signaling
Technology, Inc.), IL-6 (1:100; cat. no. 12153; Cell Signaling
Technology, Inc.), NF-κB (1:500; cat. no. 8242; Cell Signaling
Technology, Inc.) and β-actin (1:4,000; cat. no. 8457; Cell
Signaling Technology, Inc.). The membranes were then washed with
TBST for 5 min 3 times and incubated with horseradish
peroxidase-conjugated anti-mouse or rabbit secondary antibody
(1:1,000; cat. no. sc-2030; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). The proteins were detected by a chemiluminescence system
using an enhanced chemiluminescence (ECL) reagent Pierce ECL
Western Blotting Substrate (Thermo Fisher Scientific, Inc.).
Intensity of the bands was quantified using Quantity One software
(version 4.2.3; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were expressed as means ± standard deviation.
Statistical analysis was performed with SPSS 15.0 statistical
software (SPSS Inc., Chicago, IL, USA). Comparisons between two
groups were made using Student's t-test. One-way analysis of
variance followed by Student-Newman-Kuels test was utilized to
determine the significant difference between multiple groups.
P<0.05 was considered to indicate a statistically significant
result.
Results
Effect of OPN and its phosphorylation
on the relative mRNA expression levels of pro-inflammatory
factors
As shown in Fig. 1,
the relative mRNA expression levels of IL-1β, TNF-α, IL-6 and NF-κB
in the OPN group (1.744±0.125-fold, 1.522±0.086-fold,
1.204±0.027-fold and 1.880±0.052-fold, respectively) were
significantly higher compared with the control group (P<0.05).
Furthermore, p-OPN-treated chondrocytes exhibited enhanced relative
mRNA expression levels of IL-1β, TNF-α, IL-6 and NF-κB
(2.295±0.087-fold, 1.761±0.076-fold, 1.444±0.076-fold and
2.423±0.083-fold, respectively) compared with the buffer control
group (P<0.05). In addition, Fig.
1 shows that chondrocytes treated with p-OPN exhibited a
significant increase the relative mRNA expression levels of IL-1β,
TNF-α, IL-6 and NF-κB compared with cells in the OPN group
(P<0.05). Therefore, OPN upregulates the expression of
pro-inflammatory factors (IL-1β, TNF-α, IL-6 and NF-κB) at the gene
level and the effects are associated with the state of
phosphorylation.
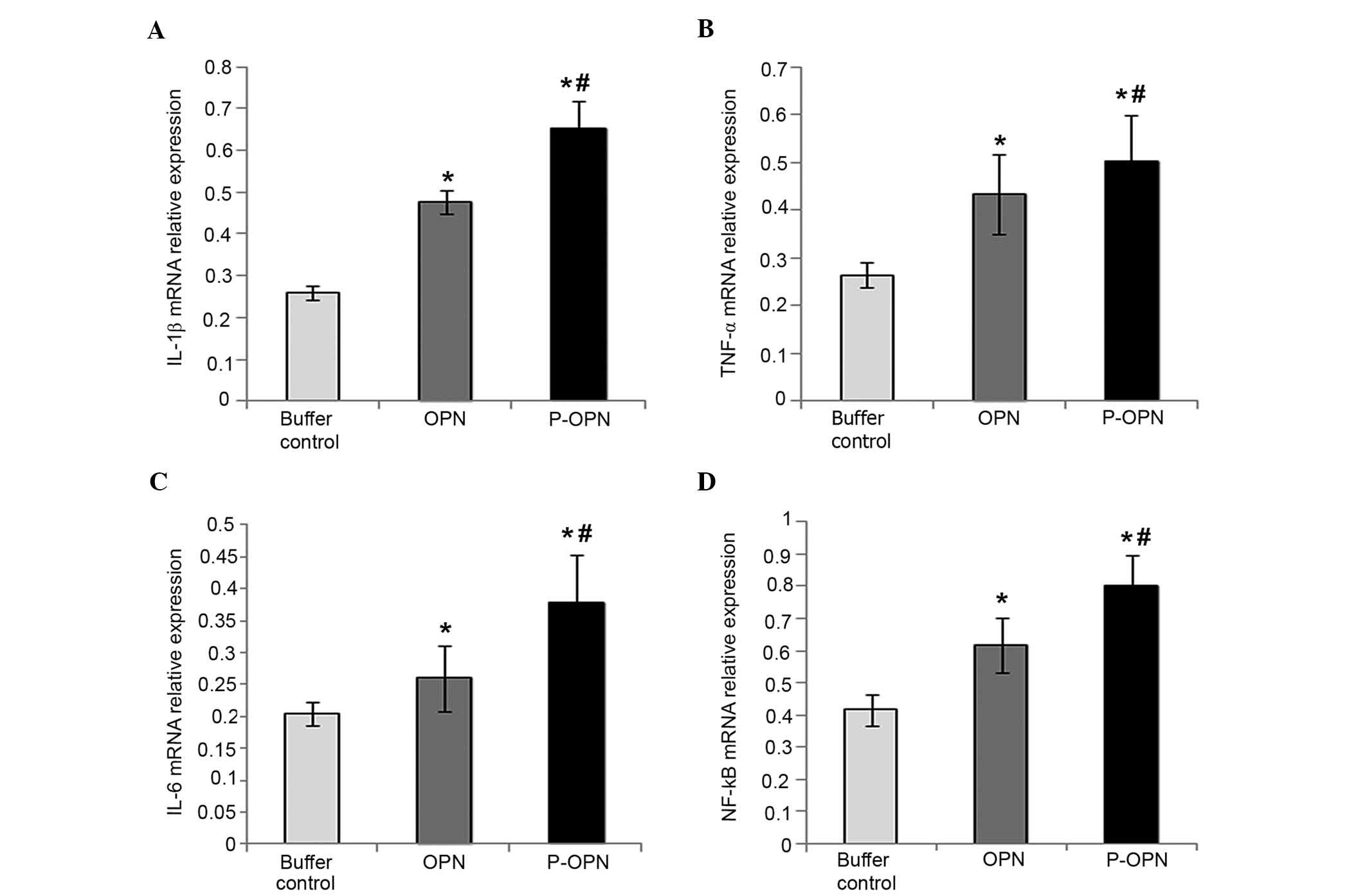 | Figure 1.p-OPN and OPN increase the relative
mRNA expression levels of (A) IL-1β, (B) TNF-α, (C) IL-6 and (D)
NF-κB in OA chondrocytes. Reverse transcription-quantitative
polymerase chain reaction was carried out to determine the relative
mRNA expression levels of pro-inflammatory factors (IL-1β, TNF-α,
IL-6 and NF-κB) in chondrocytes treated for 48 h with p-OPN, OPN
and buffer. For comparative purposes, mRNA expression levels in
buffer-treated cells were normalized to 1. Error bars represented
fold changes in the mRNA expression of IL-1β, TNF-α, IL-6 and NF-κB
following normalization with the expression levels of
buffer-treated OA chondrocytes. Samples from three independent
experiments were measured. Results were provided as means ±
standard deviation. *P<0.05, vs. the control;
#P<0.05, vs. the OPN group. p-OPN, phosphorylated
osteopontin; IL, interleukin; TNF, tumor necrosis factor; NF,
nuclear factor; OA, osteoarthritis. |
Effect of OPN and its phosphorylation
on the protein expression levels of pro-inflammatory factors
To determine the effect of OPN phosphorylation on
the protein expression of pro-inflammatory factors, western
blotting was employed to measure the protein expression levels of
IL-1β, TNF-α, IL-6 and NF-κB in all three groups of OA chondrocytes
(Fig. 2). The highest protein
expression levels of pro-inflammatory factors were found in
chondrocytes treated with p-OPN (P<0.05), although OPN-treated
chondrocytes also exhibited significantly increased
pro-inflammatory factor expression levels (P<0.05). Upregulation
of the protein expression of pro-inflammatory factors detected in
chondrocytes treated with OPN (whether phosphorylated or not)
suggests that OPN activates the protein expression of
pro-inflammatory factors. However, the 2–3-fold upregulation of the
protein expression levels of pro-inflammatory factors detected in
chondrocytes treated with p-OPN suggests the activation of
pro-inflammatory factors not only relies on the quantity of OPN,
but also on post-translational phosphorylation. Therefore, OPN
upregulates the expression of pro-inflammatory factors (IL-1β,
TNF-α, IL-6 and NF-κB) at the protein level and the effects are
associated with the state of phosphorylation.
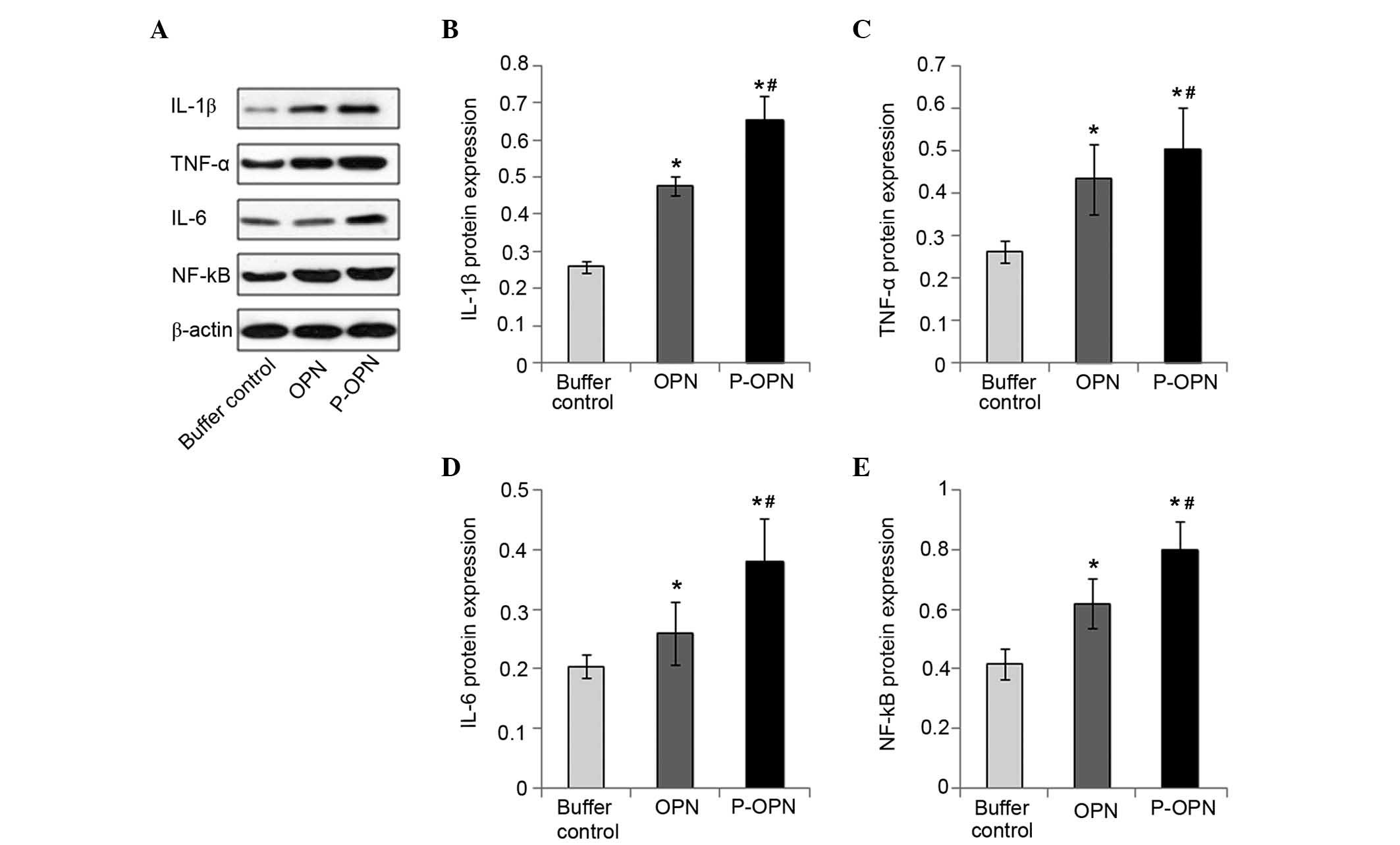 | Figure 2.p-OPN and OPN increase the protein
expression levels of pro-inflammatory factors in OA chondrocytes.
(A) Western blotting was carried out to determine the protein
expression levels of IL-1β, TNF-α, IL-6 and NF-κB in chondrocytes
treated for 48 h with p-OPN, OPN and buffer. Bars represented the
fold changes in protein expression of (B) IL-1β, (C) TNF-α, (D)
IL-6 and (E) NF-κB following normalization with the expression
levels of β-actin in the OA chondrocytes. Samples from three
independent experiments were measured. Results were given as means
± standard deviation. *P<0.05, vs. the control;
#P<0.05, vs. the OPN group. p-OPN, phosphorylated
osteopontin; IL, interleukin; TNF, tumor necrosis factor; NF,
nuclear factor; OA, osteoarthritis. |
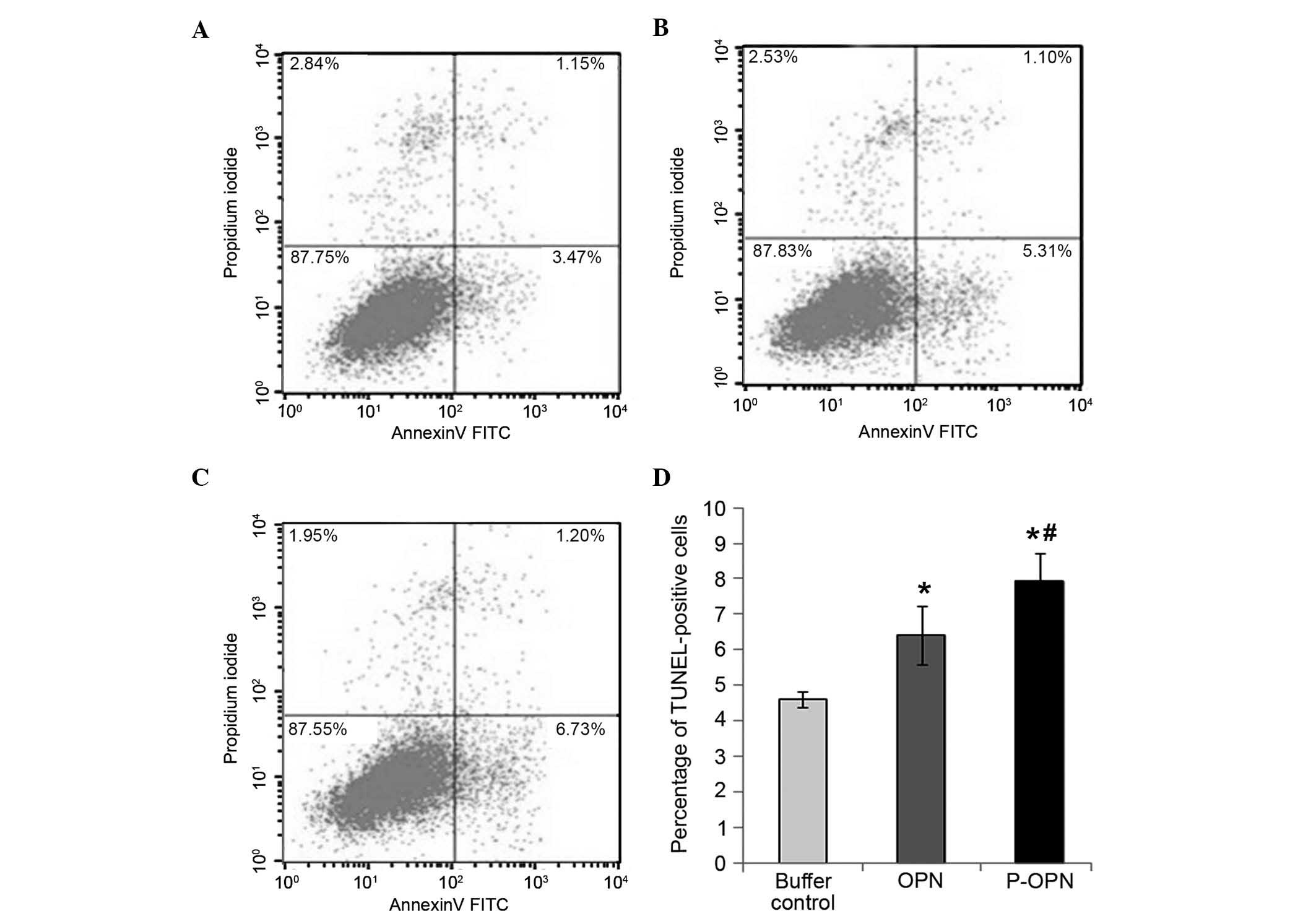
Effect of OPN and its phosphorylation
on the apoptosis of human OA chondrocytes
To examine the effect of OPN phosphorylation on OA
chondrocyte apoptosis, flow cytometry staining with Annexin
V-FITC/PI was employed to detect chondrocyte apoptosis in all three
groups (Fig. 3). Compared with the
buffer control group, treatment with OPN or p-OPN for 48 h caused
an increase in the percentage of Annexin V-positive cells
(P<0.05; Fig. 3). Furthermore,
the percentage of Annexin V-positive cells in the p-OPN group was
higher compared with that of the OPN group (P<0.05). Therefore,
OPN treatment increases human OA chondrocyte apoptosis and the
effects are associated with the state of phosphorylation.
Discussion
OPN is found predominantly as a secreted protein
expressed by various types of cells, and is present in the majority
of tissues and body fluids. Post-translational modifications (such
as sulfation, O-glycosylation and phosphorylation) modulate the
protein function of OPN (30).
Previous studies (6–21) predominantly focused on the amount of
OPN expression and demonstrated that the gene were associated with
the susceptibility and severity of OA. Osteopontin has an important
role in OA progression. Morimoto et al (31) considered that the role of OPN is
dependent on its phosphorylation state in rheumatoid arthritis. Our
previous study (22) revealed that
p-OPN led to higher levels of MMP-13 expression than OPN. This
prompted further investigation to determine whether phosphorylated
modification of OPN has a role in apoptosis and pro-inflammatory
cytokine expression in human knee OA chondrocytes. The results of
the present study demonstrated that p-OPN causes cell apoptosis and
production of inflammatory mediators in articular chondrocytes, two
primary features of OA cartilage pathology.
Chronic, low-grade inflammation in OA contributes to
the severity and symptoms of OA, as well as its progression
(32). Inflammatory cytokines (such
as TNF-α, IL-1β, IL-6 and multiple chemokines) released from
various cell types are able to promote disease progression of OA
by, for example, altering chondrocyte differentiation and function,
and promoting synovitis and subchondral bone turnover (33,34).
Previously, it was observed that OPN enhanced Th1 cytokine
(interferon γ and TNF) levels and inhibited Th2 cytokine (IL-4 and
IL-10) levels (35). We report
herein a proinflammatory response of human knee OA chondrocytes to
OPN treatment, as evidenced by the upregulation of IL-1β, TNF-α,
IL-6 and NF-κB expression at the gene and protein levels.
Furthermore, p-OPN exhibited more marked proinflammatory effects on
human knee OA chondrocytes through upregulation of proinflammatory
cytokines (IL-1β, TNF-α, IL-6, NF-κB) at the gene and protein level
compared with non-phosphorylated OPN. These results indicate that
OPN or p-OPN in synovial fluid is able to initiate joint
inflammation and/or aggravate the inflammatory process, potentially
contributing to the development and progression of OA.
Chondrocytes are the single cell type responsible
for preserving the integrity and function of articular cartilage by
synthesizing and maintaining the ECM and providing a structural
framework; reduced cartilage cellularity is a hallmark of OA
(36). Several processes (such as
reactive oxygen species accumulation, death receptor activation,
mitochondrial dysfunction and mechanical stress) are capable of
causing cellular apoptosis (37).
Previously, it was observed that neither 100 ng/ml nor 1 µg/ml
rhOPN caused cytotoxicity or chondrocyte apoptosis, and that
treatment with 1 µg/ml rhOPN significantly increased the relative
mRNA expression levels of tissue inhibitor of metalloproteinase
(TIMP)-1 and TIMP-2 (16). These
results suggest that OPN may exert protective effects against
pathological changes in advanced-stage OA. To examine the mechanism
underlying OPN or p-OPN regulation of the OA associated changes in
human articular chondrocytes, the present study investigated
whether OPN or p-OPN modulates apoptosis. The results demonstrated
that 4 ng/ml OPN induces human articular chondrocyte apoptosis. In
addition, proapoptotic and proinflammatory effects are markedly
enhanced when OPN is in a phosphorylated state.
The present study had several limitations. Firstly,
the potential phosphokinases of OPN were located in the consensus
sequence for the mammary gland casein kinase (CK) and the CKII
consensus sequence (37), both of
which are from the CK family. MAPKs were employed to act as the
phosphokinase of OPN, and this may have resulted in the
mis-phosphorylation of the non-OA specific phosphorylation sites.
Secondly, as the native human OPN has 36 potential phosphoric sites
and is highly tissue- and cell-specific for phosphorylation
(37), site-specific
characterization of O-glycosylation in human OPN remains poorly
understood. Therefore, it is difficult to determine whether the
occurrence of OA is the result of the phosphorylation of specific
sites. Post-translational modifications of OPN may affect its
structure and biological properties. Further investigations of
phosphorylation on specific sites may provide more detailed
information regarding the possible mechanism underlying the effect
of OPN in OA.
In summary, OPN increases the expression levels of
pro-inflammatory factors (IL-1β, TNF-α, IL-6, NF-κB) and induces
chondrocyte apoptosis. This effect can be greatly increased by OPN
phosphorylation, which suggests that p-OPN may contribute to the
causes and pathogenesis of knee OA. Inhibition of p-OPN may provide
a novel effective strategy to slow or halt OA progression.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81672225, 81201420,
81272034 and 81472130), the Natural Science Foundation of Hunan
Province (grant no. 14JJ3032), the Shenhua Yuying Talent Plan of
Central South University and the Huxiang Youth Talent Program. The
authors are also grateful for the support of the Orthopedics
Research Institute of Xiangya Hospital.
References
|
1
|
Lawrence RC, Felson DT, Helmick CG, Arnold
LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG,
et al: Estimates of the prevalence of arthritis and other rheumatic
conditions in the United States. Part II. Arthritis Rheum.
58:26–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu M and Hu C: Association of MIF in
serum and synovial fluid with severity of knee osteoarthritis. Clin
Biochem. 45:737–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holt HL, Katz JN, Reichmann WM, Gerlovin
H, Wright EA, Hunter DJ, Jordan JM, Kessler CL and Losina E:
Forecasting the burden of advanced knee osteoarthritis over a 10
year period in a cohort of 60–64 year-old US adults. Osteoarthritis
Cartilage. 19:44–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sodek J, Ganss B and McKee MD:
Osteopontin. Crit Rev Oral Biol Med. 11:279–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie Y, Sakatsume M, Nishi S, Narita I,
Arakawa M and Gejyo F: Expression, roles, receptors and regulation
of osteopontin in the kidney. Kidney Int. 60:1645–1657. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pullig O, Weseloh G, Gauer S and Swoboda
B: Osteopontin is expressed by adult human osteoarthritic
chondrocytes: Protein and mRNA analysis of normal and
osteoarthritic cartilage. Matrix Biol. 19:245–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Attur MG, Dave MN, Stuchin S, Kowalski AJ,
Steiner G, Abramson SB, Denhardt DT and Amin AR: Osteopontin: An
intrinsic inhibitor of inflammation in cartilage. Arthritis Rheum.
44:578–584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakata M, Tsuruha JI, Masuko-Hongo K,
Nakamura H, Matsui T, Sudo A, Nishioka K and Kato T: Autoantibodies
to osteopontin in patients with osteoarthritis and rheumatoid
arthritis. J Rheumatol. 28:1492–1495. 2001.PubMed/NCBI
|
|
9
|
Honsawek S, Tanavalee A, Sakdinakiattikoon
M, Chayanupatkul M and Yuktanandana P: Correlation of plasma and
synovial fluid osteopontin with disease severity in knee
osteoarthritis. Clin Biochem. 42:808–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsui Y, Iwasaki N, Kon S, Takahashi D,
Morimoto J, Matsui Y, Denhardt DT, Rittling S, Minami A and Uede T:
Accelerated development of aging-associated and instability-induced
osteoarthritis in osteopontin-deficient mice. Arthritis Rheum.
60:2362–2371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao SG, Li KH, Zeng KB, Tu M, Xu M and Lei
GH: Elevated osteopontin level of synovial fluid and articular
cartilage is associated with disease severity in knee
osteoarthritis patients. Osteoarthritis Cartilage. 18:82–87. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hasegawa M, Segawa T, Maeda M, Yoshida T
and Sudo A: Thrombin-cleaved osteopontin levels in synovial fluid
correlate with disease severity of knee osteoarthritis. J
Rheumatol. 38:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Y, Yao M, Liu Q and Zhou C: OPN gene
polymorphisms influence the risk of knee OA and OPN levels in
synovial fluid in a Chinese population. Arthritis Res Ther.
15:R32013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao SG, Cheng L, Zeng C, Wei LC, Zhang FJ,
Tian J, Tu M, Luo W and Lei GH: Usefulness of specific OA
biomarkers, thrombin-cleaved osteopontin, in the posterior cruciate
ligament OA rabbit model. Osteoarthritis Cartilage. 21:144–550.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang FJ, Yu WB, Luo W, Gao SG, Li YS and
Lei GH: Effect of osteopontin on TIMP-1 and TIMP-2 mRNA in
chondrocytes of human knee osteoarthritis in vitro. Exp Ther Med.
8:391–394. 2014.PubMed/NCBI
|
|
16
|
Yang Y, Gao SG, Zhang FJ, Luo W, Xue JX
and Lei GH: Effects of osteopontin on the expression of IL-6 and
IL-8 inflammatory factors in human knee osteoarthritis
chondrocytes. Eur Rev Med Pharmacol Sci. 18:3580–3586.
2014.PubMed/NCBI
|
|
17
|
Martínez-Calleja A, Velasquillo C,
Vega-López M, Arellano-Jiménez MJ, Tsutsumi-Fujiyoshi VK,
Mondragón-Flores R and Kouri-Flores JB: Osteopontin expression and
localization of Ca++ deposits in early stages of osteoarthritis in
a rat model. Histol Histopathol. 29:925–933. 2014.PubMed/NCBI
|
|
18
|
Weber GF, Zawaideh S, Hikita S, Kumar VA,
Cantor H and Ashkar S: Phosphorylation-dependent interaction of
osteopontin with its receptors regulates macrophage migration and
activation. J Leukoc Biol. 72:752–761. 2002.PubMed/NCBI
|
|
19
|
Al-Shami R, Sorensen ES, Ek-Rylander B,
Andersson G, Carson DD and Farach-Carson MC: Phosphorylated
osteopontin promotes migration of human choriocarcinoma cells via a
p70 S6 kinase-dependent pathway. J Cell Biochem. 94:1218–1233.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ek-Rylander B, Flores M, Wendel M,
Heinegárd D and Andersson G: Dephosphorylation of osteopontin and
bone sialoprotein by osteoclastic tartrate-resistant acid
phosphatase. J Biol Chem. 269:14853–14856. 1994.PubMed/NCBI
|
|
21
|
Ek-Rylander B and Andersson G: Osteoclast
migration on phosphorylated osteopontin is regulated by endogenous
tartrateresistant acid phosphatase. Exp Cell Res. 316:443–451.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu M, Zhang L, Zhao L, Gao S, Han R, Su D
and Lei G: Phosphorylation of osteopontin in osteoarthritis
degenerative cartilage and its effect on matrix metalloprotease 13.
Rheumatol Int. 33:1313–1319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blanco FJ, Guitian R, Vazquez-Martul E, de
Toro FJ and Galdo F: Osteoarthritis chondrocytes die by apoptosis:
A possible pathway for osteoarthritis pathology. Arthritis Rheum.
41:284–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HA, Lee YJ, Seong SC, Choe KW and Song
YW: Apoptotic chondrocyte death in human osteoarthritis. J
Rheumatol. 27:455–562. 2000.PubMed/NCBI
|
|
25
|
Aigner T, Soder S, Gebhard PM, McAlinden A
and Haag J: Mechanisms of disease: Role of chondrocytes in the
pathogenesis of osteoarthritis-structure, chaos and senescence. Nat
Clin Pract Rheumatol. 3:391–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dalal S, Zha Q, Daniels CR, Steagall RJ,
Joyner WL, Gadeau AP, Singh M and Singh K: Osteopontin stimulates
apoptosis in adult cardiac myocytes via the involvement of CD44
receptors, mitochondrial death pathway and endoplasmic reticulum
stress. Am J Physiol Heart Circ Physiol. 306:H1182–H1191. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katayama Y, House CM, Udagawa N, Kazama
JJ, McFarland RJ, Martin TJ and Findlay DM: Casein kinase 2
phosphorylation of recombinant rat osteopontin enhances adhesion of
osteoclasts but not osteoblasts. J Cell Physiol. 176:179–187. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anborgh PH, Mutrie JC, Tuck AB and
Chambers AF: Pre- and post-translational regulation of osteopontin
in cancer. J Cell Commun Signal. 5:111–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morimoto J, Kon S, Matsui Y and Uede T:
Osteopontin; as a target molecule for the treatment of inflammatory
diseases. Curr Drug Targets. 11:494–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chevalier X, Eymard F and Richette P:
Biologic agents in osteoarthritis: Hopes and disappointments. Nat
Rev Rheumatol. 9:400–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Husa M, Liu-Bryan R and Terkeltaub R:
Shifting HIFs in osteoarthritis. Nat Med. 16:641–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O'Regan A and Berman JS: Osteopontin: A
key cytokine in cell-mediated and granulomatous inflammation. Int J
Exp Pathol. 81:373–390. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuhn K, D'Lima DD, Hashimoto S and Lotz M:
Cell death in cartilage. Osteoarthritis Cartilage. 12:1–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Christensen B, Nielsen MS, Haselmann KF,
Petersen TE and Sorensen ES: Post-translationally modified residues
of native human osteopontin are located in clusters: Identification
of 36 phosphorylation and five O-glycosylation sites and their
biological implications. Biochem J. 390:285–292. 2005. View Article : Google Scholar : PubMed/NCBI
|