Introduction
Environmental pollution, food additives, aging,
injuries and trauma induced by cancer chemotherapy may all lead to
androgen deficiency or nonexistence of testicular cases, which are
increasing each year (1). The
congenital abnormalities and other causative factors underlying
these diseases are also common among children (2). One clinical treatment method involves
the transplantation of Leydig cells (3). Leydig cells can secrete 95% of the
body's testosterone in the adult testes and serves a crucial
function in testosterone generation (4). Although this treatment avoids certain
complications by using exogenous androgen therapy, it may lead to
immunological rejection by the host body. Furthermore, the supply
of testicular interstitial cells is limited, which restricts the
transplantation of Leydig cells in clinical applications (5,6).
Mesenchymal stem cells (MSCs) have demonstrated
marked self-renewal and pluripotency characteristics clinically and
have been increasingly used in tissue engineering and regenerative
medicine seed cell research in recent years (7). Bone marrow MSCs have been thoroughly
characterized (8,9); however, their acquisition can be
harmful, and their differentiation capability decreases with age
(10). Thus, human umbilical cord
MSCs (HUMSCs) have been investigated as an alternative due to the
ease with which they are non-invasively obtained and their low
immunogenicity and higher differentiation capacity (11).
Studies have shown that, under certain
microenvironment conditions or through addition of special inducing
factors, HUMSCs can be differentiated into cartilage cells, matrix
cells, musculoskeletal and endothelial cells (12–15).
However, the induction of HUMSC differentiation into Leydig cells
by changing the culture conditions in vitro has not been
reported. Apart from this, one study demonstrated that stem Leydig
cells can differentiate into mature Leydig cells by the application
of differentiation-inducing medium (DIM) (16). Currently, the differentiation of stem
cells into Leydig cells with gene transfection technology is
mature, but its reliability is controversial (17).
In the present study, HUMSC differentiation into
steroidogenic cells was induced using DIM prepared by adding
multiple inducible factors. However, as the DIM preparation process
is complex, the efficacy of Leydig cell-derived conditioned medium
(LC-CM) for HUMSC differentiation was also tested in order to
simplify the experimental methods. The present data demonstrate
that HUMSCs may differentiate into steroidogenic cells by adding
cytokines in the absence of gene transfection conditions; moreover,
LC-CM may induce HUMSC differentiation into steroidogenic
cells.
Materials and methods
Ethics statement
In this study, all experiments involving human
participants were approved by the institutional review board of the
Chinese Academy of Medical Science and Medical School of Shanghai
Jiaotong University (Shanghai, China). All subjects provided
written informed consent for participation in the study and
approved publication of their case details.
This study was conducted in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of Shanghai Jiaotong University and the protocol was
approved by the Committee on the Ethics of Animal Experiments of
Shanghai Jiaotong University. All surgical procedures on animals
were performed under sodium pentobarbital anesthesia (P3767;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and all efforts were
made to minimize suffering.
Isolation and culture of HUMSCs
With parental consent, human umbilical cords were
aseptically obtained from 12 full-term newborn male infants
delivered by cesarean section at Shanghai Jiaotong University
School of Medicine affiliated with Renji Hospital. Umbilical cords
were preserved in cold low glucose Dulbecco's modified Eagle's
medium (DMEM-LG; Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA), and cellular isolation began within 3 h after
delivery. HUMSC isolation was performed using the tissue block
culture attachment method (18). The
blood vessels were removed, and the cord was sheared into
2-3-mm3 pieces, which were transferred to petri dishes
and cultured in a 37°C incubator with 5% CO2 in DMEM-LG
containing 10% fetal bovine serum (FBS; Invitrogen), 1% penicillin
and streptomycin (Gibco; Thermo Fisher Scientific, Inc.). The
medium was changed every 2 days after planting and 10 days later,
the tissue blocks were removed. When the cells reached 70–80%
confluence, they were harvested and cultured at a density of
1×104 cells/cm2. Only cells from passages 3–5
were used for cell differentiation. The surface markers of HUMSCs
were analyzed by flow cytometry.
Identification of MSC marker
expression by flow cytometry
Third passage cells were collected and washed twice
in phosphate-buffered saline (PBS; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA). Cells were harvested using 0.25% trypsin
(Invitrogen), washed in PBS and incubated for 30 min at 4°C in the
dark with the following anti-human antibodies conjugated with
fluorescein isothiocyanate (FITC), phycoerythrin (PE) or
allophycocyanin (APC): Anti-CD31-PE (FAB3567P), -CD45-PE
(FAB1430P), -CD34-PE (FAB72271P) and-CD105-APC (FAB10971A) from
R&D Systems, Inc. (Minneapolis, MN, USA), and anti-CD44-FITC
(ab27285) and -CD90-FITC (ab11155) from Abcam (Cambridge, UK).
PE-conjugated IgG1 (IC002P; R&D Systems, Inc.) and
FITC-conjugated IgG1 (sc-2078; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) were used as isotype controls. After washing the
cells with PBS, the expression of the markers on the cells was
analyzed using flow cytometry, as previously described (16).
Differentiation potential of MSCs
The osteogenic, adipogenic and chondrogenic
differentiation capacity of isolated HUMSCs were assessed using
third passage MSCs.
For osteogenic differentiation, cells were plated at
a density of 2×104 cells/cm2 and cultured in
DMEM-LG supplemented with 10% FBS until reaching 80–90% confluence.
The DMEM-LG was then removed and Human Umbilical Cord MSC
Osteogenic Differentiation Medium (Cyagen Biosciences, Inc., Santa
Clara, CA, USA) was added and cells were cultured for 28 days. To
detect osteogenesis, Alizarin Red S staining (Cyagen Biosciences,
Inc.) was performed after 28 days of culture. The cells were then
washed with PBS and fixed with 4% formaldehyde solution for 30 min.
The wells were rinsed twice with 1X PBS and cells were then stained
with Alizarin Red working solution for 3–5 min.
For adipogenic differentiation, cells were plated at
a density of 2×104 cells/cm2 and cultured in
DMEM-LG supplemented with 10% FBS until reaching 100–120%
confluence. After the DMEM-LG was removed, Human Umbilical Cord MSC
Adipogenic Differentiation Medium A and Medium B (Cyagen
Biosciences, Inc.) were used to culture cells successively for 28
days. Cells were processed for Oil Red O staining (Cyagen
Biosciences, Inc.) to detect adipogenesis. The staining process was
similar to that used for detection of osteogenesis.
For chondrogenic differentiation, 2.5×105
cells were seeded into 15 ml polypropylene culture tubes. The cells
were centrifuged at 150 × g for 5 min at room temperature. The caps
of the tubes were loosened by one half turn to allow gas exchange,
and the tubes were incubated at 37°C, in a humidified atmosphere of
5% CO2. After 24 h, freshly prepared Complete
Chondrogenic Medium (Cyagen Biosciences, Inc.) was to each tube.
After 28 days culture, the chondrogenic pellets were harvested,
formalin fixed and paraffin embedded for Alcian blue staining
(Cyagen Biosciences, Inc.).
Isolation and culture of Leydig
cells
Testes were obtained from 8-week-old mice, which
were sacrificed by cervical dislocation. Leydig cells were isolated
as described previously (19)
digested by combining testes with 0.03% collagenase NB4 (SERVA
Electrophoresis GmbH, Heidelberg, Germany) in a centrifuge tube.
The supernatant was collected after digestion for 15 min in a 37°C
shaker at 150 rpm. Cells in the supernatant were centrifuged and
cultured in DMEM/F12 (Invitrogen) with 10% FBS. After 4 days, cells
were harvested with 0.25% trypsin treatment. First passage cells
were preserved at −80°C. The surface makers of Leydig cells were
detected by immunofluorescence staining (20).
Preparation of Leydig cell-conditioned
medium (LC-CM)
Second passage Leydig cells were plated on a Petri
dish at a density of 1×104 cells/cm2. Leydig
cells were cultured in DMEM/F12 containing 10% FBS, 1% penicillin
and streptomycin. The medium was collected daily and replaced with
fresh medium every 3 days. The medium was centrifuged at 500 × g
for 5 min, and the supernatant was filtered with a 0.22 µm filter.
The filtered medium and normal DMEM/F12 medium constitute the
LC-CM, which was prepared by diluting the collected conditioned
medium with an equal volume of DMEM/F12 containing 10% FBS.
Preparation of DIM
DIM (16) was
prepared by adding several hormones to phenol red-free DMEM/F12 as
follows: 5% FBS, 1 ng/ml luteinizing hormone (LH; American Research
Products, Inc., Waltham, MA, USA), 1 nM thyroid hormone (Gibco), 70
ng/ml insulin-like growth factor 1 (IGF-1), 10 ng platelet-derived
factor (PDGF)-BB (American Research Products, Inc.) and
insulin-transferrin-selenium cell culture supplement containing 5
mg/l insulin, 5 mg/l transferrin and 5 µg/l selenium (17-838Z;
Lonza Walkersville, Inc., Walkersville, MD, USA).
HUMSC differentiation into
steroidogenic cells in vitro
For differentiation into steroidogenic cells, three
experimental groups of HUMSCs were established and cultured
initially at 2,000–4,000 cells/cm2 in DMEM-LG with 10%
FBS for 24 h. After 24 h, media from the three groups were replaced
with LC-CM, DIM or DMEM/F12 with 10% FBS (control group). The
medium was changed daily.
Immunofluorescence staining
After 14 days of exposure to LC-CM or DIM, the
HUMSCs were fixed with 4% polyoxymethylene for 20 min at room
temperature and permeabilized with 0.25% Triton X-100
(Sigma-Aldrich) and blocked with 0.1% bovine serum albumin (Roche,
Basel, Switzerland). Subsequently, cells were incubated overnight
at 4°C with the following primary antibodies: Cholesterol
side-chain cleavage enzyme CYP17A1 (1:200; ABC392; Merck Millipore,
Merck KGaA), CYP11A1 (1:200; ab75497; Abcam) and 3β-hydroxysteroid
dehydrogenase (3β-HSD; 1:200; ab154385; Abcam). After washing three
times with PBS, the cells were reacted with the appropriate
fluorescent dye-conjugated secondary antibody for 30 min at 37°C.
The cell nuclei were stained with 4′,6-diamidino-2-phenylindole
(DAPI; Invitrogen) and visualized by fluorescence microscopy (Leica
Camera AG, Wetzlar, Germany). The untreated HUMSCs were used as
negative controls. All antibodies were diluted in PBS.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total cellular RNA was extracted from LC-CM and DIM
induced HUMSCs at days 3, 7 and 10 using TRIzol reagent
(Invitrogen) according to the manufacturer's recommendations. Phase
Lock Gel (Tiangen Biotech Co., Ltd., Beijing, China) was used to
remove genomic DNA according to the manufacturer's protocol. The
concentration of RNA was measured by using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.) and then 900 ng
RNA was reversely transcribed using a reverse transcriptase kit
(Takara Bio, Inc., Shiga, Japan) for 15 min at 42°C and 5 sec at
85°C. The cDNA was amplified using a PCR kit (Takara Bio, Inc.)
with the following primers:: Luteinizing hormone receptor (LHR),
forward 5′-GTGGCTGGGACTATGAATATGGTTT-3′ and reverse
5′-CTAGAGTGATGACGGTGAGGGTGTA-3′; Steroidogenic acute regulatory
protein (StAR), forward 5′-ATGGGTGGGGTTCGTGTTTAGA-3′ and reverse
5′-TGGGGTGCTGCTTGTTCTGTG-3′; HSD3B2, forward
5′-TCCAAGCCAGTGTGCCAGTCT-3′ and reverse
5′-CTTTGGTGAGGCGTGTCATCTG-3′; CYP17A1, forward
5′-GCAAGAGATAACACAAAGTCAAGGT-3′ and reverse
5′-TGCCCATACGAACCGAATAGAT-3′; CYP11A1, forward
5′-CCAACCCAGAACGATTCCTCAT-3′ and reverse
5′-CACTACTTCCTCCAGCATCCCCT-3′; and β-actin, forward
5′-CGGGAAATCGTGCGTGACAT-3′ and reverse
5′-CGGACTCGTCATACTCCTGCTTG-3′. PCR assays were conducted by
denaturation at 95°C for 30 sec followed by 30 cycles of annealing
at 58°C for 30 sec and extension at 72°C for 60 sec. Finally, PCR
products were separated by 2% agarose gel electrophoresis and
observed under ultraviolet illumination. The expected product sizes
from these primers were 454, 334, 408, 332, 387 and 481 bp for
HSD3B2, CYP17A1, LHR, CYP11A1, StAR and β-actin, respectively.
Protein extraction and western blot
analysis
After 3 weeks of exposure to LC-CM or DIM, cells
were washed twice with cold PBS and harvested by trypsinization.
For total protein isolation, cells were suspended in cell lysis
buffer (Invitrogen) and placed on ice for 30 min. The suspension
was collected after centrifugation at 15,000 × g for 30 min at 4°C.
Protein concentrations were measured using a bicinchoninic acid
protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to the manufacturer's instruction. Proteins (20 µg) were
denatured by boiling at 100°C for 5 min after the addition of a
loading buffer (CWbio, Beijing, China). Equivalent quantities of
protein were loaded and electrophoresed in 6–15% SDS-PAGE gels.
After electrophoresis, gels were transferred to nitrocellulose (NC;
Merck Millipore) membranes, and membranes were blocked with 5%
non-fat milk in Tris-buffered saline containing 0.1% Tween 20 for 1
h at room temperature. Membranes were incubated with primary
antibodies targeting CYP17A1 (1:1,000; ABC392), CYP11A1 (1:1,000;
ab75497), 3β-HSD (1:1,000; ab154385) or β-actin (1:1,000; ab8227;
Abcam) overnight at 4°C, then with IRDye 800-conjugated goat
anti-rabbit IgG secondary antibody (1:20,000; A50984P; Rockland
Immunochemicals, Inc., Pottstown, PA, USA) for 2 h at room
temperature. Immunoreactive bands were visualized using a LI-COR
Odyssey (LI-COR Biosciences, Lincoln, NE, USA).
Results
Isolation and morphological features
of HUMSCs
After three days in culture, the cells isolated from
the human umbilical cord blocks were scarce and scattered,
principally exhibiting a spindle-shaped morphology (Fig. 1A). After 10 days, the number of cells
increased, and they grew in a parallel arrangement (Fig. 1B). Once passaged, the cells expanded
rapidly with no significant changes in morphology (Fig. 1C).
Identification of HUMSCs
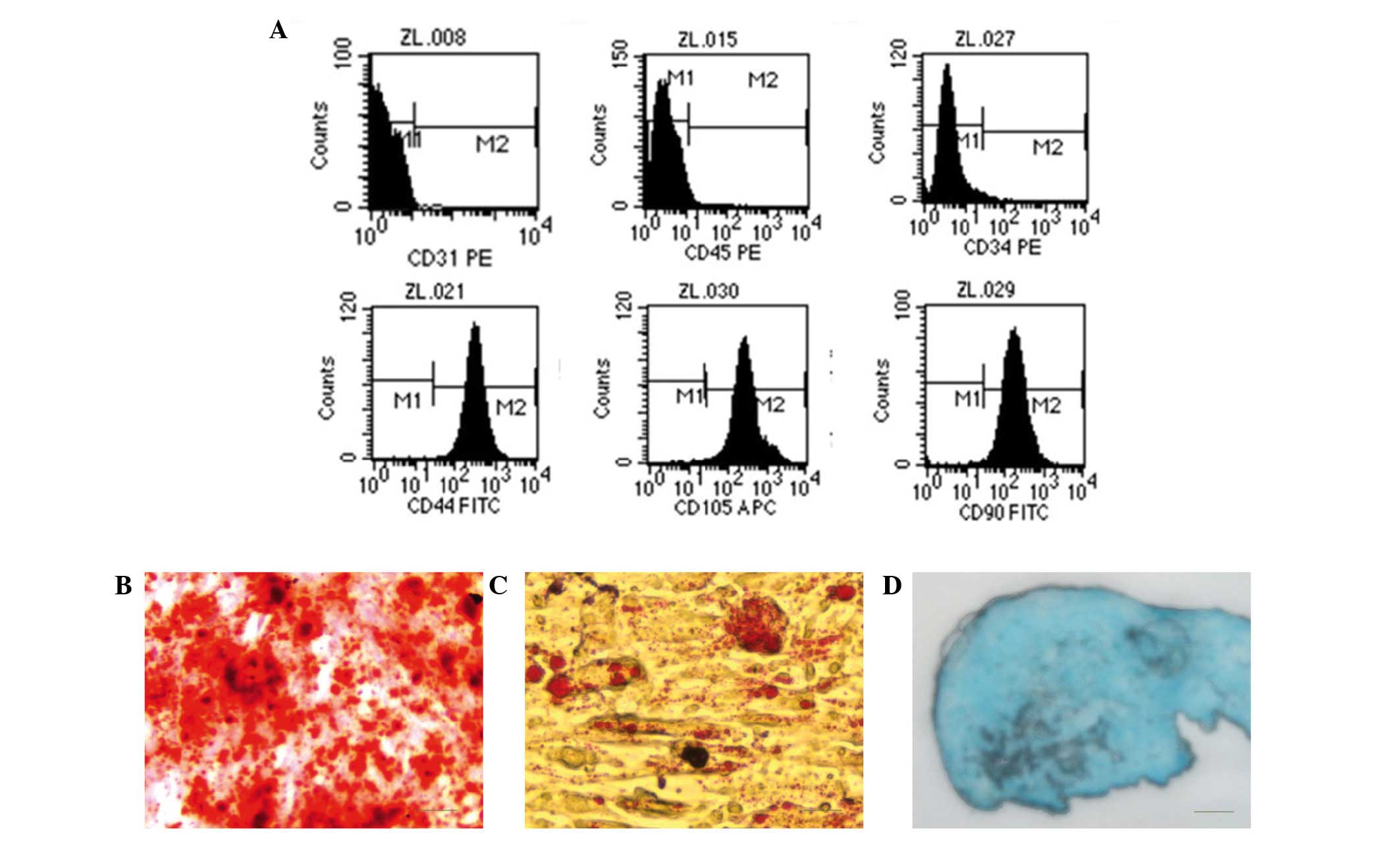
After two passages, the cells exhibited positive
expression of the MSC surface markers CD44, CD90 and CD105.
However, the cells were negative for hematopoietic lineage markers
(CD34, CD45 and CD31). These results indicated that the isolated
cells in this study were HUMSCs (Fig.
2A). After 28 days of osteogenic induction, the deposition of
calcium of cells could be visualized by Alizarin Red S staining
(Fig. 2B). When cells were cultured
in adipogenic medium for 28 days, numerous lipid droplets were
observed following staining with Oil Red O solution, which used to
identify adipocytes (Fig. 2C). Blue
staining indicates synthesis of proteoglycans by chondrocytes after
28 days of induction (Fig. 2D).
Isolation and morphological features
of Leydig cells
Leydig cells predominantly exhibited round shapes
with large lipid drops and tended to be arranged in clusters
(Fig. 3), as previously reported
(21). Immunofluorescence staining
of Leydig cells for CYP17A1 and 3β-HSD was positive (Fig. 4).
Expression of Leydig cell lineage
markers in induced HUMSCs analyzed by immunofluorescence
staining
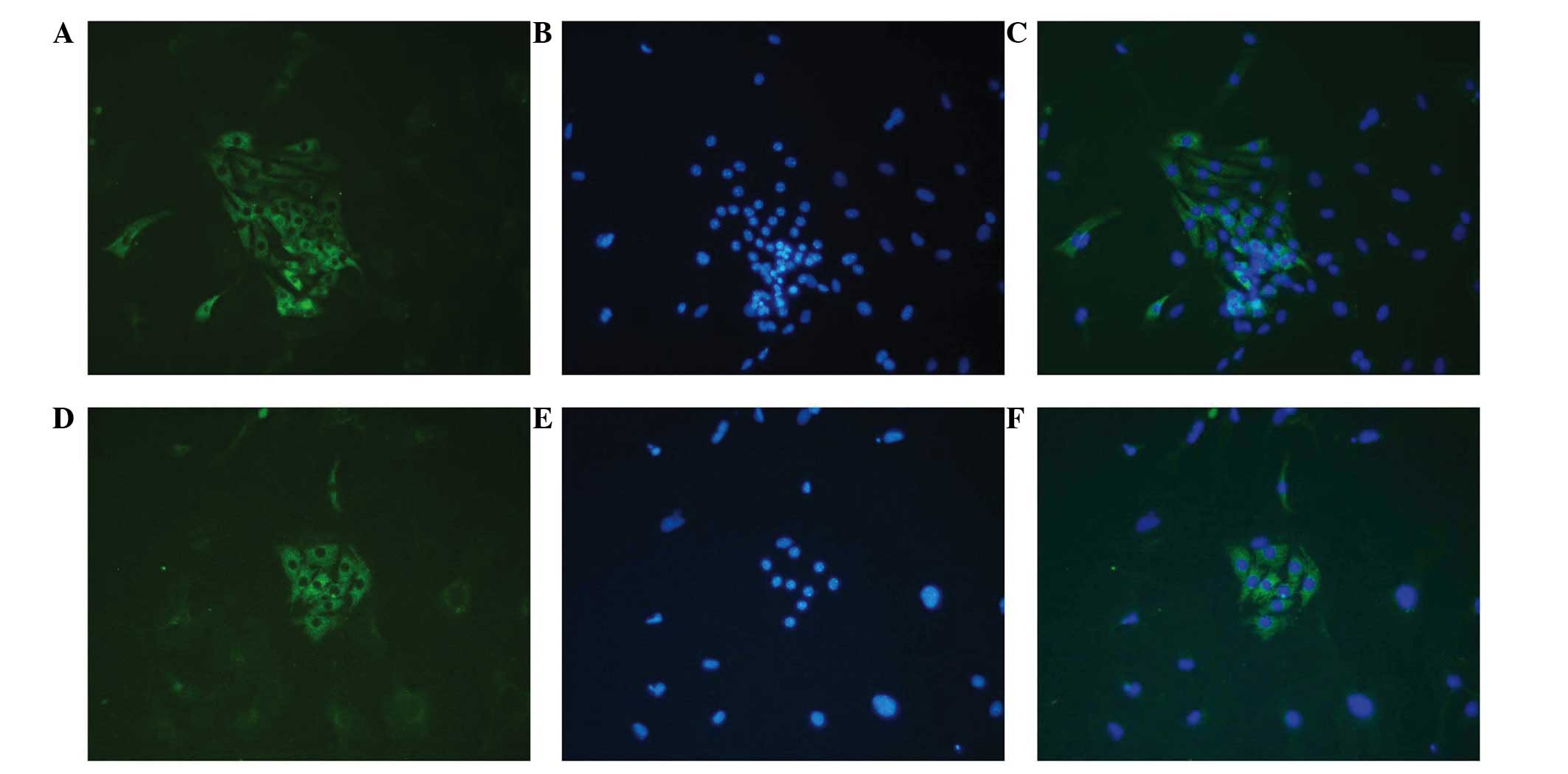
To detect the Leydig cell phenotype in induced
HUMSCs, immunofluorescence staining for CYP11A1, CYP17A1 and 3β-HSD
was performed on cells from all three groups following 14 days of
culture. Positive staining for CYP11A1, CYP17A1 and 3β-HSD was
observed in the HUMSCs treated with either LC-CM or DIM. However,
no positive staining was observed in the control cells (Fig. 5).
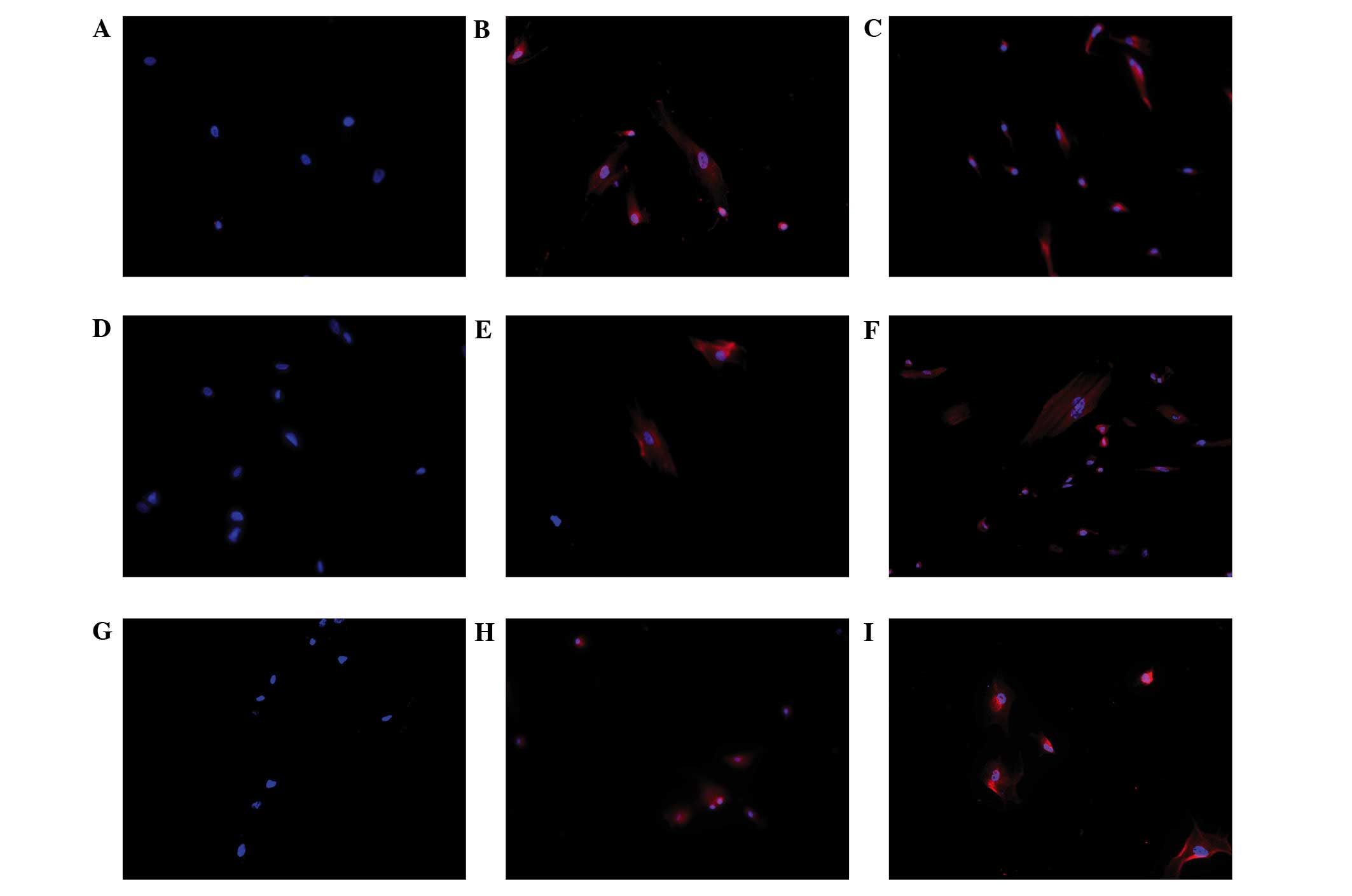 | Figure 5.Immunofluorescence staining of Leydig
cell differentiation of human umbilical cord mesenchymal stem cells
(HUMSCs) induced in DIM and LC-CM. HUMSCs induced by DIM and LC-CM
were positively stained for CYP11A1, CYPY17A1 and 3β-HSD.
Immunofluorescence for 3β-HSD was (A) negative in control cells and
positive in (B) LC-CM and (C) DIM-induced cells. Blue, nuclei; red,
3β-HSD in cytoplasm. Immunofluorescence for CYP11A1 was (D)
negative in control cells and positive in (E) LC-CM and (F)
DIM-induced cells: Blue, nuclei; red CYP11A1 in cytoplasm.
Immunofluorescence for CYP17A1 was (G) negative in control cells
and positive in (H) LC-CM and (I) DIM-induced cells: Blue, nuclei;
red, CYP17A1 in cytoplasm. (Scale bar, 100 µm.) CM, conditioned
medium; DIM, differentiation-inducing medium; 3β-HSD,
3β-hydroxysteroid dehydrogenase. |
Expression of Leydig cell lineage
markers in induced HUMSCs analyzed by RT-PCR
To assess the possibility of Leydig cell
differentiation, HUMSCs were evaluated by RT-PCR analysis of
specific testosterone-producing genes. After 7 days of culture, the
results demonstrated that HUMSCs induced with LC-CM or DIM
positively expressed Leydig cell lineage marker genes (Fig. 6).
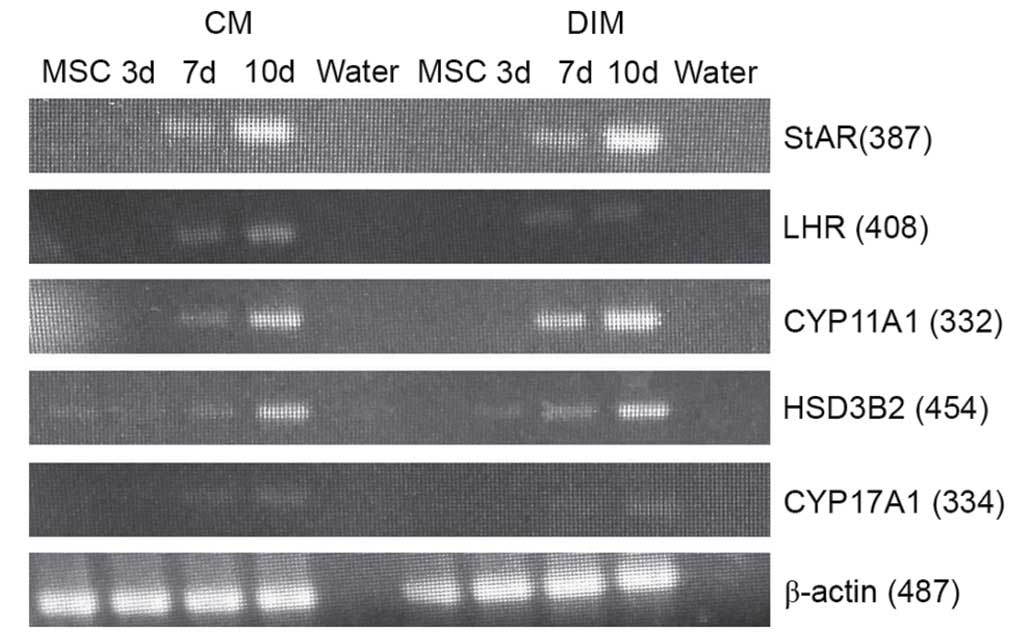 | Figure 6.Reverse transcription-polymerase
chain reaction analysis results for LC-CM and DIM-treated HUMSCs.
LC-CM and DIM-treated HUMSCs were positive for StAR, LHR, 3β-HSD,
CYP11A1, CYP17A1 and β-actin while control cells were negative. CM,
conditioned medium; DIM, differentiation-inducing medium; StAR,
steroidogenic acute regulatory protein; LHR, luteinizing hormone
receptor; 3β-HSD, 3β-hydroxysteroid dehydrogenase; HUMSC, human
umbilical cord mesenchymal stem cell; d, day. |
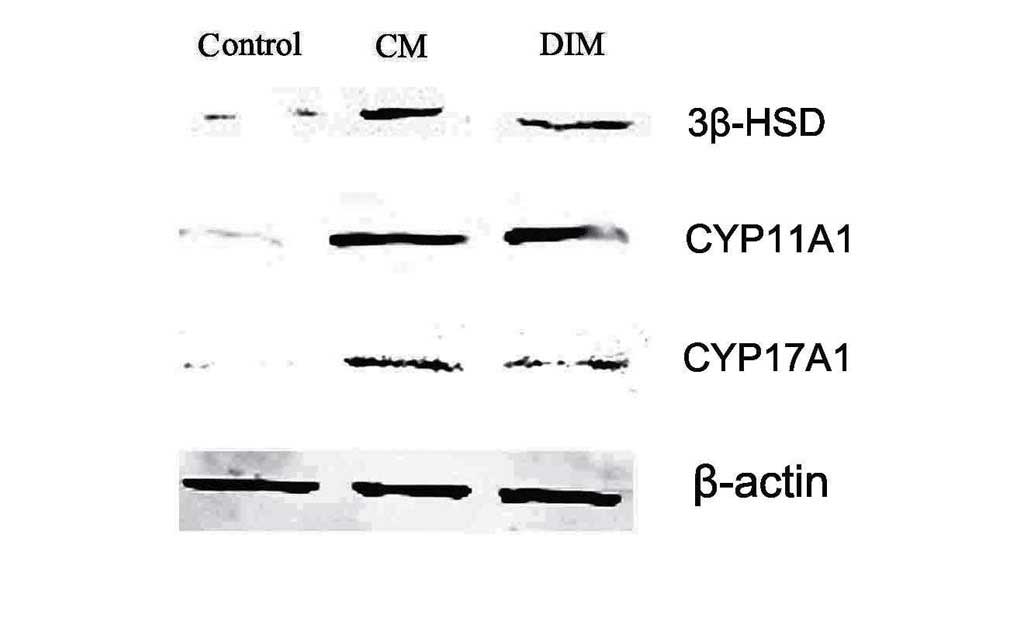
Expression of Leydig cell lineage
markers in induced HUMSCs analyzed by western blot analysis
Next, whether whether Leydig cell lineage enzymes
can be expressed after induction was examined. 3β-HSD, CYP11A1 and
CYP17A1 were induced 3 weeks after LC-CM or DIM treatment. In the
testes, the production of testosterone is dependent on 3β-HSD
activity (22). Additionally, 3β-HSD
enzymes are essential for the production of progesterone, and
CYP17A1 is required for the production of testosterone (23). These results demonstrate that HUMSCs
have properties indicating their differentiation into steroidogenic
cells, which are similar to those of Leydig cells (Fig. 7).
Discussion
MSCs have a great capacity for self-renewal while
maintaining multipotency. Previous experiments have shown that
HUMSCs have the advantages of easy accessibility, high multipotency
and low immunogenicity, and they are gradually replacing bone
marrow MSCs as an ideal clinical source of MSCs for cell therapy
and tissue engineering (24). The
tissue block attachment method and mechanical removal of the
epithelia and vessels may avoid contamination while maintaining
cellular viability and function (25). The present study was conducted under
strict quality control to ensure proper expression of multiple
markers of MSCs via flow cytometry. Positive expression of the MSC
markers CD44, CD90 and CD105 was detected, and negative expression
of the hematopoietic stem cell markers CD31, CD34 and CD45. As a
result, the adherent cells, which were isolated and cultured, were
considered to be HUMSCs.
During development, Leydig cells arise sequentially,
first as stem Leydig cells (SLC) then as progenitor Leydig cells
(PLC), immature Leydig cells (ILC) and mature Leydig cells (MLCs).
Various cytokines, such as insulin-like factor 3 (INSL3), stem cell
factor (SCF), platelet-derived factor (PDGFAA) and Müllerian
inhibiting substance can regulate Leydig cell development (26–29).
Cellular synthesis of testosterone also requires the application of
numerous enzymes. StAR plays an important role in the synthesis of
testosterone, which is a rate-limiting enzyme for steroid hormone
synthesis (30). LHRs first appear
as PLCs differentiate, and the development of the steroidogenic
capacity of PLCs needs stimulation by LH (31). Leydig cell lineage specific markers,
such as CYP11A1, CYP17A1 and 3β-HSD, are essential for steroid
synthesis in adrenal gland and gonads. In this study, the positive
expression of Leydig cell lineage markers indicate that HUMSCs
could differentiate into Leydig cells (32).
MLCs can be considered a terminally differentiated
state; however, previous studies have reported that, in an
appropriate microenvironment, certain cytokines can regulate Leydig
cell proliferation (33). Ge et
al cultured SLCs in DIM and successfully induced their
differentiation into ALCs (16,34).
Microarray analysis was conducted using purified SLCs, PLCs, ILCs,
MLCs and bone marrow stem cells (BSCs); highly correlated gene
expression patterns were seen between BSCs and SLCs, and greater
differences were seen in gene expression between SLCs and PLCs and
between SLCs and MLCs, indicating that the transcriptome of the
SLCs is similar to that of stem cells (35). In view of these results, the same
inducible factors were used in this study to induce HUMSC
differentiation into Leydig cells.
DIM contains several cytokines important for Leydig
cell development. LH serves a crucial function in inducing
differentiation of ILCs into MLCs. LH is also crucial for MLC
proliferation, as binding of LH to LHR increases testosterone
synthesis by Leydig cells (36).
Thyroid hormone T3 is critical to initiate the onset of mesenchymal
cell differentiation into adult Leydig cells, while IGF-I and
PDGF-BB can promote cell proliferation and differentiation
(31,37,38).
In the present study, under the effect of DIM,
HUMSCs demonstrate the ability to synthesize steroid hormones.
However, this approach has certain disadvantages, as the cytokine
concentration and media preparation method is complicated and the
cost of purchasing inducible factors is high. Therefore, LC-CM use
as a new induction method was investigated.
Previous studies have shown that cells and their
secreted proteins can form a functional niche in vivo, and
the balance of self-renewal and differentiation of all stem cells
is affected under the influence of this niche. Similarly, all
functional factors contained in the conditioned medium, including
proteins or ions, may also determine the direction of stem cell
differentiation (39–42). The present experimental data show
that provision of a suitable microenvironment from LC-CM may
directly promote HUMSC differentiation into Leydig cells.
Previous research has shown that two methods can
induce MSCs differentiation towards Leydig cells: Infection of MSCs
with an adenovirus-containing steroidogenesis-related gene in
vitro or interstitial testicular transplantation of MSCs in
vivo (43–46). However, both methods are limited by
the difficultly of obtaining a large number of safe and effective
seed cells for tissue reconstruction. The present experiments for
the first time induced HUMSCs differentiation into steroidogenic
cells, which may have the ability to synthesize testosterone in
vitro. Nevertheless, further studies are required to
investigate the underlying mechanism of DIM and LC-CM-mediated
induction of HUMSC differentiation into Leydig cells.
In summary, the present results suggest that HUMSCs
are able to differentiate into steroidogenic cells in an
appropriate microenvironment in vitro. DIM and LC-CM may
constitute a functional niche to promote the differentiation of
HUMSCs.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (grant no. 81270689) and Research Program of
Science and Technology Commission of Shanghai Municipality (grant
no. 12ZR1419200).
References
|
1
|
Wohlfahrt-Veje C, Main KM and Skakkebaek
NE: Testicular dysgenesis syndrome: Foetal origin of adult
reproductive problems. Clin Endocrinol (Oxf). 71:459–465. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meikle AW: The interrelationships between
thyroid dysfunction and hypogonadism in men and boys. Thyroid.
14:(Suppl 1). S17–S25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun J, Xi YB, Zhang ZD, Shen P, Li HY, Yin
MZ, Li WY and Shi CR: Leydig cell transplantation restores androgen
production in surgically castrated prepubertal rats. Asian J
Androl. 11:405–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zirkin BR: Where do adult Leydig cells
come from? Biol Reprod. 82:1019–1020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jahnukainen K, Ehmcke J, Hou M and Schlatt
S: Testicular function and fertility preservation in male cancer
patients. Best Pract Res Clin Endocrinol Metab. 25:287–302. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lo KC, Lei Z, Rao ChV, Beck J and Lamb DJ:
De novo testosterone production in luteinizing hormone receptor
knockout mice after transplantation of leydig stem cells.
Endocrinology. 145:4011–4015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mobasheri A, Csaki C, Clutterbuck AL,
Rahmanzadeh M and Shakibaei M: Mesenchymal stem cells in connective
tissue engineering and regenerative medicine: Applications in
cartilage repair and osteoarthritis therapy. Histol Histopathol.
24:347–366. 2009.PubMed/NCBI
|
|
8
|
Charbord P: Bone marrow mesenchymal stem
cells: Historical overview and concepts. Hum Gene Ther.
21:1045–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL
and Chen TH: Isolation of multipotent mesenchymal stem cells from
umbilical cord blood. Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stenderup K, Justesen J, Clausen C and
Kassem M: Aging is associated with decreased maximal life span and
accelerated senescence of bone marrow stromal cells. Bone.
33:919–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HS, Hung SC, Peng ST, Huang CC, Wei
HM, Guo YJ, Fu YS, Lai MC and Chen CC: Mesenchymal stem cells in
the Wharton's jelly of the human umbilical cord. Stem Cells.
22:1330–1337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh JY, Fu YS, Chang SJ, Tsuang YH and
Wang HW: Functional module analysis reveals differential osteogenic
and stemness potentials in human mesenchymal stem cells from bone
marrow and Wharton's jelly of umbilical cord. Stem Cells Dev.
19:1895–1910. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitchell KE, Weiss ML, Mitchell BM, Martin
P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K,
Hildreth T, et al: Matrix cells from Wharton's jelly form neurons
and glia. Stem Cells. 21:50–60. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Ott L, Seshareddy K, Weiss ML and
Detamore MS: Musculoskeletal tissue engineering with human
umbilical cord mesenchymal stromal cells. Regen Med. 6:95–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen MY, Lie PC, Li ZL and Wei X:
Endothelial differentiation of Wharton's jelly-derived mesenchymal
stem cells in comparison with bone marrow-derived mesenchymal stem
cells. Exp Hematol. 37:629–640. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ge RS, Dong Q, Sottas CM, Papadopoulos V,
Zirkin BR and Hardy MP: In search of rat stem Leydig cells:
Identification, isolation, and lineage-specific development. Proc
Natl Acad Sci USA. 103:2719–2724. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wegman F, Oner FC, Dhert WJ and Alblas J:
Non-viral gene therapy for bone tissue engineering. Biotechnol
Genet Eng Rev. 29:206–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weiss ML, Medicetty S, Bledsoe AR,
Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G and
Troyer D: Human umbilical cord matrix stem cells: Preliminary
characterization and effect of transplantation in a rodent model of
Parkinson's disease. Stem cells. 24:781–792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Zhong L, Zhu Y and Liu G: Research
on the isolation of mouse Leydig cells using differential digestion
with a low concentration of collagenase. J Reprod Dev. 57:433–436.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du T, Cheng J, Zhong L, Zhao XF, Zhu J,
Zhu YJ and Liu GH: The alleviation of acute and chronic kidney
injury by human Wharton's jelly-derived mesenchymal stromal cells
triggered by ischemia-reperfusion injury via an endocrine
mechanism. Cytotherapy. 14:1215–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teerds KJ and Huhtaniemi IT: Morphological
and functional maturation of Leydig cells: From rodent models to
primates. Hum Reprod Update. 21:310–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klinefelter GR, Hall PF and Ewing LL:
Effect of luteinizing hormone deprivation in situ on
steroidogenesis of rat Leydig cells purified by a multistep
procedure. Biol Reprod. 36:769–783. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shima Y, Miyabayashi K, Haraguchi S,
Arakawa T, Otake H, Baba T, Matsuzaki S, Shishido Y, Akiyama H,
Tachibana T, et al: Contribution of Leydig and Sertoli cells to
testosterone production in mouse fetal testes. Mol Endocrinology.
27:63–73. 2013. View Article : Google Scholar
|
|
24
|
Li X, Bai J, Ji X, Li R, Xuan Y and Wang
Y: Comprehensive characterization of four different populations of
human mesenchymal stem cells as regards their immune properties,
proliferation and differentiation. Int J Mol Med. 34:695–704.
2014.PubMed/NCBI
|
|
25
|
De Bruyn C, Najar M, Raicevic G, Meuleman
N, Pieters K, Stamatopoulos B, Delforge A, Bron D and Lagneaux L: A
rapid, simple, and reproducible method for the isolation of
mesenchymal stromal cells from Wharton's jelly without enzymatic
treatment. Stem Cells Dev. 20:547–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen H, Ge RS and Zirkin BR: Leydig cells:
From stem cells to aging. Mol Cell Endocrinol. 306:9–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsuchida J, Dohmae K, Kitamura Y and
Nishimune Y: The role of the c-kit receptor in the regenerative
differentiation of rat Leydig cells. Int J Androl. 26:121–125.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferlin A and Foresta C: Insulin-like
factor 3: A novel circulating hormone of testicular origin in
humans. Ann N Y Acad Sci. 1041:497–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu JH, Fei J, Hong X, Chen JF, Gu AH, Sun
H, Xu XL, Song L, Wang SL and Wang XR: Involvement of IGF-I
signaling pathway in the regulation of steroidogenesis in mouse
Leydig cells treated with fenvalerate. Toxicology. 292:151–155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo L, Chen H and Zirkin BR: Leydig cell
aging: Steroidogenic acute regulatory protein (StAR) and
cholesterol side-chain cleavage enzyme. J Androl. 22:149–156.
2001.PubMed/NCBI
|
|
31
|
Ariyaratne HB, Mills N, Mason JI and
Mendis-Handagama SM: Effects of thyroid hormone on Leydig cell
regeneration in the adult rat following ethane dimethane sulphonate
treatment. Biol Reprod. 63:1115–1123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luu-The V: Assessment of steroidogenesis
and steroidogenic enzyme functions. J Steroid Biochem Mol Biol.
137:176–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu X, Zhang N and Lee MM: Mullerian
inhibiting substance recruits ALK3 to regulate Leydig cell
differentiation. Endocrinology. 153:4929–4937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Svechnikov K, Landreh L, Weisser J, Izzo
G, Colón E, Svechnikova I and Söder O: Origin, development and
regulation of human Leydig cells. Horm Res Paediatr. 73:93–101.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stanley EL, Johnston DS, Fan J,
Papadopoulos V, Chen H, Ge RS, Zirkin BR and Jelinsky SA: Stem
Leydig cell differentiation: Gene expression during development of
the adult rat population of Leydig cells. Biol Reprod.
85:1161–1166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stocco DM, Wang X, Jo Y and Manna PR:
Multiple signaling pathways regulating steroidogenesis and
steroidogenic acute regulatory protein expression: More complicated
than we thought. Mol Endocrinol. 19:2647–2659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Teerds KJ, Rijntjes E, Veldhuizen-Tsoerkan
MB, Rommerts FF and de Boer-Brouwer M: The development of rat
Leydig cell progenitors in vitro: How essential is luteinising
hormone? J Endocrinol. 194:579–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bjelic MM, Stojkov NJ, Baburski AZ,
Sokanovic SJ, Mihajlovic AI, Janjic MM, Kostic TS and Andric SA:
Molecular adaptations of testosterone-producing Leydig cells during
systemic in vivo blockade of the androgen receptor. Mol Cell
Endocrinol. 396:10–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu S, Cheng Z, Liu G, Zhao X, Zhong L, Zhu
Y and Zhu J: Urothelial differentiation of human umbilical
cord-derived mesenchymal stromal cells in vitro. Anal Cell Pathol
(Amst). 36:63–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li TX, Yuan J, Chen Y, Pan LJ, Song C, Bi
LJ and Jiao XH: Differentiation of mesenchymal stem cells from
human umbilical cord tissue into odontoblast-like cells using the
conditioned medium of tooth germ cells in vitro. Biomed Res Int.
2013:2185432013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fuchs E, Tumbar T and Guasch G:
Socializing with the neighbors: Stem cells and their niche. Cell.
116:769–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moore KA and Lemischka IR: Stem cells and
their niches. Science. 311:1880–1885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei X, Peng G, Zheng S and Wu X:
Differentiation of umbilical cord mesenchymal stem cells into
steroidogenic cells in comparison to bone marrow mesenchymal stem
cells. Cell Prolif. 45:101–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sonoyama T, Sone M, Honda K, Taura D,
Kojima K, Inuzuka M, Kanamoto N, Tamura N and Nakao K:
Differentiation of human embryonic stem cells and human induced
pluripotent stem cells into steroid-producing cells. Endocrinology.
153:4336–4345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gondo S, Okabe T, Tanaka T, Morinaga H,
Nomura M, Takayanagi R, Nawata H and Yanase T: Adipose
tissue-derived and bone marrow-derived mesenchymal cells develop
into different lineage of steroidogenic cells by forced expression
of steroidogenic factor 1. Endocrinology. 149:4717–4725. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lue Y, Erkkila K, Liu PY, Ma K, Wang C,
Hikim AS and Swerdloff RS: Fate of bone marrow stem cells
transplanted into the testis: Potential implication for men with
testicular failure. Am J Pathol. 170:899–908. 2007. View Article : Google Scholar : PubMed/NCBI
|