Introduction
As an important adjunct to anesthesia, muscle
relaxants ensure that body movement does not occur in anesthetized
patients and provide improved surgical conditions. Muscle relaxants
are able to not only reduce the use of other anesthesia drugs, but
also decrease the deleterious effects of deep anesthesia on the
cardiovascular system (1). However,
previous studies demonstrated that the probability of
intraoperative awareness in patients administered muscle relaxants
under general anesthesia was higher compared with patients who were
not administered muscle relaxants (2,3). In
addition, patients treated with muscle relaxants showed increased
postoperative injuries following intraoperative awareness during
general anesthesia, as compared with patients who were not treated
with muscle relaxants (4). Using
electroencephalograph (EEG) algorithms to determine muscle
activity, it has been observed that muscle relaxants exert some
interference on the judgment and detection of anesthetic depth.
Bispectral index (BIS) is a signal processing
technique combining EEG, electromyography (EMG) and a previously
collected statistical database of patterns (3,5). The BIS
monitor reports a number from 0–100, with 100 representing the
awake state and 0 representing complete EEG inactivity (6). BIS is used to monitor the depth of
anesthesia (4).
Two primary mechanisms have been proposed to
underlie the effects of muscle relaxants on the depth of
anesthesia: Firstly, the neuromuscular inhibition caused by muscle
relaxants may directly affect the depth of anesthesia. Secondly,
muscle relaxants inhibited EMG activities and influenced the
monitoring of the depth of anesthesia, although muscle relaxants
did not directly affect the depth of anesthesia. The first
mechanism may be associated with a decrease in muscle-generated
sensory inputs to the brain, and explains why rocuronium has been
demonstrated to reduce halothane requirement and increase the depth
of anesthesia (7). The second
mechanism suggested that EMG activity just increased BIS values for
general anesthetic patients, but their actual sedation depth was
not changed (8,9). In addition, the BIS significantly
decreased and sedate status did not changed in patients in
intensive care units, which may further confirm the validity of
this mechanism (10).
Our previous studies demonstrated that propofol
concentration and BIS correlated with the depth of sedation when
propofol was used alone and in the absence of noxious stimulation,
compound analgesics and muscle relaxants (4,11).
Muscle relaxants as anesthesia depth indicators may influence or
interfere with the anesthetic dose and depth of sedation (5). Under a state of deep sedation, due to
the inhibitions of EMG activity and afferent signals, muscle
relaxants may lose their indicative role for anesthetic depth.
Kawaguchi et al (12)
reported that in the case of anesthesia using only propofol, a
non-depolarizing muscle relaxant rocuronium would cause a
dose-dependent inhibitory effect on the increase of entropy and BIS
values following intubation.
The study hypothesized that under general
anesthesia, propofol only induces both normal sedation and deep
sedation. After various doses of muscle relaxants were administered
and tracheal intubation was carried out, the changes in BIS and EMG
were compared during the process of anesthesia induction, and prior
to and following tracheal intubation. In addition, the mechanism
underlying the effects of muscle relaxants on anesthesia depth was
discussed.
Materials and methods
Patients
Ethical approval was obtained from the ethics
committee of the Tianjin Medical University Cancer Institute and
Hospital (Tianjin, China), and informed consents was obtained from
the patients. A total of 72 patients (American Society of
Anesthesiologists physical status I–II; age, 20–60 years) underwent
anesthesia via endotracheal intubation for elective surgery. The
exclusion criteria were as follows: Pregnancy; neurological,
psychiatric and endocrine system diseases; cardiovascular and
respiratory diseases; history of drug or alcohol dependency;
neuromuscular blocking drug allergies; liver and kidney
dysfunction; patients who were difficult to intubate and ventilate;
and a body weight >120 or <80% of the ideal body weight.
Data acquisition
None of the patients were medicated under any drugs.
Following opening of venous access, administration of intravenous
drip Lactated Ringer's solution (Otsuka Pharmaceutical Co., Ltd.,
Tianjin, China) and connection to Datex-Ohmeda S/5 monitoring
equipment, electrocardiogram, non-invasive blood pressure and pulse
oximetry finger were routinely monitored (13,14).
Subsequently, the foreheads of the patients were treated with
alcohol, and a BIS VISTA™ monitoring system-specific electrode was
placed. Skin electrical impedance was maintained at <5 ka. A BIS
VISTA™ monitor (Aspect Medical Systems, Inc., Natick, MA, USA) was
connected to monitor the BIS. BIS and raw EEG data were collected
in real-time using a RS232 serial port, with a sampling frequency
of 128 samples/sec. The acquired data were used for subsequent
analysis.
Anesthesia
Propofol (AstraZeneca, Basiglio, Italy) alone was
administered by target-controlled infusion for anesthesia induction
in all patients. The initial target concentration was 3.0 µg/ml,
until the patients lost consciousness and underwent mask
ventilation. Target concentration increased to 0.5 µg/ml/4 min to
adjust the BIS value. The patients were randomly divided into 2
groups with 36 patients in each group. The BIS value was stable at
40–60 in group 1, which was termed the normal sedation group; the
BIS value was <20 or with burst suppression in group 2, which
was termed the deep sedation group. After maintaining the target
BIS for 4 min in the 2 groups, the target plasma concentration and
effect-site concentration reached a steady state, following which
the target concentration of propofol did not change.
When they reached a steady state, the patients in
the 2 groups were randomly divided into A, B, C and D subgroups
(n=9) according to the various doses (0.3, 0.6, 0.9 and 1.2 mg/kg)
of rocuronium (Merck & Co., Inc., Kenilworth, NJ, USA).
Tracheal intubation was carried out 2 min after rocuronium
administration. The BIS, EMG, heart rate (HR) and mean arterial
pressure (MAP) were recorded prior to induction of anesthesia (T1),
during the steady state (T2), following administration of
rocuronium for 2 min (T3), and 0, 2, and 5 min following intubation
(L0, L2 and L3, respectively). The target concentration of propofol
was adjusted 5 min following the completion of intubation, and the
BIS was maintained at 40–60 prior to the surgical procedure.
Ephedrine (Northeast Pharmaceutical Group Co., Ltd., Shenyang,
China) was administered to increase blood pressure when MAP was
<70 mmHg and atropine (Tianjin Jinyao Amino Acid Co., Ltd.,
Tianjin, China) was administered to reduce the target concentration
of intravenous anesthetics when HR <50 beats/min during
induction. All the patients were kept at stable circulation with
stable vital signs. The patients who showed marked body movement
during intubation and those with an intubation time >1 min were
not included in the statistical analysis.
Statistical analysis
Statistical analyses were performed using SPSS l7.0
(SPSS, Inc., Chicago, IL, USA). The data were expressed as means ±
standard deviation, and non-match two-tailed t-tests or two-way
analysis of variance were used to analyze the difference. A
Student-Newman-Keuls-q test was used to compare two groups;
however, if the data was not normally distributed or exhibited
heterogeneity of variance, a non-parametric test was used. The
change in BIS and the doses of rocuronium were analyzed by the
curve fitting. P<0.05 was considered to indicate a statistically
significant result.
Results
Patient information including age, weight and gender
are presented in Table I, and were
not significantly different between patients (P>0.05). Changes
in HR and MAP prior to induction of anesthesia (T1), during steady
state (T2), following administration of rocuronium for 2 min (T3),
and 0, 2 and 5 min following intubation (L0, L2 and L3,
respectively) are presented in Tables
II and III. The results
demonstrated that compared with T1, HR and MAP at T2 and T3
significantly decreased (P<0.05). However, HR and MAP
significantly increased at L0 compared with T2 and T3 (P<0.05).
The change rate of hemodynamics in the deep sedation group was less
marked compared with the normal sedation group. HR and MAP in each
group returned to levels to those prior to intubation after 5
min.
 | Table I.Patient information including age,
weight and gender. |
Table I.
Patient information including age,
weight and gender.
| Group | Age | Weight (kg) | Gender
(man/woman) |
|---|
| 1A | 48±4 | 63.4±5.8 | 5/4 |
| 1B | 41±8 | 66.5±5.0 | 6/3 |
| 1C | 44±8 | 67.6±4.2 | 6/3 |
| 1D | 43±6 | 61.8±6.3 | 4/5 |
| 2A | 46±4 | 67.2±5.5 | 5/4 |
| 2B | 45±8 | 68.5±4.3 | 6/3 |
| 2C | 46±5 | 69.1±3.2 | 5/4 |
| 2D | 50±3 | 66.7±4.90 | 6/3 |
 | Table II.Changes in HR prior to induction of
anesthesia (T1), during steady state (T2), following administration
of rocuronium for 2 min (T3), and 0, 2 and 5 min after intubation
(L0, L2 and L3, respectively). |
Table II.
Changes in HR prior to induction of
anesthesia (T1), during steady state (T2), following administration
of rocuronium for 2 min (T3), and 0, 2 and 5 min after intubation
(L0, L2 and L3, respectively).
|
|
|
|
| Following
intubation |
|---|
|
|
|
|
|
|
|---|
| HR (beats/min) | T1 | T2 | T3 | L0 | L2 | L5 |
|---|
| 1A | 78±5 | 70±10a | 72±3a | 92±7b,c | 80±8 | 72±8 |
| 1B | 82±3 | 73±5a | 74±8a | 90±10b,c | 78±6 | 74±5 |
| 1C | 72±6 | 66±4a | 70±3a | 85±6b,c | 76±7 | 68±7 |
| 1D | 76±7 | 68±6a | 69±4a | 89±5b,c | 70±3 | 65±8 |
| 2A | 79±8 | 62±5a | 64±8a | 74±3b,c | 57±5 | 52±4 |
| 2B | 80±5 | 60±4a | 62±3a | 75±4b,c | 64±7 | 60±6 |
| 2C | 74±7 | 55±7a | 59±6a | 69±7b,c | 56±8 | 58±3 |
| 2D | 70±6 | 56±5a | 55±4a | 70±4b,c | 54±5 | 51±7 |
 | Table III.Changes in HR prior to induction of
anesthesia (T1), during steady state (T2), following administration
of rocuronium for 2 min (T3), and 0, 2, and 5 min following
intubation (L0, L2 and L3, respectively). |
Table III.
Changes in HR prior to induction of
anesthesia (T1), during steady state (T2), following administration
of rocuronium for 2 min (T3), and 0, 2, and 5 min following
intubation (L0, L2 and L3, respectively).
|
|
|
|
| Following
intubation |
|---|
|
|
|
|
|
|
|---|
| MAP (mmHg) | T1 | T2 | T3 | L0 | L2 | L5 |
|---|
| 1A | 109±5 | 96±5a | 93±6a | 110±7b,c | 100±6 | 98±4 |
| 1B | 105±7 | 89±2a | 90±7a | 108±8b,c | 90±9 | 86±3 |
| 1C | 103±4 | 85±5a | 83±8a | 112±7b,c | 93±4 | 86±6 |
| 1D | 104±5 | 86±4a | 87±6a | 115±5b,c | 89±7 | 84±4 |
| 2A | 101±8 | 72±3a | 73±9a | 94±4b,c | 84±5 | 79±7 |
| 2B | 105±10 | 77±6a | 75±4a | 96±8b,c | 81±6 | 76±5 |
| 2C | 103±4 | 74±4a | 77±6a | 101±4b,c | 85±4 | 73±3 |
| 2D | 101±6 | 73±5a | 71±3a | 92±6b,c | 87±6 | 72±5 |
The changes in BIS in each group are presented in
Fig. 1. The results demonstrated
that BIS at T1 was significantly higher compared with the other
time points (P<0.05). The BIS of groups 1C and 1D at T3 were
significantly lower compared with T2 (P<0.05). The BIS of groups
1A and 1B at L0 were significantly higher compared with T3
(P<0.05). These results suggested that the BIS of groups 1A and
1B increased immediately following intubation.
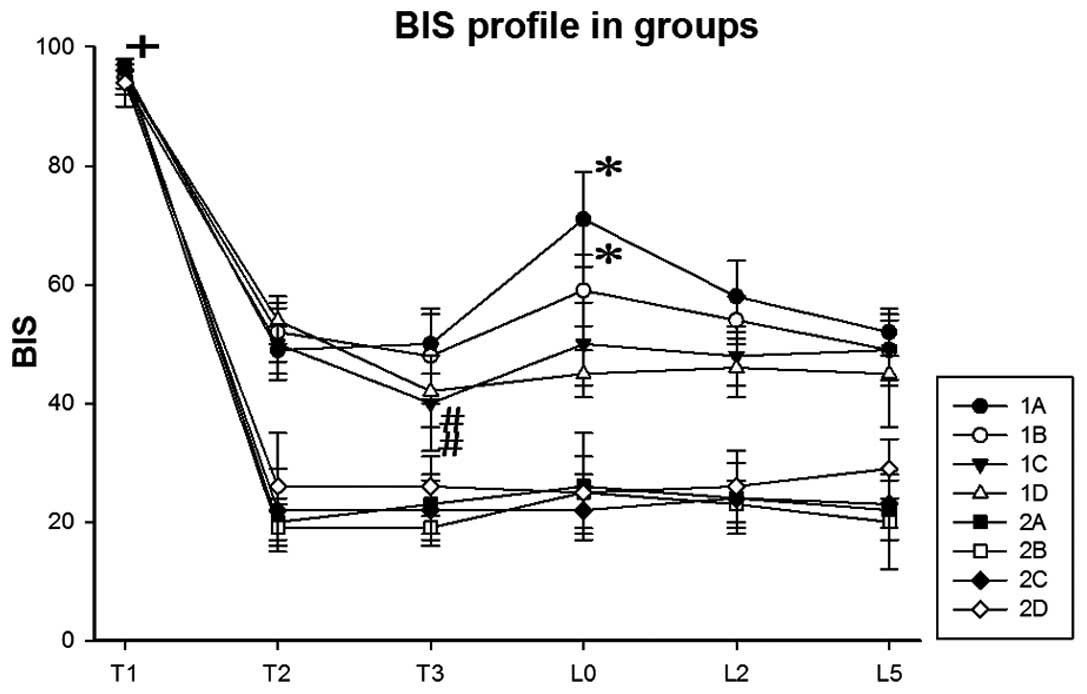 | Figure 1.Changes in BIS in each group (means ±
standard deviation; n=9) prior to induction of anesthesia (T1),
during steady state (T2), following administration of rocuronium
for 2 min (T3), and 0, 2, and 5 min following intubation (L0, L2
and L3, respectively). +P<0.05, BIS at T1 was
significantly higher compared with the other time points;
#P<0.05, BIS of groups 1C and 1D at T3 were
significantly lower compared with T2; *P<0.05, BIS of groups 1A
and 1B at L0 were significantly higher compared with T3. BIS,
bispectral index. |
The changes in EMG in each group are presented in
Fig. 2. The results demonstrated
that the EMG values at T1 were significantly higher compared with
other time points (P<0.05). The EMG value of group 1D was
significantly lower at T3, compared with T2 (P<0.05). The EMG
values of groups 1A and 1B at L0 were significantly higher compared
with T3 (P<0.05). These results suggested that the EMG of groups
1A and 1B increased immediately following intubation.
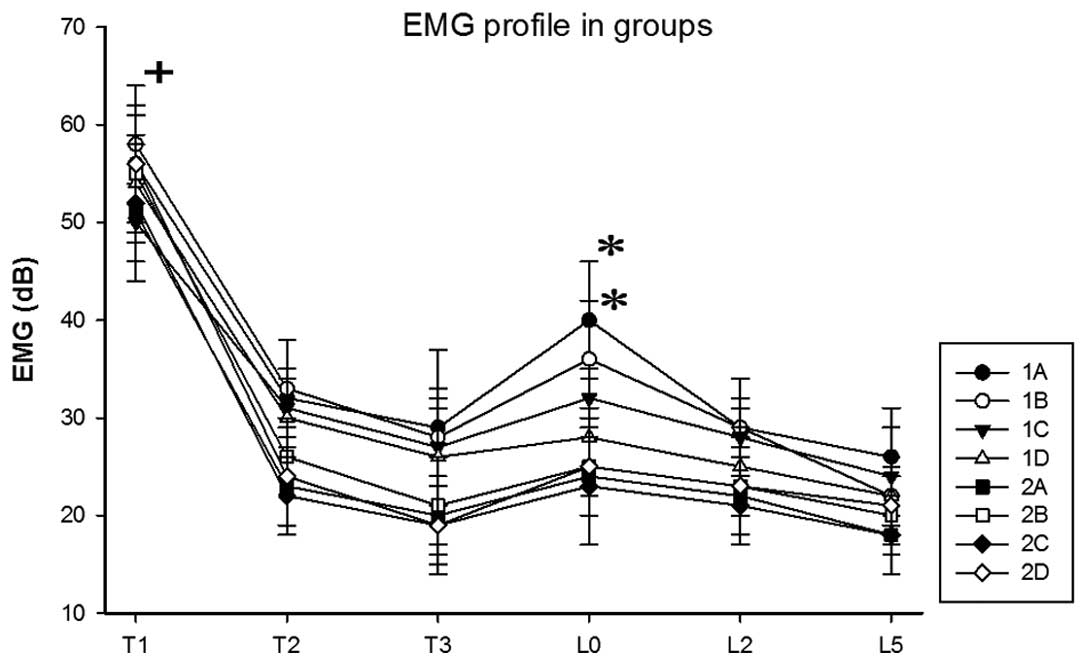 | Figure 2.Changes in EMG in each group (means ±
standard deviation; n=9) prior to induction of anesthesia (T1),
during steady state (T2), following administration of rocuronium
for 2 min (T3), and 0, 2, and 5 min following intubation (L0, L2
and L3, respectively). +P<0.05, EMG at T1 was
significantly higher compared with other time points; *P<0.05,
EMG of groups 1A and 1B at L0 were significantly higher compared
with T3. EMG, electromyography. |
Discussion
With the development of various anesthesia drugs,
novel muscle relaxants and anesthetic techniques have also been
developed and applied; numerous clinical signs of general
anesthesia are no longer regular and specific. Therefore, it is
difficult to define the concept of general anesthesia and provide
clear indications of a sedated state. Muscle relaxation lead to the
decrease or disappearance of EMG near the electrodes, which in turn
decreases the BIS. The indicative role of BIS on anesthesia depth
is then affected. When monitoring indicators of depth of
anesthesia, the numerical range of the depth of anesthesia (80–100,
awake; 60–80, mild sedation; 40–60, anesthesia; <40, deep
sedation) reflects the state of consciousness of a patient. A
larger fluctuation range makes it more difficult to determine the
state of consciousness of the patient using the index (15). Therefore, the present study aimed to
study the impact and possible mechanism underlying the effects of
muscle relaxants on depth of anesthesia.
Morimoto et al (16) investigated the association between
BIS and EEG using nitrous oxide/isoflurane anesthesia. The authors
developed a new index called burst-compensated spectral edge
frequency 95%, derived from the burst suppression ratio and
spectral edge frequency, which is the product of the power spectrum
analysis (16). The results revealed
that BIS values may be calculated from Beta Ratios under shallow
sedation (16). However, during deep
anesthesia, synch fast slow (17)
reflected power changes in EEG that were superior to changes in
phase coupling in EEG. This suggests that synch fast slow
predominantly reflected power changes in EEG, and BIS changes were
largely due to power changes in EEG during deep anesthesia; and the
impact of the EEG phase coupling is less marked than the EEG
power.
Non-depolarizing muscle relaxants competed with
acetylcholine for the nicotinic receptors, which are located at the
motorial end-plate of striated muscles. Muscle relaxants cause
muscle fibers to be in the polarization state and thus to have
blocked ion channels. There were no end-plate potential activated
excitement-contraction couplings, which suggests the blocked
conduction between motor nerve terminals and striated muscles
induced muscle relaxation. Previous studies have suggested that
non-depolarizing muscle relaxants may have sedative effects
(18,19). Muscle stretch receptors activate the
cerebral center, and non-depolarizing muscle relaxants inhibit the
activity of stretch receptors, which results in sedation (20).
In the present study, patients were anesthetized
with propofol using a target-controlled infusion, and these
patients were randomly divided into 2 sedation groups. Each group
was randomly divided into 4 subgroups A-D (n=9), according to the
various doses of rocuronium used (0.3, 0.6, 0.9 and 1.2 mg/kg). The
results of the present study revealed that the BIS of groups 1C and
1D at T3 were significantly lower compared with T2 (P<0.05), and
EMG changes were not statistically significant. This suggests that
high doses of rocuronium effectively inhibited the activity of
muscle stretch receptors, and afferent impulses from the muscle
spindle to the wake center decreased. This resulted in the absence
of painful stimulus and decreased BIS. These results were
concordant with the hypothesis of muscle spindle afferents
(21). Since there were no
significant differences in EMG, the interference of frontal
electromyography (fEMG) on BIS did not occur. Although EMG and BIS
values in the other two groups (0.3 and 0.6 mg/kg rocuronium)
decreased, the differences were not statistically significant.
Therefore, it may be hypothesized that rocuronium causes an
afferent decrease in impulses from the muscle spindle to the wake
center, which results in deeper sedation and decreased BIS.
EMG and BIS in the deep sedation group (group 2) at
T2 and T3 exhibited no significant changes (P>0.05). This
suggests that under deep sedation and in the absence of harmful
stimuli, the inhibition of the central awakening by propofol was
stronger than muscle spindle afferent impulses. The other
possibility is that muscle relaxants eliminate EMG artifacts, which
results in decreased BIS (22). fEMG
may be inhibited by deep sedation which in turn counteracts
rocuronium, thereby producing no statistically significant
different in EMG and BIS. Muscle relaxants had no effect on BIS in
the absence of EMG artifacts during deep sedation.
Endotracheal intubation is the most important
harmful stimuli during anesthesia induction. This causes
circulatory system fluctuations in the brain subcortical central
(23). Cortical excitability awakes
the patient (24), and may even lead
to intraoperative awareness (25).
Intubation during propofol anesthesia significantly increased BIS
(4). However, other investigators
observed that intubation did not have an impact on BIS, and may
lead BIS to increase (26,27). When sevoflurane is used alone, BIS
was able to better monitor changes in the depth of anesthesia
during endotracheal intubation; when combined with rocuronium,
intubation conditions improved, the stress response caused by
endotracheal intubation decreased, and the sensitivity of BIS
decreased (28).
In the present study, BIS and EMG in groups 1A and
1B at L0 were significantly higher than at T3 (P<0.05), and
conversely EMG and BIS in groups 1C and 1D exhibited no
statistically different changes (P>0.05). Various doses of
rocuronium had different muscle paralysis effects. Rocuronium
inhibited myoelectric activities following intubation, which
suggested the increased BIS may be associated with rocuronium.
Therefore, high doses of rocuronium did not significantly increase
BIS; increased BIS caused by low doses of rocuronium may be
associated with intubation stimulation and fEMG. Furthermore, when
muscle activity was excited, the stretching or contraction of
muscle fibers continued to increase muscle afferent activities, and
cause afferent impulses caused excitation in the brain.
BIS and EMG in each group did not significantly
change (P>0.05) under deep sedation. This suggested that deep
sedation inhibited the effects of intubation on BIS, results which
are concordant with those of Morimoto et al (16). Under deep sedation, brain cell
activity was inhibited, brain efficiency was decreased, and the
inhibitory effect of propofol on brain awakening was significantly
increased. Muscle spindle afferent impulses at this time may have
been under the central brain arousal threshold, and the incoming
excitatory stimulus was not sufficient to awaken the central
nervous system (16).
BIS and hemodynamics are sensitive to stress
response triggered by noxious stimuli such as endotracheal
intubation and skin incisions (29).
Guignard et al (24) also
demonstrated that BIS was able to accurately reflect the degree of
noxious stimuli during tracheal intubation. The results of the
current study revealed that HR and MAP decreased in each group at
T2 and T3, and significantly increased at L0 compared with T3
(P<0.05). However, changes in hemodynamics in the normal
sedation group were significantly better compared with the deep
sedation group. Rate-pressure in each group returned to the levels
similar to those prior to intubation 5 min after intubation. This
suggests that endotracheal intubation may result in significant
hemodynamic changes, and HR and MAP increases both under general
sedation and deep sedation. In addition, the various doses of
rocuronium had no significant effect on hemodynamics.
Since this study was based on clinical research,
following experimentation the patients remained sedated and
underwent surgical procedures. Therefore, an awake rocuronium test
group was not established. However, previous studies have reported
that under BIS and EMG simultaneous monitoring, patients were able
to communicate with the researchers when succinylcholine was given
without any other anesthetics. These results revealed that BIS
values decreased rapidly following administration of
succinylcholine, which is similar to sedation under anesthesia. At
the same time, fEMG activity disappeared. BIS recovery exhibited a
correlation with EMG activity recovery (29). These results revealed that muscle
relaxants alone are unable to sedate patients, however BIS
decreased and induced a state of sedation.
References
|
1
|
Huang YF, Pryor ME, Mather LE and Veering
BT: Cardiovascular and central nervous system effects of
intravenous levobupivacaine and bupivacaine in sheep. Anesth Analg.
86:797–804. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gazzanelli S, Vari A, Tarquini S,
Fermariello A, Caputo M, Almansour M, Costi U, Basso L, Casullo L
and Izzo L: Monitoring of consciousness with BIS during induction
of anesthesia. G Chir. 26:163–169. 2005.PubMed/NCBI
|
|
3
|
Gao JD, Zhao YJ, Xu CS, Zhao J, Huang YG,
Wang TL, Pei L, Wang J, Yao LN, Ding Q, et al: Evaluation of
entropy for monitoring the depth of anesthesia compared with
bispectral index: A multicenter clinical trial. Chin Med J (Engl).
125:1389–1392. 2012.PubMed/NCBI
|
|
4
|
Hans P, Giwer J, Brichant JF, Dewandre PY
and Bonhomme V: Effect of an intubation dose of rocuronium on
spectral entropy and bispectral index responses to laryngoscopy
during propofol anaesthesia. Br J Anaesth. 97:842–847. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pomfrett CJ: Heart rate variability, BIS
and 'depth of anaesthesia'. Br J Anaesth. 82:659–662. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sie MY, Goh PK, Chan L and Ong SY:
Bispectral index during modified rapid sequence induction using
thiopentone or propofol and rocuronium. Anaesth Intensive Care.
32:28–30. 2004.PubMed/NCBI
|
|
7
|
Forbes A, Cohen N and Eger EI II:
Pancuronium reduces halothane requirement in man. Anesth Analg.
58:497–499. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riess ML, Graefe UA, Goeters C, Van Aken H
and Bone HG: Sedation assessment in critically ill patients with
bispectral index. Eur J Anaesthesiol. 19:18–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prell J, Rampp S, Ache J, Laule S,
Rachinger J, Scheller C, Alfieri A and Strauss C: The potential of
quantified lower cranial nerve EMG for monitoring of anesthetic
depth. J Neurosurg Anesthesiol. 24:139–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vivien B, Di Maria S, Ouattara A, Langeron
O, Coriat P and Riou B: Overestimation of Bispectral Index in
sedated intensive care unit patients revealed by administration of
muscle relaxant. Anesthesiology. 99:9–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SJ, Cho YJ, Oh JH, Hwang JW, Do SH
and Na HS: Pretreatment of magnesium sulphate improves intubating
conditions of rapid sequence tracheal intubation using alfentanil,
propofol, and rocuronium-a randomized trial. Korean J Anesthesiol.
65:221–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawaguchi M, Takamatsu I and Kazama T:
Rocuronium dose-dependently suppresses the spectral entropy
response to tracheal intubation during propofol anaesthesia. Br J
Anaesth. 102:667–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pearsall FJ, Davidson JA and Asbury AJ:
Attitudes to the Association of Anaesthetists recommendations for
standards of monitoring during anaesthesia and recovery.
Anaesthesia. 50:649–653. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chun H, Kang J, Kim KJ, Park KS and Kim
HC: IT-based diagnostic instrumentation systems for personalized
healthcare services. Stud Health Technol Inform. 117:180–190.
2005.PubMed/NCBI
|
|
15
|
Iannuzzi M, Iannuzzi E, Rossi F, Berrino L
and Chiefari M: Relationship between bispectral index,
electroencephalographic state entropy and effect-site EC50 for
propofol at different clinical endpoints. Br J Anaesth. 94:492–495.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morimoto Y, Hagihira S, Koizumi Y, Ishida
K, Matsumoto M and Sakabe T: The relationship between bispectral
index and electroencephalographic parameters during isoflurane
anesthesia. Anesth Analg. 98:1336–1340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rampil IJ: A primer for EEG signal
processing in anesthesia. Anesthesiology. 89:980–1002. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hosseinzadeh H and Asl M Nassiri:
Anticonvulsant, sedative and muscle relaxant effects of
carbenoxolone in mice. BMC Pharmacol. 3:32003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanford TJ Jr, Weinger MB, Smith NT,
Benthuysen JL, Head N, Silver H and Blasco TA: Pretreatment with
sedative-hypnotics, but not with nondepolarizing muscle relaxants,
attenuates alfentanil-induced muscle rigidity. J Clin Anesth.
6:473–480. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dahaba AA, Mattweber M, Fuchs A, Zenz W,
Rehak PH, List WF and Metzler H: The effect of different stages of
neuromuscular block on the bispectral index and the bispectral
index-XP under remifentanil/propofol anesthesia. Anesth Analg.
99:781–787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dahaba AA, Bornemann H, Hopfgartner E,
Ohran M, Kocher K, Liebmann M, Wilfinger G and Metzler H: Effect of
sugammadex or neostigmine neuromuscular block reversal on
bispectral index monitoring of propofol/remifentanil anaesthesia.
Br J Anaesth. 108:602–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aho AJ, Kamata K, Yli-Hankala A,
Lyytikäinen LP, Kulkas A and Jäntti V: Elevated BIS and Entropy
values after sugammadex or neostigmine: An electroencephalographic
or electromyographic phenomenon? Acta Anaesthesiol Scand.
56:465–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mi WD, Sakai T, Takahashi S and Matsuki A:
Haemodynamic and electroencephalograph responses to intubation
during induction with propofol or propofol/fentanyl. Can J Anaesth.
45:19–22. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guignard B, Menigaux C, Dupont X, Fletcher
D and Chauvin M: The effect of remifentanil on the bispectral index
change and hemodynamic responses after orotracheal intubation.
Anesth Analg. 90:161–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ekman A, Lindholm ML, Lennmarken C and
Sandin R: Reduction in the incidence of awareness using BIS
monitoring. Acta Anaesthesiol Scand. 48:20–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugiura S, Hidaka K, Seki S and Tsuchida
H: Comparative study of two beta adrenergic antagonists on
hemodynamics at the endotracheal intubation. Anesthesiology.
107:A3092007.
|
|
27
|
Messieha ZS, Guirguis S and Hanna S:
Bispectral index monitoring (BIS) as a guide for intubation without
neuromuscular blockade in office-based pediatric general
anesthesia: A retrospective evaluation. Anesth Prog. 58:3–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Messner M, Beese U, Romstöck J, Dinkel M
and Tschaikowsky K: The bispectral index declines during
neuromuscular block in fully awake persons. Anesth Analg.
97:488–491. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Albertin A, Casati A, Federica L, Roberto
V, Travaglini V, Bergonzi P and Torri G: The effect-site
concentration of remifentanil blunting cardiovascular responses to
tracheal intubation and skin incision during bispectral
index-guided propofol anesthesia. Anesth Analg. 101:125–130. 2005.
View Article : Google Scholar : PubMed/NCBI
|
















