Introduction
Skeletal muscle atrophy occurs under various
pathophysiological conditions, such as aging, starvation, disuse,
severe injury, cancer and other cachectic diseases (1–3).
Previous studies have been concerned with denervation-induced
skeletal muscle atrophy following peripheral nerve injury (4,5).
Therefore, isobaric tags for relative and absolute quantification
coupled with two-dimensional liquid chromatography-tandem mass
spectrometry were performed in order to identify the differentially
expressed proteins in the tibialis anterior (TA) muscle 1 and 4
weeks following sciatic nerve transection in rats (4). The results demonstrated that tumor
necrosis factor receptor-associated factor 6 (TRAF6) was
upregulated in denervated TA muscle (4). Conversely, the results also
demonstrated that knockdown of TRAF6 significantly attenuated
glucocorticoid-induced myotube atrophy in vitro and in
vivo (5).
As is well known, TRAF6 is a unique E3 ubiquitin
ligase and adaptor protein. It is involved in activation of various
signaling cascades including mitogen-activated protein kinase,
nuclear factor-κB, and phosphoinositide 3-kinase/protein kinase B
in response to various cytokines (6–8), and
also important for the interaction with multiple components of the
ubiquitin-proteasome system (UPS) in some cell types (9–11). Due
to its biological traits, TRAF6 regulates skeletal muscle mass and
UPS activation in denervation or starvation-induced muscle atrophy
(6,12), and catalyzes lysine 63-linked
polyubiquitination of several target proteins through its
association with the dimeric ubiquitin-conjugating enzyme
Ubc13/Uev1A (13).
MicroRNAs (miRNAs, miRs) are a novel class of small,
non-coding RNAs of ~22 nucleotides in length. miRNAs negatively
regulate the expression of target genes through interaction with
the 3′-untranslated regions (3′-UTR) of the target mRNAs in many
biological processes (14). There
have been extensive studies of the functions of miRNAs in normal
physiological phenomena and numerous diseases (15,16). For
instance, previous studies have demonstrated that targeting miR-122
in patients with chronic HCV genotype 1 infection caused prolonged
dose-dependent reductions in HCV RNA levels without evidence of
viral resistance, which indicted that targeting miR-122 may be an
effective and safe treatment strategy for HCV infection (17,18).
However, the involvement of miRNAs in muscle-associated biological
processes has not been fully investigated. To our knowledge, it has
only been reported that miR-1 and miR-133 are able to promote
differentiation and proliferation of the skeletal and cardiac
muscle cells (19), and the
expression of miR-351 in differentiated C2C12 cells is upregulated
following treatment with dexamethasone (20). The underlying mechanisms, however,
are still to be further explored. The present study aimed to
examine whether certain miRNAs, such as miR-351, are able to
regulate denervation-induced skeletal atrophy in a model of rat
sciatic nerve injury and TA muscle denervation, providing a
potential novel molecular target for the clinical treatment of
denervation-induced muscle atrophy.
Materials and methods
Animal surgery and treatments
A total of 126 adult male Sprague-Dawley rats
(weight, 200 g; age, 2 months) were supplied by the Experimental
Animal Center of Nantong University (Nantong, China) with routine
provision of food and water, and were maintained at 24°C with a
12:12 h light/dark cycle. All animal experiments were carried out
in accordance with the institutional animal care guidelines of
Nantong University and approved by the Administration Committee of
Experimental Animals, Jiangsu Province.
A total of 72 rats underwent surgery for sciatic
nerve transection as described previously (21). The TA muscle was harvested from a
cohort of rats at 0, 3, 7 and 14 days post-surgery. Another cohort
of 54 rats was randomly divided into two groups to receive a
multi-point injection of miR-351 agomir and negative control agomir
(both at 2.5 nmol in 50 µl vehicle; Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) into the TA muscle. The injection was
administered a total of 5 times at a regular interval of 2 days,
the rats were sacrificed by cervical dislocation and the TA muscle
was dissected.
Dual luciferase reporter assay
HEK-293T cells were cultured in growth medium
consisting of Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
µg/ml of streptomycin (Sigma-Aldrich, St. Louis, MO, USA), and 100
U/ml of penicillin (Sigma-Aldrich) in a 37°C, 5% CO2
incubator. The cells were co-transfected with 600 ng of the
wild-type or mutant 3′-UTR luciferase reporter (Guangzhou RiboBio
Co., Ltd., Guangzhou, China) and 20 µM of the miR-351 agomir or the
negative control duplexes using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) in 24-well plates. The
sequences of these reporters were as follows: TRAF6 3′-UTR:
5′-ACGCTCATCAATTTGTCAGGGAA; TRAF6 3′-UTR mutant:
5′-ACGCTCATCAATTTGAGTCCCTA. Following transfection for 24 h, the
cells were harvested by adding 100 µl of lysis buffer (Promega
Corporation, Madison, WI, USA). The activity of firefly and Renilla
luciferases was measured from the cell lysates using the dual
luciferase reporter assay system (Promega Corporation). TargetScan
prediction (www.targetscan.org/vert_71) was then used to deduce
the biological targets of miRNAs.
Muscle morphological observation
At 14 days post surgery, the animals in the two
groups that had been injected with miR-351 agomir and negative
control agomir were anesthetized with 30 mg/kg pentobarbital (Merck
Millipore, Darmstadt, Germany) and perfused through the left
ventricle sequentially with saline and 4% paraformaldehyde. The TA
muscle injected with either miR-351 agomir or negative control
agomir was harvested and weighed, in order to calculate the wet
weight ratio of the muscle.
For Masson's trichrome staining, the harvested TA
muscle were post-fixed in 4% paraformaldehyde at 4°C for 24 h,
dehydrated in a graded ethanol series, cleared in xylene, embedded
in paraffin, and cut into 5 µm-thick transverse sections using a
freezing microtome. Hematoxylin and eosin staining was applied to
every section, and images were captured under a light microscope
(magnification, ×40). The average cross-sectional area (CSA) of the
TA muscle were then determined using a Leica Q5501 W image analysis
system (Leica Mycrosystems GmbH, Wetzlar, Germany) as described
previously (22).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the TA muscle (100
mg/group), harvested as described previously, using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). RT to cDNA was
carried out with a SuperScript First-Strand Synthesis system
(Invitrogen; Thermo Fisher Scientific, Inc.). All primers were
purchased from Generay Biotech Co., Ltd. (Shanghai, China). The
primers used in this study were as follows: TRAF6 forward,
5′-GCAGAGGAATCACTTGGCACG-3′ and reverse,
5′-CACGGACGCAAAGCAAGGTT-3′; miR-351 forward,
5′-ACACTCCAGCTGGGTCCCTGAGGAGCCCTTGG-3′ and reverse,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAGGCTC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
and GAPDH forward, 5′-CAACGGGAAACCCATCACCA-3′ and reverse,
5′-ACGCCAGTAGACTCCACGACAT-3′. RT-qPCR was conducted in a StepOne™
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using FastStart® SYBR Green qPCR Master Mix (Roche
Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's instructions. The reaction mixture contained 1 µl
cDNA from each sample that was mixed with 10 µl 2X Fast
Start® SYBR Green qPCR Master Mix, 2 µl 10X ROX of the
assays-on-demand kit (Biotium, Fremont, CA, USA), 1 µl primer, and
6 µl of RNase/DNase-free water (Biotium). A three-step fast cycle
protocol was used, with cycling conditions as follows: Denaturation
at 95°C, 10 sec; annealing at 60°C, 30 sec; extension at 70°C, 10
sec. The relative mRNA expression was measured through the
2−ΔΔCq method (23). U6
snRNA and GAPDH served as an internal control.
Western blot analysis
The total protein of the TA muscles, harvested as
previously described, was extracted using a T-PER tissue protein
extraction reagent (Pierce Biotechnology; Thermo Fisher Scientific,
Inc.) and quantified using a Coomassie Plus Bradford assay kit
(Pierce Biotechnology; Thermo Fisher Scientific, Inc.). Samples
containing 15 µg total protein were separated by 12% (w/v) SDS-PAGE
and transferred to a polyvinylidene difluoride membrane (EMD
Millipore, Bedford, MA, USA). Following incubation for 1 h with 5%
(w/v) non-fat dry milk in Tris-buffered saline with Tween-20
[TBS-T; 0.05% (v/v) Tween-20], the membrane was probed with the
following primary antibodies diluted in blocking buffer overnight
at 4°C: Rabbit anti-TRAF6 polyclonal antibody (1:1,000; Abgent,
Inc., San Diego, CA, USA), goat anti-muscle ring finger 1 (MuRF1)
polyclonal antibody (1:1,000; R&D System, Minneapolis, MN,
USA), rabbit anti-muscle atrophy F-box (MAFBx) polyclonal antibody
(1:1,000; LifeSpan BioSciences, Inc., Seattle, WA, USA), and rabbit
anti-β-tubulin polyclonal antibody (1:2,000; Abcam, Cambridge, MA,
USA). Following washing with TBS-T, the membrane was incubated with
secondary antibodies diluted in blocking buffer (LI-COR
Biosciences, Lincoln, NE, USA) at room temperature for 1 h. These
were anti-rabbit (dilution, 1:5,000; cat. no. 611-701-127; Rockland
Immunochemicals, Inc., Pottstown, PA, USA) or anti-goat IgG
(dilution, 1:6,000; cat. no. 605-706-125, Rockland Immunochemicals,
Inc.). The images were scanned with the Odyssey infrared image
system (LI-COR Biosciences), and the absorbance data were analyzed
using PDQuest 7.2.0 software (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analyses
All data are presented as means ± standard error of
the mean. Comparison between groups was assessed by unpaired
Student's t test using SPSS 10.0 software (SPSS, Inc., Chicago, IL,
USA). Unless otherwise specified, all assays were performed in
triplicate. P<0.05 was considered to indicate a statistically
significant result.
Results
Time-dependent expression profile of
miR-351 and TRAF6 in TA muscle following sciatic nerve
transection
RT-qPCR indicated that the expression levels of
miR-351 in the TA muscle were gradually decreased with time
following sciatic nerve transection (Fig. 1A). The mRNA and protein expression
levels of TRAF6 in the TA muscle were upregulated at various times
following sciatic nerve transaction, as determined by RT-qPCR and
western blot analysis (Fig. 1B-D).
Notably, the temporal expression profile of miR-351 in the TA
muscle was inversely correlated with that of TRAF6 during the
period of post-sciatic nerve injury.
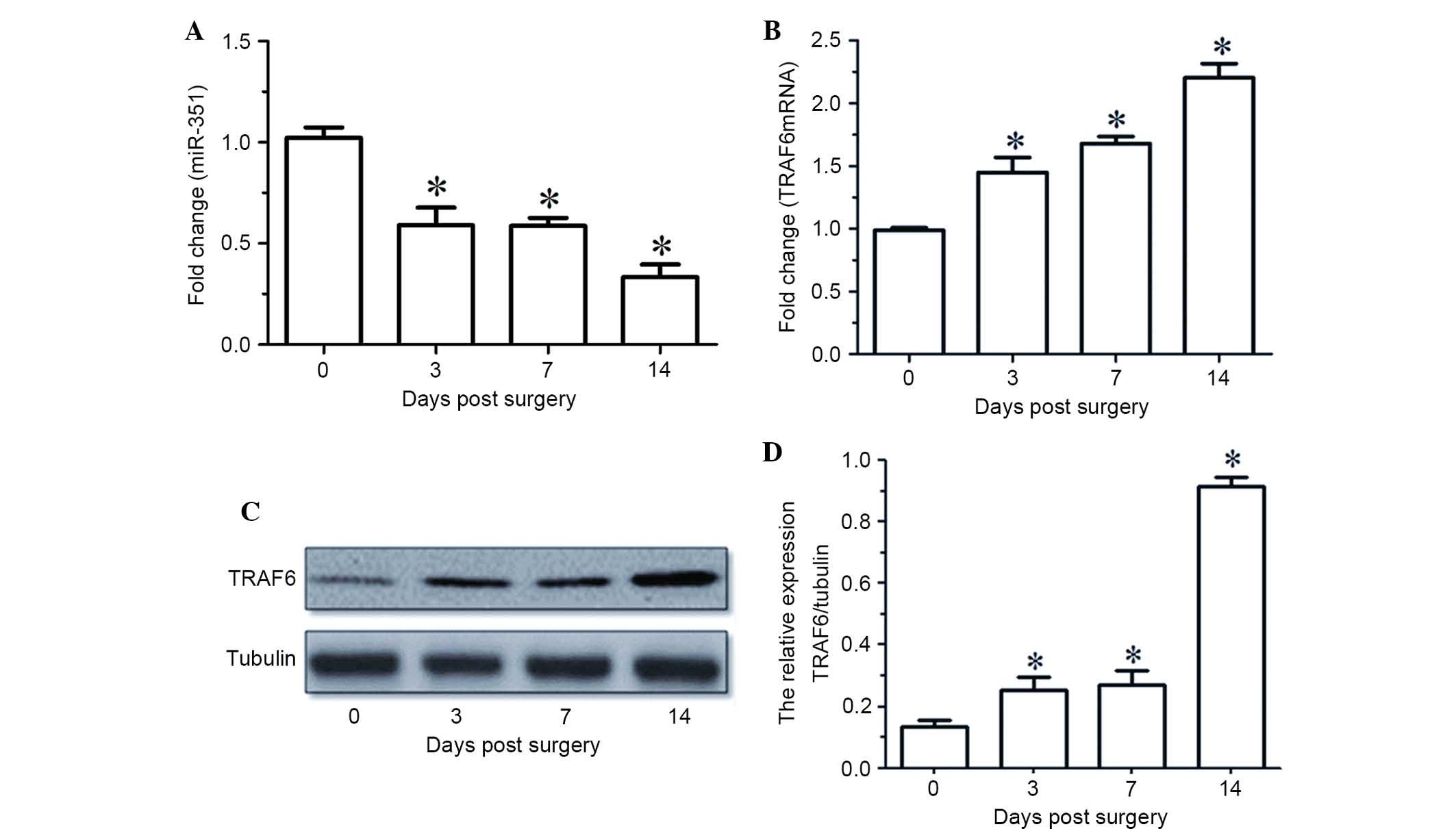 | Figure 1.(A and B) miR-351 and TRAF6 expression
levels in TA muscles at various time points (0, 3, 7, 14 days)
following sciatic nerve transection, as determined by reverse
transcription-quantitative polymerase chain reaction. (C) Western
blot analysis and (D) quantified results demonstrating the protein
expression levels of TRAF6 in the TA muscles at various time points
(0, 3, 7, 14 days) following sciatic nerve transection. Data are
presented as means ± standard deviation, n=9 per animal group.
*P<0.05, vs. 0 days post sciatic nerve transection. Tubulin was
used as a loading control in the western blot analysis. miR,
microRNA; TRAF6, tumor necrosis factor receptor-associated factor
6; TA, tibialis anterior. |
Post-transcriptional downregulation of
TRAF6 expression by miR-351
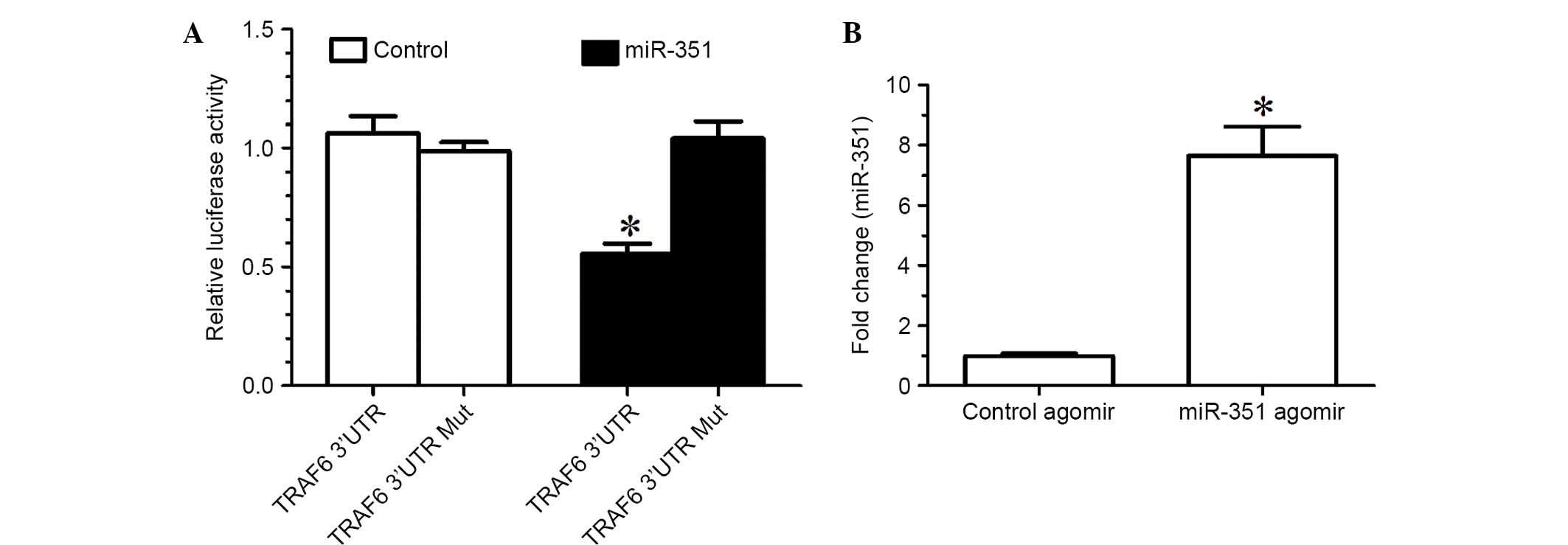
TargetScan prediction determined that the TRAF6
3′-UTR and miR-351 had a conservative matching area. To confirm
that TRAF6 was negatively regulated by miR-351 through direct
binding to the 3′-UTR of TRAF6, the wild-type 3′-UTR and mutant
3′-UTR of TRAF6 were constructed and inserted into the downstream
region of the luciferase reporter gene, respectively. In the
luciferase activity assay, miR-351 agomir or negative control
agomir with p-Luc-UTR luciferase reporter were co-transfected into
HEK 293T cells. Due to the presence of the wild-type 3′-UTR of
TRAF6, miR-351 was able to significantly decrease the relative
luciferase activity by >50%, whereas the relative luciferase
activity of mutant 3′-UTR was not decreased by miR-353, suggesting
that miR-353-induced decrease in the relative luciferase activity
was sequence-specific (Fig. 2A). In
other words, miR-351 is able to downregulate TRAF6 expression by
directly targeting the 3′-UTR of TRAF6.
Inhibition of denervation-induced TA
muscle atrophy by overexpression of miR-351
The expression levels of miR-351 in the TA muscle of
rats treated with miR-351 agomir were significantly increased
compared with those treated with control agomir (Fig. 2B), suggesting that injection of
miR-351 agomir into the TA muscle was an effective method for
increasing the expression levels of miR-351 in the muscle.
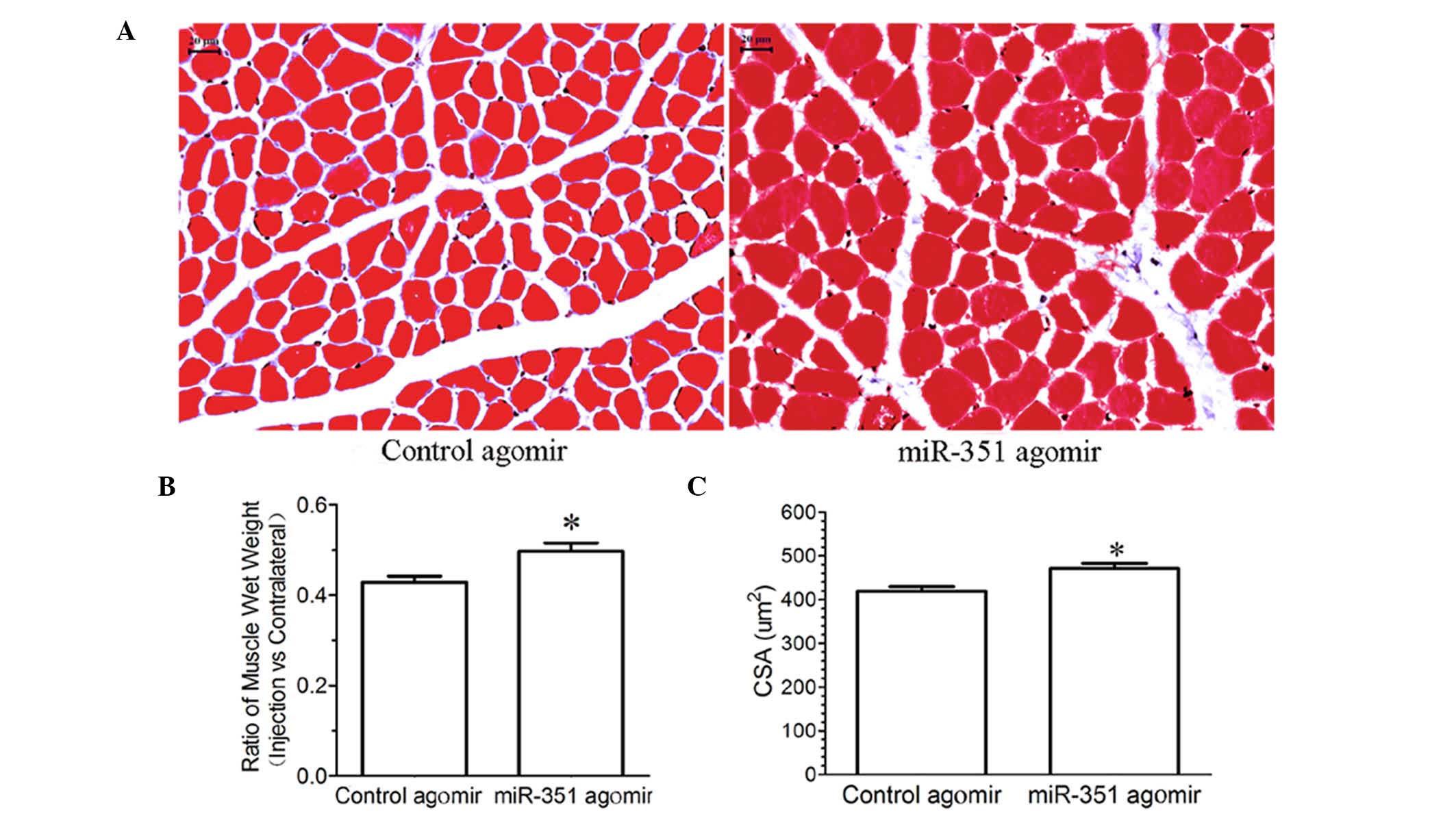
Morphological observation showed that treatment with
miR-351 agomir inhibited a significant decrease in either the wet
weight ratio or CSA of the TA muscle compared with control agomir
(Fig. 3), suggesting that
overexpression of miR-351 may inhibit denervation-induced muscle
atrophy.
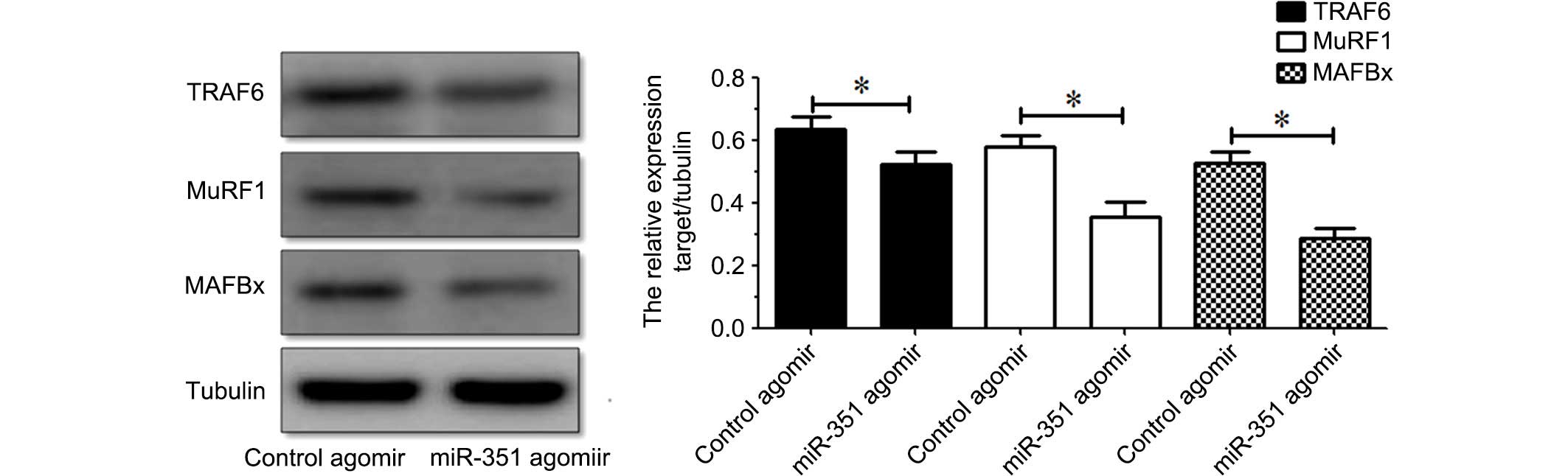
Suppression of the expression of
TRAF6, MuRF1 and MAFBx in denervated TA muscle by miR-351
Western blot analysis indicated that the protein
expression levels of TRAF6, MuRF1 or MAFBx in the TA muscle were
inhibited following treatment with miR-351 agomir compared with
those following treatment with control agomir (Fig. 4). The results indicated that
overexpression of miR-351 is able to suppress the protein
expression of TRAF6, MuRF1 or MAFBx in denervated TA muscles.
Discussion
Recent evidence suggests that miRNAs are involved in
modulation of atrophy, proliferation and differentiation of
skeletal muscles (20,24,25). A
previous study observed that miR-351 is transiently increased in
early days of muscle regeneration (26). miR-351 inhibits the expression of
E2f3, a key regulator of cell cycle progression and proliferation,
promotes myogenic progenitor cell proliferation and protects early
differentiating myogenic progenitor cell from apoptosis (26). These findings revealed that miR-351
is involved in muscle atrophy. The results of the present study
identified miR-351 as a novel regulator of denervation-induced
muscle atrophy. miR-351 was differentially expressed in the TA
muscle at various times following sciatic nerve transection, and
the temporal expression profile of miR-351 in the TA muscle was
inversely correlated with that of TRAF6 at the mRNA and protein
levels. The dual luciferase reporter assay indicated that miR-351
was able to significantly downregulate TRAF6 expression by directly
targeting the 3′-UTR of TRAF6. The results further demonstrated
that overexpression of miR-351 inhibited a significant decrease in
the wet weight ratio or CSA of the TA muscle following sciatic
nerve transection.
Studies have determined that TRAF6 expression is
significantly upregulated in denervation- or starvation-induced
muscle atrophy and TRAF6 affects denervation- or starvation-induced
muscle atrophy through regulation of muscle-specific ubiquitin
ligases MuRF1 and MAFBx (5,27). In addition, the E3 ubiquitin ligase
activity of TRAF6 has proven essential for starvation-induced
muscle atrophy (6,13). In this study, therefore, it was also
determined whether MuRF1 and MAFBx, as two downstream signaling
molecules of TRAF6, were regulated by miR-351. Western blot
analysis indicated that the protein expression levels of MuRF1 and
MAFBx as well as TRAF6 were all inhibited by overexpression of
miR-351.
In conclusion, the results of the present study
demonstrated that miR-351 has an inhibitory role in
denervation-induced muscle atrophy through, at least in part,
negative regulation of TRAF6 and MuRF1 and MAFBx (two
muscle-specific ubiquitin ligases). Further research, however, is
required in order to provide a good understanding of the detailed
mechanism underlying miR-351 regulation in muscle atrophy.
Acknowledgements
This study was supported by the 863 Program (grant
no. 2012AA020502), the 973 Program (grant nos. 2014CB542202 and
2014CB542203), the NSFC (grant nos. 81130080, 81171180 and
81301628), China Postdoctoral Science Foundation (grant no.
2016M591894), Fund of Doctoral Start-up of Nantong University
(grant no. 15B18), the Priority Academic Program Development of
Jiangsu Higher Education Institutions, the Natural Science
Foundation of Jiangsu Province (grant no. BK2012230), the
Collegiate Natural Science Foundation of Jiangsu Province (grant
nos. 13KJB180018 and 13KJ180005), the Natural Science Foundation of
Nantong University (grant no. 142014). We thank Professor Jie Liu
for assistance in the preparation of the manuscript.
References
|
1
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kandarian S: The molecular basis of
skeletal muscle atrophy-parallels with osteoporotic signaling. J
Musculoskelet Neuronal Interact. 8:340–341. 2008.PubMed/NCBI
|
|
3
|
Piccirillo R, Demontis F, Perrimon N and
Goldberg AL: Mechanisms of muscle growth and atrophy in mammals and
Drosophila. Dev Dyn. 243:201–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun H, Qiu J, Chen Y, Yu M, Ding F and Gu
X: Proteomic and bioinformatic analysis of differentially expressed
proteins in denervated skeletal muscle. Int J Mol Med.
33:1586–1596. 2014.PubMed/NCBI
|
|
5
|
Sun H, Gong Y, Qiu J, Chen Y, Ding F and
Zhao Q: TRAF6 inhibition rescues dexamethasone-induced muscle
atrophy. Int J Mol Sci. 15:11126–11141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paul PK, Bhatnagar S, Mishra V, Srivastava
S, Darnay BG, Choi Y and Kumar A: The E3 ubiquitin ligase TRAF6
intercedes in starvation-induced skeletal muscle atrophy through
multiple mechanisms. Mol Cell Biol. 32:1248–1259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H and Arron JR: TRAF6, a molecular
bridge spanning adaptive immunity, innate immunity and
osteoimmunology. Bioessays. 25:1096–1105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Zhang J, Zhang L, van Dam H and
ten Dijke P: UBE2O negatively regulates TRAF6-mediated NF-κB
activation by inhibiting TRAF6 polyubiquitination. Cell Res.
23:366–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura K, Kimple AJ, Siderovski DP and
Johnson GL: PB1 domain interaction of p62/sequestosome 1 and MEKK3
regulates NF-kappaB activation. J Biol Chem. 285:2077–2089. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamothe B, Campos AD, Webster WK,
Gopinathan A, Hur L and Darnay BG: The RING domain and first zinc
finger of TRAF6 coordinate signaling by interleukin-1,
lipopolysaccharide and RANKL. J Biol Chem. 283:24871–24880. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi CS and Kehrl JH: TRAF6 and A20
regulate lysine 63-linked ubiquitination of Beclin-1 to control
TLR4-induced autophagy. Sci Signal. 3:ra422010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paul PK and Kumar A: TRAF6 coordinates the
activation of autophagy and ubiquitin-proteasome systems in
atrophying skeletal muscle. Autophagy. 7:555–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paul PK, Gupta SK, Bhatnagar S, Panguluri
SK, Darnay BG, Choi Y and Kumar A: Targeted ablation of TRAF6
inhibits skeletal muscle wasting in mice. J Cell Biol.
191:1395–1411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ouyang YB, Xu L, Yue S, Liu S and Giffard
RG: Neuroprotection by astrocytes in brain ischemia: Importance of
microRNAs. Neurosci Lett. 565:53–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soares RJ, Cagnin S, Chemello F,
Silvestrin M, Musaro A, De Pitta C, Lanfranchi G and Sandri M:
Involvement of microRNAs in the regulation of muscle wasting during
catabolic conditions. J Biol Chem. 289:21909–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirby TJ, Chaillou T and McCarthy JJ: The
role of microRNAs in skeletal muscle health and disease. Front
Biosci (Landmark Ed). 20:37–77. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janssen HL, Reesink HW, Lawitz EJ, Zeuzem
S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A,
Zhou Y, Persson R, et al: Treatment of HCV infection by targeting
microRNA. N Engl J Med. 368:1685–1694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Ree MH, van der Meer AJ, de
Bruijne J, Maan R, van Vliet A, Welzel TM, Zeuzem S, Lawitz EJ,
Rodriguez-Torres M, Kupcova V, Wiercinska-Drapalo A, et al:
Long-term safety and efficacy of microRNA-targeted therapy in
chronic hepatitis C patients. Antiviral Res. 111:53–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen JF, Mandel EM, Thomson JM, Wu Q,
Callis TE, Hammond SM, Conlon FL and Wang DZ: The role of
microRNA-1 and microRNA-133 in skeletal muscle proliferation and
differentiation. Nat Genet. 38:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen H, Liu T, Fu L, Zhao S, Fan B, Cao J
and Li X: Identification of microRNAs involved in
dexamethasone-induced muscle atrophy. Molecular and cellular
biochemistry. 381:105–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu B, Qian T, Wang Y, Zhou S, Ding G, Ding
F and Gu X: miR-182 inhibits Schwann cell proliferation and
migration by targeting FGF9 and NTM, respectively at an early stage
following sciatic nerve injury. Nucleic Acids Res. 40:10356–10365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang D, Liu M, Ding F and Gu X:
Expression of myostatin RNA transcript and protein in gastrocnemius
muscle of rats after sciatic nerve resection. J Muscle Res Cell
Motil. 27:37–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen JF, Callis TE and Wang DZ: microRNAs
and muscle disorders. J Cell Sci. 122:13–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eisenberg I, Alexander MS and Kunkel LM:
miRNAS in normal and diseased skeletal muscle. J Cell Mol Med.
13:2–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Melton DW, Gelfond JA, McManus LM
and Shireman PK: MiR-351 transiently increases during muscle
regeneration and promotes progenitor cell proliferation and
survival upon differentiation. Physiol Genomics. 44:1042–1051.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar A, Bhatnagar S and Paul PK: TWEAK
and TRAF6 regulate skeletal muscle atrophy. Curr Opin Clin Nutr
Metab Care. 15:233–9. 2012. View Article : Google Scholar : PubMed/NCBI
|