Introduction
ST-elevation myocardial infarction (STEMI) is
defined as a clinical characteristic symptom of myocardial
ischemia. It is association with persistent electrocardiographic ST
elevation and subsequent release of biomarkers of myocardial
necrosis (1). A high percentage
(40–70%) of patients presenting with STEMI have been diagnosed with
multivessel coronary artery disease (CAD) with ≥1 additional severe
non-infarct-related lesion in an artery (2,3). These
patients have worse outcomes with >2-fold increase in mortality
at 1 year compared with patients who have single vessel disease
(4,5).
Previous studies have been reported the treatment of
STEMI. For example, primary percutaneous coronary intervention
(PPCI) has been considered as the treatment of choice for patients
with STEMI (6). In addition,
complete revascularization during PPCI for STEMI has been verified
safely with improved clinical results (7). Other studies have suggested that
medical therapy with fondaparinux (8) or aspirin (with the addition of
clopidogrel) (9) is effective and
can reduce risk of complications and mortality. However, it still
remains controversial regarding the optimal management of patients
with STEMI and multivessel CAD in spite of the recent Preventive
Angioplasty in Myocardial Infarction (PRAMI) trial and Complete vs.
Lesion-Only Primary PCI trial (CvLPRIT) (10,11).
The present prospective, open-label, randomized
study aimed to further evaluate the effect of conservative
pharmacotherapy (CP) and staged percutaneous coronary intervention
(SPCI) on significant non-culprit vessel lesions following PPCI in
patients with STEMI by comparing the incidence rates of major
adverse cardiovascular events (MACE), recurrent myocardial
infarction, recurrent angina pectoris and MACE-free survival rates
at a 180- and 360-day follow-up.
Subjects and methods
Subjects and criteria
This prospective study enrolled a total of 306
patients with acute STEMI (266 male and 40 female; mean age,
56.4±10 years) treated with PPCI in Beijing Shijitan Hospital
(Beijing, China) between April 2011 and December 2012. Patients
were included on the basis of the following criteria as described
previously (12–14): i) Patients aged between 18 and 75
years; ii) patients underwent continuous ischemic chest pain for
≥30 min; iii) the electrocardiogram examination revealed
ST-elevation ≥0.2 mV in leads V2 and V3 or ST-elevation ≥0.1 mV in
at least two other continuous leads, and cardiac troponin-I
(cTn-I)-elevation >0.05 ng/ml; iv) the coronary arteriography
performed within 12 h following the onset of symptoms indicated
thrombolysis in myocardial infarction bleeding grade 0–1 in culprit
vessels; v) the PPCI was performed only in culprit vessels; and vi)
the presence of coronary arteriography corroborated lesions in
non-culprit vessel. Non-culprit lesions were defined as
angiostenosis ≥70% in one or more coronary vessels with a diameter
≥2 mm vessels other than culprit vessels.
Patients were excluded for the following criteria:
i) Patients with left main coronary artery disease, cardiogenic
shock or complete left bundle branch block; ii) PPCI performed for
both culprit and non-culprit vessels at the same time or a PPCI
performance failure; iii) patients who have experienced hemodynamic
instability or spontaneous ischemia following PPCI; iv) lesions and
angiostenosis ≥70% in vessels remaining following SPCI; v) patients
who had a history of coronary artery bypass grafting (CABG) or
chronic cardiac failure; and vi) bleeding corporeality, prior
administration of thrombolytic therapy, known thrombopenia or
leucopenia, sever liver and renal function abnormalities, active
infection, immune system and connective tissue diseases, known
contraindications to aspirin or heparin, pregnancy, life expectancy
<1 year, major operation within 3 months, uncontrolled
hypertension, a history of ischemic or hemorrhagic stroke,
intracranial diseases including aneurysm and arteriovenous
malformation within 30 days, extensive trauma within 6 weeks, oral
anticoagulant therapy, severe ongoing myocardial infarction-related
complications or perioperative mortality.
The ethical committee of Beijing Shijitan Hospital
approved the study protocol and detailed informed consent was
obtained from all of the patients or their family members.
Study procedures and drug
treatment
Patients were randomly divided into CR and CP groups
using a table of random digits. Patients in the CR group underwent
SPCI for non-culprit lesions after 7–10 days of PPCI. The PPCI was
performed according to standard techniques, during which a
drug-eluting stent was used. Patients in the CP group were only
treated with CP following PPCI. Echocardiography was performed in
both groups using a Doppler echocardiograph (Vivid; GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA), and left ventricular function
with preserved ejection fraction was diagnosed according to the
study of Paulus et al (15).
Then, an intra-aortic balloon pump was inserted in patients with
poor left ventricular function at the lesion site of
infarct-related artery following PPCI. In addition, all patients
received a single dose of oral aspirin (300 mg; Bayer Healthcare
Co., Ltd., Beijing, China) and clopidogrel (300 mg; Sanofi-Aventis,
Paris, France) prior to PPCI, and 100 mg/d aspirin, 75 mg/d
clopidogrel orally for ≥1 year. Low molecular weight heparin was
administered for 3 days following PPCI, as well as other drugs such
as statins, β receptor blocker, angiotensin-converting enzyme
inhibitor/angiotensin II receptor blocker, calcium antagonists,
nitrates or tirofiban.
Experimental endpoints
The primary endpoints were adjudicated by the
incidence of MACE at the 180- and 360-day follow-up, including
cardiac death, recurrent myocardial infarction and repeated
revascularization of the target-vessel. The secondary endpoints
were the recurrence of angina pectoris, newly developed heart
failure and bleeding events, and MACE-free survival at the 180- and
360-day follow-up. All the events were adjudicated according to the
Bleeding Academic Research Consortium proposed standardized
bleeding definitions (16).
Data collection and follow-up
Baseline demographics, including gender, age,
height, weight, case history, previous medication and other
clinical data of patients were collected. The content of cTn-I was
recorded prior to PPCI, and thereafter every 8 h after the
procedure. The levels of fasting blood glucose, hepatorenal
function, blood fat, C-reactive protein and brain natriuretic
peptide were detected on the second day following PPCI. The
cumulative incidence of MACE, mortality, repeated revascularization
of the target-vessel, recurrent myocardial infarction, recurrent
angina pectoris, new heart failure and major bleeding were recorded
at the 180- and 360-day follow-up. Follow-up was performed by
interview.
Statistical analysis
Qualitative data are expressed as percentages, and
differences between groups were analyzed using Fisher's test.
Quantitative data showing normal distribution are expressed as the
mean ± standard deviation, and differences between the two groups
were determined by independent t-test. Quantitative data showing
abnormal distribution were expressed by median (interquartile
range), and differences between groups were determined by the
Mann-Whitney U-test. The survival curves during the 180- and
360-day follow-up were plotted using the Kaplan-Meier method and
the analyses were determined using pairwise log-rank test. All
statistical tests were two-sided and the analyses were performed
using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
General characteristics and
examination of patients
A total of 306 patients in the trial were divided
into two groups following PPCI: 148 patients (48%) in the CR group,
and 158 patients (52%) in the CP group. As shown in Table I, baseline clinical characteristics
were similar in the two groups, except for the mean age (58±10 vs.
55±9 years; P=0.006), patient weight (75±13 vs. 72±10 kg; P=0.024),
length of hospital stay (10±6 vs. 8±7 days; P=0.008), disease time
(6±4 vs. 5±3 h; P=0.014), body temperature (36.3±0.3 vs.
36.4±0.4°C; P=0.014) and left ventricular end-diastolic dimension
(LVEDD; 50±4 vs. 48±4 mm; P<0.001) in the CR and CP groups,
respectively. During the hospital stay, patients in the CR and CP
groups received similar intra-aortic balloon counterpulsation and
administration of drugs (Table II),
except that significantly more CP patients were administered
nitrates compared with the CR cases (94.3 vs. 75.7%, respectively;
P<0.001). The information collected prior to and following PPCI
did not differ between the CP and CR groups (all P>0.05;
Table III).
 | Table I.Clinical characteristics of the
patients at baseline. |
Table I.
Clinical characteristics of the
patients at baseline.
| Characteristics | CR group | CP group | P-value |
|---|
| Female, n (%) | 22 (14.9) | 18 (11.4) | 0.368 |
| Mean age, years | 58±10 | 55±9 | 0.006 |
| Height, cm | 169±7 | 169±7 | 1.000 |
| Weight, kg | 75±13 | 72±10 | 0.024 |
| Smoking history, n
(%) | 100 (67.6) | 108 (68.4) | 0.883 |
| Drinking history, n
(%) | 50 (33.8) | 55 (34.8) | 0.850 |
| Case history, n
(%) |
|
|
|
| Previous
stenocardia | 76 (51.4) | 80 (50.6) | 0.900 |
| Previous
myocardial infarction | 2 (1.4) | 3 (1.9) | 0.706 |
| Family
history of coronary heart disease | 30 (20.3) | 27 (17.1) | 0.475 |
|
Hypertension | 86 (58.1) | 80 (50.6) | 0.190 |
|
Diabetes | 43 (29.1) | 45 (28.5) | 0.912 |
|
Hyperlipidemia | 72 (48.6) | 76 (48.1) | 0.924 |
|
Stroke | 8 (5.4) | 8 (5.1) | 0.893 |
|
Peripheral vascular
disease | 2 (1.4) | 4 (2.5) | 0.740 |
| Chronic
renal insufficiency | 1 (0.7) | 2 (1.3) | 1.000 |
|
Alimentary tract
hemorrhage | 4 (2.7) | 4 (2.5) | 1.000 |
| Previous medical
therapy, n (%) |
|
|
|
|
Aspirin | 21 (14.2) | 16 (10.1) | 0.276 |
|
Clopidogrel | 1 (0.7) | 1 (0.6) | 1.000 |
|
Warfarin | 1 (0.7) | 1 (0.6) | 1.000 |
| β
blocker | 15 (10.1) | 11 (7.0) | 0.320 |
|
ACEI/ARB | 24 (16.2) | 19 (12.0) | 0.292 |
|
Statins | 10 (6.8) | 13 (8.2) | 0.626 |
| Calcium
antagonists | 32 (21.6) | 27 (17.1) | 0.315 |
| Hospital stay,
days | 10±6 | 8±7 | 0.008 |
| Duration of disease,
h | 6±4 | 5±3 | 0.0135 |
| Killip grade III/IV,
n (%) | 2 (1.4) | 3 (1.9) | 1.000 |
| Body temperature,
°C | 36.3±0.3 | 36.4±0.4 | 0.014 |
| Heart rate,
times/min | 77±13 | 80±14 | 0.053 |
| Respiration,
times/min | 18±2 | 18±2 | 1.000 |
| LVEF, % | 54±8 | 53±8 | 0.275 |
| LVEDD, mm | 50±4 | 48±4 | <0.001 |
| Convict vessel site,
n (%) |
|
|
|
| LAD | 71 (48.0) | 85 (53.8) | 0.308 |
| LCX | 9 (6.1) | 10 (6.3) | 0.928 |
| RCA | 68 (45.9) | 63 (39.9) | 0.283 |
| Non-culprit lesions,
n (%) |
|
|
|
|
Single-vessel | 99 (66.9) | 105 (66.5) | 0.936 |
|
Double-vessel | 49 (33.1) | 53 (33.5) | 0.936 |
| Emergency stents | 1.3±0.6 | 1.3±0.5 | 1.000 |
| Severe ventricular
arrhythmias, n (%) | 1 (0.7) | 2 (1.3) | 1.000 |
| Slow blood flow or no
reflow, n (%) | 8 (5.4) | 10 (6.3) | 0.731 |
 | Table II.Treatment administered during the
hospital stay. |
Table II.
Treatment administered during the
hospital stay.
| Item | CR group | CP group | P-value |
|---|
| Intra-aortic
balloon counterpulsation |
|
|
|
| Case, n
(%) | 12 (8.1) | 14 (8.9) | 0.813 |
| Time,
h | 6.4±18.8 | 10.4±28.2 | 0.148 |
| Medical therapy, n
(%) |
|
|
|
|
Aspirin | 148 (100) | 158 (100) | – |
|
Clopidogrel | 148 (100) | 158 (100) | – |
|
Cilostazol | 2 (1.4) | 2 (1.3) | 1.000 |
| Low
molecular weight heparin | 100 (67.6) | 101 (63.9) | 0.502 |
|
βreceptor blocker | 128 (86.5) | 135 (85.4) | 0.793 |
|
ACEI/ARB | 125 (84.5) | 137 (86.7) | 0.575 |
|
Statins | 148 (100) | 158 (100) | – |
| Calcium
antagonists | 1 (0.7) | 1 (0.6) | 1.000 |
|
Nitrates | 112 (75.7) | 149 (94.3) | <0.001 |
|
Tirofiban | 50 (33.8) | 55 (34.8) | 0.850 |
 | Table III.Data collected prior to and following
surgery. |
Table III.
Data collected prior to and following
surgery.
| Terms | CR group | CP group | P-value |
|---|
| Before surgery |
|
|
|
| AUC of
CK-MB, ×103 ng/(ml*h) | 5.7 (4.0–10.9) | 5.5 (3.7–8.9) | 0.473 |
| AUC of
cTnI, ×103 ng/(ml*h) | 2.9 (1.3–5.4) | 2.8 (1.6–4.7) | 0.891 |
| Peak of
CK-MB, ng/ml | 252 (169–459) | 260 (191–423) | 0.875 |
| Peak of
cTn-I, ng/ml | 77 (53–185) | 104 (61–182) | 0.227 |
| Time to
CK-MB peak, h | 11 (9–15) | 10 (8–14) | 0.116 |
| Time to
cTnI peak, h | 12 (9–16) | 11 (8–16) | 0.910 |
| After surgery |
|
|
|
| ALT,
U/l | 19±8 | 21±7 | 0.511 |
| AST,
U/l | 17±10 | 18±8 | 0.237 |
| CRP,
mg/l | 7.7±3 | 8.5±2.6 | 0.682 |
| BNP,
pg/ml | 896.5±102.3 | 937.3±98.5 | 0.149 |
| White
blood cell, ×109/l | 11.0±3.6 | 12.2±4.4 | 0.446 |
| Red
blood cell, ×1012/l | 4.5±0.5 | 4.6±0.4 | 0.271 |
|
Hemoglobin, g/l | 142±17 | 143±16 | 0.862 |
|
Hematocrit, % | 41±4 | 42±3 | 0.179 |
| Blood
platelets, ×109/l | 218±52 | 217±51 | 0.864 |
| Blood
urea nitrogen, mmol/l | 6.3±1.9 | 6.0±1.7 | 0.142 |
| Serum
creatinine, µmol/l | 83.2±20.2 | 83.5±24 | 0.357 |
| Blood
uric acid, µmol/l | 326±108 | 303±91 | 0.226 |
| Blood
sugar, mmol/l | 8.7±2.3 | 9.0±3 | 0.073 |
|
Triglyceride, mmol/l | 1.8±1.6 | 1.7±1.2 | 0.557 |
| Total
cholesterol, mmol/l | 4.7±1 | 4.5±1 | 0.315 |
|
High-density lipoprotein,
mmol/l | 1.1±0.4 | 1.1±0.3 | 0.782 |
|
Low-density lipoprotein,
mmol/l | 2.9±0.9 | 2.7±0.8 | 0.163 |
MACE and recurrent angina pectoris
during follow-up
All patients were followed-up and the clinical
outcomes of the 180- and 360-day follow-up after PPCI are presented
in Table IV. The incidence of
recurrent angina pectoris in the CP group was significantly higher
compared with that in the CR group at the 180-day follow-up (13.9
vs. 5.4%, respectively; P=0.012) and the 360-day follow-up (18.4
vs. 8.1%, respectively; P=0.009). A total of 29 cases had developed
MACE symptoms by the 360-day follow-up, and the incidence of MACE
in the CP group was significantly higher compared with that in the
CR group (6.1 vs. 12.7%; P=0.050), and the same tendency was
detected in the incidence of recurrent myocardial infarction (CP
group vs. CR group, 10.1 vs. 4.1%; P=0.040). The incidence of
mortality, repeated revascularization of the target-vessel, newly
developed heart failure and major bleeding did not differ between
the two groups (all P>0.05).
 | Table IV.Clinical outcomes during the
follow-up. |
Table IV.
Clinical outcomes during the
follow-up.
|
| 180 days
follow-up |
| 360 days
follow-up |
|
|---|
|
|
|
|
|
|
|---|
| Outcome | CR group | CP group | P-value | CR group | CP group | P-value |
|---|
| Primary outcome, n
(%) |
|
|
MACE | 4 (2.7) | 12 (7.6) | 0.055 | 9 (6.1) | 20 (12.7) | 0.050 |
|
Mortality | 1 (0.7) | 2 (1.3) | 1.000 | 3 (2.0) | 4 (2.5) | 1.000 |
|
Repeated revascularization of
target-vessel | 2 (1.4) | 5 (3.2) | 0.289 | 4 (2.7) | 7 (4.4) | 0.417 |
|
Recurrent myocardial
infarction | 3 (2.0) | 10 (6.3) | 0.062 | 6 (4.1) | 16 (10.1) | 0.040 |
| Secondary outcome,
n (%) |
|
|
Recurrent angina pectoris | 8 (5.4) | 22 (13.9) | 0.012 | 12 (8.1) | 29 (18.4) | 0.009 |
| New
heart failure | 2 (1.4) | 2 (1.3) | 1.000 | 2 (1.4) | 3 (1.9) | 1.000 |
| Major
bleeding | 8 (5.4) | 7 (4.4) | 0.693 | 11 (7.4) | 10 (6.3) | 0.703 |
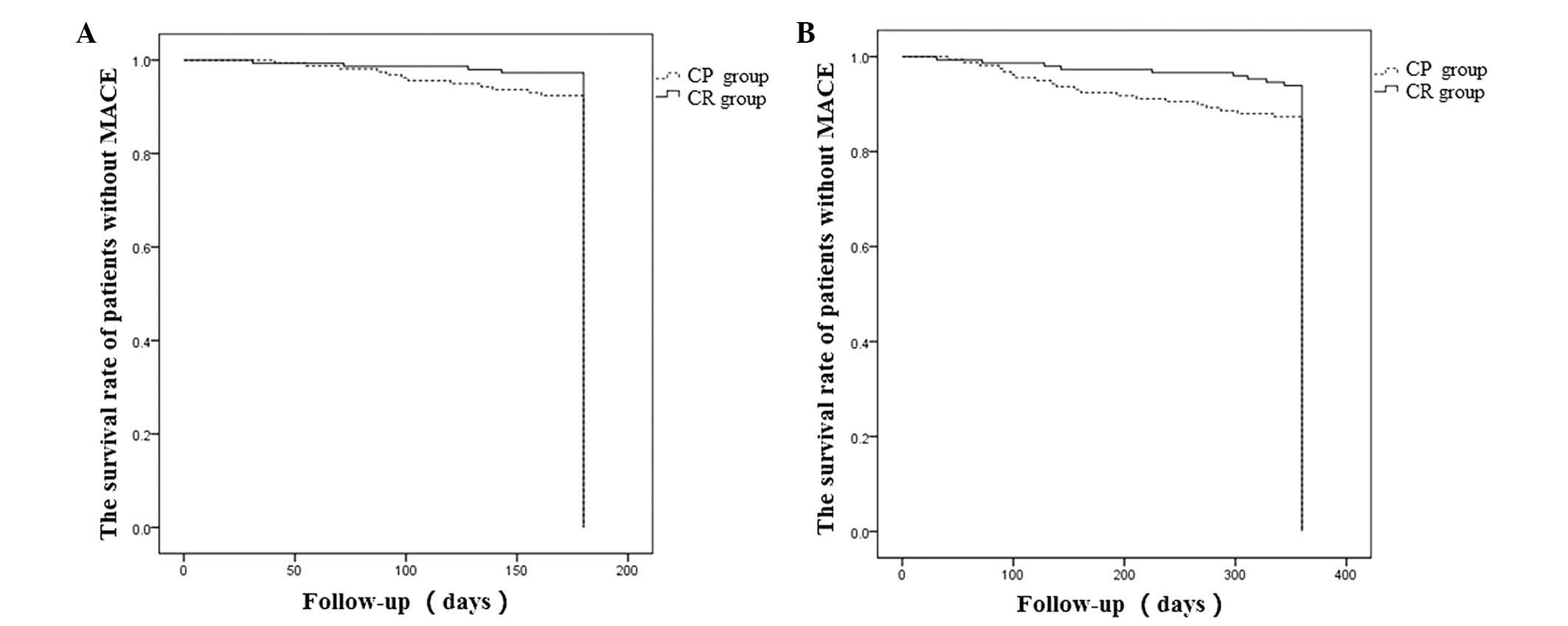
Patient survival rate
The Kaplan-Meier analysis revealed no difference in
the MACE-free survival rate of patients between the CR and CP
groups at the 180-day follow-up (97.3 vs. 92.4%; P>0.05;
Fig. 1A). However, the MACE-free
survival rate of the CR group was significantly higher compared
with that of the CP group at the 360-day follow-up (93.9 vs. 87.3%;
P<0.05; Fig. 1B).
Discussion
In the present study, a prospective trial analysis
was performed and the outcomes of SPCI and CP on non-culprit
vessels in STEMI following treatment with PPCI are compared. The
results of the 360-day follow-up indicated that the incidence of
MACE and recurrent myocardial infarction in the CP group were
higher compared with those in the CR group. In addition, the
incidence of recurrent angina pectoris in the CP group was higher
compared with that in the CR group during the 180-and 360-day
follow-up. At the 360-day follow-up, the MACE-free survival rate of
the CR group was significantly higher compared with that in the CP
group.
In the current study, all of the decisions regarding
the treatment of patients were made based on the discretion of the
clinicians involved. The performance of intra-aortic balloon
counterpulsation and medical therapy were similar in the two
groups, expect for the administration of nitrates. At the 360-day
follow-up, repeated revascularization of the target vessel was
performed in 11 patients: 4 in the CR group and 7 in the CP group.
Despite no statistically significant difference, the findings
suggested that SPCI on non-culprit lesions may result in less
repeated revascularization of the target vessel.
A previous study by Di Mario et al (17) investigated the safety of the primary
PCI procedure and identified that CR was not associated with excess
in-hospital or 1-year MACE (defined as mortality, repeat myocardial
infarction, urgent percutaneous transluminal coronary angioplasty
or CABG). Qarawani et al (7)
demonstrated that CR facilitated the reduced incidence of major
cardiac events, including recurrent ischemia, acute heart failure,
re-infarction and in-hospital mortality during PPCI. The analyses
of the 360-day follow-up data in the present study revealed a
significantly higher incidence of MACE in the CP group compared
with the CR group. In addition, patients in the CR group
experienced a lower incidence of recurrent angina pectoris compared
with patients in the CP group, particularly at the 360-day
follow-up. These findings are in agreement with two recent
randomized trials (PRAMI and CVLPRIT) which showed reduced
incidence of MACE with CR compared with CP in non-infarct coronary
arteries with major stenosis (10,11).
It has been hypothesized that for patients with
STEMI, PPCI of the non-culprit vessel in the acute phase could
reduce ischemia and improve survival rate (18). Vlaar et al (19) performed a pairwise meta-analysis by a
primary end point of short-term mortality and identified a benefit
with a staged approach in STEMI with multi-vessel disease. In
addition, Bainey et al (20)
performed a randomized and observational meta-analysis by adding
further long-term survival (14.5 months), supporting SPCI in STEMI
therapy. Furthermore, a study on the safety and efficacy of
complete vs. culprit-only revascularization in patients with STEMI
identified that SPCI can improve short- and long-term survival and
reduce repeat PPCI (21).
Consistently, patients in the present study had a higher MACE-free
survival rate in the CR group compared with in the CP group at the
360 day follow-up.
A number of limitations of the current study must be
addressed. First, although patients in each group were matched by
demographic and baseline clinical characteristics, there was a
significant difference in the age, disease time, hospital stay,
body temperature and LVEDD between the patients in the two groups.
Furthermore, the CP group appeared to have more severe coronary
artery disease and more impaired cardiac function compared with
patients in the CR group, although statistical significance was not
reached. Second, significance levels were borderline in the
results, which may be due to the small number of patients enrolled
in the study. Third, the limited data collected during the short
follow-up in this study result in the need of larger trials that
can confirm the benefits of staged multivessel PPCI in patients
with STEMI.
In conclusion, the results of the present study
demonstrate that in patients with STEMI, SPCI for non-culprit
lesions results in improved clinical outcomes compared with CP, as
evidenced by reduced incidence of subsequent MACE, recurrent
myocardial infarction at 360-day follow-up, low incidence of
recurrent angina pectoris at 180- and 360-day follow-up and a
higher MACE-free survival rate at 360-day follow-up.
Acknowledgements
The present study was supported by the Found of the
Capital Medicine Developing Research, Beijing (grant no.
2009-2076).
References
|
1
|
American College of Emergency Physicians;
Society for Cardiovascular Angiography and Interventions, ; O'Gara
PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA,
Ettinger SM, Fang JC, et al: American College of Cardiology
Foundation/American Heart Association Task Force on Practice
Guidelines: 2013 ACCF/AHA guideline for the management of
ST-elevation myocardial infarction: A report of the American
college of cardiology foundation/American heart association task
force on practice guidelines. J Am Coll Cardiol. 61:e78–e140.
2013.PubMed/NCBI
|
|
2
|
de Waha S, Eitel I, Desch S, Fuernau G,
Pöss J, Schuler G and Thiele H: Impact of multivessel coronary
artery disease on reperfusion success in patients with ST-elevation
myocardial infarction-insights from cardiac magnetic resonance
imaging. J Cardiovasc Magn Reson. Dec 21–2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toma M, Buller CE, Westerhout CM, Fu Y,
O'Neill WW, Holmes DR Jr, Hamm CW, Granger CB and Armstrong PW:
APEX-AMI Investigators: Non-culprit coronary artery percutaneous
coronary intervention during acute ST-segment elevation myocardial
infarction: Insights from the APEX-AMI trial. Eur Heart J.
31:1701–1707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaski BE, Cohen JD, Trausch J, Marsh DG,
Bail GR, Overlie PA, Skowronski EW and Smith SC Jr: Outcome of
urgent percutaneous transluminal coronary angioplasty in acute
myocardial infarction: Comparison of single-vessel versus
multivessel coronary artery disease. Am Heart J. 124:1427–1433.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sorajja P, Gersh BJ, Cox DA, McLaughlin
MG, Zimetbaum P, Costantini C, Stuckey T, Tcheng JE, Mehran R,
Lansky AJ, et al: Impact of multivessel disease on reperfusion
success and clinical outcomes in patients undergoing primary
percutaneous coronary intervention for acute myocardial infarction.
Eur Heart J. 28:1709–1716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
King SB III, Smith SC Jr, Hirshfeld JW Jr,
Jacobs AK, Morrison DA, Williams DO; 2005 WRITING COMMITTEE
MEMBERS, ; Feldman TE, Kern MJ, O'Neill WW, et al: 2007 Focused
update of the ACC/AHA/SCAI 2005 guideline update for percutaneous
coronary intervention: A report of the American college of
cardiology/American heart association task force on practice
guidelines: 2007 Writing group to review new evidence and update
the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary
intervention, writing on behalf of the 2005 writing committee.
Circulation. 117:261–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qarawani D, Nahir M, Abboud M, Hazanov Y
and Hasin Y: Culprit only versus complete coronary
revascularization during primary PCI. Int J Cardiol. 123:288–292.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yusuf S, Mehta SR, Chrolavicius S, Afzal
R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin
L, et al: OASIS-6 Trial Group: Effects of fondaparinux on mortality
and reinfarction in patients with acute ST-segment elevation
myocardial infarction: The OASIS-6 randomized trial. JAMA.
295:1519–1530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sabatine MS, Cannon CP, Gibson CM,
López-Sendón JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill
KA, Skene AM, et al: CLARITY-TIMI 28 Investigators: Addition of
clopidogrel to aspirin and fibrinolytic therapy for myocardial
infarction with ST-segment elevation. N Engl J Med. 352:1179–1189.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wald DS, Morris JK, Wald NJ, Chase AJ,
Edwards RJ, Hughes LO, Berry C and Oldroyd KG: PRAMI Investigators:
Randomized trial of preventive angioplasty in myocardial
infarction. N Engl J Med. 369:1115–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gershlick AH, Khan JN, Kelly DJ, Greenwood
JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL,
Banya W, et al: Randomized trial of complete versus lesion-only
revascularization in patients undergoing primary percutaneous
coronary intervention for STEMI and multivessel disease: The
CvLPRIT trial. J Am Coll Cardiol. 65:963–972. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stone GW, Witzenbichler B, Guagliumi G,
Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ,
Pocock SJ, et al: HORIZONS-AMI Trial Investigators: Bivalirudin
during primary PCI in acute myocardial infarction. N Engl J Med.
358:2218–2230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stone GW, Lansky AJ, Pocock SJ, Gersh BJ,
Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie
BR, et al: HORIZONS-AMI Trial Investigators: Paclitaxel-eluting
stents versus bare-metal stents in acute myocardial infarction. N
Engl J Med. 360:1946–1959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehran R, Lansky AJ, Witzenbichler B,
Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann
F, Gersh BJ, et al: HORIZONS-AMI Trial Investigators: Bivalirudin
in patients undergoing primary angioplasty for acute myocardial
infarction (HORIZONS-AMI): 1-year results of a randomised
controlled trial. Lancet. 374:1149–1159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paulus WJ, Tschöpe C, Sanderson JE,
Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De
Keulenaer G, Leite-Moreira AF, et al: How to diagnose diastolic
heart failure: A consensus statement on the diagnosis of heart
failure with normal left ventricular ejection fraction by the heart
failure and echocardiography associations of the european society
of cardiology. Eur Heart J. 28:2539–2550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cutlip DE, Windecker S, Mehran R, Boam A,
Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, et al:
Academic Research Consortium: Clinical end points in coronary stent
trials: A case for standardized definitions. Circulation.
115:2344–2351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Mario C, Mara S, Flavio A, Imad S,
Antonio M, Anna P, Emanuela P, Stefano DS, Angelo R, Stefania C, et
al: Single vs multivessel treatment during primary angioplasty:
Results of the multicentre randomised HEpacoat™ for cuLPrit or
multivessel stenting for acute myocardial infarction (HELP AMI)
Study. Int J Cardiovas Interve. 6:128–133. 2004. View Article : Google Scholar
|
|
18
|
Hochman JS, Sleeper LA, Webb JG, Dzavik V,
Buller CE, Aylward P, Col J and White HD: SHOCK Investigators:
Early revascularization and long-term survival in cardiogenic shock
complicating acute myocardial infarction. JAMA. 295:2511–2515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vlaar PJ, Mahmoud KD, Holmes DR Jr, van
Valkenhoef G, Hillege HL, van der Horst IC, Zijlstra F and de Smet
BJ: Culprit vessel only versus multivessel and staged percutaneous
coronary intervention for multivessel disease in patients
presenting with ST-segment elevation myocardial infarctiona
pairwise and network meta-analysis. J Am Coll Cardiol. 58:692–703.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bainey KR, Mehta SR, Lai T and Welsh RC:
Complete vs culprit-only revascularization for patients with
multivessel disease undergoing primary percutaneous coronary
intervention for ST-segment elevation myocardial infarction: A
systematic review and meta-analysis. Am Heart J. 167:1.e12–14.e12.
2014. View Article : Google Scholar
|
|
21
|
Sethi A, Bahekar A, Bhuriya R, Singh S,
Ahmed A and Khosla S: Complete versus culprit only
revascularization in acute ST elevation myocardial infarction: A
meta-analysis. Catheter Cardiovasc Interv. 77:163–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|