Introduction
Traditionally, the therapeutic effects of anti-tumor
therapies are determined by detecting reductions in tumor size via
ultrasonography, computed tomography (CT), and magnetic resonance
imaging. However, this process requires a lengthy time interval of
several months on average. For some intractable tumors, early
treatment response evaluation can help to adjust the therapeutic
schedule prior to changes in tumor size, which may avoid
unnecessary toxic reactions and economic waste. Tumor cell
proliferative activity is an important biological characteristic of
malignant tumors that has been used to evaluate the responses of
tumors to various treatments (1).
However, not all tumors can be evaluated clinically via biopsy to
detect alterations in the tumor cell proliferative activity during
treatment. Therefore, a noninvasive, simple, and accurate in
vivo method to detect tumor cell proliferative activity is
required. Given the development of clinical applications for
positron emission tomography (PET)-CT imaging, it is possible to
use positron imaging agents to evaluate the effects of treatments
on tumors, particularly with respect to early curative effects.
Currently, F-18-fluoro-deoxyglucose (18F-FDG) is the
most widely applied clinical positron imaging agent. This agent
reflects glucose metabolism in organs and tissues and thereby is
widely used in tumor diagnosis, staging, curative effect
determination, and recurrence monitoring (2). However, some metabolically active
tissues or lesions, including the myocardium, brain tissue,
inflammatory areas, and certain benign tumors, may also exhibit
increased uptake of imaging agents, leading to poor
18F-FDG imaging specificity (2). Previous preclinical and clinical
studies have shown that F-18-fluoro-3′-deoxy-3′-L-fluorothymidine
(18F-FLT), which is a pyrimidine analogue, reflects
changes in the tumor cell proliferative activity and its uptake in
tumor tissues is associated with this activity (3–13).
Therefore, 18F-FLT may be used as a positron imaging
agent for early evaluation of the curative effects of therapies on
tumors. However, some studies have indicated that there may not be
an association between 18F-FLT uptake and tumor cell
proliferative activity (14,15).
Although 18F-FLT shares some biochemical
characteristics with thymidine, it serves as a terminator of DNA
strand synthesis and is not incorporated into the DNA strand. In
addition, the dynamic differences between 18F-FLT and
thymidine have not yet been clearly defined (16). The underlying mechanism of
18F-FLT, as well as its significance with respect to
tumor diagnosis, also remains unclear. However, 18F-FLT
is expected to be of value in terms of tumor diagnosis and curative
effect evaluations. In the present study, 18F-FLT and
18F-FDG uptake were used to evaluate the early
therapeutic effects of chemotherapy in Walker 256 tumor-bearing
Wistar rats, and the correlations between uptake and tumor cell
proliferative activity were analyzed.
Materials and methods
Establishment of the rat model
A total of 3 walker 256 ascites tumor-bearing Wistar
rats (mean age, 6 weeks; mean weight, 135 g) were obtained from the
Institute of Oncology, Chinese Academy of Medical Science (Beijing,
China). All rats were pathogen-free and were housed in a specific
pathogen-free room at a constant temperature of 25°C and humidity
of 45%, with a 12 h light/dark cycle and ad libitum access
to food and water. When the rats exhibited abdominal bulging, 5–6
ml of flaxen-colored ascites were collected and diluted with
physiological saline to a 4×107 cells/ml suspension. A
total of 30 additional healthy female Wistar rats (age, 5 weeks;
weight, 120±20 g) were obtained from the Laboratory Animal Center
of China Medical University (Shenyang, China). The prepared Walker
256 cell suspension was subcutaneously inoculated into the right
axilla of healthy rats (0.2 ml/rat). Tumors grew within 7–10 days.
Rats were used in the following experiments once the maximum tumor
diameters at the inoculation sites grew to 1.5–2.0 cm. The present
study was conducted in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee
(IACUC) of China Medical University.
Animal grouping and treatment
Walker 256 tumor model rats were randomly divided
into two groups, a control group (n=10) and a chemotherapy group
(n=20). Chemotherapy was performed via tail intravenous injection
of epirubicin (Zhejiang Hisun Pharmaceutical Co., Ltd., Shanghai,
China). Epirubicin was diluted to a concentration of 1 mg/ml, and
each rat was administered a volume of 0.5–0.7 ml at a dose of 5
mg/kg body weight. At 24 h after chemotherapy, five rats each
underwent 18F-FLT PET-CT or 18F-FDG PET-CT
imaging. At 48 h after chemotherapy, five each of the remaining 10
rats underwent 18F-FLT PET-CT and 18F-FDG
PET-CT imaging. Rats in the control group received tail intravenous
injections of equivalent volumes of physiological saline and
subsequently underwent 18F-FLT or 18F-FDG
PET-CT imaging (n=5 for each treatment).
18F-FLT and
18F-FDG PET-CT imaging
18F-FLT and 18F-FDG were
synthesized at the PET-CT Center of the Affiliated Shengjing
Hospital of China Medical University with a Minitrace drug
synthesis system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
The FLT precursor was produced by Jiangsu Huayi Chemical Co., Ltd.,
(Changshu, China). A 0.2-ml volume of 18F-FLT or
18F-FDG (0.5 mCi) was administered to each rat via
caudal vein injection. The radioactive intensity of the syringe was
detected prior to and following injection. Following a period of 40
min, the rats were anesthetized via intraperitoneal injection of
chloral hydrate (Chinese Medicine Group Chemical Reagent Co., Ltd.,
Shanghai, China) at a dose of 3 ml/kg body weight. Each rat was
subjected to PET-CT imaging 1 h after imaging agent injection. Each
rat was fixed on a foam plate and placed in a PET-CT imaging system
(Discovery ST16 PET-CT; GE Healthcare Bio-Sciences). CT acquisition
conditions were set to 120 kV, 60 mA, and 5-mm layer thickness. A
3-dimensional acquisition model was used for PET. Each bed was
scanned for 3 min from head to tail. Images were subsequently
processed on a Xeleris workstation (GE Healthcare Bio-Sciences).
The ratio of ROI radioactivity counts in the tumor tissue to those
in the same area of the contralateral soft tissue (T/M) was
quantitatively determined.
Tumor tissue processing
Following imaging, the rats were immediately
sacrificed by cervical dislocation and weighed. Tumor tissues were
removed, flushed with physiological saline, and wiped dry to
determine the radioactive intensities and weights. The percentage
intake of radioactivity per gram of tumor tissue (% ID/g) was
calculated. Tumor tissues were subsequently fixed in 10% neutral
formaldehyde and processed into paraffin-embedding blocks within 24
h. Embedded tissues were sectioned into slides and stained with
hematoxylin and eosin. Immunohistochemical Ki-67 staining of the
tumor tissues was performed using an Envision kit (cat. no.
D-C1-08C22C; Wuhan Boster Biological Technology Ltd., Wuhan, China)
according to the manufacturer's instructions. Ki-67 expression was
determined via light microscopy by an experienced pathologist.
Samples were considered to be Ki-67-positive if the tumor cell
nuclei and occasional cytoplasm were stained brown. If only the
cytoplasm but no nuclei were stained, the sample was considered
negative. The positive percentage of Ki-67, which was termed as the
labeling index (LI-Ki-67), was identified by scanning 10 random
high-powered (magnification, ×400) fields per tumor section.
Statistical analysis
Data processing was performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). Measurement
data are shown as the mean ± standard deviation. Mann-Whitney tests
were used to compare the data collected before and after
chemotherapy, and Pearson correlation analysis was implemented.
P<0.05 was considered to indicate a statistically significant
difference.
Results
18F-FLT and
18F-FDG radioactivity distribution
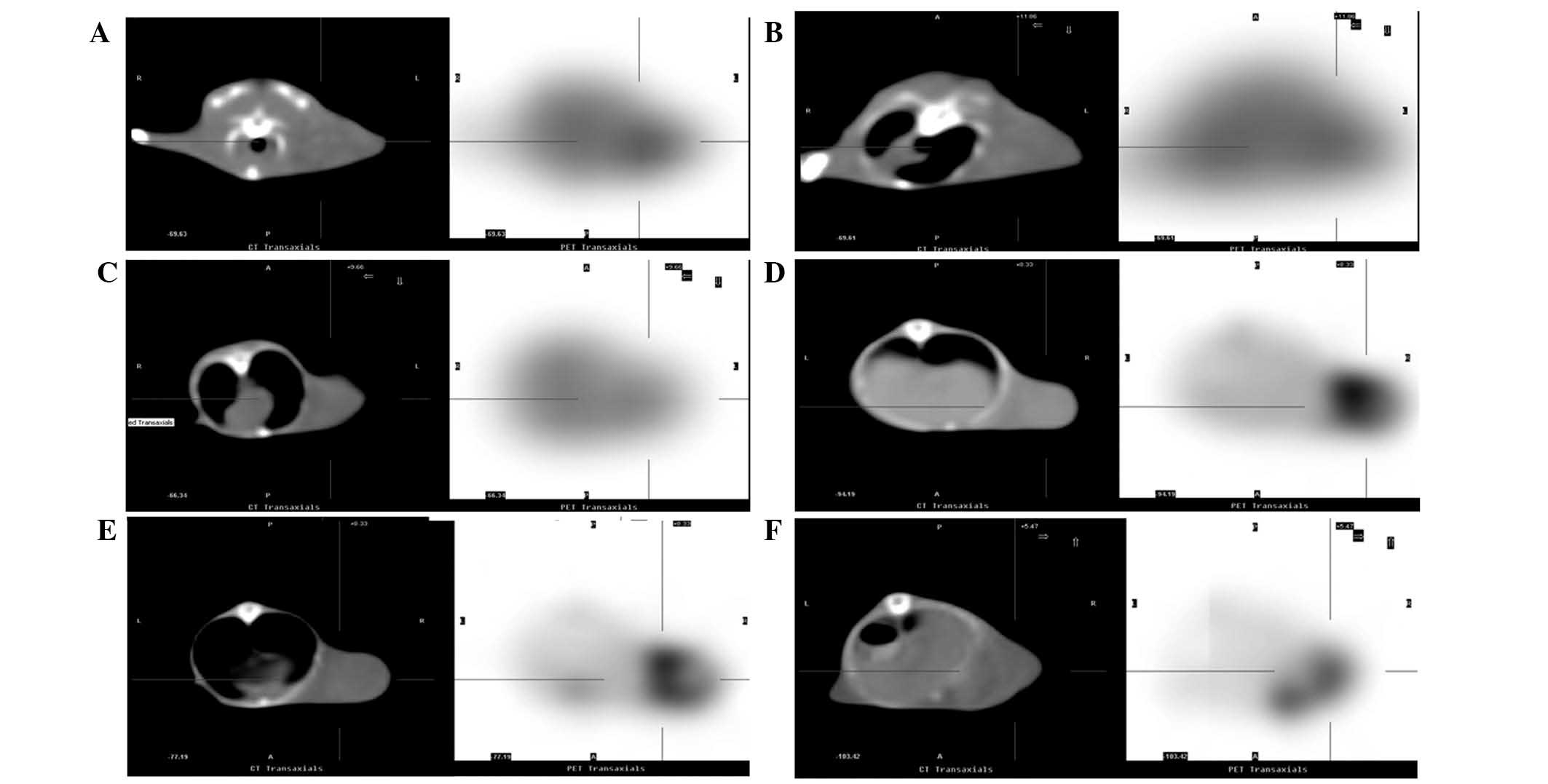
PET-CT imaging demonstrated significant reductions
in the distribution of 18F-FLT radioactivity in
tumor-bearing mice at 24 and 48 h after chemotherapy, as compared
with the control group (Fig. 1A-C).
The T/M ratios were also significantly reduced (P<0.01, Table I). Similarly, the distribution of
18F-FDG radioactivity and T/M ratio significantly
reduced at 24 and 48 h after chemotherapy (Fig. 1D-F; P<0.05; Table I).
 | Table I.18F-FLT and
18F-FDG uptake and LI-Ki-67 in tumor tissues. |
Table I.
18F-FLT and
18F-FDG uptake and LI-Ki-67 in tumor tissues.
|
| 18F-FLT
uptake | 18F-FDG
uptake |
|
|---|
|
|
|
|
|
|---|
| Groups | T/M | % ID/g | T/M | % ID/g | LI-Ki-67 |
|---|
| Before
chemotherapy | 2.128±0.145 | 0.334±0.034 | 6.374±0.649 | 4.100±0.573 | 58.92±4.85 |
| 24 h after
chemotherapy | 1.572±0.181 | 0.244±0.032 | 5.482±0.459 | 3.232±0.441 | 41.00±5.70 |
| 48 h after
chemotherapy | 1.156±0.221 | 0.168, 0.030 | 4.744±0.309 | 2.636±0.364 | 25.96±3.42 |
|
P-valuea | 0.001 | 0.003 | 0.036 | 0.028 | 0.001 |
| Fb | 8.221 | 8.198 | 5.704 | 4.819 | 12.419 |
|
P-valueb | <0.001 | <0.001 | 0.001 | 0.001 | <0.001 |
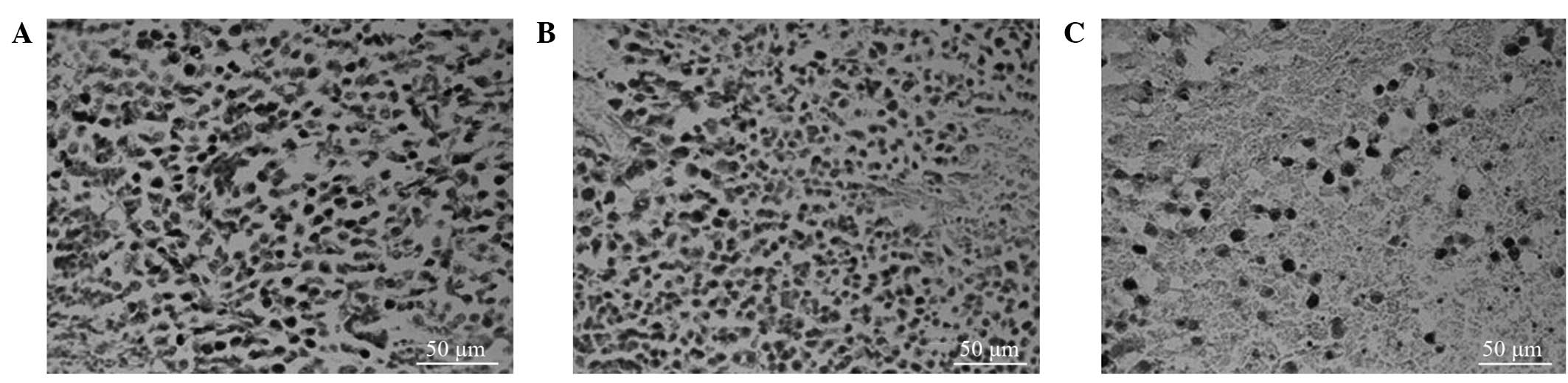
Ki-67 labeling index
Tumor cells with brown-stained nuclei were
considered to be proliferating cells. Compared with the LI-Ki-67 of
the control group, the LI-Ki-67 values at 24 and 48 h after
chemotherapy were significantly reduced in the tumor-bearing mice
(58.92±4.85% vs. 41.00±5.70% and 58.92±4.85% vs. 25.96±3.42%,
respectively; P<0.001; Fig. 2A-C;
Table I).
Correlation analysis
18F-FLT uptakes (T/M) at both 24 and 48 h
after chemotherapy correlated positively with the LI-Ki-67
(r=0.899). Meanwhile, the 18F-FDG uptake (T/M) after
chemotherapy also correlated positively with the LI-Ki-67
(r=0.813). In both the 18F-FLT and 18F-FDG
groups, the T/M correlated positively with the % ID/g (r=0.898 and
0.843, respectively).
Discussion
The present study primarily investigated whether
changes in the 18F-FLT and 18F-FDG uptake in
Walker 256 tumors during the early post-chemotherapy stage may
reflect alterations in the tumor cell proliferative activity. The
applicability of 18F-FLT for evaluating the early
efficacy of tumor treatment was also assessed. The results
demonstrated that alterations in the uptake of 18F-FLT
and 18F-FDG after chemotherapy correlated positively
with changes in tumor cell proliferation.
The present findings showed that the uptake of
18F-FLT by Walker 256 tumors was reduced during the
early stage after epirubicin chemotherapy (24 h) and that
18F-FLT uptake exhibited an additional significant
reduction 48 h after chemotherapy. Immunohistochemistry also
demonstrated downregulated Ki-67 expression in the tumor tissues,
suggesting that epirubicin may inhibit tumor proliferative
activity, a process that could also be detected via
18F-FLT PET-CT imaging.
An alteration in the hydroxyl group of 4′-epirubicin
from a cis- to a trans-form renders epirubicin a cell
cycle-nonspecific drug, and its main mechanism of action is
mediated through DNA binding (17,18). A
previous in vitro experiment demonstrated that epirubicin
was able to rapidly enter cells and bind to DNA, thereby inhibiting
the nucleic acid synthesis and mitosis (19). From its mechanism of function, we
concluded that since epirubicin inhibits cellular DNA synthesis,
tumor cells use less thymidine and thus take up smaller amounts of
the 18F-FLT thymidine analogue. The pyrimidine analogue
18F-FLT is involved in DNA synthesis, but not RNA
synthesis, during the process of cell proliferation (20). 18F-FLT is transported into
cells via passive diffusion and facilitated by
Na+-dependent carrier transport. It is subsequently
phosphorylated by thymidine kinase 1 (TK1) to yield
18F-FLT-monophosphate and is retained in the cell. TK1
is among the key enzymes necessary for the DNA salvage pathway,
which is inactive in resting cells but exerts maximal activity in
the late G1 and S phases of proliferating cells (21). Therefore, TK1 catalytic
phosphorylation is the basis for the use of 18F-FLT as a
tracer. It has previously been reported that TK1 activity is
markedly increased in malignant tumor cells, when compared with
benign cells (22). Furthermore, in
tumor cells, mutations occur at the carboxy terminus of TK1, which
inhibits the normal degradation of TK1 during the M phase; as a
result, the activity of TK1 increases abnormally in tumor cells
(23). Therefore, actively
proliferating tumors are able to take up 18F-FLT and
exhibit focally concentrated radioactivity on PET-CT. Accordingly,
the reduced uptake of 18F-FLT by Walker 256 tumors
following epirubicin chemotherapy may have been associated with
inhibited cell proliferative activity. The amount of
18F-FLT uptake may be used to reflect tumor cell
proliferation during the early stage after chemotherapy.
Although the study demonstrated an association
between 18F-FLT uptake and cell proliferation, in
contrast to the opposing results achieved by other scholars
(14,15), it is important to note that there are
two tumor DNA synthesis pathways, de novo synthesis and
salvage synthesis. Different tumors may employ different DNA
synthesis pathways. Some tumors predominantly use salvage
synthesis, whereas others typically use de novo synthesis
(24). TK1 is an enzyme that only
acts within DNA salvage synthesis, and thus it can only reflect DNA
salvage synthesis activity in tumors. Chemotherapy drugs can induce
a transformation between the two synthetic routes. Certain
chemotherapy drugs are able to activate the DNA salvage synthesis
pathway by inhibiting the de novo synthesis pathway, thus
increasing TK1 activity and 18F-FLT uptake. For
instance, the chemotherapy drug 5-fluorouracil (5-FU) can transform
into its derivatives fluorodeoxyuridine monophosphate and
fluorouridine triphosphate in vivo and thus block the
synthesis of dTMP (25).
Accordingly, 5-FU can affect DNA biosynthesis, induce S phase
stasis, and subsequently activate the DNA salvage synthesis route.
Eventually, 5-FU therapy can result in increased TK1 activity. This
increased TK1 activity promotes 18F-FLT uptake but
downregulates DNA synthesis and subsequently reduces the cell
proliferation rate (26). In such
situations, 18F-FLT uptake does not reflect tumor cell
proliferation. Despite this finding, the role of 18F-FLT
in tumor research cannot currently be denied. Its association with
tumor cell proliferation requires more in-depth and detailed
studies.
Different drugs inhibit tumor growth via various
mechanisms at different cell cycle phases. 18F-FLT is
only involved in the DNA salvage synthesis pathway, and thus, it
does not reflect the proliferation of tumors that predominantly
employ the de novo synthesis pathway. Therefore, when
determining the effects of 18F-FLT uptake on tumor cell
proliferation, the anti-tumor drugs and their mechanisms of action
should be considered to ensure that 18F-FLT uptake
provides a true reflection of tumor cell proliferation. Clinically,
combination chemotherapy is used to treat patients with tumors,
which leads to complicated changes in tumor DNA synthesis. In this
situation, 18F-FLT uptake can hardly reflect tumor cell
proliferation. Accordingly, additional preclinical and clinical
research is required to eludicate the roles of 18F-FLT
in different tumors treated via different methods (27).
18F-FDG uptake by tumors also decreased
during the early stage after chemotherapy in the present study, a
phenomenon that may have been associated with the mechanism of
18F-FDG uptake. In cells, 18F-FDG uptake
predominantly depends on glucose transporter expression and hexose
phospho-kinase activity on the cell membrane (2). Potential explanations for the decreased
18F-FDG uptake observed in tumors after epirubicin
chemotherapy may include the following: Epirubicin may reduce the
activity of hexose phospho-kinase and thus reduce
18F-FDG uptake by the tumor, and epirubicin may inhibit
tumor cell division and thus lead to a lower energy requirement in
these cells. Glucose is the main source of energy for tumor growth.
Therefore, 18F-FDG uptake decreases if glucose
utilization decreases. A decrease in 18F-FDG uptake is
an indirect result of reduced tumor cell proliferation, whereas a
decrease in 18F-FLT uptake by tumor cells is directly
associated with reduced tumor cell proliferation. Therefore,
18F-FDG metabolism may also indirectly reflect tumor
cell proliferation to some extent, although this metabolism may be
influenced by various factors. For instance, blood sugar levels,
the functional status of the brain tissue, and other factors may
induce changes in the 18F-FDG uptake of tumor tissues.
Conversely, cell proliferative activity may be among the factors
influencing 18F-FDG uptake.
In conclusion, the results of the present study
showed that 18F-FLT and 18F-FDG uptake by
tumors correlated positively with the tumor cell proliferative
activity during the early stage after chemotherapy. In other words,
18F-FLT and 18F-FDG uptake by tumors may
reflect changes in the tumor cell proliferative activity during the
early stage after treatment and may be an effective index for
evaluating the early therapeutic effects of chemotherapy. In the
present study, small animals were subjected to clinical PET-CT,
resulting in poor image resolution. However, the correlation
between the tumor uptake (T/M) as determined via PET-CT imaging and
the actual tumor uptake (% ID/g) was also analyzed and the findings
showed a good positive correlation between these factors,
suggesting that PET-CT-determined T/M may reflect the actual uptake
in small animal tumor tissues.
References
|
1
|
Colozza M, Azambuja E, Cardoso F, Sotiriou
C, Larsimont D and Piccart MJ: Proliferative markers as prognostic
and predictive tools in early breast cancer: Where are we now? Ann
Oncol. 16:1723–1739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mamede M, Higashi T, Kitaichi M, Ishizu K,
Ishimori T, Nakamoto Y, Yanagihara K, Li M, Tanaka F, Wada H, et
al: [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions
in cancers and inflammatory lesions of the lung. Neoplasia.
7:369–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shields AF, Grierson JR, Dohmen BM,
Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O
and Mangner TJ: Imaging proliferation in vivo with [F-18]FLT and
positron emission tomography. Nat Med. 4:1334–1336. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grierson JR and Shields AF: Radiosynthesis
of 3′-deoxy-3′-[18F]fluorothymidine: [18F]FLT for imaging of
cellular proliferation in vivo. Nucl Med Biol. 27:143–156. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rasey JS, Grierson JR, Wiens LW, Kolb PD
and Schwartz JL: Validation of FLT uptake as a measure of thymidine
kinase-1 activity in A549 carcinoma cells. J Nucl Med.
438:1210–1217. 2002.
|
|
6
|
Buck AK, Halter G, Schirrmeister H,
Kotzerke J, Wurziger I, Glatting G, Mattfeldt T, Neumaier B, Reske
SN and Hetzel M: Imaging proliferation in lung tumours with PET:
18F-FLT versus 18F-FDG. J Nucl Med. 44:1426–1431. 2003.PubMed/NCBI
|
|
7
|
Mier W, Haberkorn U and Eisenhut M:
[18F]FLT; portrait of a proliferation marker. Eur J Nucl Med Mol
Imaging. 29:165–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shields AF: Positron emission tomography
measurement of tumour metabolism and growth: Its expanding role in
oncology. Mol Imaging Biol. 8:141–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McKinley ET, Smith RA, Zhao P, Fu A, Saleh
SA, Uddin MI, Washington MK, Coffey RJ and Manning HC:
3′-Deoxy-3′-18F-fluorothymidine PET predicts response to
(V600E)BRAF-targeted therapy in preclinical models of colorectal
cancer. J Nucl Med. 54:424–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mason NS, Lopresti BJ, Ruszkiewicz J, Dong
X, Joyce S, Leef G, Sen M, Wahed AS, Mathis CA, Grandis JR and
Thomas SM: Utility of 3′-[(18)F]fluoro-3′-deoxythymidine as a PET
tracer to monitor response to gene therapy in a xenograft model of
head and neck carcinoma. Am J Nucl Med Mol Imaging. 3:16–31.
2013.PubMed/NCBI
|
|
11
|
Hoeben BA, Troost EG, Span PN, van Herpen
CM, Bussink J, Oyen WJ and Kaanders JH: 18F-FLT PET during
radiotherapy or chemoradiotherapy in head and neck squamous cell
carcinoma is an early predictor of outcome. J Nucl Med. 54:532–540.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Liu B, Tian J, Xu B, Zhang J, Qu B
and Chen Y: Evaluation of 18F-FDG and 18F-FLT
for monitoring therapeutic responses of colorectal cancer cells to
radiotherapy. Eur J Radiol. 82:e484–e491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Herrmann K, Pirsig S,
Philipp-Abbrederis K, Henninger M, Aichler M, Feuchtinger A, Walch
A, Beer AJ, Ringshausen I, et al: Molecular imaging for early
prediction of response to Sorafenib treatment in sarcoma. Am J Nucl
Med Mol Imaging. 4:70–79. 2013.PubMed/NCBI
|
|
14
|
Geven EJ, Evers S, Nayak TK, Bergström M,
Su F, Gerrits D, Franssen GM and Boerman OC: Therapy response
monitoring of the early effects of a new BRAF inhibitor on melanoma
xenograft in mice: Evaluation of (18) F-FDG-PET and (18) F-FLT-PET.
Contrast Media Mol Imaging. 10:203–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keen HG, Ricketts SA, Maynard J, Logie A,
Odedra R, Shannon AM, Wedge SR and Guichard SM: Examining changes
in [18 F]FDG and [18 F]FLT uptake in U87-MG glioma xenografts as
early response biomarkers to treatment with the dual mTOR1/2
inhibitor AZD8055. Mol Imaging Biol. 16:421–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krohn KA, Mankoff DA, Muzi M, Link JM and
Spence AM: Ture tracers: Compraring FDG with glucose and FLT with
thymidine. Nucl Med Biol. 32:663–671. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grasl-Kraupp B, Ruttkay-Nedecky B,
Müllauer L, Taper H, Huber W, Bursch W and Schulte-Hermann R:
Inherent increase of apoptosis in liver tumors: implications for
carcinogenesis and tumor regression. Hepatology. 25:906–912. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kolaja KL, Stevenson DE, Walborg EF Jr and
Klaunig JE: Reversibility of promoter induced hepatic focal lesion
growth in mice. Carcinogenesis. 17:1403–1407. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Monteiro LJ, Khongkow P, Kongsema M,
Morris JR, Man C, Weekes D, Koo CY, Gomes AR, Pinto PH, Varghese V,
et al: The Forkhead Box M1 protein regulates BRIP1 expression and
DNA damage repair in epirubicin treatment. Oncogene. 32:4634–4645.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Been LB, Suurmeijer AJ, Cobben DC, Jager
PL, Hoekstra HJ and Elsinga PH: [18F]FLT-PET in oncology: Current
status and opportunities. Eur J Nucl Med Mol Imaging. 31:1659–1672.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Munch-Petersen B, Cloos L, Jensen HK and
Tyrsted G: Human thymidine kinase 1. Regulation in normal and
malignant cells. Adv Enzyme Regul. 35:69–89. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boothman DA, Davis TW and Sahijdak WM:
Enhanced expression of thymidine kinase in human cells following
ionizing radiation. Int J Radiat Oncol Biol Phys. 30:391–398. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kauffman MG and Kelly TJ: Cell cycle
regulation of thymidine kinase: Residues near the carboxyl terminus
are essential for the specific degradation of the enzyme at
mitosis. Mol Cell Biol. 11:2538–2546. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cole PD, Smith AK and Kamen BA:
Osteosarcoma cells, resistant to methotrexate due to nucleoside and
nucleobase salvage, are sensitive to nucleoside analogs. Cancer
Chemother Pharmacol. 50:111–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Foekens JA, Romain S, Look MP, Martin PM
and Klijn JG: Thymidine kinase and thymidylate synthase in advanced
breast cancer: Response to tamoxifen and chemotherapy. Cancer Res.
61:1421–1425. 2001.PubMed/NCBI
|
|
26
|
Dittmann H, Dohmen BM, Kehlbach R,
Bartusek G, Pritzkow M, Sarbia M and Bares R: Early changes in
[18F]FLT uptake after chemotherapy: An experimental study. Eur J
Nucl Med Mol Imaging. 29:1462–1469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dittmann H, Dohmen BM, Paulsen F, Eichhorn
K, Eschmann SM, Horger M, Wehrmann M, Machulla HJ and Bares R:
[18F]FLT PET for diagnosis and staging of thoracic tumours. Eur J
Nucl Med Mol Imaging. 30:1407–1412. 2003. View Article : Google Scholar : PubMed/NCBI
|