Introduction
The liver is the primary organ responsible for the
metabolism of numerous xenobiotics, including drugs and toxic
chemicals (1). Carbon tetrachloride
(CCl4), an industrial solvent, cleaner and degreaser,
has been used extensively in models of xenobiotic-induced
hepatotoxicity (2).
CCl4-induced liver damage is characterized by
progressive tissue injury, starting with inflammation and followed
by necrosis, fibrosis and, finally, cirrhosis (2,3). Acute
inflammation triggers further inflammatory processes, initiated by
cytokines released from activated Kupffer cells. This represents a
key event in the induction of liver damage. Previous studies have
demonstrated that inflammation is initiated by the release of
pro-inflammatory mediators, including TNF-α, cyclooxygenase-2 and
interleukin-6, in response to oxidative stress conditions, such as
those during CCl4-induced hepatotoxicity, and occurs in
parallel with increasing apoptosis (4,5). TNF-α,
the primary pro-inflammatory protein synthesized by Kupffer cells,
initiates a cascade of cytokines that mediate the inflammatory
response (6).
A previous study found an elevated expression of
TNF-α in the early phases of liver damage (7). The next stage in
CCl4-induced liver damage is activation of hepatic
stellate cells (HSCs) (8). HSCs are
myofibroblasts that reside in a heterogeneous cell population
originating from liver fibroblasts and bone marrow-derived
circulating fibroblasts. Active HSCs are characterized by a high
rate of proliferation, migration and contractility (9). In addition, HSCs are the primary
producers of α-SMA, which activates the production of transforming
growth factor-b1, the primary pro-fibrogenic cytokine (10). These events result in collagen
deposition and the release of other matrix proteins into the
extracellular space, promoting liver fibrosis.
Natural antioxidants can prevent liver damage by
scavenging free radicals and other reactive oxygen species, or by
modulation of the inflammatory response (11). Additionally, a previous study has
shown that xenobiotic-induced hepatotoxicity is diminished by
flavonoids, such as silymarin (12).
Silymarin, the primary bioactive compound of Silybum
marianum, is a complex mixture of flavonolignans, which has
protective effects against xenobiotics, particularly in the liver
(13). The hepatoprotective activity
of silymarin is a result of its antioxidant properties, lipid
peroxidation inhibition and cell membrane preservation (14). Chrysin (5,7-dihydroxyflavone) is
another natural flavonoid. Chrysin has not been as well-studied as
silymarin, but is known to be present in high levels in honey,
propolis and numerous plant extracts (15). Chrysin has been identified to possess
antioxidant (16–18), anti-allergic (19), anti-inflammatory (20), anti-fibrotic (21) and anti-cancer (22,23)
properties. However, there are previous reports in the literature
regarding the hepatoprotective activity of chrysin, but these did
not reveal how its protective activity is initiated in acute liver
damage condition.
In the present study, the hepatoprotective effects
of chrysin against acute CCl4-induced liver damage are
investigated and the results used to postulate a possible mechanism
by which this occurs. In addition, the interaction between chrysin
and TNF-α was evaluated by computational molecular modeling.
Materials and methods
Chemicals and reagents
Chrysin (97%), silymarin (98%) and carboxymethyl
cellulose were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany). Anti-TNF-α and anti-α-SMA, antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The
Novolink Max Polymer Detection System for immunohistochemistry was
purchased from Leica Microsystems GmbH (Wetzlar, Germany).
Experimental animals
A total of 50 male CD-1 mice (weight, 20±3 g; age,
8–10 weeks), supplied by the Animal House of the Vasile Goldis
Western University of Arad (Arad, Romania) were used in the present
study. The animals were maintained in an environment at a constant
temperature of 20±1°C and 50±5% humidity, with a 12-h light/dark
cycle and ad libitum access to food and water. All
experimental procedures were approved by the Ethical Committee of
Vasile Goldis Western University of Arad (Arad, Romania).
Treatments and experimental
design
A 50 mg/kg body weight dose of chrysin was chosen,
as it was previously proven to be protective against oxidative
damage caused by toxicants in rodents (24). Silymarin (50 mg/kg) was used as the
positive control drug. Chrysin and silymarin were dissolved in 0.5%
sodium carboxymethylcellulose (Sigma-Aldrich; Merck Millipore) and
given orally. CCl4 (1.0 ml/kg, in a 1:1 ratio with 50% olive oil),
injected intraperitoneally, was used to induce acute liver
damage.
The 50 mice were divided into 5 groups of 10 mice.
Group 1 (control) received only the vehicle daily for 7 days. Group
2 (CCl4) received the vehicle daily for 7 days, followed
by CCl4 the next day. Group 3 (chrysin pre-treatment
group; CHR+CCl4) received chrysin for 7 days, followed
by CCl4 the next day. Group 4 (silymarin pre-treatment
group; Sy+CCl4) received silymarin for 7 days, followed
by CCl4 the next day. Group 5 (CHR) group, received
chrysin alone for 7 days.
Serum and liver sample collection
The mice were sacrificed with 2.5 ml/l/min
isoflurane on day 9 and blood collected from the venae cavae. The
collected blood was placed in heparinized tubes and centrifuged at
room temperature for 15 min at 366 × g in order to obtain
serum samples for biochemical analysis. Liver samples (2-cm
samples) were preserved in a buffered formalin solution for
histology and immunohistochemistry and glutaraldehyde solution for
electron microscopy processing.
Biochemical analysis of the activity
of serum markers of hepatic function
The activities of serum aspartate aminotransferase
(AST) and alanine aminotransferase (ALT) were evaluated by
spectrophotometry using a commercially available detection kits
(cat. no. 11876805216; Roche Applied Science, Penzberg, Germany)
according to the manufacturer's instructions.
Histopathology
Liver sections (5-µm) were deparaffinized and
processed routinely for hematoxylin and eosin staining. The extent
of CCl4-induced hepatic damage was then evaluated by assessing
morphological changes to the liver sections. Frozen sections were
cut to 8 µm using an MNT cryostat (SLEE medical GmbH, Mainz,
Germany), fixed in 10% buffered formaldehyde and stained with Oil
Red O according to the manufacturer's instructions. Mounted sample
slides were examined under a BX43 light microscope and images
captured using an XC30 digital camera (both Olympus Corporation,
Tokyo, Japan).
Immunohistochemistry
Immunohistochemistry analysis was performed on
paraffin-embedded 4-µm-thick liver tissue sections. Liver sections
were deparaffinized in toluene and rehydrated prior to epitope
retrieval in Novocastra Epitope Retrieval Solution (Leica
Biosystems, Nussloch, Germany). Following neutralization of
endogenous peroxidase with 3% H2O2 for 10 min, sections were
incubated with BSA blocking solution (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 30 min at room temperature, and then
incubated at 4°C overnight with anti-TNF-α (cat. no. sc-52746;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or anti-α-SMA
(cat. no. ab32575; Abcam, Cambridge, UK) primary antibodies
(1:100). Detection was then performed using a polymer detection
system (cat. no. RE7280-K; Novolink Max Polymer Detection system)
and 3,3′-diaminobenzidine as chromogenic substrate (both Leica
Biosystems). Tissues were stained with hematoxylin, dehydrated in a
gradient of alcohol and mounted onto slides. Negative control
sections were processed in the same way, but with the primary
antibodies substituted for immunoglobulins of the same isotype.
Slides were examined and images captured as described previously.
The intensity of TNF-α and α-SMA immunopositivity was analyzed with
ImageJ (64-bit) software v.2.1.4.6. (U.S. National Institutes of
Health, Bethesda, Maryland, USA). Five fields were selected
randomly from each liver section. Results are presented as the
percentage of brown-stained TNF-α and α-SMA positive fields
compared with the control group (set to 100%).
Electron microscopy
Liver sections for electron microscopy were prefixed
in 2.7% glutaraldehyde solution in 0.1 M phosphate buffer at 4°C
for 1.5 h. Then, specimens were washed in 0.15 M phosphate buffer
(pH 7.2) and fixed in 2% osmium tetroxide in 0.15 M phosphate
buffer at 4°C for 1 h. Dehydration was performed in acetone and
samples embedded in epoxy resin Epon 812. Embedded samples were
then cut into 70-nm-thick sections with the Leica EM UC7
ultramicrotome (Leica Microsystems GmbH, Wetzlar, Germany), treated
with uranyl acetate and lead citrate solutions for double contrast
and analyzed with a Tecnai transmission electron microscope (FEI;
Hillsboro, OR, USA).
Molecular modeling of
chrysin-TNF-α-converting enzyme (TACE or ADAM17) binding
To understand the protective effects of chrysin on
CCl4-induced TNF-α expression, the present study performed
molecular modeling to study the interaction between chrysin and
TACE, the proteinase responsible for cleaving pro-TNF-α to generate
TNF-α. The crystal structure of the catalytic domain of TACE with a
bound inhibitor (IK682) is solved and available in Protein Data
Bank (PDB; PDB ID: 2FV5) (25). In
the present study, the inhibitor was considered a reference ligand
and the active site of the enzyme was defined as the residues
within 10 Å of the reference ligand.
The 3D structure of chrysin was built using the
molecular builder interface of HYPERCHEM (version 8.0) (26) and optimized using the PM3
semi-empirical method to a root mean square (RMS) gradient of 0.01
kcal/Å.mol with the Polak-Ribière conjugate gradient algorithm and
imported in a mol2 file format. The chrysin structure modeled was
‘docked’ into the TACE active site (described above) using the
FlexX program (27), included in
LeadIT Molecular Viewer, version 2.6 (28). FlexX uses a defragmentation procedure
on the ligand, places the anchor fragment within the binding site
and then incrementally builds the entire ligand. A total of 377
possible docking solutions were generated and ranked using a
scoring function that estimates the free energy of binding of the
protein-ligand complexes. Preparation of the receptor, ligand and
all the post docking interactions were analyzed using the LeadIT
molecular viewer. Docking procedures were validated using a
re-docking approach, where the reference ligand was docked into the
PDB 2FV5 crystal structure using the same procedure and the docking
orientation compared with the crystal orientation to judge the
reliability of the docking procedure.
Statistical analysis
Statistical analysis was performed with a one-way
analysis of the variance procedure using Stata software (version
13; StataCorp LP, College Station, TX, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
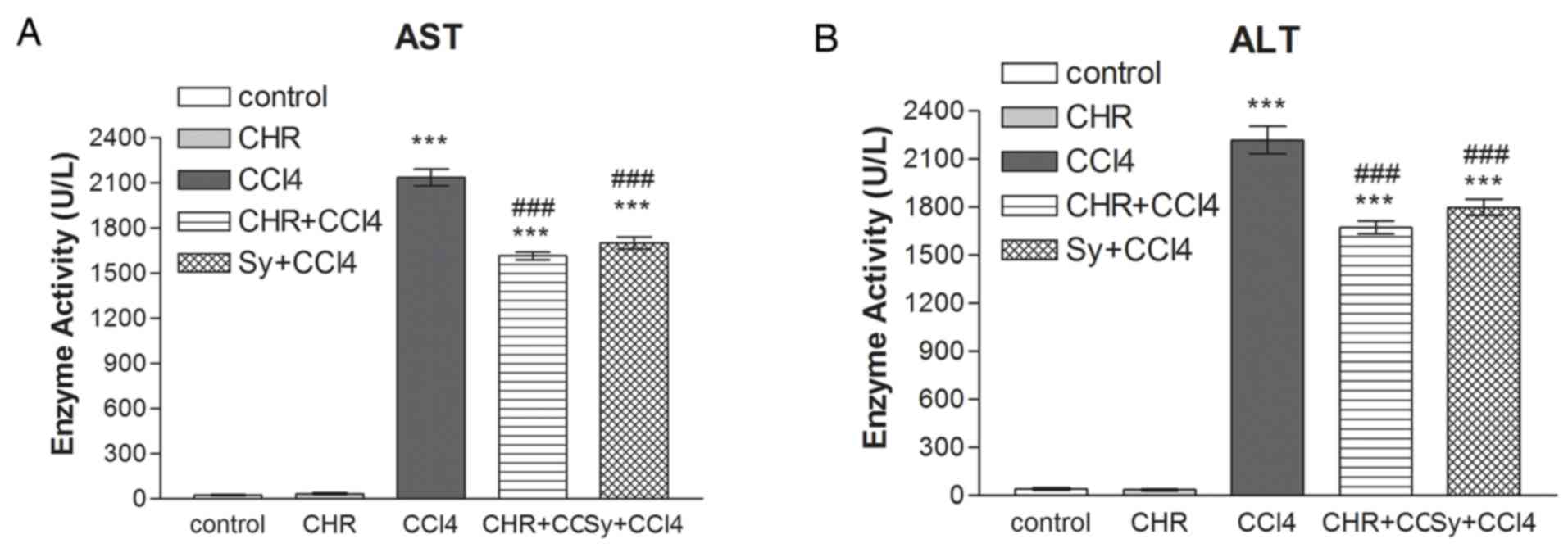
Serum ALT and AST activity
The results of 7 days of chrysin administration on
serum ALT and AST activities in mice from various treatment groups
are shown in Fig. 1. Serum AST and
ALT activity was significantly increased (P<0.001) 24 h
following CCl4 treatment (CCl4 group) compared with the control
group. Pre-treatment with chrysin (CHR+CCl4) significantly
decreased serum aminotransferase activity compared with the CCl4
group (P<0.001). This result was similar to that of the
silymarin pre-treated group (Sy+CCl4; P<0.001 compared with the
CCl4 group). The group treated with chrysin and not CCl4 showed no
increase in ALT and AST activity compared with the control
group.
It is well known that the hepatotoxic agent
CCl4 is metabolized to a trichloromethyl radical
(CCl3•), which interacts with oxygen to form more free radicals,
which damage the liver. Increased membrane permeability from
hepatocyte injury leads to leakage of liver enzymes, which causes
increased activity of the serum transaminases (2,29). The
present study found a significant increase in serum AST and ALT
serum activity in mice following CCl4 administration
(P<0.001). Chrysin (50 mg/kg) pre-treatment significantly
decreased serum AST and ALT activity in CCl4-treated
mice (P<0.001), which indicates that chrysin preserves the
structural integrity of membranes. This is consistent with the
finding of a previous report (16).
Histopathology
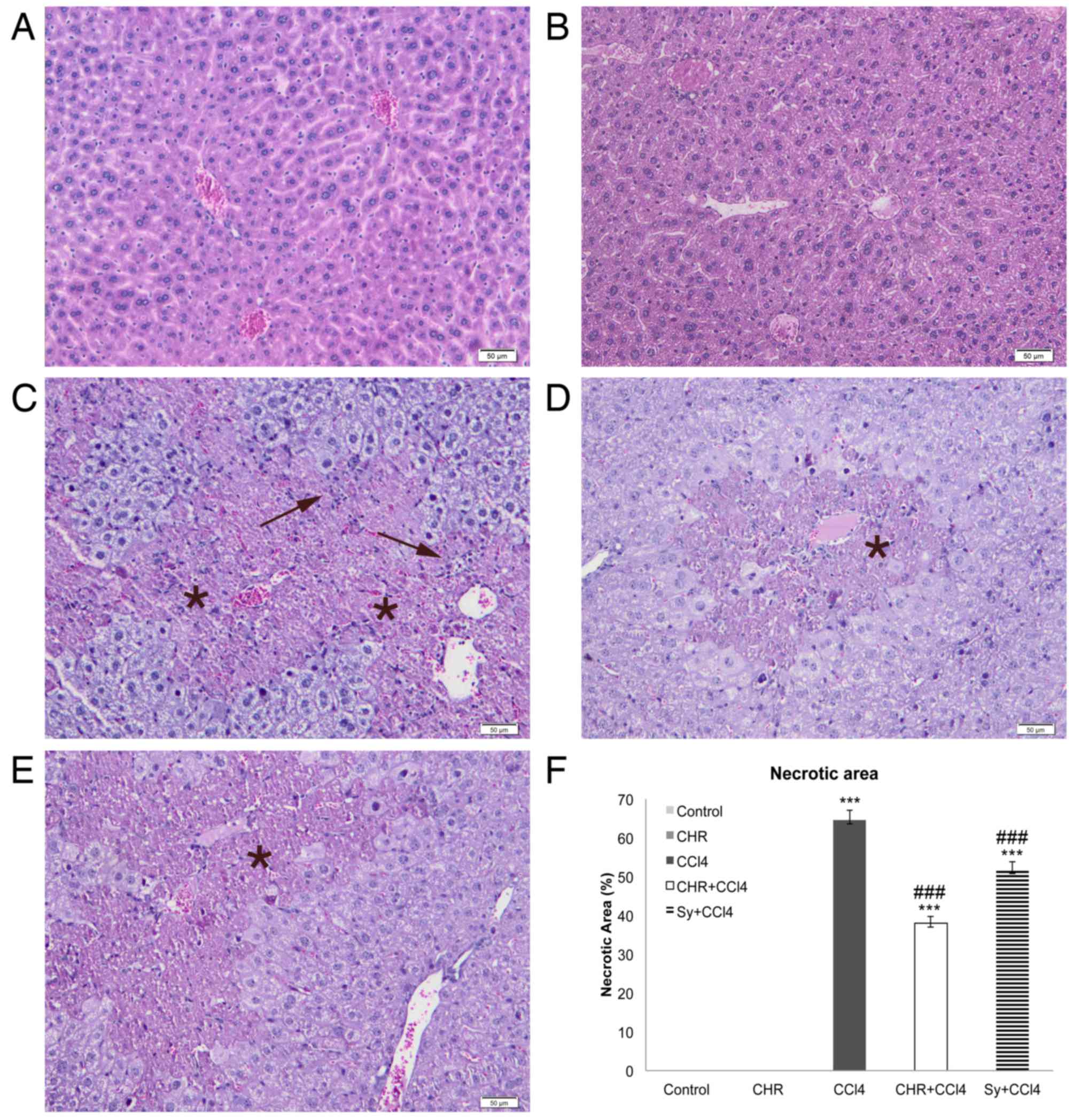
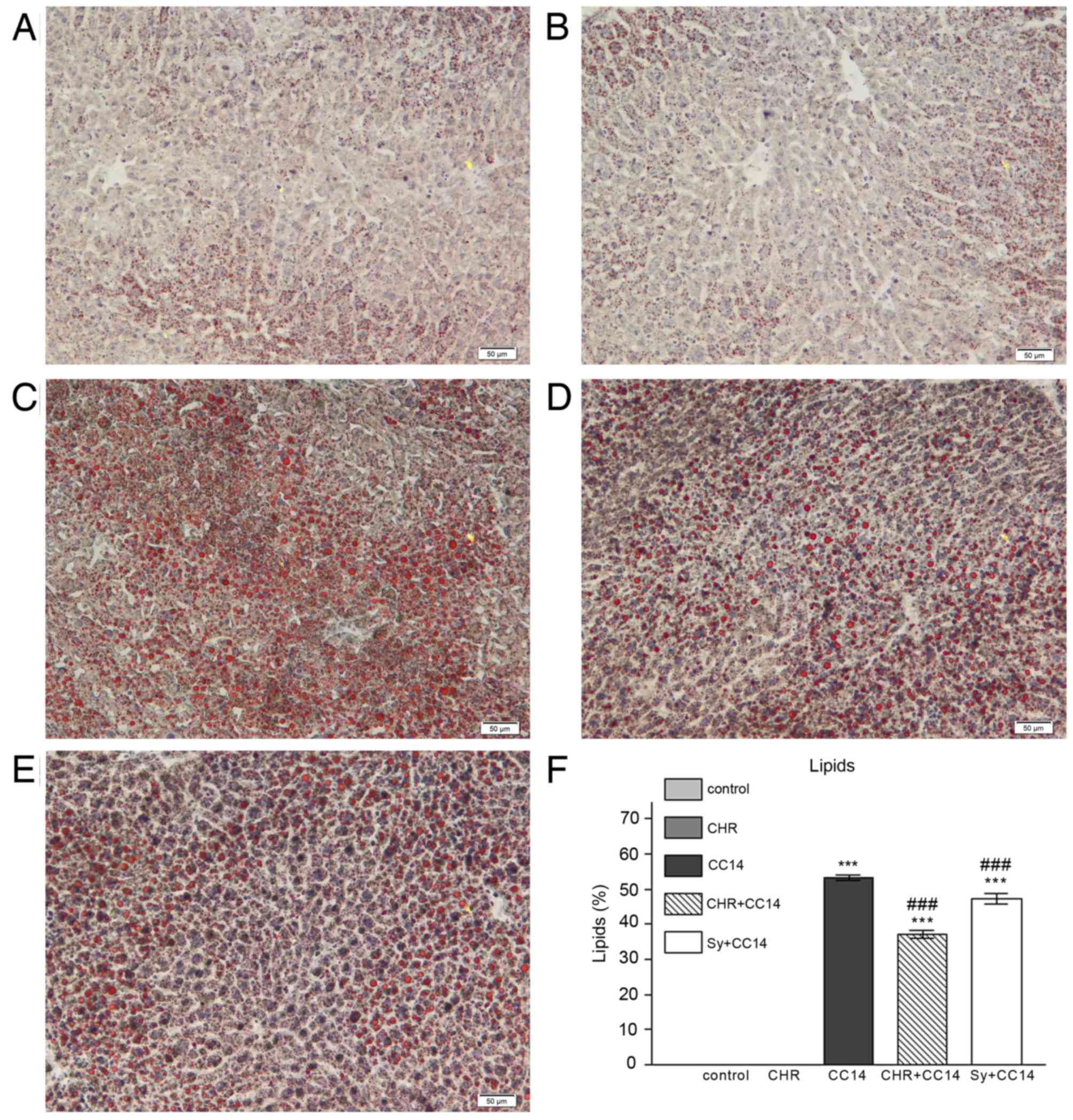
The effect of chrysin on histological changes and
lipid accumulation in the liver was investigated (Figs. 2 and 3). Evaluation of liver tissues by light
microscopy identified that, compared with the normal liver
architecture of the control group (Figs.
2A and 3A), livers of the CCl4
group showed necrotic changes to hepatocytes, which were
particularly pronounced in the centrilobular area (Fig. 2C). In addition, inflammatory cell
infiltration (Fig. 2C) and
microvesicular steatosis of the hepatocytes (Fig. 3C) were detected in the CCl4 group.
The group pre-treated with chrysin prior to CCl4 injection
(CHR+CCl4; Figs. 2D and 3D), showed a significant reduction in
hepatocellular necrosis and steatosis compared with the CCl4 group
(P<0.001; Figs. 2F and 3F). This reduction was greater than that
seen in the silymarin group (Figs. 2E
and F, and 3E and F). The liver
morphology of the group treated with chrysin alone (Figs. 2B and 3B) was comparable with that of the control
(Figs. 2A and 3A).
Increased deposition of neutral lipids in
hepatocytes is caused by an imbalance between the gain and loss of
fatty acids, and the synthesis and excretion of triglycerides
(30). This imbalance can be induced
by exposure to CCl4, causing micro- and macro-vesicular
steatosis (30). The reduction in
liver triglyceride accumulation seen in the group pre-treated with
chrysin suggests that this flavonoid is able to restore the lipid
balance in hepatocytes and thus hepatic function.
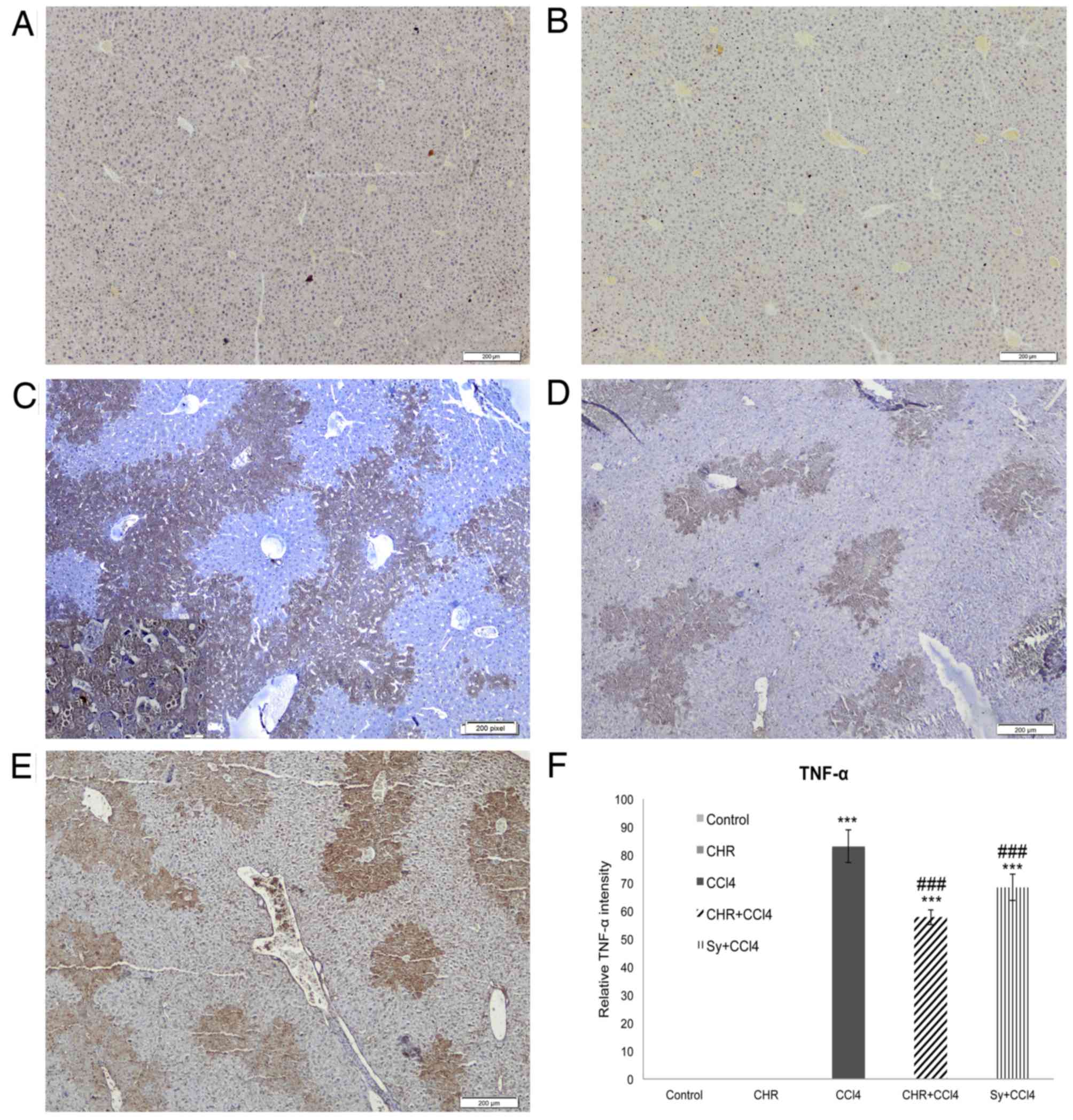
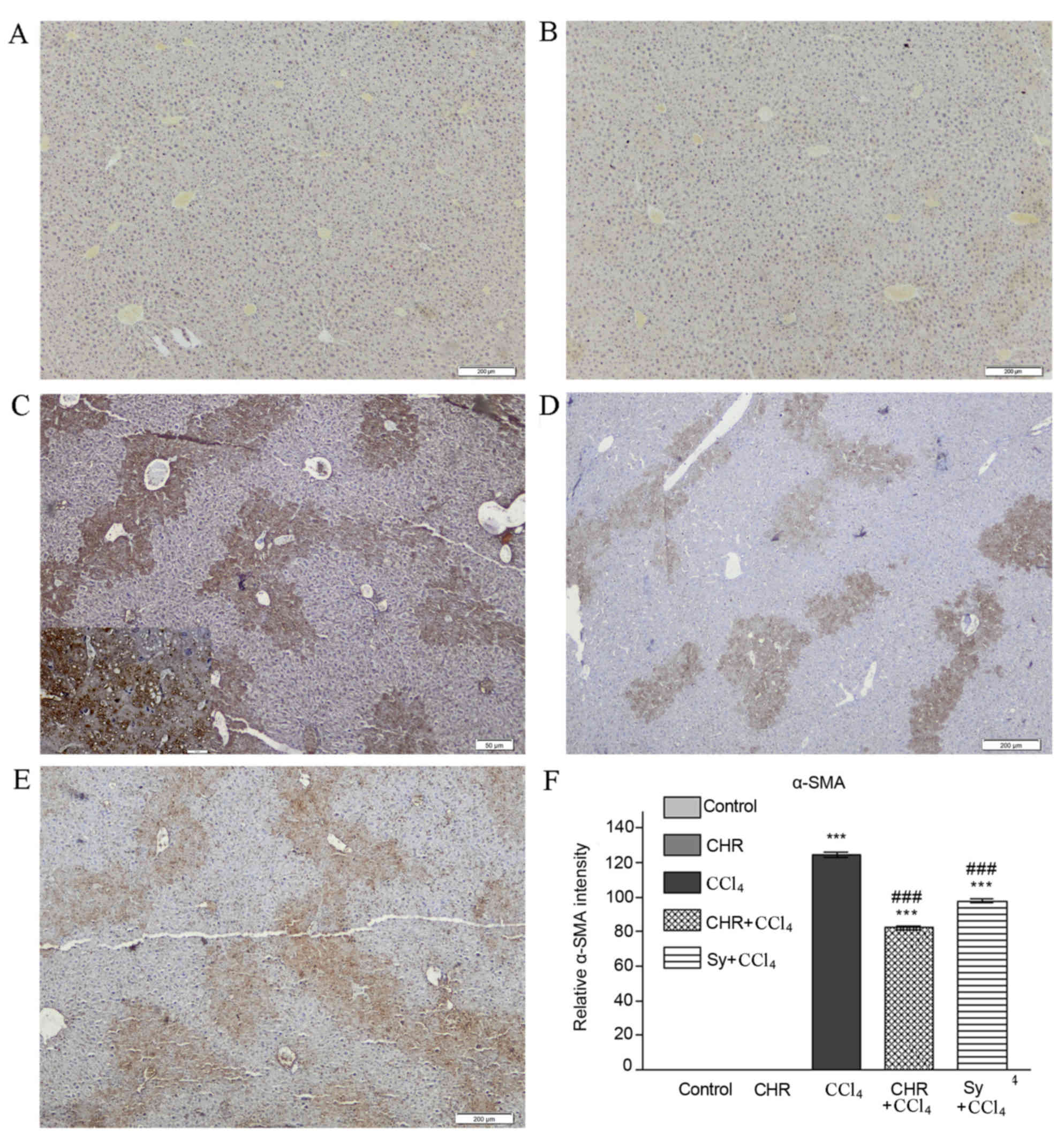
Liver expression of TNF-α and α-SMA
proteins
The effect of chrysin on liver TNF-α and α-SMA
expression, which is elevated by CCl4, a proinflammatory and
profibrotic agent, is shown in Figs.
4 and 5. Significantly increased
TNF-α (Fig. 4C) and α-SMA (Fig. 5C) expression was observed in the CCl4
group (both P<0.001 compared with the control), particularly in
the areas surrounding the centrilobular veins, forming bridges
between neighboring veins. TNF-α and α-SMA expression was
significantly decreased in the livers of chrysin pre-treated mice
(Figs. 4D and Fig. 5D) compared with the CCl4 group
(P<0.001; Figs. 4F and 5F). This decrease was greater than that
seen with silymarin pre-treatment (Figs.
4E and 4F, and 5E and F). No expression of TNF-α or α-SMA
was detected in the group treated with chrysin alone (Figs. 4B and 5B) or the control group (Figs. 4A and 5A).
In the present study, chrysin pre-treatment reduced
CCl4-induced TNF-α expression. TNF-α is a
pro-inflammatory cytokine produced by Kupffer cells, which has been
found to be elevated in acute liver diseases and following exposure
to hepatotoxic chemicals, including CCl4 (31,32). In
this study, the reduction in TNF-α expression caused by chrysin
suggests that it serves an important role in attenuating the
CCl4-induced inflammatory cascade in the liver. In
agreement with the findings of the current study, Ai et al
(33) determined potential
inhibition of the pro-inflammatory TNF-α pathway by other
flavonoids.
Strong hepatic inflammatory responses are
accompanied by the necrosis of large areas and the formation of
bridges between centrilobular veins (34), causing extended damage to the liver
parenchyma. In the present study, these changes were observed in
the in the CCl4 group and were reduced by chrysin
pre-treatment.
Chronic or severe inflammation can stimulate a
fibrotic response, characterized by an irreversible decline in
liver function (35). In the present
study, chrysin pre-treatment reduced the activation of HSCs, as
determined by α-SMA expression and the progression of acute hepatic
damage into liver fibrosis. α-SMA is considered an important factor
in the development of liver fibrosis and is thus a marker for HSC
(the primary producers of α-SMA) activation and fibrous tissue
deposition. Therefore, the expression of α-SMA is a useful marker
for monitoring the efficacy of hepatoprotective therapy. The
results of the present study identified that α-SMA expression in
liver tissue from the CCl4 group was significantly
increased compared with the control group (P<0.001). A previous
study reported that a reduction in α-SMA expression was accompanied
by a decrease in the quantity of activated HSCs (36). The results of the present study
showed that α-SMA expression in CCl4-injured livers was
reduced by chrysin, which indicates that chrysin deactivates
HSCs.
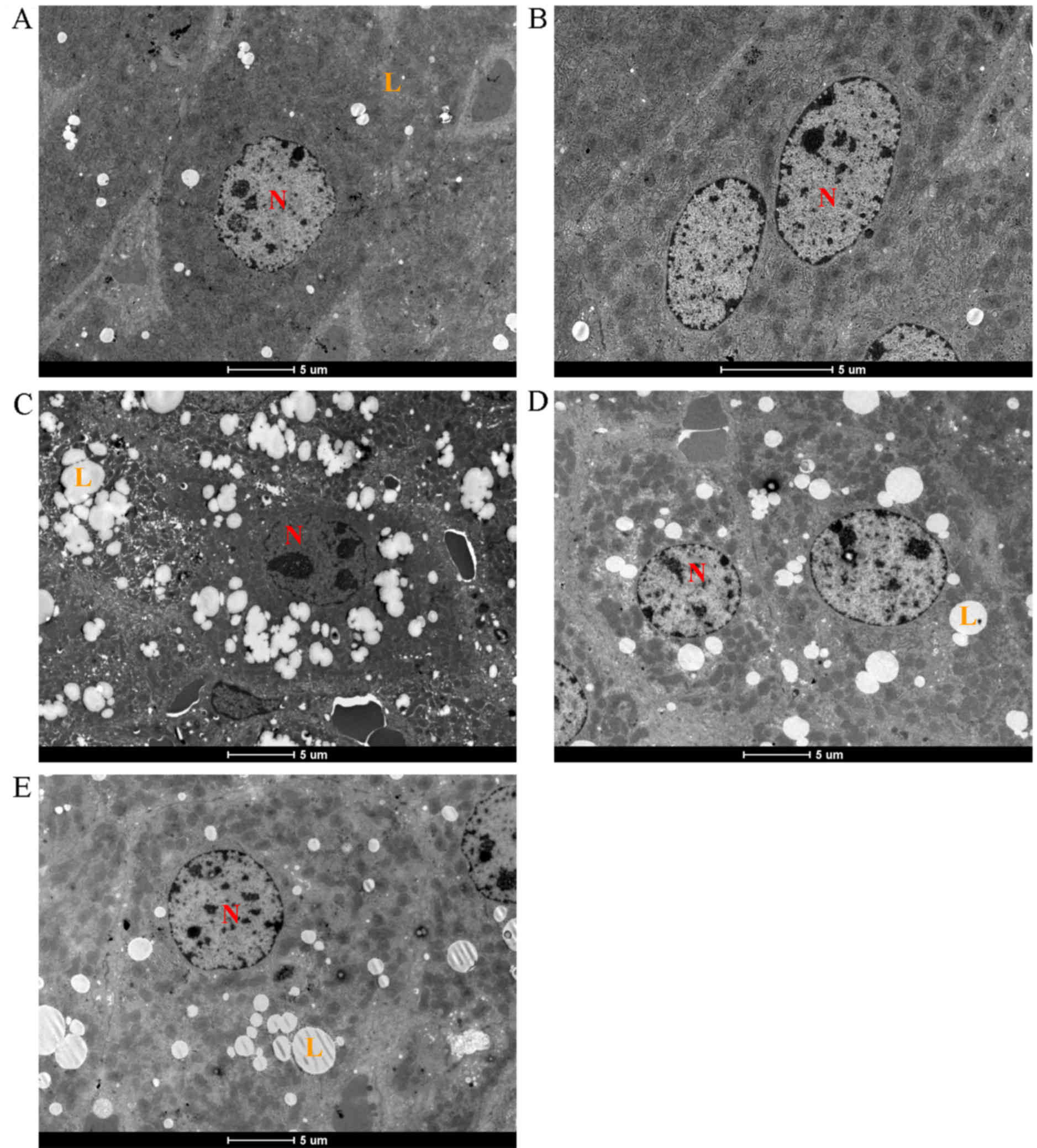
Electron microscopy
Electron microscopy revealed a normal hepatocyte
ultrastructure in the control group (Fig. 6A) and chrysin alone (Fig. 6B), with regularly shaped nuclei and
rough endoplasmic reticuli (rER), and few lipid globules. However,
hepatocytes of the CCl4 group showed lipid globule accumulation,
organelle degeneration and proliferation of smooth ER (sER)
vesicles (Fig. 6C). Pre-treatment
with chrysin markedly reduced lipid globule enlargement and
quantity (Fig. 6D), similarly to the
silymarin pre-treated group (Fig.
6E).
The results of the present study identified that
organelle and cytoplasmic structures were protected against
hepatotoxic effects of CCl4 by chrysin pre-treatment,
including membranes preservation. A previous study demonstrated
that membrane damage causes alterations in lipoprotein and lipid
droplet accumulation in hepatocytes (37). Lipid accumulation is accompanied by
dilatations and focal breaks of rER cisternae, likely due to
membrane structure damage caused by lipid peroxidation (38). In addition, a previous study showed
that the flavonoids provided protection against free radicals
generated by xenobiotic biotransformation and lipid peroxidation
(16).
Numerous xenobiotics are metabolized in oxidase
chain reactions, including that of the cytochrome P450 system.
Located primarily in centrlobular hepatocytes, cytochrome P450
enzymes are associated with drug-induced sER proliferation
(39). In the present study, sER
proliferation was evident in electron microscopy micrographs of the
CCl4 treated group. Furthermore, chrysin reduced
CCl4-induced effects on hepatocyte ultrastructure,
similarly to silymarin at the same dose.
Molecular modeling
The present study demonstrated that the
hepatoprotective activity of chrysin is mediated through TNF-α.
Chrysin pre-treatment significantly reduced CCl4-induced TNF-α
protein expression (P<0.001). This indicates that chrysin may
modulate TNF-α processing, reducing soluble TNF-α generation. TNF-α
is synthesized as a membrane-anchored precursor and the soluble
form of TNF-α is released into the extracellular space through
limited proteolysis by the zinc-endopeptidase TACE (40). TACE is a multi-domain peptidase
consisting of an extracellular region, a transmembrane helix and an
intracellular C-terminal tail. The extracellular region of TACE
comprises an N-terminal pro-domain, a 259 amino acid residue
catalytic domain and a disintegrin-like cysteine-rich domain
(40). The catalytic domain of TACE
recognizes the pro-TNF-α cleavage site (Ala76-Val77) to generate
TNF-α.
The present study investigated the theory of chrysin
interacting with the TACE active site using the FlexX program. The
crystal structure of TACE in complex with the inhibitor IK682 (PDB
ID: 2FV5) (25) was used for a
docking study (results shown in Fig.
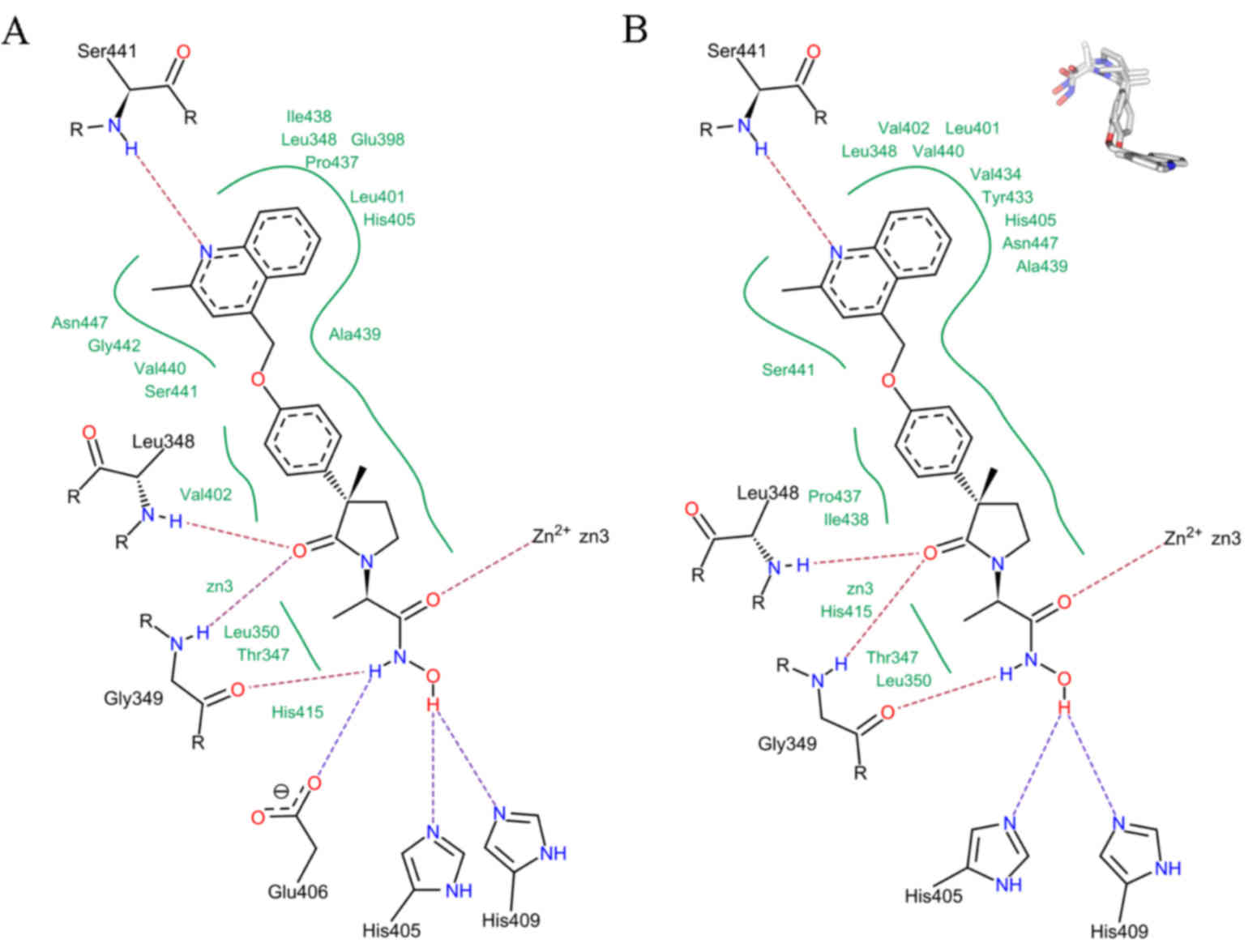
7), which was validated by a re-docking procedure. FlexX
reliably reproduced the crystallographic orientation of bound IK682
with an RMSD of 2.2 Å (Fig. 7).
Comparison of the pharmacophoric features of the ligand-enzyme
interaction in FlexX revealed the most favorable binding energy
solution (Fig. 7A), which elucidated
the pharmacophoric interaction for TACE inhibition as observed in
the crystal structure (Fig. 7B).
Therefore, FlexX accurately docked IK682 within the TACE active
site (Fig. 7, upper right). This
methodology was then applied to investigate docking of chrysin
within the TACE active site.
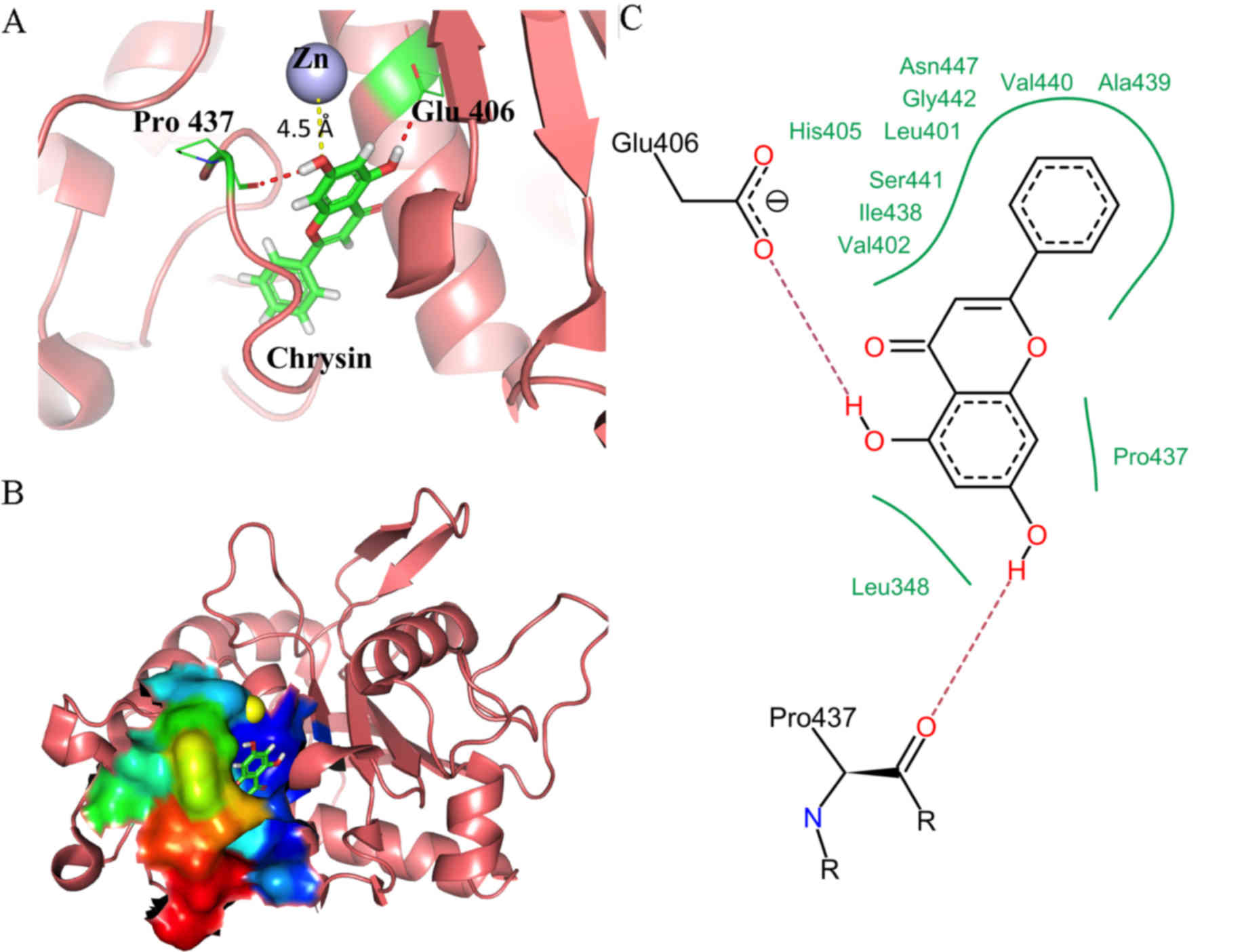
Molecular docking of chrysin revealed that chrysin
favorably binds to the active site of TACE, with an estimated
binding energy of −23.5 kJ/mol. In addition, chrysin was found to
dock close to the catalytic region of TACE. The lowest energy
docking solution revealed that the chromone moiety of chrysin,
composed of A-, B- and C-rings, was oriented towards the catalytic
zinc residue in the hydrophobic S1 sub-pocket of TACE, with the
B-ring deep in the hydrophobic S3 sub-pocket (Fig. 8A). This binding of chrysin is
non-planar, with the B-ring tilted ~45° in respect to the chromone
plane (Fig. 8A). Fig. 8A shows essential interactions of
chrysin with key catalytic residues of TACE. The catalytic zinc
residue of TACE is coordinated by three imidazole nitrogen atoms of
His-405, His-409 and His-415. The 7-OH group of TACE-docked chrysin
was 4.5 Å away from the catalytic zinc and thus able to coordinate
with it. This interaction may displace the ‘catalytic’ water
molecule from the active site of the enzyme, explaining the
inhibition of TACE activity by chrysin. In addition, the 5-OH group
of chrysin forms a hydrogen bond with the carboxylate oxygen of
Glu-406 in TACE, which acts as a base during catalysis (41). Furthermore, the 7-OH group of chrysin
forms a hydrogen bond with Pro-437 of TACE, which serves a key role
in reversing the Met-turn produced by Tyr-433, Val-434, Met-435 and
Tyr-436 to form the outer wall of the S1 crevice (39). The hydrogen bonding seen positioned
the outer wall loop of the S1 sub-pocket in such a way that it
essentially blocked the cavity opening. All these interactions
stabilize the TACE-chrysin closed complex.
Fig. 8B represents
the surface of the chrysin-bound TACE active site, colored
according to B-factor value (flexibility). Chrysin was found to
bind deep inside the hydrophobic cavity and the flexible Met-turn
closes the cavity opening, which is bridged by Ala-439 and Leu-348.
The essential pharmacophoric interaction of TACE inhibition by
chrysin is shown in Fig. 8C.
Excluding the two hydrogen bonds (described above), the majority of
the interactions were hydrophobic. The hydrophobic B-ring of
chrysin is oriented inside the S3 sub-pocket framed by a number of
hydrophobic residues, including Leu-401, Val-402, Ile-438, Ala-439
and Val-440.
In conclusion, the results of the present study
suggest that inflammation signaling pathways were activated in the
pathogenesis of CCl4-induced acute hepatic damage and
that this could be counteracted with seven days of chrysin
pre-treatment. In addition, the hepatoprotective activity of
chrysin was identified to be mediated through TNF-α, via chrysin
reducing soluble TNF-α generation via inhibiting TACE. Furthermore,
chrysin was demonstrated to be a potent hepatoprotective agent,
with similar effects to silymarin at the same dose, and should be
investigated as a targeted drug to maintain a healthy liver and
prevent liver damage.
Acknowledgements
The authors thank Dr Soumalee Basu, Coordinator of
the Centre for High Performance Computing for Modern Biology,
Ballygunge Science College, University of Calcutta, Kolkata, West
Bengal, India, for access to HYPERCHEM version 8.0 and the FlexX
docking package. The present study was supported by the Fundación
Séneca del Centro de Coordinación de la Investigación, Murcia,
Spain (grant no 18946/JLI/13) and the NILS Science and
Sustainability Individual Mobility of Researchers grant, funded by
the European Economic Area financial mechanism (grant no.
012-ABEL-CM-2014A).
References
|
1
|
Kaminski M and Wiaderkiewicz R: The role
of the liver in xenobiotic biotransformation. Part I. The role of
the liver and its cells and their interactions. Problems of
Forensic Sciences. LXXII:357–378. 2007.
|
|
2
|
Manibusan MK, Odin M and Eastmon DA:
Postulated carbon tetrachloride mode of action: A review. J Environ
Sci Health C Environ Carcinog Ecotoxicol Rev. 25:185–209. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber LW, Boll M and Stampfl A:
Hepatotoxicity and mechanism of action of haloalkanes: Carbon
tetrachloride as a toxicological model. Crit Rev Toxicol.
33:105–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong D, Zhang S, Yin L, Tang X, Xu Y, Han
X, Qi Y and Peng J: Protective effects of the total saponins from
Rosa laevigata Michx fruit against carbon tetrachloride-induced
acute liver injury in mice. Food Chem Toxicol. 62:120–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamel R and El Morsy EM: Hepatoprotective
effect of methylsulfonylmethane against carbon
tetrachloride-induced acute liver injury in rats. Arch Pharm Res.
36:1140–1148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pfeffer K: Biological functions of tumor
necrosis factor cytokines and their receptors. Cytokine Growth
Factor Rev. 14:185–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alric L, Pinelli E, Carrera G, Vinel JP,
Beraud M, Duffaut M, Pascal JP and Pipy B: Involvement of calcium
in macrophage leukotriene release during experimental cirrhosis.
Hepatology. 23:614–622. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tarras N, Moles A, Morales A, García-Ruiz
C, Fernández-Checa JC and Marí M: Critical role of tumor necrosis
factor receptor 1, but not 2, in hepatic stellate cell
proliferation, extracellular matrix remodeling and liver
fibrogenesis. Hepatology. 54:319–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leask A and Abraham D: TGF-beta signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lobo V, Patil A, Phatak A and Chandra N:
Free radicals, antioxidants and functional foods: Impact on human
health. Pharmacogn Rev. 4:118–126. 2014. View Article : Google Scholar
|
|
12
|
Pietta PG: Flavonoids as Antioxidants. J
Nat Prod. 63:1035–1042. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abascal K and Yarnell E: The many faces of
Silybum marianum (Milk Thistle): Part 2- clinical uses, safety and
types of preparations. Alternative and Complementary Therapies.
9:251–256. 2003. View Article : Google Scholar
|
|
14
|
Kshirsagar A, Ingawale D, Ashok P and
Vyawahare N: Silymarin: A comprehensive review. Phcog Rev.
3:126–134. 2009.
|
|
15
|
Siess MH, Le Bon AM, Canivenc-Lavier MC,
Amiot MJ, Sabatier S, Aubert SY and Suschetet M: Flavonoids of
honey and propolis: Characterization and effects on hepatic drug
metabolizing enzymes and benzo[a]pyrene-DNA binding in rats. J
Agric Food Chem. 44:2297–2301. 1996. View Article : Google Scholar
|
|
16
|
Anand KV, Anandhi R, Pakkiyaraj M and
Geraldine P: Protective effect of chrysin on carbon tetrachloride
(CCl4)-induced tissue injury in male Wistar rats. Toxicol Ind
Health. 27:923–933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pushpavalli G, Kalaiarasi P, Veeramani C
and Pugalendi KV: Effect of chrysin on hepatoprotective and
antioxidant status in D-galactosamine-induced hepatitis in rats.
Eur J Pharmacol. 631:36–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brechbuhl HM, Kachadourian R, Min E, Chan
D and Day BJ: Chrysin enhances doxorubicin-induced cytotoxicity in
human lung epithelial cancer cell lines: The role of glutathione.
Toxicol Appl Pharmacol. 258:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bae Y, Lee S and Kim SH: Chrysin
suppresses mast cell-mediated allergic inflammation: Involvement of
calcium, caspase-1 and nuclear factor-κB. Toxicol Appl Pharmacol.
254:56–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin EK, Kwon HS, Kim YH, Shin HK and Kim
JK: Chrysin, a natural flavone, improves murine inflammatory bowel
diseases. Biochem Biophys Res Commun. 381:502–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balta C, Herman H, Boldura OM, Gasca I,
Rosu M, Ardelean A and Hermenean A: Chrysin attenuates liver
fibrosis and hepatic stellate cell activation through TGF-b/Smad
signaling pathway. Chem Biol Interact. 240:94–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan MS, Devaraj H and Devaraj N: Chrysin
abrogates early hepatocarcinogenesis and induces apoptosis in
N-nitrosodiethylamine-induced preneoplastic nodules in rats.
Toxicol Appl Pharmacol. 251:85–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phan TA, Yu XM, Kunnimalaiyaan M and Chen
H: Antiproliferative effect of chrysin on anaplastic thyroid
cancer. J Surg Res. 170:84–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khan R, Khan AQ, Qamar W, Lateef A, Tahir
M, Rehman MU, Ali F and Sultana S: Chrysin protects against
cisplatin-induced colon. toxicity via amelioration of oxidative
stress and apoptosis: Probable role of p38MAPK and p53. Toxicol
Appl Pharmacol. 258:315–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niu X, Umland S, Ingram R, Beyer BM, Liu
YH, Sun J, Lundell D and Orth P: IK682, a tight binding inhibitor
of TACE. Arch Biochem Biophys. 451:43–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hyperchem, Hypercube, Inc.; USA: 2002
|
|
27
|
Rarey M, Kramer B, Lengauer T and Klebe G:
A fast flexible docking method using an incremental construction
algorithm. J Mol Biol. 261:470–489. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patel DS and Bharatam PV: New leads for
selective GSK-3 inhibition: Pharmacophore mapping and virtual
screening studies. J Comput Aided Mol Des. 20:55–66. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clawson GA: Mechanisms of carbon
tetrachloride hepatotoxicity. Pathol Immunopathol Res. 8:104–112.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wahlang B, Beier J, Clair H, Bellis-Jones
HJ, Falkner KC, McClain CJ and Cave MC: Toxicant-associated
steatohepatitis. Toxicol Pathol. 41:343–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brenner C, Galluzzi L, Kepp O and Kroemer
G: Decoding cell death signals in liver inflammation. J Hepatol.
59:583–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orfila C, Lepert JC, Alric L, Carrera G,
Beraud M, Vinel JP and Pipy B: Expression of TNF-alpha and
immunohistochemical distribution of hepatic macrophage surface
markers in carbon tetrachloride-induced chronic liver injury in
rats. Histochem J. 31:677–685. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ai G, Liu Q, Hua W, Huang Z and Wang D:
Hepatoprotective evaluation of the total flavonoids extracted from
flowers of Abelmoschus manihot (L.) Medic: In vitro and in vivo
studies. J Ethnopharmacol. 146:794–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Domitrović R, Jakovac H and Blagojević G:
Hepatoprotective activity of berberine is mediated by inhibition of
TNF-α, COX-2, and iNOS expression in CCl(4)-intoxicated mice.
Toxicology. 280:33–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim M, Yang SG, Kim JM, Lee JW, Kim YS and
Lee JI: Silymarin suppresses hepatic stellate cell activation in a
dietary rat model of non-alcoholic steatohepatitis: Αnalysis of
isolated hepatic stellate cells. Int J Mol Med. 30:473–479.
2012.PubMed/NCBI
|
|
37
|
Ozturk F, Gul M, Ates B, Ozturk IC, Cetin
A, Vardi N, Otlu A and Yilmaz I: Protective effect of apricot
(Prunus armeniaca L.) on hepatic steatosis and damage induced by
carbon tetrachloride in Wistar rats. Br J Nutr. 102:1767–1775.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tasci I, Mas N, Mas MR, Tuncer M and
Comert B: Ultrastructural changes in hepatocytes after taurine
treatment in CCl4 induced liver injury. World J
Gastroenterol. 14:4897–4902. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheville N: Ultrastructural pathology and
interorgannelle cross talk in hepatotoxicity. Toxicol Pathol.
41:210–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maskos K, Fernandez-Catalan C, Huber R,
Bourenkov GP, Bartunik H, Ellestad GA, Reddy P, Wolfson MF, Rauch
CT, Castner BJ, et al: Crystal structure of the catalytic domain of
human tumor necrosis factor-αlpha-converting enzyme. Proc Natl Acad
Sci USA. 95:3408–3412. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grams F, Reinemer P, Powers JC, Kleine T,
Pieper M, Tschesche H, Huber R and Bode W: X-ray structures of
human neutrophil collagenase complexed with peptide hydroxamate and
peptide thiol inhibitors. Implications for substrate binding and
rational drug design. Eur J Biochem. 228:830–841. 1995. View Article : Google Scholar : PubMed/NCBI
|