Introduction
Infertility is a serious public health issue,
estimated to affect 15% of the reproductive aged population. It has
gained much attention worldwide. In vitro fertilization
(IVF) has appeared as a powerful solution in the aspect of
infertility treatment (1). The
process of IVF involves monitoring and stimulating a woman's
ovulatory process and further removing an ovum or ova (egg or eggs)
from the woman's ovaries for IVF with sperm in a laboratory. The
fertilized egg (zygote) is cultured for 2 to 6 days in a growth
medium and then implanted in the woman's uterus with intention of
establishing a successful pregnancy (2). Accordingly, morphological assessment
was the primary method used in the fertility clinics to select
in vitro generated embryo(s) for the transfer into the
uterus (3). However, it was widely
known that the correlation between embryo morphology and
implantation potential is also elusive (4). Indeed, embryo morphological grading
methods mainly depends on the observer experience and the
assessment criteria is rather subjective. As a milestone technique
in reproductive medicine, the first time-lapse microscopy system
for IVF was approved in 2009 for clinical use. Embryo morphokinetic
scoring system has been shown to further improve the rates of
pregnancy (5). The images were
compiled using specialized software to create a time-lapse sequence
of embryo development. Consequently, the study negated the need for
the embryologist to remove the embryos from the incubator for
morphological assessment. Additionally, widely using this technique
also addresses some of the encountered obstacles presently due to
its high cost. Therefore, in order to increase the rates of
implantation, the improved assessment methodology was required.
Accordingly, many different approaches have been suggested to
reflect the embryo's function or physiological state. Several
non-invasive methods assessing embryo viability and the
potentiality of development have been established, such as
proteomics (6), measurement of
respiration rate (7), soluble human
leukocyte antigen-G (8), pyruvate
uptake (9) and glucose uptake
(10). However, all of these methods
have not been widely used in clinic because they were either
expensive and required dedicated equipment, technical staff or do
not provide results quickly enough to be used within the time frame
of clinical IVF.
However, the Raman spectroscopy technique, used to
observe vibration of bonds, rotational, and other low-frequency
modes in a system, has been commonly used in chemistry to provide a
fingerprint by which molecules can be identified (11) and serves as an are of interest.
Indeed, over the past few years, Raman spectroscopy has become a
powerful diagnostic tool in the life sciences (12). Currently, in the field of assisted
reproduction technology, there are few studies using the Raman
technique to investigate the correlation between the metabonomics
profile and the embryo quality as well as the developmental
potential and the outcome of pregnancy. Seli et al reported
that the non-invasive metabonomics profiling of embryo culture
media using Raman and near infrared (NIR) spectroscopy were
correlated with the pregnancy outcome in women undergoing IVF
(13). They also carried out two
studies demonstrating that metabonomics models developed using NIR
or Raman may predict embryo viability (14,15). The
same group conducted an analogous prospective study assessing day 3
and 5 culture media which confirmed the strong association of the
metabonomics profile and clinical outcome, high sensitivity as well
as the specificity and accuracy of the profiling (15). Recently, these findings have been
extended by Zhao et al who found that a combination of
embryo morphology scores and Raman determined that sodium pyruvate
and phenylalanine levels in culture medium were indicative of high
reproductive potential (16).
Although the abovementioned reports demonstrated that Raman
spectroscopy was a potential technique for the assessment of the
embryo quality, the results from the studies were not consistent
for the diversity of the Raman machine, embryo culture media and
the study protocol. The development and validation of the method of
assessment for embryo quality with Raman spectroscopy in various
reproductive centers is needed to prove the reliability of this
assay method.
Thus, the current study aimed to assess the
metabonomics profile of embryo culture media using Raman
spectroscopy comparing with conventional morphologic methods. We
also aimed to develop a rapid, non-invasive method for the
assessment of the developmental potential of embryos.
Materials and methods
Patients and ethics approval
All the patients enrolled in our study were treated
at the Department of Reproductive Medicine of Nantong Maternity and
Child Health Hospital, in Nantong, China. Ethical approval for the
protocol was received from the Medical Ethics Committee of Nantong
Maternity and Child Health Hospital. Each couple whose embryos were
enrolled into the study signed a written informed consent.
Patient treatment
Ten patients who underwent ovarian stimulation using
either a long gonadotropin-releasing hormone (GnRH) agonist
protocol or a flexible GnRH antagonist protocol were selected to
our study. Oocyte retrieval was performed 36 h after the human
chorionic gonadotropin injection by transvaginal ultrasound-guided
aspiration. The oocyte cumulus complexes were isolated, the
majority of the cumulus were stripped mechanically and the oocytes
were placed into individual 25 µl droplets of media (Quinn's
advantage cleavage medium (SAGE In-Vitro Fertilization, Inc.,
Trumbull, CT, USA) supplemented with 10% serum substitute
supplement (Irvine Scientific, Santa Ana, CA, USA). Fertilized
oocytes were individually cultured for 3 days in 25 µl
pre-equilibrated drops of sequential culture media.
Embryo assessment
A standard embryo scoring system was used for the
evaluation of the quality of embryo based on the rate of cleavage
and morphology (17). The
morphological criteria considered include: i) The number of
pronuclei and polar bodies (zygotes); ii) cell number; iii)
evenness of mitotic divisions; iv) presence of micronuclei; and v)
amount of cellular fragmentation (cleavage embryos). The embryo
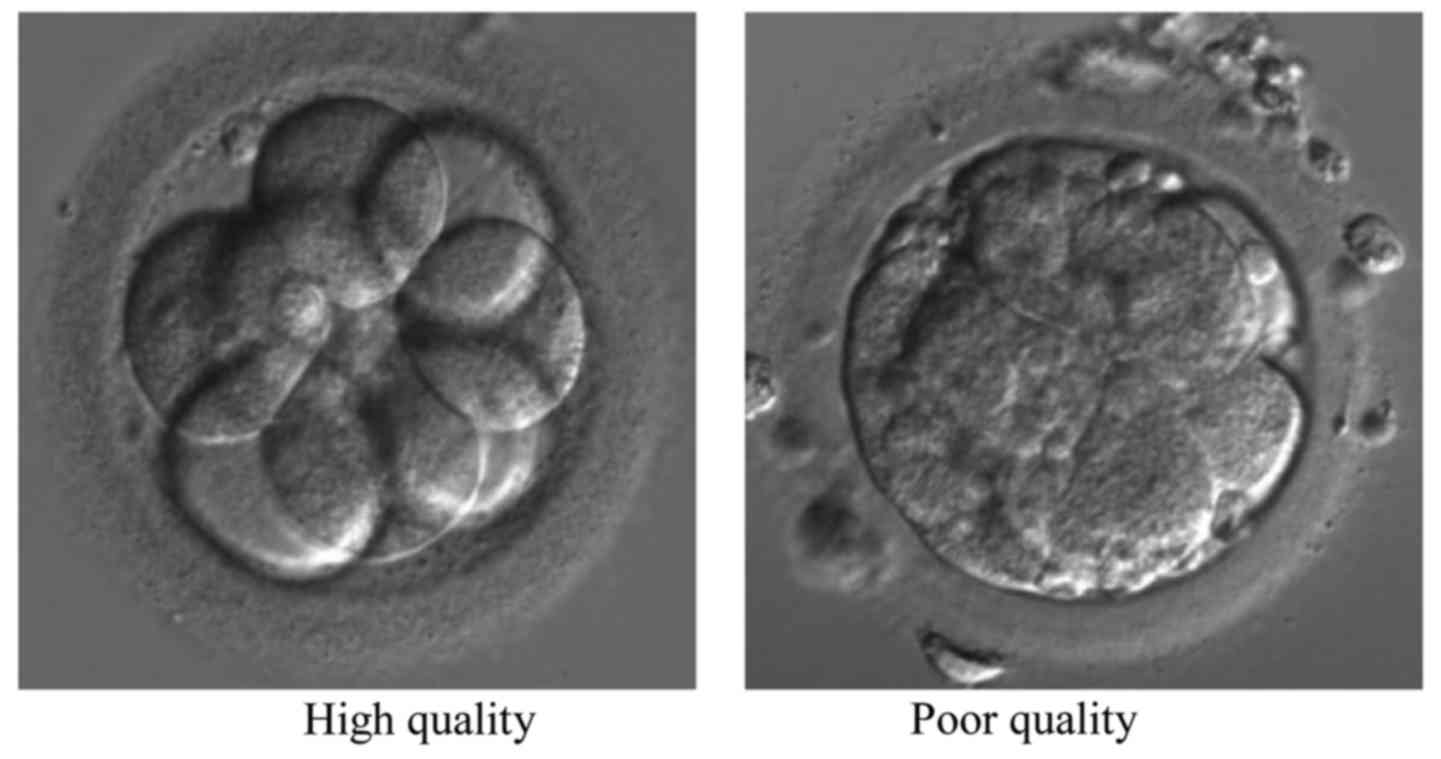
grade A and B were defined as high-quality embryo and embryo grade
C was considered as poor quality embryo (Table I). The same embryologist preformed
all embryology and embryo scoring in this study to ensure the
consistency.
 | Table I.Embryo assessment criteria. |
Table I.
Embryo assessment criteria.
| Classification | Grade | Cell no. | Fragmentation
(%) | Symmetry | Multi-nucleation | Vacuoles | Zona pellucida |
|---|
| High quality | A | 4(d2) → 7-8(d3) | <10 | Even | No | No | Normal |
| 3–4 | B | 4(d2) → 7-8(d3) | 11–25 | Even | No | No | Normal |
|
|
| 4(d2) → ≥9(d3) | 11–25 | Even | No | No | Normal |
| Poor quality | C | 2,4,6(d2) →
>7(d3) | 26–35 | Uneven | No | Few | Abnormal |
| 4–5 |
| 6(d2) →
>8(d3) | <35 | Uneven | No | Few | Abnormal |
|
|
| 2 or 4(d2) →
6(d3) | <35 | Uneven | No | Few | Abnormal |
|
|
| 3(d2) →
>6(d3) | <35 | Uneven | No | Few | Abnormal |
Culture medium specimens
The embryo selection for implantation was based on
embryo morphology at day 3. Following embryo transfer, the spent
culture media were collected individually and stored at −80°C. The
equilibrated culture media without embryos was also collected and
used for normalization. During each experimental step, laboratory
personnel wore gloves and coat, and the physical isolation was
guaranteed by working in clean air hoods.
Raman spectroscopy
The spent culture media were thawed at room
temperature (25°C) for 30 min before analysis. The mineral oil on
the surface of the spent media was removed by capillary siphon
until there was no visual stratification. After which the media
were vortexed for 10 sec and centrifuged for 10 min at 11,000 × g.
Next, 20 µl of embryo culture medium, taken from the cryovials by
pipette gun, were dropped into a round glass with a thin layer of
gold film on the surface, and the glass was placed onto a slide.
Ten points were selected and Raman spectroscopy was measured for
each sample. Raman analysis was conducted using a Raman
spectrometer (BWTEK; B&W Tek, Inc. Newark, DE, USA). The
spectra were recorded from 600 to 2,000 cm−1. The signal
acquisition time was 60 sec. Raman spectroscopy was collected at
10–15 points/sample. Laser Raman spectroscopy of the samples from
10 patients was conducted under the parameters above. The original
spectra were preprocessed automatically by Origin 6.0 software.
Results and Discussion
Although numerous studies have verified the
feasibility of metabonomics profiling of spent media to select the
high quality embryo, the validation from other laboratories is
needed for the consistency of results on the diversity of the
medium, Raman instrument and protocol of analysis. In the present
study, we analyzed spent culture media of embryos using Raman
spectroscopy, trying to evaluate the embryo, and compare with the
conventional morphological methods. The IVF spent culture media
were thawed at room temperature for 30 min. The mineral oil layer
on the surface, which act as the protecting shell to prevent
moisture volatilizing were taken away with physical siphon until
there was no visual stratification. Our preliminary results showed
that there is no effect on spectral signals of embryo media for
mineral oil. To verify the consistency of measurement, different
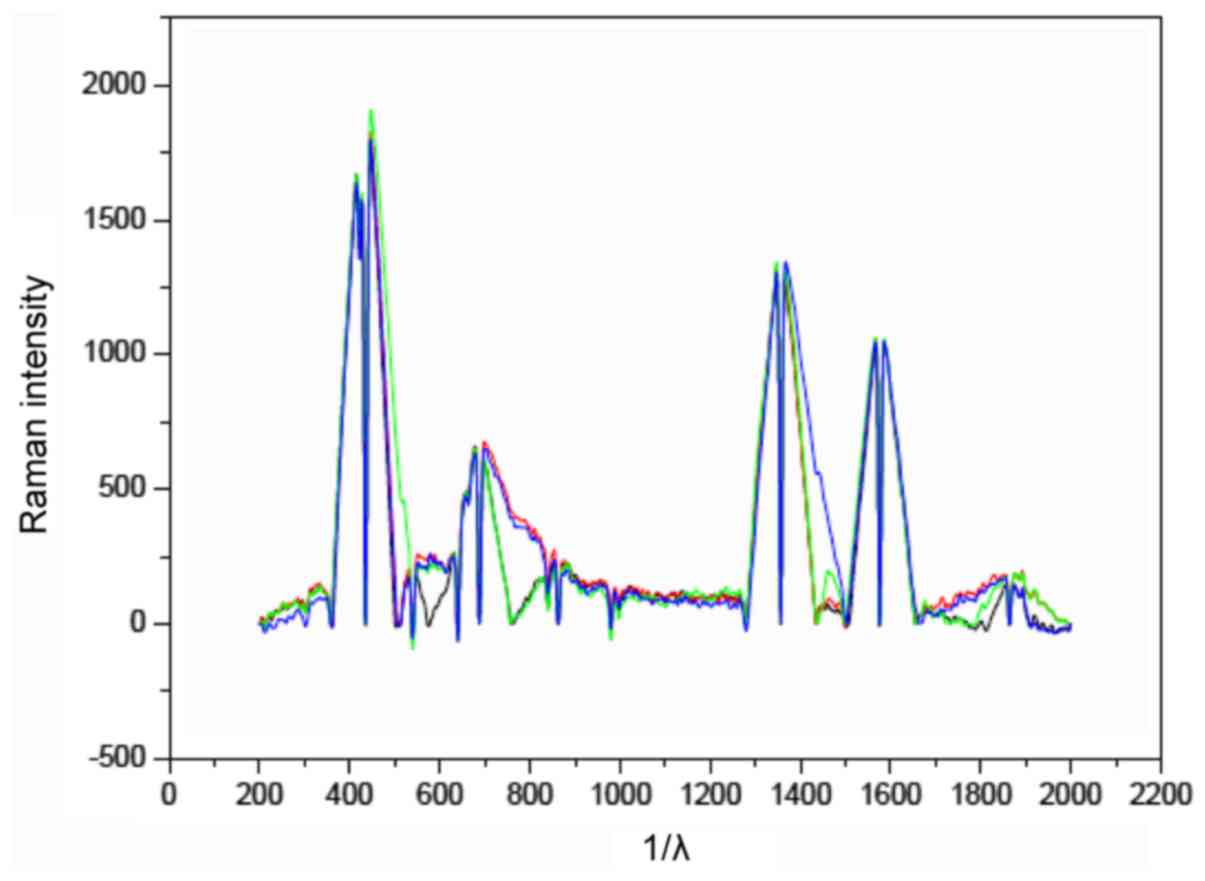
grade embryos from the same couple were analyzed. Figs. 1 and 2
show the typical Raman spectrograms and microphotography of embryos
of day 3 in different grade by morphological scoring, respectively.
The peak area of at Raman shifting 755 cm−1 from the
high quality embryo was obviously different to the poor one
(Fig. 1). Raman shifting 750
cm−1 was the characteristic peak for tryptophan, an
essential amino acid which cannot be synthesized by the organism
and must be provided by the exterior nutrition (15). In the fresh media for embryo culture,
tryptophan is 0.02 mM in general. More tryptophan was consumed by
the high quality embryo for their development comparing to the poor
embryo. Thus, the metabolism of a healthy embryo may alter the
surrounding environment differently from one that is less healthy
and thus possessing less reproductive potential. It may produce a
profile that may more clearly be associated with embryo
viability.
Amino acids have numerous roles in early embryo
development in addition to protein synthesis. Preimplantation
embryos can consume and produce amino acids in a manner dependent
on the stage of development that may be predictive of subsequent
viability. Tryptophan has a variety of metabolic functions within
the cell. It is incorporated into the polypeptide chains of enzymes
and proteins, and it is a biosynthetic precursor of the cofactor
NAD, the siderophore quinolobactin, and the neuron transmitters
serotonin and melatonin (18).
Numerous studies have analyzed the remaining media
to determine novel assessing and screening methods to select the
greatest potential embryo (16,19).
Different from the former studies, the spent media from
conventional IVF were analyzed with Raman spectroscopy in our
research. Although to the best of our knowledge, there is no
research or epidemiology study indicating that intracytoplasmic
sperm injection could impair the embryo development and the
pregnancy outcome to this day, the different protocol of
fertilization may have led to the different metabolites. Thus, only
the remaining conventional IVF media were collected and analyzed in
this study.
There are several studies trying to understand the
correlation between Raman spectrum of spent media and the outcome
of pregnancy of a woman implanted with the embryo (20). It is clear that the quality of the
embryo is not an independent factor contributing to the pregnancy
outcome. There are other reasons which may impair the outcome of
the embryo transfer, such as the endometrium situation,
psychological state, or hormone level. Therefore, we only focused
on the correlation between the Raman spectrograms and morphological
grading. In the present study, we compared the metabolic profile
with the morphological classification. However, we did not
calculate the correlation index, because of the tiny number of
samples in the study. In subsequent investigations, we intend to
include a larger number of samples.
In conclusion, the profile of Raman spectrum of the
spent embryo culture media was presented comparing the
morphological assessment in our study. Without any doubt, an
appropriate evaluation method can improve the rates of embryo
implantation and pregnancy. To substantiate the results in our
study, additional investigations of the correlation between the
Raman spectrum and rate of pregnancy should be conducted in the
near future.
Acknowledgements
The study was supported by the Jiangsu Maternity and
Child Health Research Project (F201347) and the Nantong Science and
Technology Project (HS2013012).
References
|
1
|
Ombelet W, Cooke I, Dyer S, Serour G and
Devroey P: Infertility and the provision of infertility medical
services in developing countries. Hum Reprod Update. 14:605–621.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ying LY, Wu LH and Loke AY: The experience
of Chinese couples dndergoing in vitro fertilization treatment:
perception of the treatment process and partner support. PLoS One.
10:e01396912015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nasiri N and Eftekhari-Yazdi P: An
overview of the available methods for morphological scoring of
pre-implantation embryos in in vitro fertilization. Cell J.
16:392–405. 2015.PubMed/NCBI
|
|
4
|
Filho Santos E, Noble JA, Poli M,
Griffiths T, Emerson G and Wells D: A method for semi-automatic
grading of human blastocyst microscope images. Hum Reprod.
27:2641–2648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kovacs P: Embryo selection: the role of
time-lapse monitoring. Reprod Biol Endocrinol. 12:1242014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nyalwidhe J, Burch T, Bocca S, Cazares L,
Green-Mitchell S, Cooke M, Birdsall P, Basu G, Semmes OJ and
Oehninger S: The search for biomarkers of human embryo
developmental potential in IVF: a comprehensive proteomic approach.
Mol Hum Reprod. 19:250–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilding M, Dale B, Marino M, di Matteo L,
Alviggi C, Pisaturo ML, Lombardi L and De Placido G: Mitochondrial
aggregation patterns and activity in human oocytes and
preimplantation embryos. Hum Reprod. 16:909–917. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vercammen MJ, Verloes A, Van de Velde H
and Haentjens P: Accuracy of soluble human leukocyte antigen-G for
predicting pregnancy among women undergoing infertility treatment:
meta-analysis. Hum Reprod Update. 14:209–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ray PF, Conaghan J, Winston RM and
Handyside AH: Increased number of cells and metabolic activity in
male human preimplantation embryos following in vitro
fertilization. J Reprod Fertil. 104:165–171. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gardner DK, Wale PL, Collins R and Lane M:
Glucose consumption of single post-compaction human embryos is
predictive of embryo sex and live birth outcome. Hum Reprod.
26:1981–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Votteler M, DA Berrio Carvajal, Pudlas M,
Walles H and Schenke-Layland K: Non-contact, label-free monitoring
of cells and extracellular matrix using Raman spectroscopy. J Vis
Exp. 63:39772012.
|
|
12
|
Brauchle E and Schenke-Layland K: Raman
spectroscopy in biomedicine - non-invasive in vitro analysis of
cells and extracellular matrix components in tissues. Biotechnol J.
8:288–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seli E, Vergouw CG, Morita H, Botros L,
Roos P, Lambalk CB, Yamashita N, Kato O and Sakkas D: Noninvasive
metabolomic profiling as an adjunct to morphology for noninvasive
embryo assessment in women undergoing single embryo transfer.
Fertil Steril. 94:535–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seli E, Sakkas D, Scott R, Kwok SC,
Rosendahl SM and Burns DH: Noninvasive metabolomic profiling of
embryo culture media using Raman and near-infrared spectroscopy
correlates with reproductive potential of embryos in women
undergoing in vitro fertilization. Fertil Steril. 88:1350–1357.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scott R, Seli E, Miller K, Sakkas D, Scott
K and Burns DH: Noninvasive metabolomic profiling of human embryo
culture media using Raman spectroscopy predicts embryonic
reproductive potential: a prospective blinded pilot study. Fertil
Steril. 90:77–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Q, Yin T, Peng J, Zou Y, Yang J, Shen
A and Hu J: Noninvasive metabolomic profling of human embryo
culture media using a simple spectroscopy adjunct to morphology for
embryo assessment in in vitro fertilization (IVF). Int J Mol Sci.
14:6556–6570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alpha Scientists in Reproductive Medicine
and ESHRE Special Interest Group of Embryology: The Istanbul
consensus workshop on embryo assessment: proceedings of an expert
meeting. Hum Reprod. 26:1270–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Radwanski ER and Last RL: Tryptophan
biosynthesis and metabolism: biochemical and molecular genetics.
Plant Cell. 7:921–934. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Telford NA, Watson AJ and Schultz GA:
Transition from maternal to embryonic control in early mammalian
development: a comparison of several species. Mol Reprod Dev.
26:90–100. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JY, Schopf JW, Bottjer DJ, Zhang CY,
Kudryavtsev AB, Tripathi AB, Wang XQ, Yang YH, Gao X and Yang Y:
Raman spectra of a Lower Cambrian ctenophore embryo from
southwestern Shaanxi, China. Proc Natl Acad Sci U S A.
104:6289–6292. 2007. View Article : Google Scholar : PubMed/NCBI
|