Introduction
Spinal cord injury (SCI), which can result from
traumatic injuries and progressive neuropathies, is a devastating
condition that causes physical and emotional damage to individuals,
and places an economic burden on society (1). SCI triggers a cascade of secondary
damage, including ischemia, hypoxia, necrocytosis, inflammation and
exacerbation of axon demyelination, which causes substantial
neurological disability in the affected areas of the extremities
and/or trunk, such as loss of bowel, bladder and sexual functions
(2,3). Despite advances in medical and surgical
technique and regenerative engineering, no current treatments can
reverse the devastating consequences of SCI (4).
Previous studies in rodent models identified that
transplantation of stem cells could ameliorate secondary damage
caused by SCI and facilitate functional recovery (5). The capacity of stem cells to
initiatively home into regional tissue or passively home into
lesion area supports the principle for the targeted delivery
(6). Among candidate stem cells,
BMSCs have emerged as a promising alternative to adipose-derived
stem cells induced pluripotent stem cells and embryonic stem cells,
demonstrating good axonal sprouting and regrowth in a SCI (7). Accumulating evidence indicates that
BMSCs can promote the repair and regeneration of neurons, and
promote functional rehabilitation, in animal models of SCI
(8). A number of characteristics of
BMSCs make them suitable for transplantation, including being easy
to isolate and obtain, their multi-lineage potential,
immunosuppression and suitability for autologous transplantation
meaning there are no ethical issues (9). Despite the advances in BMSC
transplantation in the last decades, only a minority of grafted
cells migrate to target areas and BMSC survival is decreased due to
the route of administration (venous system transplantation) as,
after being filtered by circulatory system and blood brain barrier,
only a small portion of transplanted BMSCs are able to reach the
injured spinal cord (10). A
previous study determined that free radical generation, deficiency
in trophic factors and cell apoptosis result in poor survival of
transplanted BMSCs (11). Therefore,
more efficient strategies for BMSC delivery need to be explored in
order to obtain the best possible therapeutic outcome.
Erythropoietin (EPO), which is known for its tissue
protective and regenerative capabilities for a number of organs,
such as the heart, spinal cord, brain and kidneys, has been shown
to facilitate BMSC recruitment and angiogenesis (12–15).
Interestingly, the results of a recent study demonstrate that the
EPO receptor is expressed on the surface of BMSCs and via this EPO
could promote the proliferation of BMSCs in acute kidney injury and
reverse their low secretion (BMSCs can be secreted autologously by
rats with acute kidney injury, but this rare, and EPO may promote
the secretion of BMSC) (16). In
addition, a recent study found that EPO promote the mobilization of
BMSCs to damaged bone tissue and trigger BMSC differentiation in
osteogenesis (17). The current
study hypothesized that BMSC targeting to sites of SCI could be
enhanced by co-transplantation with EPO, facilitating enhanced
functional recovery. To investigate this hypothesis and, if true,
explore the possible mechanisms through which it occurs, a rat
model of SCI was created through the improved Allen method
(18).
Materials and methods
Experimental animals
Pathogen-free female Sprague Dawley rats (n=60; age,
8 weeks old; weight, 200–250 g) were provided by the Animal
Experiment Center at Hubei University of Medicine (Shiyan, China).
The rats were housed with 3 animals cage from ~1 week prior to the
commencement of the experiments, with free access to food and water
and were maintained in a suitable environment at 21°C, 60% air
humidity and a 12 h light/dark cycle. The majority (n=45) of the
rats were used to establish models of SCI and the remaining rats
(n=15) were used to derive BMSCs for culture. All procedures
involving animals were approved by Laboratory Animal Management
Committee of Hubei University of Medicine (Shiyan, China).
Derivation and culture of BMSCs
BMSCs were derivatized according to a previously
described method (19). Briefly,
rats were sacrificed by decapitation following anesthesia (0.8 ml
10% chloral hydrate; SouthernBiotech, Birmingham, AL, USA). Then,
femurs and tibiae were removed and conterminal tissues withdrawn in
a sterile environment. Bone marrow tissues were rinsed with
phosphate buffer saline (PBS) and the BMSCs were isolated.
Retrieved BMSCs were centrifuged at 1,000 × g for 5 min at room
temperature and cultured in Dulbecco's modified Eagle medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA). Culture media was
replaced every 3 days. BMSCs were sub-cultured when they were
between 80 and 90% confluence. To track the in vivo
migration of BMSCs towards the site of SCI, plasmid transfection of
a recombinant adenovirus encoding green fluorescent protein (GFP)
was performed according to the manufacturer's instruction (Ad-GFP;
Cyagen, Sunnyvale, CA, USA). Culture media was replaced 6 h
following transfection and expression of GFP was confirmed 2 days
following the media being replaced via fluorescence microscopy.
Establishing the rat model of SCI
Rats were anesthetized with an intraperitoneal
injection of 10% chloral hydrate (0.3 ml/100 g) and fixed in a
prone position using a vertical locator (W.M. Keck Center for
Collaborative Neuroscience, Rutgers University, New Brunswick, NJ,
USA). The ninth thoracic vertebra crest was identified and ~2 cm of
the skin and superficial fascia over this region was incised along
the posterior median line under aseptic conditions. A dorsal
laminectomy was performed on the ninth thoracic vertebra crest and
the corresponding spinal cord unfolded. Subsequently, a moderate
compressional SCI was imposed on each rat by perpendicularly
descending a spinal cord impactor (W.M. Keck Center for
Collaborative Neuroscience Rutgers, New Brunswick, NJ, USA) with a
10 g impact rod from a height of 5 cm onto the spinal cord and
compress it for 10 sec. The following signs indicated that this
procedure was successful: Rat tails twitched involuntary, bilateral
hind limbs and truncus convulsed, both hind limbs were flaccid and
there was paralysis of the hind limbs. Following surgery, the
incision was irrigated with penicillin and saline and the muscles
and skin were stitched in their correct anatomical layers.
Groupings and treatment
SCI model rats (n=45) were randomly distributed into
the following three groups: A, the control group, treated once with
10 µl PBS by intraspinal injection; B, the BMSC group, treated with
10 µl of BMSC suspension containing 3×105 cells by
intraspinal injection; group C, the BMSC + EPO group, treated with
10 µl of BMSCs suspension containing 3×105 cells and
recombinant human EPO (rhEPO; Beijing Four Rings Bio-Pharmaceutical
Co., Ltd, Beijing, China) through intraperitoneal injection
(5×103 IU/kg). Rats were injected intramuscularly with
sulfentanil (0.05 µg/kg) and penicillin (3×104 U/kg) for
the first 3 days after SCI. The bladders of the rats were pressed
by hand twice daily until automatic micturition function was
restored. Food and water were places in areas accessible to the
rats.
Evaluation of neurological
function
Hind limb motor function was quantified and recorded
at 1, 7, 14, 21 and 28 days following SCI with reference to the
Basso, Beattie and Bresnahan (BBB) locomotor scale (20). The BBB scale range is between 0 and
21, where 0 stands for complete loss of locomotive function and 21
stands for normal locomotive function. A 120×30 cm foam-padded box
with a flat bottom was used for the BBB test and rats were placed
in the box 2 days prior to evaluation to acclimatize to their
surroundings.
Additionally, a grid walk test was performed to
evaluate motor sensitivity and capacity for precise control of the
hind limbs. Rats were allowed to crawl freely in a 120×120 cm grid
structure, which was divided into 10 mm squares. Missteps were
tracked as the rats mislaid the paw through the squares. The tests
was terminated when a maximum of 20 missteps were achieved. Rats
that failed to accomplish a coherent gait in the BBB scale test
were not put through the grid walk test and were instead assigned
the maximum score (21). The
individuals recording the movements were blinded to the study to
reduce error and raise accuracy.
Detection of in vitro BMSC
migration
BMSC migration was detected using the transwell
migration assay (cat no. 3413; Beijing Unique Biotechnology Co.,
Ltd, Beijing, China). In the chemotaxis group, serum-free medium
(200 µl) with 1×105 (cells/ml) BMSCs was placed into the
upper chambers, while DMEM (800 µl) with 10 units rhEPO and 10% FBS
was put into the lower chambers. In the control group, the same
procedure was followed, except that rhEPO was excluded from the
media in the lower chamber. Following 18 h of incubation as
previously described, cells that failed to migrate from the upper
chamber to the lower chamber were washed away. Then, cells that had
migrated on to the membranes were fixed with 4% paraformaldehyde
for 30 min, stained with 5% crystal violet for 20 min, washed with
PBS, and observed and imaged using an optical microscope. Cells
were counted three times, and an average was taken.
Detection of apoptosis at the site of
SCI
The apoptotic index of the SCI lesion site was
determined using the terminal deoxynucleotidyl transferase dUTP
nick end labeling (TUNEL) assay using an in situ cell death
detection kit (cat no. 40306ES50; Po Valley Biotechnology,
Shanghai, China) according to the manufacturer's instructions. The
specimens from the spinal cord were frozen and embedded in optical
coherence tomography (Leica Biosystems, Shanghai, China), and 7 µm
longitudinal cryostat sections were cut. Subsequently they were
dewaxed, hydrated with a series of graded alcohol (99.5%) and
co-cultured with proteinase K at room temperature for 30 min,
followed by devitalization of endogenous peroxidases with 30%
H2O2 for 5 min. Then, sections were incubated
with the TUNEL reaction mixture in a 60% air humidity atmosphere at
37°C for 60 min, followed by suspension of the reaction using the
stop buffer. Subsequent staining with hematoxylin dyed the nuclei
of apoptotic cells brown. The apoptotic index was calculated as the
percentage of TUNEL-positive (apoptotic) cells out of the total
number of nucleated cells. Cells were counted three times, and an
average was taken.
Detection of in vivo BMSC
distribution
To detect the distribution of BMSCs in the injured
spinal cord of rats, fluorescence microscopy scanning was conducted
on samples taken 4 weeks following surgery. Samples (as previously
described) were fixed with acetone for 20–30 min and then embedded
with a 1×1×1 cm foil paper mold, which was filled with frozen
embedding agent. Subsequently, they were frozen at −80°C in liquid
nitrogen, and then cut into slices with freezing microtome (Thermo
Fisher Scientific, Inc.). Then, sections were blocked with 5%
normal goat serum (Beyotime Institute of Biotechnology, Beijing,
China) at room temperature for 30 min. Sections were blotted using
bibulous paper to remove excess liquid and mounted onto slides with
an anti-fluorescent quencher (Antifade Mounting Medium; Leica
Biosystems, Shanghai, China). The distribution of BMSCs was
determined through visualization of GFP signals using fluorescence
microscopy (Shanghai Wanheng Precision Instruments Co., Ltd.,
Shanghai, China).
Western blot analysis of the
expression of vascular endothelial growth factor (VEGF) and brain
derived neurotrophic factor (BDNF) in vivo
Samples (as previously described) were homogenized
using Tissue Tearor (BioSpec Products, Inc., Bartlesville, OK, USA)
for 1 min at 4°C, following lysis in radioimmunoprecipitation
buffer (Boster Biological Technology, Ltd., Wuhan, China). Then,
samples underwent centrifugation (12,000 × g) at 4°C for 40 min and
50 µg of total protein was extracted from the supernatant for
separation by 10% SDS-PAGE, followed by transfer to a PVDF membrane
(HD Biosciences Co., Ltd., Shanghai, China). After being blocked
with Tris Buffered Saline Tween-20 (TBST; EMD Millipore, Billerica,
MA, USA) for 30 min, the PVDF membranes were incubated at 4°C
overnight with primary monoclonal antibodies as follows: Rabbit
anti-VEGF (cat no. CL-1313T), rabbit anti-BDNF (cat no. AHP1831) or
rabbit anti-GAPDH antibodies (cat no. A01020) (all diluted 1:200;
HD Biosciences Co., Ltd., Shanghai, China). Subsequently, they were
washed with TBST for 30 min, incubated with horseradish
peroxidase-labeled goat anti-rabbit antibody (1:5,000; cat no.
DC06L-200UG; Shanghai Biological Technology Co., Ltd., Shanghai,
China) at 26°C for 2 h. Bands were then visualized using an
enhanced chemiluminescence detection kit (cat no. 36222ES60; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and X-ray film. The
densitometry of the bands was analyzed using Image-Pro Plus 6.0
software (Media Cybernetics, USA). Cells were counted three times,
and an average was taken.
Statistical analysis
All results are expressed as the mean ± standard
deviation. One-way analysis of the variance was used to compare the
results of different groups. Multiple comparison between the groups
was performed using Student Newman Keuls method. Statistical
analysis was performed using SPSS software (version 21.0; IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
significant difference.
Results
EPO enhances the recovery of
neurological function in rat models of SCI treated with BMSCs
Functional hind limb movement was measured using two
ethological examinations. The BBB locomotor rating scale was
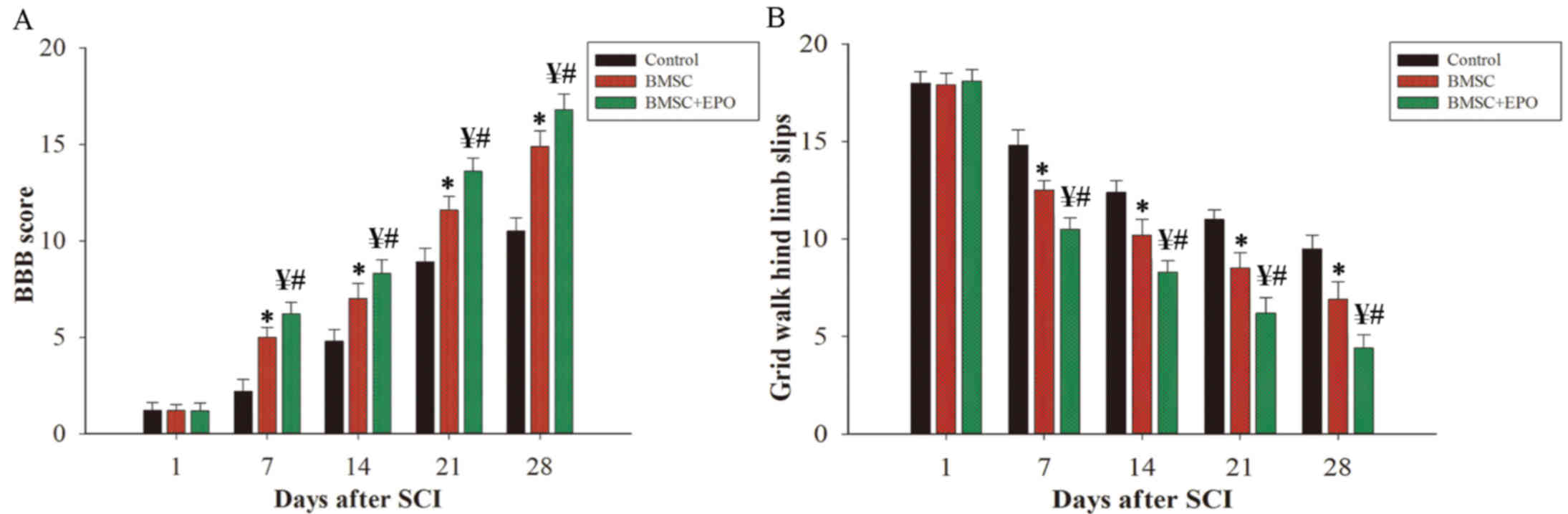
employed to access motor function in each group post-SCI (Fig. 1A). Due to the effect of the
anesthetic used prior to surgery, no significant difference was
found between the three groups 1 day following SCI (Fig. 1A). As time passed, the groups all
showed increasing motor function, however, the BBB score of the
BMSC + EPO group was significantly higher compared with that of the
control (P<0.01) and BMSC alone groups (P<0.05) (Fig. 1A). In addition, the grid walk test
was performed to access the coordination and accuracy of hind limb
motor function (Fig. 1B). BMSC + EPO
treated rats showed significantly less missteps from 7 days
post-SCI compared with the control (P<0.01) and BMSC alone
groups (P<0.05) (Fig. 1B).
Effect of EPO on the migration
capacity of BMSC in vitro
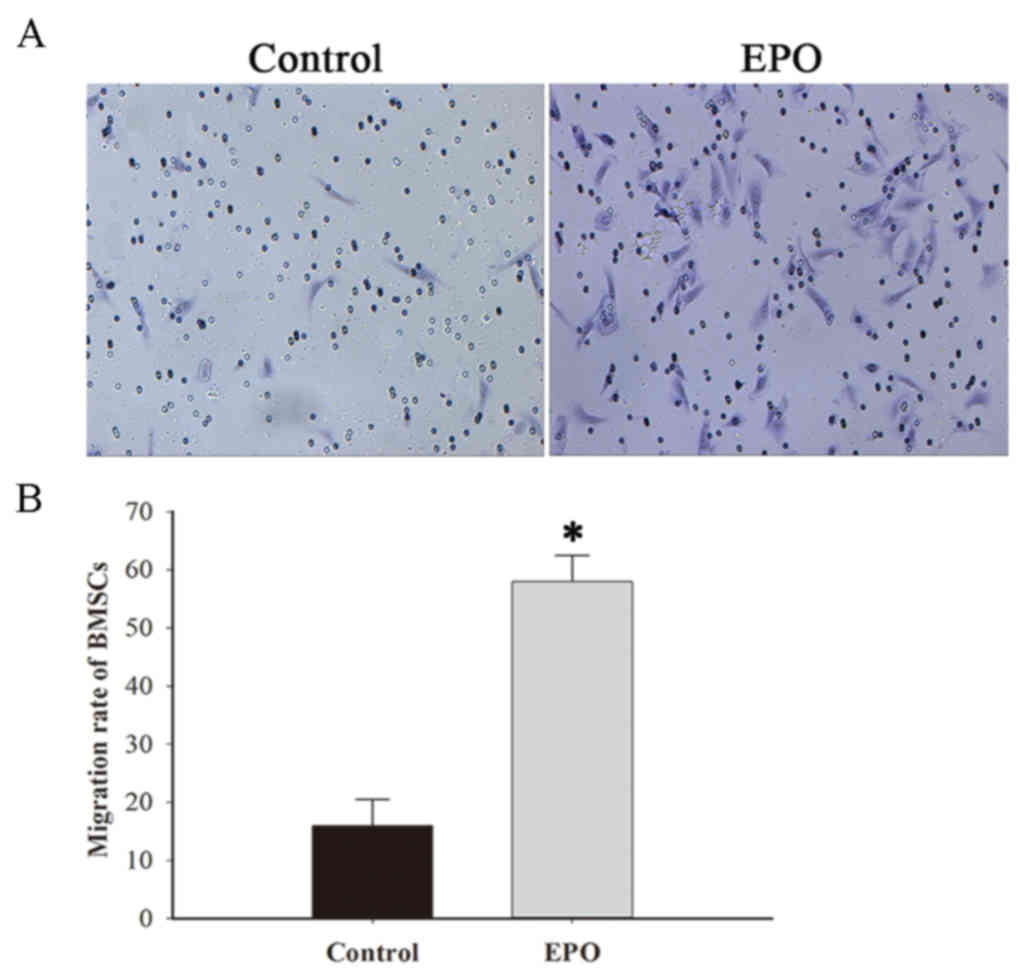
The transwell migration assay was performed to
access the effect of EPO on the in vitro migration capacity
of BMSCs (Fig. 2A). The results
identified that EPO significantly increased the migration capacity
of BMSCs compared with the BMSCs alone group (P<0.001; Fig. 2B).
Effect of EPO on the apoptotic index
of SCI sites
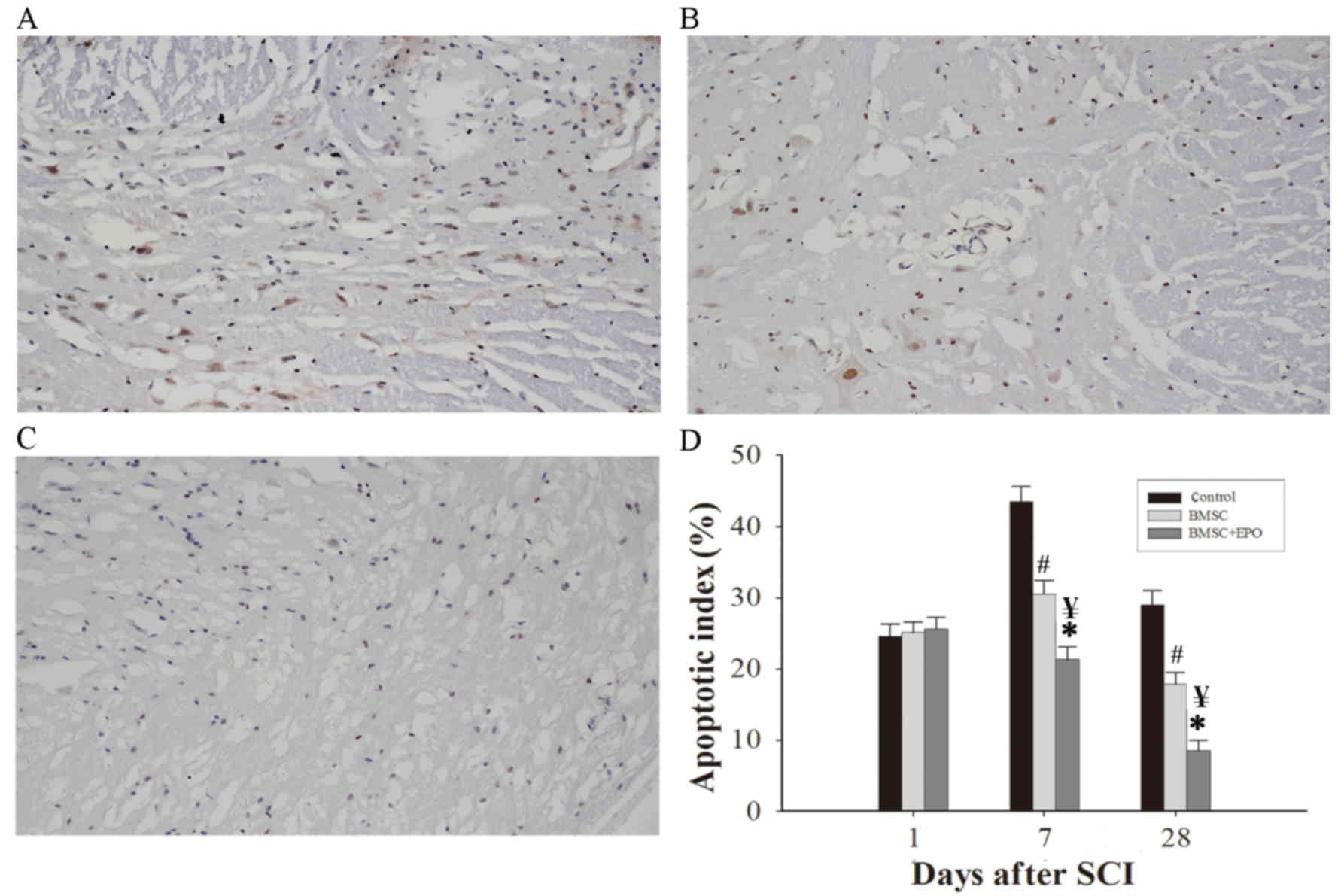
The TUNEL assay was used to identify apoptotic cells
and apoptotic cells were stained brown (Fig. 3A-C). This was used to determine the
apoptotic index of the groups following SCI (Fig. 3D). There was no significant
difference in the apoptotic indexes of the three groups 1 day
following SCI (Fig. 3D). However, 7
and 28 days following SCI, the apoptotic index of the BMSC group
was significantly decreased compared with the control group
(P<0.05) and the apoptotic index of the BMSCs + EPO group was
significantly decreased compared with the control (P<0.01) and
BMSC treatment groups (P<0.05) (Fig.
3D). These results demonstrate that EPO may increase the
anti-apoptotic effect of BMSCs in SCIs.
EPO facilitates the recruitment of
BMSCs to sites of SCI
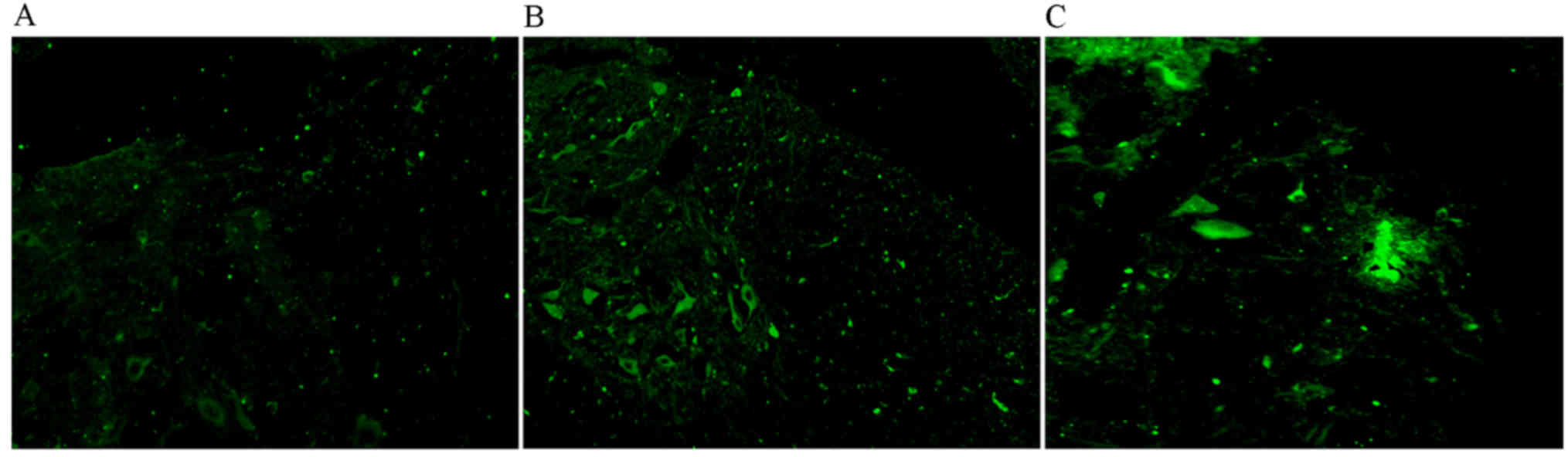
An immunofluorescence assay was performed on SCI
samples taken 4 weeks following SCI (Fig. 4). Fluorescence signals were almost
undetectable in the control group (Fig.
4A), while GFP-labeled BMSCs were detected at the site of the
SCI in the BMSC treatment (Fig. 4B)
and BMSC + EPO groups (Fig. 4C).
However, the signal was stronger in the BMSC + EPO group (Fig. 4C). These results indicate that EPO
facilitates the migration of BMSCs to sites of SCI.
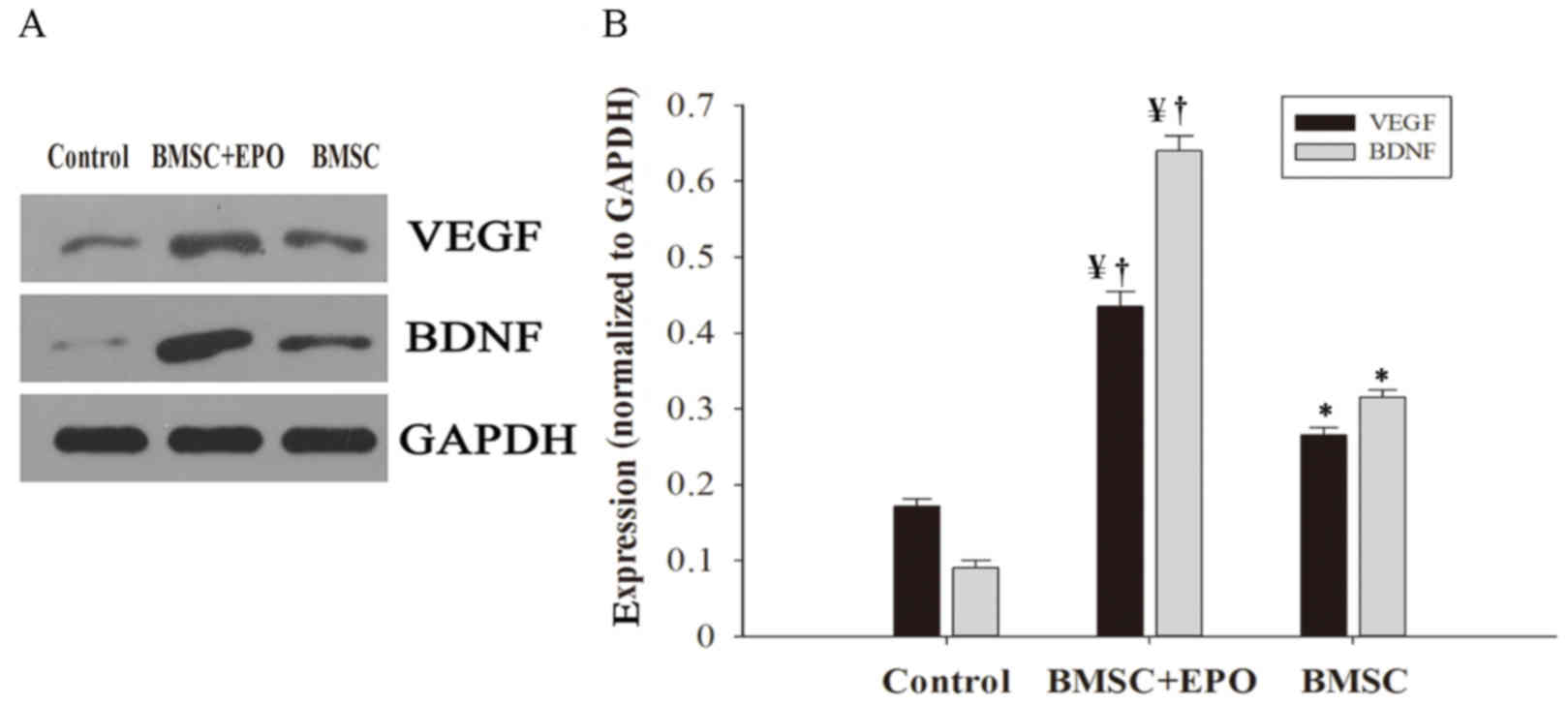
EPO increases in vivo expression of
VEGF and BDNF
The expression of VEGF and BDNF was determined
through western blotting (Fig. 5A)
and subsequent image analysis (Fig.
5B). The BMSC treatment group had significantly increased VEGF
and BDNF expression levels compared with the control group
(P<0.05), and expression levels in the BMSC + EPO group were
significantly higher compared with those of the control (P<0.01)
and BMSC treatment groups (P<0.05).
Discussion
The determining factor for the therapeutic efficacy
of BMSC transplantation is not the quantity of engraftments, but
the quantity of BMSCs that successfully migrate to the lesion site
(21). In addition, a number of
grafting routes increase the quantity of successful BMSCs and
enhance the restoration of neurological function following SCI,
such as transplanting directly into the lesion site, through the
cerebrospinal fluid or through caudal vein (22).
At the site of SCI there is upregulation in the
expression of numerous chemokines, cytokines, corresponding
receptors and growth factors, including VEGF, platelet-derived
growth factor, hepatocyte growth factor and tumor necrosis factor-α
(23). These factors, together with
signaling molecules in the local microenvironment, form a signaling
network that directs the migration of BMSCs in vivo
(24). However, it is difficult to
evaluate the validity of biomaterials and BMSC treatment for SCI,
as there is a deficiency in controlled studies and methodology
guidance.
The primary restrictions in the therapeutic
application of BMSCs for SCI are poor cell viability following
transplantation, a deficiency in neuronal differentiation,
necrosis, glial scar formation, poor microenvironment and the short
time period in which transplantation is successful (25–27). In
order to overcome these restrictions, a number of strategies have
been applied, including stimulating neuronal differentiation prior
to grafting, neurotrophic gene transfection, co-administration of
glial cells and histological engineering (28–30). In
a previous study, genetically engineered human BMSCs injected above
and below the SCI site in rats immediately following SCI
significantly ameliorated subsequent locomotor function (31). In addition, transplantation of
neurally differentiated BMSCs into the center of a contused spinal
cord in rats resulted in restoration of locomotor function and
significantly shortened primary latency of BMSCs (32). Co-transplantation of neurally
differentiated and undifferentiated autologous BMSCs into the site
of SCI in rats remarkably improved BBB locomotor scale scores
compared with controls (33).
Results of the present study indicate that EPO promotes the
migration of BMSCs to SCI sites and significantly improves
locomotor function following SCI.
Angiogenesis is essential to increase blood flow to
injured areas, a crucial component of neural reconstruction
following SCI. VEGF stimulates angiogenesis and increases vascular
permeability, which is important in the healing of SCIs (34). A previous study in rats indicated
that microvessel density is significantly increased by
administration of VEGF, which improved locomotor function (35). BMSCs secrete VEGF and BDNF, which
trigger downstream signaling by binding to the
tropomyosin-receptor-kinases A and B (36). In addition, a previous study found
that VEGF produced by BMSCs induced angiogenesis and osseous
regeneration (37). Furthermore, Han
et al (38) identified that
administration of simvastatin promotes expression of BDNF and VEGF,
which regulates the microenvironment of the injured spinal cord,
resulting in improved viability, proliferation and neuronal
differentiation of BMSCs recruited to the site of SCI. In the
present study, co-administration BMSC + EPO upregulated the
expression of BDNF and VEGF at the site of SCI and promoted
recovery of neurological function following SCI.
Neuronal apoptosis is an essential part of the
pathophysiological process following SCI. BMSCs serve a primary
role in the inhibition of apoptosis by stimulating endogenous
salvage signaling pathways and secreting anti-apoptotic factors
(39). EPO has been determined to
inhibit neuronal apoptosis in a rat model of compressional SCI
(40). Furthermore, a previous study
found that EPO significantly inhibited apoptosis of motor neurons
in transient spinal cord ischemia (41). In the present study, BMSC
transplantation significantly decreased apoptosis compared with the
control group, while co-transplantation of BMSC + EPO significantly
decreased apoptosis compared with the control and BMSC treatment
groups. This indicates that EPO enhances the anti-apoptotic effect
of BMSCs.
In conclusion, the present study suggests that EPO
facilitates the recruitment of BMSCs to sites of SCI, increases the
expression of BDNF and VEGF, enhances the anti-apoptotic effect of
BMSCs and accelerates recovery of neurological function following
SCI. These results indicate that the co-transplantation of BMSC +
EPO is a promising strategy for the treatment of traumatic
SCIs.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Hubei Province, Hubei, China (grant no.
2013CFC035).
References
|
1
|
Hagen EM: Acute complications of spinal
cord injuries. World J Orthop. 6:17–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sekhon LH and Fehlings MG: Epidemiology,
demographics, and pathophysiology of acute spinal cord injury.
Spine (Phila Pa 1976). 26:(24 Suppl). S2–S12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morawietz C and Moffat F: The effects of
locomotor training after incomplete spinal cord injury: A
systematic review. Arch Phys Med Rehabil. 94:2297–2308. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bustos ML, Huleihel L, Kapetanaki MG,
Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ,
Kaminski N, Cerdenes N, et al: Aging mesenchymal stem cells fail to
protect because of impaired migration and antiinflammatory
response. Am J Respir Crit Care Med. 189:787–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ansboro S, Greiser U, Barry F and Murphy
M: Strategies for improvement of therapeutic cells: Implications
for tissue repair. Eur Cells Mater. 23:310–319. 2012. View Article : Google Scholar
|
|
6
|
Kean TJ, Duesler L, Young RG, Dadabayev A,
Olenyik A, Penn M, Wagner J, Fink DJ, Caplan AI and Dennis JE:
Development of a peptide-targeted, myocardial ischemia-homing,
mesenchymal stem cell. J Drug Target. 20:23–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martinez AM, Goulart CO, Bdos Ramalho S,
Oliveira JT and Almeida FM: Neurotrauma and mesenchymal stem cells
treatment: From experimental studies to clinical trials. World J
Stem Cells. 6:179–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel DM, Shah J and Srivastava AS:
Therapeutic potential of mesenchymal stem cells in regenerative
medicine. Stem Cells Int. 2013:4962182013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robert CR, Sorkin M, Garg RK and Gurtner
GC: Stem cell recruitment after injury: Lessons for regenerative
medicine. Regen Med. 7:833–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Novikova LN, Brohlin M, Kingham PJ,
Novikov LN and Wiberg M: Neuroprotective and growth-promoting
effects of bone marrow stromal cells after cervical spinal cord
injury in adult rats. Cytotherapy. 13:873–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffmann J, Glassford AJ, Doyle TC,
Robbins RC, Schrepfer S and Pelletier MP: Angiogenic effects
despite limited cell survival of bone marrow-derived mesenchymal
stem cells under ischemia. Thorac Cardiovasc Surg. 58:136–142.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eliopoulos N, Zhao J, Forner K, Birman E,
Young YK and Bouchentouf M: Erythropoietin gene-enhanced marrow
mesenchymal stromal cells decrease cisplatin-induced kidney injury
and improve survival of allogeneic mice. Mol Ther. 19:2072–2083.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong M, Chen S, Yu H, Liu Z, Zeng Y and
Li F: Neuroprotection of erythropoietin and methylprednisolone
against spinal cord ischemia-reperfusion injury. J Huazhong Univ
Sci Technolog Med Sci. 31:652–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teixeira M, Rodrigues-Santos P, Garrido P,
Costa E, Parada B, Sereno J, Alves R, Belo L, Teixeira F,
Santos-Silva A and Reis F: Cardiac antiapoptotic and
proproliferative effect of recombinant human erythropoietin in a
moderate stage of chronic renal failure in the rat. J Pharm
Bioallied Sci. 4:76–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Li J, Peng H, Zhou J and Fang H:
Administration of erythropoietin exerts protective effects against
glucocorticoid-induced osteonecrosis of the femoral head in rats.
Int J Mol Med. 33:840–848. 2014.PubMed/NCBI
|
|
16
|
Liu NM, Tian J, Wang WW, Han GF, Cheng J,
Huang J and Zhang JY: Effect of erythropoietin on mesenchymal stem
cell differentiation and secretion in vitro in an acute kidney
injury microenvironment. Genet Mol Res. 12:6477–6487. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nair AM, Tsai YT, Shah KM, Shen J, Weng H,
Zhou J, Sun X, Saxena R, Borrelli J Jr and Tang L: The effect of
erythropoietin on autologous stem cell-mediated bone regeneration.
Biomaterials. 34:7364–7371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Onifer SM, Zhang O, Whitnel-Smith LK, Raza
K, O'Dell CR, Lyttle TS, Rabchevsky AG, Kitzman PH and Burke DA:
Horizontal ladder task-specific re-training in adult rats with
contusive thoracic spinal cord injury. Restor Neurol Neurosci.
29:275–286. 2011.PubMed/NCBI
|
|
19
|
Deng W, Bivalacqua TJ, Chattergoon NN,
Jeter JR Jr and Kadowitz PJ: Engineering ex vivo-expanded marrow
stromal cells to secrete calcitonin gene-related peptide using
adenoviral vector. Stem Cells. 22:1279–1291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakano N, Nakai Y, Seo TB, Homma T, Yamada
Y, Ohta M, Suzuki Y, Nakatani T, Fukushima M, Hayashibe M and Ide
C: Effects of bone marrow stromal cell transplantation through CSF
on the subacute and chronic spinal cord injury in rats. PLoS One.
8:e734942013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caplan AI and Correa D: PDGF in bone
formation and regeneration: New insights into a novel mechanism
involving MSCs. J Orthop Res. 29:1795–1803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Urdzíková LM, Růžička J, LaBagnara M,
Kárová K, Kubinová Š, Jiráková K, Murali R, Syková E,
Jhanwar-Uniyal M and Jendelová P: Human mesenchymal stem cells
modulate inflammatory cytokines after spinal cord injury in rat.
Int J Mol Sci. 15:11275–11293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia P, Pan S, Cheng J, Yang M, Qi Z, Hou T
and Yang X: Factors affecting directional migration of bone marrow
mesenchymal stem cells to the injured spinal cord. Neural Regen
Res. 9:1688–1695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakajima H, Uchida K, Guerrero AR,
Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT,
Johnson WE and Baba H: Transplantation of mesenchymal stem cells
promotes an alternative pathway of macrophage activation and
functional recovery after spinal cord injury. J Neurotrauma.
29:1614–1625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mothe AJ, Bozkurt G, Catapano J, Zabojova
J, Wang X, Keating A and Tator CH: Intrathecal transplantation of
stem cells by lumbar puncture for thoracic spinal cord injury in
the rat. Spinal Cord. 49:967–973. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boido M, Garbossa D, Fontanella M, Ducati
A and Vercelli A: Mesenchymal stem cell transplantation reduces
glial cyst and improves functional outcome after spinal cord
compression. World Neurosurg. 81:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abrams MB, Dominguez C, Pernold K, Reger
R, Wiesenfeld-Hallin Z, Olson L and Prockop D: Multipotent
mesenchymal stromal cells attenuate chronic inflammation and
injury-induced sensitivity to mechanical stimuli in experimental
spinal cord injury. Restor Neurol Neurosci. 27:307–321.
2009.PubMed/NCBI
|
|
29
|
Osaka M, Honmou O, Murakami T, Nonaka T,
Houkin K, Hamada H and Kocsis JD: Intravenous administration of
mesenchymal stem cells derived from bone marrow after contusive
spinal cord injury improves functional outcome. Brain Res.
1343:226–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho SR, Kim YR, Kang HS, Yim SH, Park CI,
Min YH, Lee BH, Shin JC and Lim JB: Functional recovery after the
transplantation of neurally differentiated mesenchymal stem cells
derived from bone barrow in a rat model of spinal cord injury. Cell
Transplant. 18:1359–1368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang YJ, Zhang W, Lin CG, Ding Y, Huang
SF, Wu JL, Li Y, Dong H and Zeng YS: Neurotrophin-3 gene modified
mesenchymal stem cells promote remyelination and functional
recovery in the demyelinated spinal cord of rats. J Neurol Sci.
313:64–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng Y, Wang J, Ling S, Li Z, Li M, Li Q,
Ma Z and Yu S: Differentiation of mesenchymal stem cells into
neuronal cells on fetal bovine acellular dermal matrix as a tissue
engineered nerve scaffold. Neural Regen Res. 9:1968–1978.
2014.PubMed/NCBI
|
|
33
|
Pedram MS, Dehghan MM, Soleimani M,
Sharifi D, Marjanmehr SH and Nasiri Z: Transplantation of a
combination of autologous neural differentiated and
undifferentiated mesenchymal stem cells into injured spinal cord of
rats. Spinal Cord. 48:457–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Canavese M and Spaccapelo R: Protective or
pathogenic effects of vascular endothelial growth factor (VEGF) as
potential biomarker in cerebral malaria. Pathog Glob Health.
108:67–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Patel CB, Cohen DM, Ahobila-Vajjula P,
Sundberg LM, Chacko T and Narayana PA: Effect of VEGF treatment on
the blood-spinal cord barrier permeability in experimental spinal
cord injury: Dynamic contrast-enhanced magnetic resonance imaging.
J Neurotrauma. 26:1005–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wyse RD, Dunbar GL and Rossignol J: Use of
genetically modified mesenchymal stem cells to treat
neurodegenerative diseases. Int J Mol Sci. 15:1719–1745. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen J, Zhang C, Jiang H, Li Y, Zhang L,
Robin A, Katakowski M, Lu M and Chopp M: Atorvastatin induction of
VEGF and BDNF promotes brain plasticity after stroke in mice. J
Cereb Blood Flow Metab. 25:281–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han X, Yang N, Cui Y, Xu Y, Dang G and
Song C: Simvastatin mobilizes bone marrow stromal cells migrating
to injured areas and promotes functional recovery after spinal cord
injury in the rat. Neurosci Lett. 521:136–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Isele NB, Lee HS, Landshamer S, Straube A,
Padovan CS, Plesnila N and Culmsee C: Bone marrow stromal cells
mediate protection through stimulation of PI3-K/Akt and MAPK
signaling in neurons. Neurochem Int. 50:243–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arishima Y, Setoguchi T, Yamaura I, Yone K
and Komiya S: Preventive effect of erythropoietin on spinal cord
cell apoptosis following acute traumatic injury in rats. Spine
(Phila Pa 1976). 31:2432–2438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Celik M, Gökmen N, Erbayraktar S,
Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E,
Cerami A and Brines M: Erythropoietin prevents motor neuron
apoptosis and neurologic disability in experimental spinal cord
ischemic injury. Proc Natl Acad Sci USA. 99:2258–2263. 2002.
View Article : Google Scholar : PubMed/NCBI
|