Introduction
Functional gastrointestinal disorder (FGID) is one
of the most common gastrointestinal disorders in humans,
encompassing functional dyspepsia and irritable bowel syndrome
(IBS) (1). The pathophysiology of
FGID is complicated and there are various factors to consider,
including abnormal motility, visceral hypersensitivity,
psychological factors, and disturbed brain-gut interaction
(1). Disturbed digestive motility is
often responsible for the manifestation of gastrointestinal
symptoms in various diseases (2).
Postoperative ileus (POI) typically occurs following
abdominal surgery and is characterized by a transient hypomotility
of the gastrointestine, which may prolong hospital stays and
increase mortality (3,4). Surgical stress stimulates the release
of endogenous opioids, resulting in impaired post-surgical
gastrointestinal function (5,6).
Previous studies have suggested µ-opioid receptor antagonists,
cholinergic agonists or 5-HT4 receptor agonists as
targets for the development of a drug to improve management of POI
(7,8).
IBS is considered to be a functional
gastrointestinal disorder characterized by chronic abdominal pain
and discomfort associated with alteration in bowel habits in the
absence of demonstrable pathology (9). Evidence suggests that increased
visceral sensitivity is primarily responsible for the manifestation
of symptoms of IBS (10). At
present, it has been suggested that abnormal interactions between
normal and disordered motility or visceral hypersensitivity may be
responsible for IBS symptoms (11,12).
Prokinetic drugs are commonly prescribed for
gastrointestinal disorders in humans (13). However, two gastroprokinetic agents,
domperidone and tegaserod, are associated with serious side effects
(14,15), and the efficacy data on two others,
mosapride and itopride, are conflicting (16,17).
Therefore, there is a requirement for safer and more effective
gastroprokinetic agents to be developed. LD02GIFRO is a novel
prokinetic agent obtained from extracts of Poncirus fructus
(PF) and Zanthoxylum sp. fruits (ZF), both of which have
been used in Asian traditional medicine for the treatment of
gastrointestinal disorders. PF has been used as a traditional
medicine for the treatment of abnormal gastrointestinal motility
and gastric secretion (18–20). ZF, which belongs to the Rutaceae
family, has been used as a traditional medicine to alleviate
stomach pain, diarrhea, and jaundice (21,22).
Additionally, ZF extracts have been demonstrated to have a
prokinetic effect that is able to ameliorate delayed
gastrointestinal transit (GIT) in rats, and have been reported to
stimulate duodenal and ileal motility (23,24).
Based on these traditional herbal medicinal theories and clinical
records, PF and ZF were selected for use in the present study.
Materials and methods
Preparation of LD02GIFRO
LD02GIFRO is the standardized extract of two herbs.
PF is the immature fruit of Poncirus trifoliate, and
Zanthoxyli fructus is the pericarp of Zanthoxylum
piperitum. These herbs were purchased at a Chinese herb
medicine shop in Kyung-dong Market (Seoul, South Korea) and washed
with distilled water to remove adulterations prior to use. A total
of 100 g of dried Ponciri fructus was added to 800 ml of 70%
ethanol and 250 g of dried Zanthoxyli fructus was added to
1,750 ml of 30% ethanol. The herbs were subsequently extracted at
room temperature for 24 h, concentrated at 55–65°C under 25.5 MPa
pressure and dried to obtain the solvent extracts of Ponciri
fructus and Zanthoxyli fructus. The 70% ethanol extract
of Ponciri fructus and 30% ethanol extract of Zanthoxyli
fructus were mixed at a ratio of 1:12 by weight to prepare the
complex extracts of Ponciri fructus and Zanthoxyli
fructus.
Chemicals
Atropine sulfate, mosapride, phenol red, Evans Blue
and trinitrobenzenesulfonic acid (TNBS) were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Acetic acid
was purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan).
Alvimopan and morphine were purchased from GlaxoSmithKline PLC
(Brentford, UK) and BC World Pharm Co., Ltd. (Seoul, Korea),
respectively. GR125487 was purchased from Tocris Bioscience
(Bristol, UK).
Experimental animals
A total of 296 male Sprague-Dawley rats (age, 6
weeks; weight, 180–200 g) were obtained from Orient Bio, Inc.
(Gyeonggi, Korea). Animal care and experimental procedures were
conducted in accordance with the approval and guidelines of the
Institutional Animal Care and Use Committee of the Medical School
of Inha University (Incheon, Korea; approval ID, INHA 140618-302,
INHA 141126-340). Rats were provided standard rat chow and tap
water ad libitum and were maintained at room temperature
(25–30°C) and at 45–55% relative humidity in a 12-h light/dark
cycle. Rats were fasted for 24 h prior to experiments, with ad
libitum access to water. Rats were randomly distributed into
experimental groups (37 groups; n=8 per group).
Surgical preparation
Rats were anesthetized via intraperitoneal injection
of 100 mg/kg ketamine (Yuhan Co., Ltd., Seoul, Korea) and 4.4 mg/kg
xylazine (Bayer AG, Leverkusen, Germany). Teflon-coated electrodes
(A-M Systems, Inc., Sequim, WA, USA) were implanted in the external
oblique muscles and externalized to the back of the neck to record
electromyography (EMG). The experiment was initiated three days
post-surgery.
POI
POI was induced according to the previously reported
method (25). Control and treatment
groups were anesthetized with 3% isoflurane (Hana Pharm Co., Ltd.,
Seoul, Korea) and the abdomen was opened via a 2-cm midline
laparotomy. The small intestine and caecum were gently pulled out
of the abdominal cavity and the small intestine was gently
manipulated with the fingers for 5 min. Following manipulation, the
small intestine and caecum were replaced in the abdominal cavity,
and the surgical wound was sutured. The normal group underwent
isoflurane anesthesia only.
GIT
To study the effects of POI on GIT, 0.2 ml Evans
Blue (50 mg/ml) in saline was orally administered to each rat 4 h
post-operatively. Rats (n=8 per group) were sacrificed under 100
mg/kg ketamine (Yuhan Co., Ltd.) and 4.4 mg/kg xylazine (Bayer AG)
anesthesia 20 min after the administration of Evans Blue, and the
rate of GIT was calculated by dividing the distance of Evans Blue
migration by the total length of the small intestine.
Study 1: In the POI model group, LD02GIFRO (65, 130
or 260 mg/kg) or alvimopan (10 mg/kg) was orally administered 30
min prior to Evans Blue. Control and normal rats were orally
administered the same volume (5 ml/kg) of vehicle (5% Pluronic
F-68; Sigma-Aldrich; Merck Millipore). Rats were randomly
distributed into six groups (n=8 per group): Normal group, 3%
isoflurane anesthesia + vehicle; control group, POI + vehicle;
LD02GIFRO groups (65, 130 or 260 mg/kg), POI + LD02GIFRO; and
alvimopan group, POI + alvimopan.
Study 2: To investigate whether LD02GIFRO was able
to ameliorate delayed GIT induced by morphine (3 mg/kg), LD02GIFRO
was administered 30 min after a subcutaneous (s.c.) injection of
morphine (3 mg/kg; BC World Pharm Co., Ltd.). Rats were randomly
distributed into six groups (n=8 per group): Normal group, 3%
isoflurane anesthesia + vehicle; control group, POI + vehicle;
LD02GIFRO groups (65, 130 and 260 mg/kg), POI + LD02GIFRO; and
alvimopan group, POI + alvimopan.
Study 3: As presurgical treatment, LD02GIFRO was
administered 30 min prior to morphine (1 mg/kg, s.c.), which was in
turn administered 15 min prior to the intestinal manipulation to
induce POI. Two h post-surgery, Evans Blue was orally administered,
and rats were deeply anesthetized 20 min later under 100 mg/kg
ketamine (Yuhan Co., Ltd.) and 4.4 mg/kg xylazine (Bayer AG)
anesthesia. Subsequently, the abdominal aorta was transected to
exsanguinate the rats. Rats were randomly distributed into five
groups (n=8 per group): Normal group, 3% isoflurane anesthesia +
vehicle; control group: vehicle + morphine + POI; LD02GIFRO groups
(130 and 260 mg/kg): LD02GIFRO + morphine + POI; and alvimopan
group, alvimopan + morphine + POI.
Study 4: As postsurgical treatment, morphine (1
mg/kg, s.c.) was administered 15 min prior to POI surgery, and
LD02GIFRO was administered 30 min post-surgery. At 2 h following
the induction of POI, rats were administered with Evans Blue by
gavage, and were sacrificed 20 min later. Rats were randomly
distributed into five groups (n=8 per group): Normal group, 3%
isoflurane anesthesia + vehicle; control group, morphine + POI +
vehicle; LD02GIFRO groups (130 and 260 mg/kg), morphine + POI +
LD02GIFRO; and alvimopan group, morphine + POI + alvimopan.
Study 5: To investigate whether cholinergic or
serotonergic receptors mediate the stimulatory effect of LD02GIFRO
on POI, rats were administered with saline (1 ml/kg), atropine (2
mg/kg) or GR125487 (a 5-HT4 receptor antagonist; 2
mg/kg) via s.c. injection 15 min prior to LD02GIFRO administration.
The group that underwent both surgical and morphine treatment was
designed to mimic the clinical situation of patients receiving
opioids following abdominal surgery. Rats were randomly distributed
into five groups (n=8 per group): Normal group, 3% isoflurane
anesthesia + saline + vehicle; control group, POI + saline +
vehicle; saline group, POI + saline + LD02GIFRO; atropine group:
POI + atropine + LD02GIFRO; and GR125487 group, POI + GR125487 +
LD02GIFRO.
Visceromotor responses (VMR) to
colorectal distension (CRD)
Rats were anesthetized with 3% isoflurane and a
barostat balloon (Mui Scientific, Mississauga, ON, Canada) was
subsequently inserted into the distal colon and held in place by
taping the balloon catheter to the base of the tail. The catheter
was attached to a programmable rigid piston barostat with zero
intrinsic compliance (Distender Series II; G&J Electronics,
Inc., Toronto, ON, Canada). The Teflon coated silver wire was
connected to an alternating current (AC) amplifier system (Grass
Instrument Co., West Warwick, RI, USA). The AC amplifier was
connected to data acquisition equipment and data were analyzed
using Powerlab 8/35 (PL3508; AD Instruments, Colorado Springs, CO,
USA). The rats were left for 30 min to recover completely. The
stimulus-response of the external oblique muscle during CRD was
recorded with computerized signals in the holding cage. The CRD
procedure consisted of two series of CRDs for 3 min at constant
pressures of 15, 30, 45, 60 and 80 mmHg, separated by 3-min
intervals without distension. The stimulus-response during each
distension period was expressed as the integral.
Colonic hypersensitivity
Study 6: Acute colonic hypersensitivity was induced
as described by Langlois et al (26) and Plourde et al (27). Briefly, male SD rats (300–400 g) were
fasted overnight, anesthetized with 3% isoflurane and administered
with an intracolonic infusion of dilute acetic acid (0.6%; 1.5 ml).
Rats were randomly distributed into five groups (n=8, each group):
0.6% AA group (0.6% acetic acid + vehicle), 0.6% AA + LD02GIFRO
groups (300, 600, 900 mg/kg); Normal control group: saline +
vehicle.
Study 7: Chronic hypersensitivity was induced as
described by Greenwood et al (28). Briefly, male SD rats (180–200 g) were
anesthetized with 3% isoflurane and administered with an
intracolonic infusion of TNBS (50 mg/kg; 0.5 ml in 25% ethanol).
The experiment was initiated 30 days after infusion. Rats were
randomly distributed into five groups (n=8, each group): 50 mg/kg
TNBS group (50 mg/kg TNBS + vehicle), 50 mg/kg TNBS + LD02GIFRO
groups (300, 600, 900 mg/kg); Normal control group: 25% ethanol +
vehicle.
Experimental design and drug treatment
of colonic hypersensitivity
Experiments were designed to examine the effect of
LD02GIFRO on acute acetic acid-induced colonic sensitivity and
postinflammatory rat models. The VMRs to an initial series of CRDs
were recorded 10 min prior to the administration of LD02GIFRO or
the vehicle. A second series of CRDs was performed 10 min after
intraperitoneal administration of LD02GIFRO (300, 600 or 900 mg/kg)
or vehicle (50% propylene glycol) and the VMRs recorded.
Data analysis and statistics
All values are expressed as the mean ± standard
error of the mean. Differences among groups were examined using a
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference. Data analyses were performed
using SPSS software (version 19.0; IBM SPSS, Armonk, NY, USA).
Abdominal muscular contractions in response to CRD, were monitored
via EMG recording to determine the response to visceral pain. The
degree of EMG response was positively correlated to the CRD volume.
The EMG response was calculated as follows: Integral of EMG
activity during CRD/80 mmHg control EMG response ×100. Responses
were measured for two consecutive series of CRD at 15, 30, 45, 60
and 80 mmHg.
Results
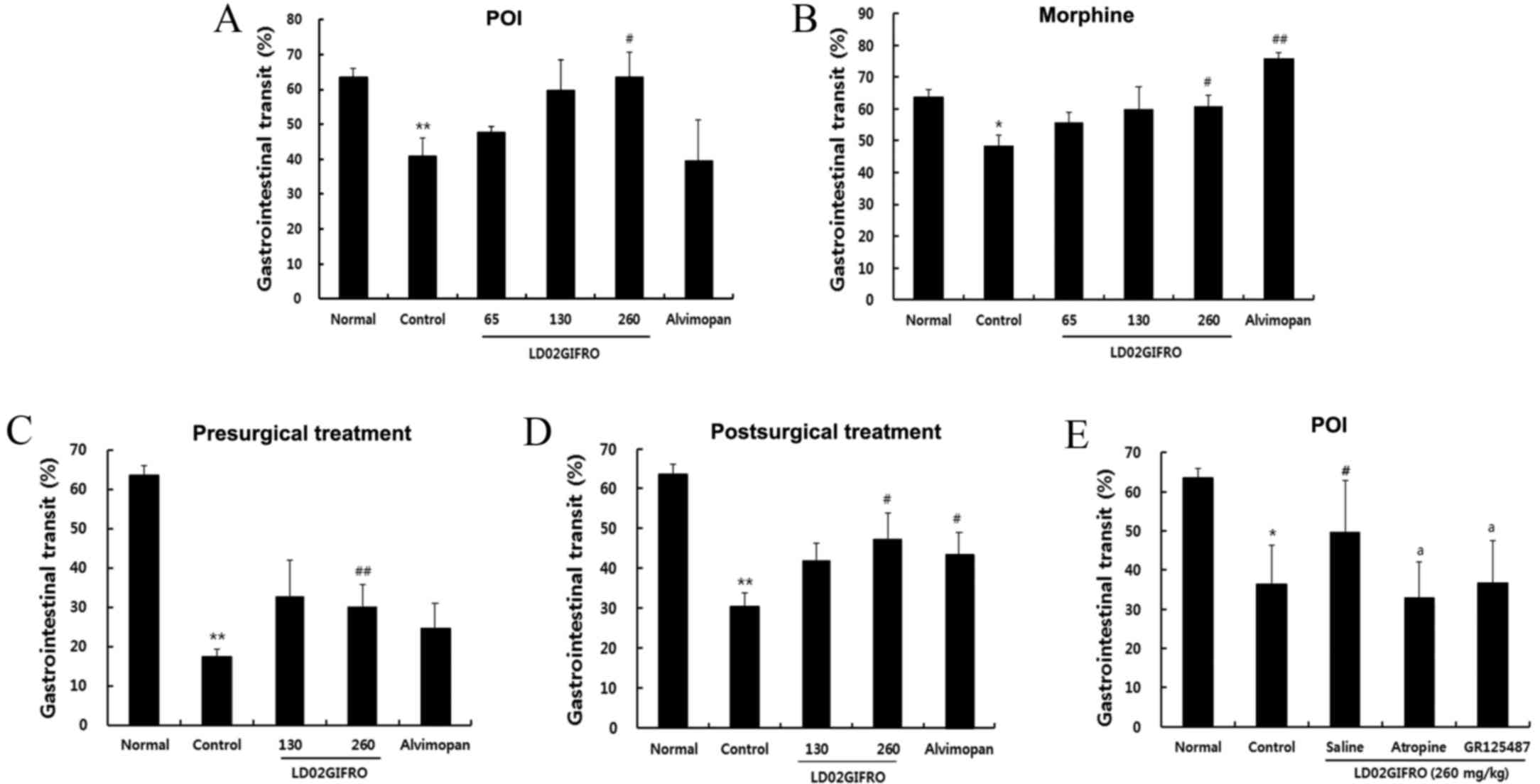
Effects of LD01GIFRO on GIT
The aim of the present study was to investigate
whether LD02GIFRO was capable of restoring delayed GIT. The rate of
GIT was significantly reduced in rats post-surgery (P<0.01);
however, administration of ≥65 mg/kg LD02GIFRO was able to
ameliorate the delayed GIT, and a significant improvement was
observed in the rats treated with 260 mg/kg (P<0.05; Fig. 1A). Furthermore, administration of 10
mg/kg morphine significantly decreased the GIT compared with the
normal group (P<0.05). The maximum effect was presented at the
dose of 260 mg/kg, with GIT of 60.7±3.5% (P<0.05; Fig. 1B). These results suggest that
LD02GIFRO is able to accelerate GIT in rats with delayed GIT
induced by laparotomy and morphine administration.
Pre-treatment with LD02GIFRO for
morphine and POI induced delayed GIT
Rats were administered with LD02GIFRO 30 min prior
to 1 mg/kg morphine injection (1 mg/kg, s.c.), which was
administered 15 min prior to mechanical stimulation. GIT was
significantly decreased in the control group with presurgical
treatment for vehicle and morphine rats (Fig. 1C; P<0.01). However, administration
of LD02GIFRO increased GIT by 32.7±9.3 and 30.2±5.7% at the doses
of 130 and 260 mg/kg, respectively. Notably, treatment with 260
mg/kg LD02GIFRO significantly restored delayed GIT (P<0.01),
whereas 10 mg/kg alvimopan had no significant effect on GIT.
Post-treatment with LD02GIFRO for
morphine and POI induced delayed GIT
Rats were treated with 1 mg/kg morphine 15 min prior
to surgery and LD02GIFRO was administered 45 min post-surgery. As
demonstrated in Fig. 1D, GIT was
significantly diminished in the control rats (30.5±3.3%;
P<0.01); however, LD02GIFRO significantly improved GIT at the
dose of 260 mg/kg compared with the controls (P<0.05). Treatment
with alvimopan also induced a significant increase in GIT rate
compared with the controls (P<0.05). As a result of these
findings, all subsequent experiments were performed using LD02GIFRO
at a dosage of 260 mg/kg.
Influence of atropine and GR125487 on
the effect of LD02GIFRO on delayed GIT in POI
GIT was markedly delayed in rats with POI compared
with normal rats (P<0.05; Fig.
1E). Administration of 260 mg/kg LD02GIFRO significantly
ameliorated the delayed GIT (P<0.05); however, this amelioration
was prevented by co-administration of 2 mg/kg atropine, a
muscarine-receptor antagonist (32.9±9.1%; P<0.05) or GR125487 (2
mg/kg), a 5-HT4 receptor antagonist (49.2±5.7%;
P<0.05).
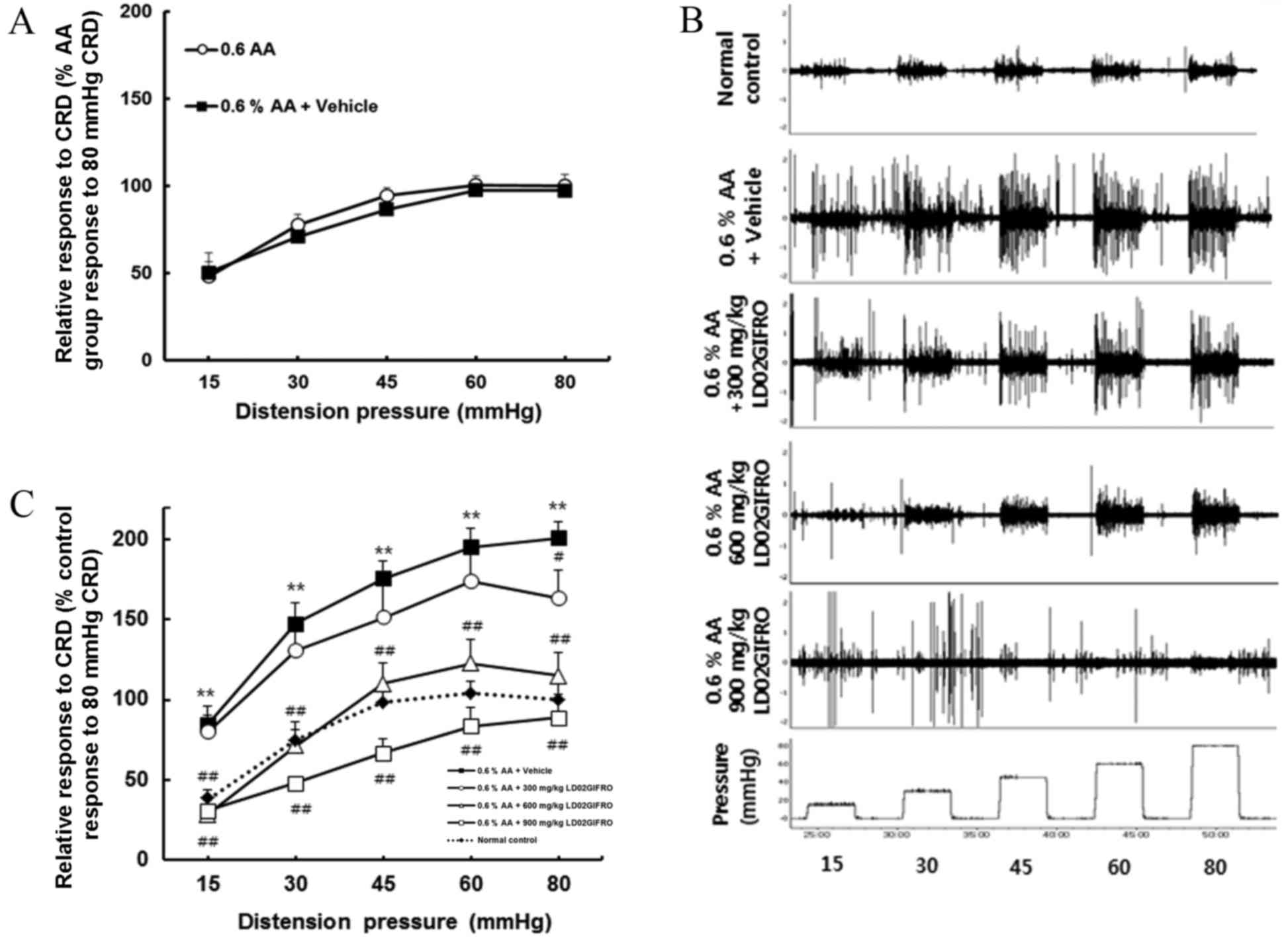
Effects of LD02GIFRO in rats with
acute colonic hypersensitivity
Acute colonic hypersensitivity in rats induced by
intra-colorectal infusion of 0.6% acetic acid was assessed by
comparing the exaggerated VMR to graded pressure of CRD with the
control rats (Fig. 2A-C). Rats with
acute colonic hypersensitivity developed significant visceral
hyperalgesia compared with the control group (P<0.01); this
hypersensitivity was evident for all CRD pressures (Fig. 2C). To investigate whether LD02GIFRO
had an inhibitory effect on the VMR in rats with hypersensitivity
to colonic stimulation, the rats were treated with LD02GIFRO (300,
600 or 900 mg/kg). The vehicle for LD02GIFRO, propylene glycol, had
no significant effect on the VMR of acetic acid-treated rats
(Fig. 2A). In contrast, when rats
were administered with LD02GIFRO, the VMR level decreased in a
dose-dependent manner. Rats treated with 300 mg/kg, LD02GIFRO had a
significantly lower VMR at CRD of 80 mmHg compared with the acetic
acid-treated response (P<0.05). At doses of 600 mg/kg the VMR
was significantly reduced at all CRD pressures to a level similar
to the corresponding control rats (P<0.01). Notably, the highest
dose of 900 mg/kg induced a significantly greater reduction in VMR
level at all distension pressures, with responses being reduced
below the VMR level recorded in normal control rats (P<0.01;
Fig. 2C). Fig. 2B demonstrates a representative EMG
response during the distension period to pressures from 15–80 mmHg
of vehicle, acetic acid-induced and following LD02GIFRO.
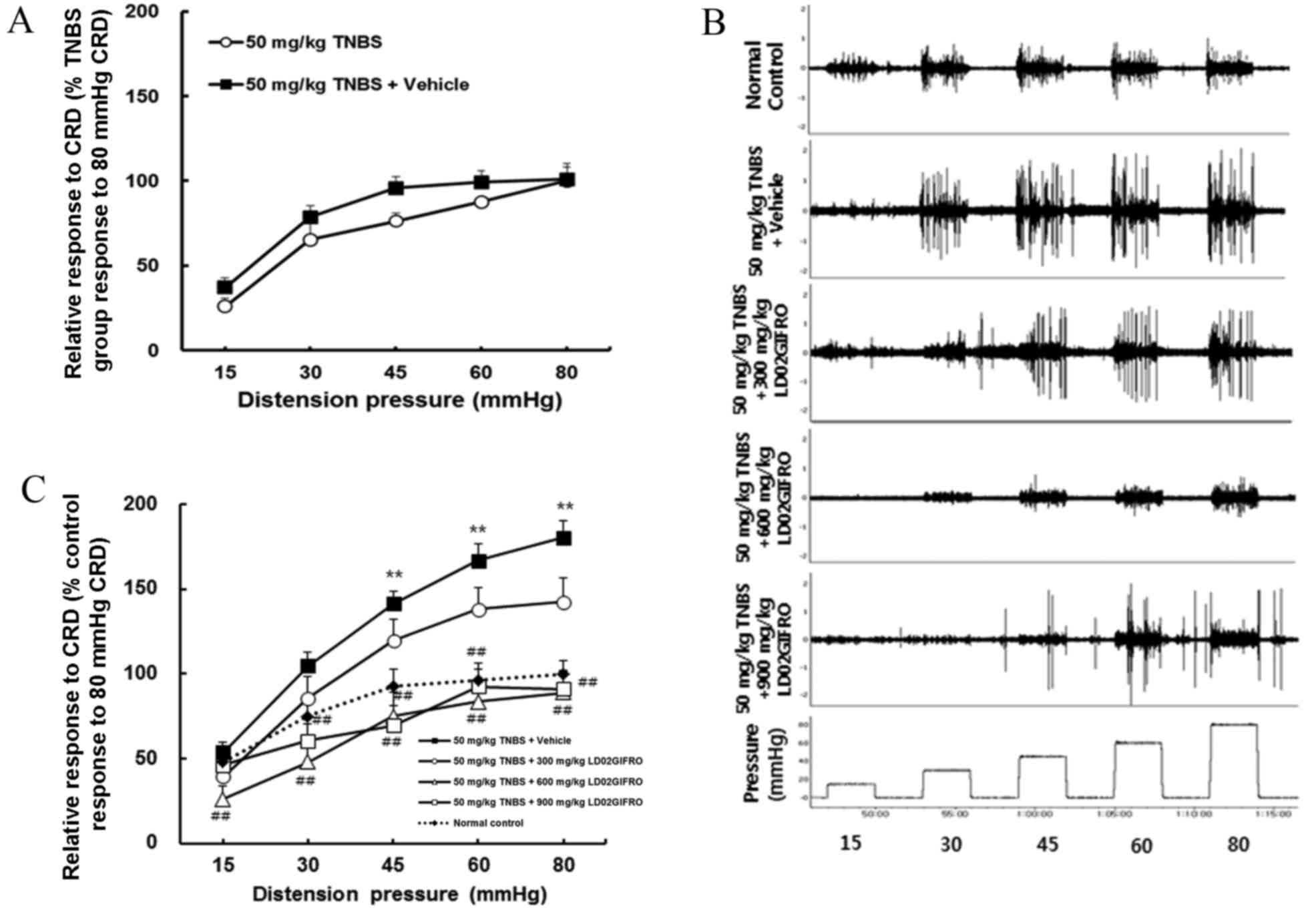
Effects of LD02GIFRO in rats with
postinflammatory colonic hypersensitivity
It was investigated whether postinflammatory colonic
hypersensitivity may be verified by recording electrical activity
in response to CRD in rats (Fig.
3A-C). Rats developed significant visceral hyperalgesia
compared with the control 30 days following the induction of TNBS
colitis (P<0.01; Fig. 3C).
Hypersensitive rats were treated with LD02GIFRO or vehicle. The
vehicle had no significant effect on the VMR at any CRD pressures
(Fig. 3A), whereas LD02GIFRO (300,
600 or 900 mg/kg) suppressed the VMR levels. At a dosage of 600 or
900 mg/kg, the VMR induced by all distension pressures were
significantly reduced to a VMR level below or similar to that
recorded at pressures in normal control rats (P<0.01; Fig. 3C). A representative EMG response to
pressures from 15 to 80 mmHg during a distension period in each
treatment group is displayed in Fig.
3B.
Discussion
FGID is one of the most prevalent gastrointestinal
disorders in humans (1). Several
herbal medicinal products have been assessed to determine whether
they may be used as an effective therapeutic treatment for patients
with FGID (29). For example, a
Chinese herbal medicine, known as Rikkunshi-to and TJ-43, has been
reported to enhance gastric emptying (30) and gastric adaptive relaxation
(31). It has been demonstrated that
daikenchuto, a Japanese herbal medicine comprised of four herbs, is
able to ameliorate morphine-induced inhibition of intestinal and
colonic transit (32). However, the
optimal therapeutic strategy for FGID remains to be determined due
to the poorly defined pathogenesis of the condition. In the present
study, the prokinetic agent LD02GIFRO was developed and its
pharmacological effects evaluated. The results suggest that
LD02GIFRO may have a prominent effect on gastrointestinal motility
by ameliorating GIT and reducing visceral hypersensitivity.
Various mechanisms have been proposed for the
pathogenesis of POI, although there is not yet a clear consensus.
Experimental and clinical ileus has been demonstrated to be
associated with the degree of surgical manipulation performed
(33), suggesting that surgical
manipulation of the intestine serves an important role in mediating
POI (34,35). Additionally, a typical factor that
contributes to post-surgical gastrointestinal dysregulation is the
use of opioids for pain management during the peri- and
post-operative periods (36).
Typically, morphine inhibits relaxation of the circular muscle and
contraction of the longitudinal muscle, resulting in delayed GIT
(37). In the present study,
treatment with 260 mg/kg LD02GIFRO was demonstrated to
significantly improve POI-induced delayed GIT. Morphine-induced
delayed GIT was also eliminated by administration of 260 mg/kg
LD02GIFRO.
Differences in the stimulatory effect were observed
between pre- and post-treatment of LD02GIFRO with morphine and POI.
GIT significantly improved with pre- and post-treatment of
LD02GIFRO, whereas administration of alvimopan had a significant
effect only when POI was accompanied with morphine.
Neural reflex pathways have previously been
implicated in postoperative dysmotility; cholinergic agonists and
5-HT4 receptor agonists are able to ameliorate
postoperative GIT dysmotility (7,38),
suggesting that activation of the cholinergic nervous system or
5-HT4 receptor agonist improves dysmotility in POI.
LD02GIFRO ameliorated the hypoperistalsis from POI, which was
completely inhibited by treatment with atropine or GR125487.
Therefore, the cholinergic nerves and 5-HT4 receptors
may be involved in this action of LD02GIFRO. Based on these
results, LD02GIFRO may be suggested as a superior treatment to
conventional therapeutics, particularly for GIT in abnormal
conditions.
Several studies have demonstrated that 5-HT has a
pivotal role in the control of gastrointestinal motility and
visceral sensations (39–41). Visceral hypersensitivity may be
induced by abnormal afferent nervous function or central
integration (42) and may be
associated with digestive dysmotility (43,44).
Several visceral hypersensitivity models have been investigated,
including that induced by acetic acid. In this model, mucosal
inflammation was induced via mucosal exposure to acetic acid,
inducing visceral hypersensitivity to rectal distension, which was
suggested to be due to the higher degranulation rate of mast cells
in the mucosa (45,46). In addition, another study previously
reported that postinflammatory visceral hypersensitivity was
induced 30 days following infusion of TNBS (28). In the present study, LD02GIFRO was
able to significantly ameliorate VMR to CRD in the acetic acid- and
TNBS-induced hypersensitivity rat model.
PF exerts its prokinetic activity via influencing
the 5-HT3 and 5-HT4 receptor mediated
pathways (47,48). In addition, ZF, has been reported to
ameliorate delayed GIT as an agonist of cholinergic nerves and the
5-HT4 receptor (22,23),
which was confirmed in the present study using LD02GIFRO.
In conclusion, the results of the present study
demonstrate that LD02GIFRO may have potential as an effective
prokinetic agent capable of ameliorating gastrointestinal disorders
and for an enhanced quality of life in patients with FGID.
Acknowledgements
The present study was supported by the Korean Health
Industry Development Institute and the T2B Infrastructure Center
for Digestive Disorders (grant no. HI15C0989).
References
|
1
|
Drossman DA: The functional
gastrointestinal disorders and the rome III process.
Gastroenterology. 130:1377–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirako M, Kamiya T, Misu N, Kobayashi Y,
Adachi H, Shikano M, Matsuhisa E and Kimura G: Impaired gastric
motility and its relationship to gastrointestinal symptoms in
patients with chronic renal failure. J Gastroenterol. 40:1116–1122.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Livingston EH and Passaro EP Jr:
Postoperative ileus. Dig Dis Sci. 35:121–132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holte K and Kehlet H: Postoperative ileus:
A preventable event. Br J Surg. 87:1480–1493. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brix-Christensen V, Tønnesen E, Sanchez
RG, Bilfinger TV and Stefano GB: Endogenous morphine levels
increase following cardiac surgery as part of the antiinflammatory
response? Int J Cardiol. 62:191–197. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taguchi A, Sharma N, Saleem RM, Sessler
DI, Carpenter RL, Seyedsadr M and Kurz A: Selective postoperative
inhibition of gastrointestinal opioid receptors. N Engl J Med.
345:935–940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holte K and Kehlet H: Postoperative ileus:
Progress towards effective management. Drugs. 62:2603–2615. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mattei P and Rombeau JL: Review of the
pathophysiology and management of postoperative ileus. World J
Surg. 30:1382–1391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whitehead WE and Drescher VM: Perception
of gastric contractions and Self-Control of gastric motility.
Psychophysiology. 17:552–558. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanazawa M, Hongo M and Fukudo S: Visceral
hypersensitivity in irritable bowel syndrome. J Gastroenterol
Hepatol. 26:(Suppl 3). S119–S121. 2011. View Article : Google Scholar
|
|
11
|
Houghton LA, Calvert EL, Jackson NA,
Cooper P and Whorwell PJ: Visceral sensation and emotion: A study
using hypnosis. Gut. 51:701–704. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coutinho SV, Plotsky PM, Sablad M, Miller
JC, Zhou H, Bayati AI, McRoberts JA and Mayer EA: Neonatal maternal
separation alters stress-induced responses to viscerosomatic
nociceptive stimuli in rat. Am J Physiol Gastrointest Liver
Physiol. 282:G307–G316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Longo WE and Vernava AM III: Prokinetic
agents for lower gastrointestinal motility disorders. Dis Colon
Rectum. 36:696–708. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drolet B, Rousseau G, Daleau P, Cardinal R
and Turgeon J: Domperidone should not be considered a no-risk
alternative to cisapride in the treatment of gastrointestinal
motility disorders. Circulation. 102:1883–1885. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tack J, Camilleri M, Chang L, Chey WD,
Galligan JJ, Lacy BE, Müller-Lissner S, Quigley EM, Schuurkes J, De
Maeyer JH and Stanghellini V: Systematic review: Cardiovascular
safety profile of 5-HT(4) agonists developed for gastrointestinal
disorders. Aliment Pharmacol Ther. 35:745–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hallerbäck B, Bommelaer G, Bredberg E,
Campbell M, Hellblom M, Lauritsen K, Wienbeck M and Holmgren LL:
Dose finding study of mosapride in functional dyspepsia: A
placebo-controlled, randomized study. Aliment Pharmacol Ther.
16:959–967. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holtmann G, Talley NJ, Liebregts T, Adam B
and Parow C: A placebo-controlled trial of itopride in functional
dyspepsia. N Engl J Med. 354:832–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim DW, Shim JE, Paik HY, Song WO and
Joung H: Nutritional intake of Korean population before and after
adjusting for within-individual variations: 2001 Korean National
Health and Nutrition Survey Data. Nutr Res Pract. 5:266–74. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi JM, Kim MS, Koo HN, Song BK, Yoo YH and
Kim HM: Poncirus trifoliata fruit induces apoptosis in human
promyelocytic leukemia cells. Clin Chim Acta. 340:179–185. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee H, Seo E, Chung S and Shim C: Effect
of an aqueous extract of dried immature fruit of poncirus
trifoliata (L.) Raf. on intestinal transit in rodents with
experimental gastrointestinal motility dysfunctions. J
Ethnopharmacol. 102:302–6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi Y: The Korean Folklore Plants.
Academybook; Seoul, South Korea: pp. 191–193. 1992
|
|
22
|
Lee J, Chang K and Kim G: Composition and
anti-inflammatory activities of Zanthoxylum schinifolium essential
oil: Suppression of inducible nitric oxide synthase,
cyclooxygenase-2, cytokines and cellular adhesion. J Sci Food
Agric. 89:1762–1769. 2009. View Article : Google Scholar
|
|
23
|
Tokita Y, Yuzurihara M, Sakaguchi M, Satoh
K and Kase Y: The pharmacological effects of daikenchuto, a
traditional herbal medicine, on delayed gastrointestinal transit in
rat postoperative ileus. J Pharmacol Sci. 104:303–310. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibata C, Sasaki I, Naito H, Ueno T and
Matsuno S: The herbal medicine dai-kenchu-tou stimulates upper gut
motility through cholinergic and 5-hydroxytryptamine 3 receptors in
conscious dogs. Surgery. 126:918–924. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Winter BY, Boeckxstaens GE, De Man JG,
Moreels TG, Herman AG and Pelckmans PA: Differential effect of
indomethacin and ketorolac on postoperative ileus in rats. Eur J
Pharmacol. 344:71–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Langlois A, Pascaud X, Junien JL, Dahl SG
and Rivière PJ: Response heterogeneity of 5-HT3 receptor
antagonists in a rat visceral hypersensitivity model. Eur J
Pharmacol. 318:141–144. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plourde V, St-Pierre S and Quirion R:
Calcitonin gene-related peptide in viscerosensitive response to
colorectal distension in rats. Am J Physiol. 273:G191–G196.
1997.PubMed/NCBI
|
|
28
|
Greenwood-Van Meerveld B, Venkova K, Hicks
G, Dennis E and Crowell MD: Activation of peripheral 5-HT4
receptors attenuates colonic sensitivity to intraluminal
distension. Neurogastroenterol Motil. 18:76–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coon Thompson J and Ernst E: Systematic
review: Herbal medicinal products for non-ulcer dyspepsia. Aliment
Pharmacol Ther. 16:1689–1699. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin X, Shibata C, Naito H, Ueno T,
Funayama Y, Fukushima K, Matsuno S and Sasaki I: Intraduodenal and
intrajejunal administration of the herbal medicine, dai-kenchu-tou,
stimulates small intestinal motility via cholinergic receptors in
conscious dogs. Dig Dis Sci. 46:1171–1176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayakawa T, Arakawa T, Kase Y, Akiyama S,
Ishige A, Takeda S, Sasaki H, Uno H, Fukuda T, Higuchi K and
Kobayashi K: Liu-Jun-Zi-Tang, a kampo medicine, promotes adaptive
relaxation in isolated guinea pig stomachs. Drugs Exp Clin Res.
25:211–218. 1999.PubMed/NCBI
|
|
32
|
Nakamura T, Sakai A, Isogami I, Noda K,
Ueno K and Yano S: Abatement of morphine-induced slowing in
gastrointestinal transit by Dai-kenchu-to, a traditional Japanese
herbal medicine. Jpn J Pharmacol. 88:217–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Satoh K, Hayakawa T, Kase Y, Ishige A,
Sasaki H, Nishikawa S, Kurosawa S, Yakabi K and Nakamura T:
Mechanisms for contractile effect of Dai-kenchu-to in isolated
guinea pig ileum. Dig Dis Sci. 46:250–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kalff JC, Buchholz BM, Eskandari MK,
Hierholzer C, Schraut WH, Simmons RL and Bauer AJ: Biphasic
response to gut manipulation and temporal correlation of cellular
infiltrates and muscle dysfunction in rat. Surgery. 126:498–509.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalff JC, Schraut WH, Simmons RL and Bauer
AJ: Surgical manipulation of the gut elicits an intestinal
muscularis inflammatory response resulting in postsurgical ileus.
Ann Surg. 228:652–663. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Panchal S, Müller-Schwefe P and Wurzelmann
J: Opioid-induced bowel dysfunction: Prevalence, pathophysiology
and burden. Int J Clin Pract. 61:1181–1187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grider JR and Makhlouf GM: Role of opioid
neurons in the regulation of intestinal peristalsis. Am J Physiol.
253:G226–G231. 1987.PubMed/NCBI
|
|
38
|
Ruwart MJ, Klepper MS and Rush BD:
Carbachol stimulation of gastrointestinal transit in the
postoperative lleus rat. J Surg Res. 26:18–26. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Talley NJ: Serotoninergic neuroenteric
modulators. Lancet. 358:2061–2068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Borman R and Burleigh D: Evidence for the
involvement of a 5-HT4 receptor in the secretory response of human
small intestine to 5-HT. Br J Pharmacol. 110:927–928. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hillier K, Tam FS, Bunce K and Grossman C:
Inhibition of motility induced by the activation of 5-HT1-like and
5-HT4-like receptor types in isolated human colon smooth muscle.
British Journal of Pharmacology-Proceedings Supplement.
112:102P1994.
|
|
42
|
Sarnelli G, Vandenberghe J and Tack J:
Visceral hypersensitivity in functional disorders of the upper
gastrointestinal tract. Digestive and Liver Disease. 36:371–376.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feinle-Bisset C, Vozzo R, Horowitz M and
Talley NJ: Diet, food intake and disturbed physiology in the
pathogenesis of symptoms in functional dyspepsia. Am J
Gastroenterol. 99:170–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nishiyama H, Mizuta Y, Isomoto H,
Takeshima F, Omagari K, Miyahara Y, Murata I and Kohno S: Chronic
visceral hypersensitivity renders defecation more susceptible to
stress via a serotonergic pathway in rats. Dig Dis Sci. 49:763–769.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
La JH, Kim TW, Sung TS, Kang JW, Kim HJ
and Yang IS: Visceral hypersensitivity and altered colonic motility
after subsidence of inflammation in a rat model of colitis. World J
Gastroenterol. 9:2791–2795. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
La JH, Kim TW, Sung TS, Kim HJ, Kim JY and
Yang IS: Role of mucosal mast cells in visceral hypersensitivity in
a rat model of irritable bowel syndrome. J Vet Sci. 5:319–324.
2004.PubMed/NCBI
|
|
47
|
Shim WS, Back H, Jung SW, Kim JW, Jang Y,
Lee B, Seo EK, Oh U and Shim CK: An aqueous extract of Poncirus
fructus activates the prokinetic activity of 5-HT receptor subtype
4 without hERG interaction. J Ethnopharmacol. 132:328–333. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim BJ, Kim HW, Lee GS, Choi S, Jun JY, So
I and Kim SJ: Poncirus trifoliate fruit modulates pacemaker
activity in interstitial cells of Cajal from the murine small
intestine. J Ethnopharmacol. 149:668–675. 2013. View Article : Google Scholar : PubMed/NCBI
|