Introduction
Focal cortical dysplasia (FCD) was initially
described by Taylor et al (1)
and is one of the most common causes of refractory epilepsy
(2). FCDs are localized regions of
malformed cerebral cortex and are often associated with epilepsy in
both adults and children (3). FCDs
are recognized by their histological attributes, which include
blurring of the cortical dyslamination, gray-white matter junction,
and/or the presence of abnormal balloon cells in the cortical and
subcortical regions (4). A broad
spectrum of histopathology has been included in the diagnosis of
FCD, and the classification method created by Blümcke et al
(5) has been applied more widely.
The classification system included aberrant radial or tangential
lamination of the neocortex (FCD Type I), cytological abnormalities
(FCD Type II) and cortical dyslamination with hippocampal
sclerosis, epilepsy-associated tumours, vascular malformations and
epileptogenic lesions acquired in early life (FCD, Type III). In
recent years, the number of patients with FCD IIa in the Second
Affiliated Hospital of Dalian Medical University (Dalian, China)
has significantly increased. The characteristic of the FCD IIa is
the presence of dysmorphic neurons, which have an enlarged cell
body and nucleus, abnormally distributed intracellular Nissl
substance and cytoplasmic accumulation of neurofilament proteins
(4). However, no balloon cells were
observed.
In the majority of patients with epilepsy, the
disease became refractory to medical therapy (6). Therefore, surgical intervention is
usually necessary. However, previous results have documented
unsatisfactory epilepsy surgery outcomes in this group of patients
(7). Due to the progress in
neuroimaging, including magnetic resonance imaging (MRI) and
electrocorticography (ECoG), the FCD becomes more visible to
delineate in preoperative assessment, and therefore the use of
surgery is once again increasing in recent years (8,9). The
outcome of surgery for epilepsy has not been studied well and there
are few long-term data that are relevant to the seizure outcome.
The identification of risk factors for seizure recurrence following
epilepsy surgery for seizures is important for preoperative and
postoperative counseling and evaluation (10). The challenge is to remove the whole
lesion, since complete resection has proven to be an essential
prognostic factor of seizure freedom (11,12).
Determination of prognostic factors for surgery for epilepsy is
important when counselling patients (13). Moreover, identification of prognostic
factors may improve the general understanding of the disease.
Hence, the purpose of the present study is to evaluate prognostic
factors that influence the epilepsy outcome by comparing the
changes of clinical characteristics, electroencephalogram (EEG),
MRI and the quality of life prior to and following the surgery.
Surgical resection has been an important alternative
treatment for patients with intractable epilepsy. Preoperative and
intraoperative ECoG provides a unique opportunity to assess the
epileptogenicity of exposed cortical areas during surgery (14). Although the EEG is important in the
diagnosis and prediction of the outcome of surgery for epilepsy,
the surgical outcome in patients with MRI abnormalities is
satisfactory compared to patients without an identifiable lesion on
presurgical MRI. Localization using MRI and ECoG can carry an
important prognostic value due to patients with identifiable,
well-defined lesions being more likely to have satisfactory
postoperative seizure-free outcomes (15,16).
Moreover, MRI results are abnormal in ~65% of patients (17). The imaging features of FCD can range
from minor gray-white junction blurring and subcortical signal
abnormalities to evident cortical malformation (18). Furthermore, the examination of MRI
and EcoG may be critical in determining the approach to resect
lesions. Following surgery for epilepsy, the EEG revealed that
epileptiform waves were significantly reduced (19). Moreover, the anxiety of patients was
gradually relieved and the quality of life was evidently
improved.
The aims of the present study were: i) To assess the
outcome of adult patients with FCD IIa after surgery for epilepsy;
ii) to investigate the clinical characteristics, EEG and MRI
changes and quality of life in adults with FCD IIa after surgery
for epilepsy; and iii) to evaluate the prognostic factors
influencing the outcome of epilepsy.
Materials and methods
Patient selection and presurgical
evaluation
A total of 110 patients were assessed between 2007
and 2015, and those with a definite pathological diagnosis of FCD
IIa were included in the present study. The data from epilepsy
surgeries at the Second Affiliated Hospital of Dalian Medical
University were analysed retrospectively. Patients with epilepsy
were eligible for inclusion in the study if they were ≥16 years
old. All surgical patients who participated in the present study
represented individuals who had undergone surgery at least one year
before completing the study materials. Moreover, all patients gave
informed consent for analysis of the clinical data. The present
study was approved by the ethics committee of the Second Affiliated
Hospital of Dalian Medical University.
Presurgical evaluation included detailed personal
history, date of seizure onset, type of seizure (simple partial,
complex partial and/or generalized), seizure frequency, seizure
duration, medications and other symptoms (such as the frequency of
AEDs). Other preoperative evaluations included EEG, ECoG,
high-resolution MRI, neuropsychological assessment and quality of
life. Preoperative MRI was independently reviewed by two
experienced neuroradiologists. Imaging features included: i) Focal
cortical thickening, often accompanied by abnormal cortical
sulcation; ii) blurring of the gray-white matter junction; iii)
abnormal signal intensity of the cortex on the T2-weighted of
fluid-attenuated inversion recovery images iv) transmantle signs;
and v) cortical thinning or atrophy. An EEG examination used the
Nicolet application where the video electroencephalogram (VEEG,
which combines the EEG monitoring system with a video recording
device, and can simultaneously record clinical manifestation and
EEG of the epilepsy patients) was monitored with a 32/64/128 leads
(all capable of recodring different waves) EEG for long-range EEG
for an average of 5 days (1–10 days). According to the video data,
we can carefully observe the clinical manifestations of these
patients. The scalp EEG is according to the standard of 10–20
electrodes placed system records, with special circumstances. For
example a sphenoid electrode could be placed in the anterior
temporal area. Moreover, the cortex EEG can be placed for parts or
figures according to the scalp EEG impression. EEG was read by at
least two experienced EEG technicians by independent reading and
reporting, and a consensus was reached. The epileptic discharge of
the intermittent periods of the seizure was then divided into four
categories from VEEG: i) Spike; ii) spine-slow; iii)
multiple-spike; and iv) fast rhythm wave. ECoG was routinely
performed during all surgeries in the epilepsy surgery program used
in the present study. In addition, ECoG provides a unique
opportunity to assess the epileptogenicity of exposed cortical
areas during surgery. This technique is based on the assumption
that epileptiform discharges recorded from the cortex are
indicators of local cortical involvement in an epileptogenic
network. After opening the dura mater, an effective number of 16
electrodes with intercontact distances of 10 mm were placed on the
exposed cortex surface. However, the electrodes were usually
repositioned during the procedure resulting in a larger sampling
area.
Surgery and postoperative outcome
There were patients who underwent complete or
partial lesionectomy. The type of surgery performed was based on
the results of the preoperative evaluation. The surgical area was
then decided based on the clinical, neuroimaging and
electrophysiological results. After a one-year follow-up, a
postoperative MRI and scalp EEG were performed. The outcomes of the
seizures were obtained by telephone interviews and from the
patients' medical charts, and the surgical outcome was classified
according to Engel's four-category classification (20). Class I, free of disabling seizures or
auras only; class II, rare seizures (≤two seizures/year or ≥90%
seizure reduction); class III, worthwhile improvement reduction of
seizure frequency ≥75%; and class IV, no worthwhile improvement,
reduction of seizure frequency <75%. Class I is free of seizures
and classes II–IV represent poorer control of seizures following
surgical treatment.
Health-related quality of life was evaluated using
the quality of life in epilepsy (QOLIE) scale (21) before and at one year after the
surgery. A 10-item questionnaire (QOLIE-10) was used for screening
patients with epilepsy about the impact of the epilepsy on their
lives. It entails a subset of items from the QOLIE-89 questionnaire
(22). The total QOLIE-10 scores
range between 5 and 50, where high scores indicate a poor quality
of life. Moreover, the QOLIE-10 was brief, simple and easy to be
completed, therefore, it was selected as a measure of
health-related quality of life.
Statistical analysis
An independent sample t-test and χ2-test
were conducted to compare the favorable (Engel class I) and the
poor (Engel class II–IV) outcomes with the following criteria: i)
Gender and age at epilepsy onset, age at epilepsy surgery, duration
of epilepsy, seizure frequency, location of resection, side of the
brain lesion, past medical history, medicine history,
anti-epileptic drug (AED) numbers and MRI; ii) cases with or
without EcoG; and iii) complete resection or none. A paired-sample
t-test was used to study the changes of EEG before and one year
after the surgery. In addition, a multivariate analysis of seizure
recurrence was conducted using Cox proportional. The probability of
remaining free of disabling seizures was calculated using
Kaplan-Meier survival analysis, and all of the analyses were
conducted using SPSS version 20.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was used to indicate a statistically significant
difference.
Results
Baseline characteristics
The baseline characteristics for the study samples
are presented in Table I. Among the
110 adult patients (≥16 years old) there were 52 males and 58
females. The age of the onset of epilepsy ranged between 1 and 53
years old (mean age, 14.06 years). Furthermore, the age of the
surgery for epilepsy ranged between 16 and 69 years old (mean age,
30.92 years). The mean duration of epilepsy before surgery was
16.86 years (range, 1–44 years). In addition, the mean duration of
their follow-up was 54.18 months (range, 12–84 months). Past
medical history existed in 37 cases (33.6%), meanwhile 16 cases had
febrile convulsion, 10 cases had hypoxia and 11 cases had
encephalitis history. The lesions were resected completely in 96
cases (87.3%) and were resected incompletely in 14 cases (12.7%).
Moreover, MRI was performed in all of the patients. In total,
68/110 patients (61.8%) demonstrated visible abnormalities on the
MRI, whereas the remaining 42 patients (38.2%) had no abnormalities
observed on an MRI. The lesions were located on the left side in 64
cases and on the right side in 46 cases. Out of all the patients,
78 (70.9%) had temporal lobe epilepsy, 15 (13.6%) had frontal lobe
epilepsy and 3 (2.7%) had parietal lobe epilepsy, and were referred
for surgical consideration following several therapeutic attempts
with different AEDs (range, 0–7) and with poor results on their
seizures.
 | Table I.Baseline characteristics of the 110
enrolled patients. |
Table I.
Baseline characteristics of the 110
enrolled patients.
| Characteristic | Value |
|---|
| Patients included,
n | 110 |
| Malea | 52 (47.3) |
| Age at epilepsy
onset, yearsb | 14.06 (10.62) |
| Age at epilepsy
surgery, yearsb | 30.92 (11.13) |
| Mean duration of
epilepsy, yearsb | 16.86 (9.74) |
| Past medical
historya | 37 (33.6) |
| Complete
resectiona | 96 (87.3) |
| Left side of
lesionsa | 64 (58.2) |
| AED
categoryb | 2.39 (1.26) |
| Location of
resectiona |
|
Temporal lobe | 78 (70.9) |
|
Extratemporal lobe | 23 (20.9) |
|
Multilobe lesionectomy | 9 (8.2) |
| Follow up,
monthsb | 54.18 (22.60) |
Histopathological features of FCD
IIa
Histopathological features of FCD IIa can be
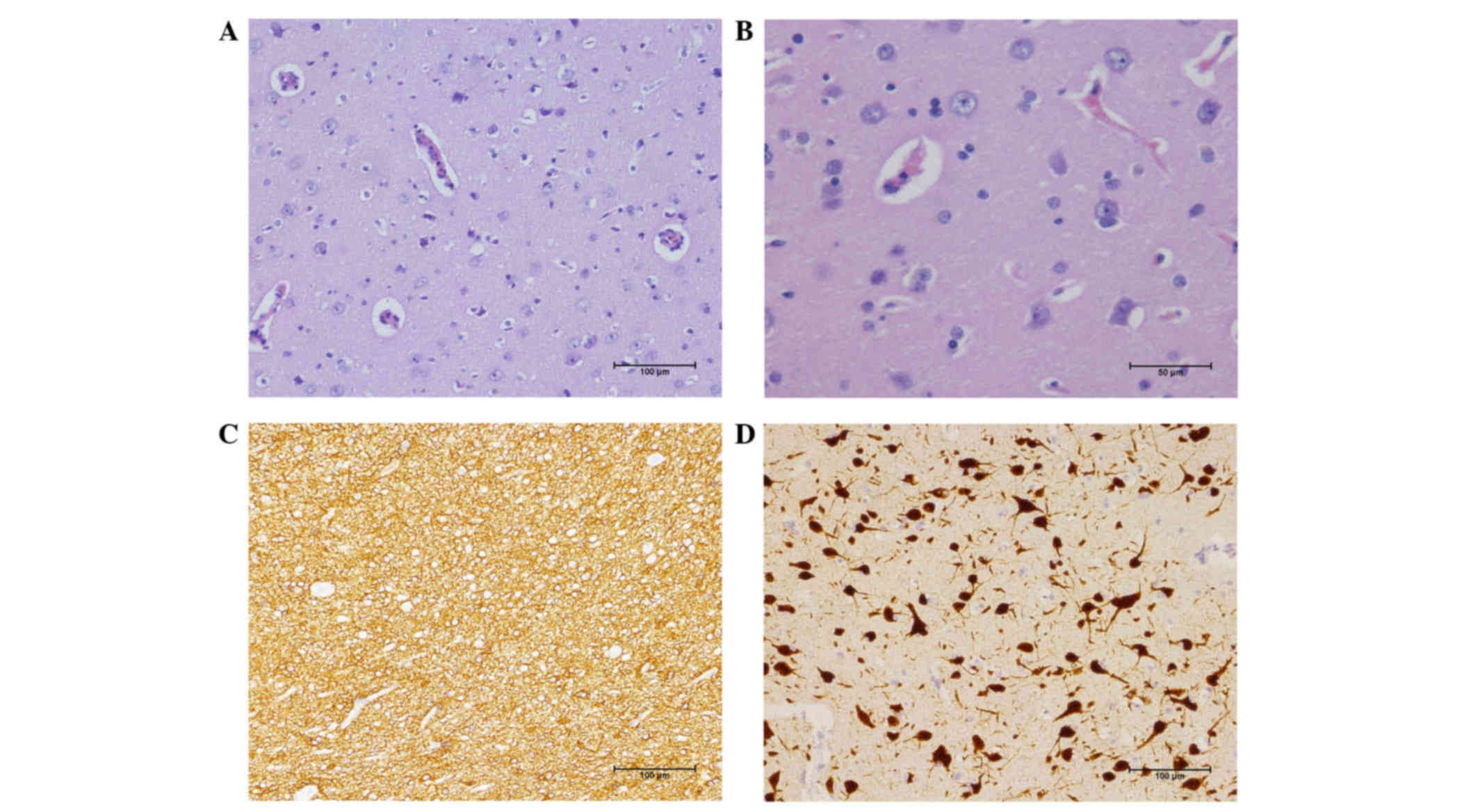
diagnosed by H&E staining and immunohistochemistry (Fig. 1). All the cases mentioned in the
present study were diagnosed by two pathologists. H&E staining,
revealed that the structure of neurons on the dysplastic temporal
lobe cortex were arranged in disorder. Moreover, architectural
abnormalities with giant cells and dysmorphic neurons but no
balloon cells are shown in Fig. 1A.
Gliosis was often observed in the brain tissues from patients with
refractory temporal lobe epilepsy together with neuronophagia and
neuronal degeneration. In dysmorphic neurons, Nissl substance was
aggregated and displaced towards the cell membrane (Fig. 1B). Furthermore, immunohistochemical
examination revealed that NeuN displayed a disordered arrangement
of neurons (Fig. 1D), GFAP(+) and
Olig-2(+) to the glial cells (Fig.
1C), as well as CD34(+) to the vascular endothelium. Finally,
all of them underwent focal cortical dysplasia (FCD IIa).
Summary of a seizure-related profile
and surgical outcome
For the 110 patients who were followed up for one
year after the last epilepsy surgery, 72 patients (65.4%) achieved
Engel class I, 20 (18.2%) Engel class II, 11 (10%) Engel class III,
and 7 (6.4%) Engel class IV. Furthermore, the Engel seizure outcome
was relevant with the extent of resection (P=0.028), presurgical
MRI (P=0.023) and presurgical ECoG (P=0.001). A complete surgical
resection and preoperative imaging evaluation in the patients may
lead to a positive operation efficacy, whereas no other
statistically significant differences were observed between the two
Engel class groups with other clinical characteristics, including
gender, age at epilepsy onset, duration of epilepsy, age at
surgery, the number of AEDs, the patient history, presurgical image
or the side and location of surgery. The seizure-related profile
and outcomes of the 30 patients are summarized in Table II.
 | Table II.Summary of seizure-related profile
and surgical outcome of the 110 patients. |
Table II.
Summary of seizure-related profile
and surgical outcome of the 110 patients.
|
| Engel class |
|
|
|---|
|
|
|
|
|
|---|
|
Characteristics | I | II–IV | Statistics | P-value |
|---|
| Age at epilepsy
surgery (years) | 30.85 | 31.05 | t=−0.092 | 0.927 |
| Age at epilepsy
onset (years) | 13.99 | 14.18 | t=−0.089 | 0.929 |
| Duration of
epilepsy (years) | 16.85 | 16.87 | t=−0.007 | 0.994 |
| AED category | 2.4 | 2.37 | t=0.135 | 0.893 |
| Gender |
|
|
| 0.677 |
|
Male | 33 | 19 |
x2=0.173 |
|
|
Female | 39 | 19 |
|
|
| Extent of
resection |
|
|
| 0.028 |
|
Complete resection | 67 | 29 |
x2=4.858 |
|
|
Incomplete resection | 5 | 9 |
|
|
| Past medical
history |
|
|
| 0.926 |
|
Yes | 24 | 13 |
x2=0.009 |
|
| No | 48 | 25 |
|
|
| Side of lesion |
|
|
| 0.652 |
|
Left | 43 | 21 |
x2=0.203 |
|
|
Right | 29 | 17 |
|
|
| Location of
resection |
|
|
| 0.597 |
|
Temporal lobe | 53 | 25 |
x2=1.032 |
|
|
Extratemporal lobe | 13 | 10 |
|
|
|
Multilobe lesionectomy | 6 | 3 |
|
|
| Presurgical
MRI |
|
|
| 0.023 |
| + | 50 | 18 |
x2=5.136 |
|
| − | 22 | 20 |
|
|
| Presurgical
ECoG |
|
|
| 0.001 |
|
Yes | 45 | 11 |
x2=11.204 |
|
| No | 27 | 27 |
|
|
ECoG is different from scalp EEG. EcoG is an
accurate and stable location method, particularly when the results
of the MRI are negative. Therefore, in order to resect chronic
lesions completely, preoperative ECoG was required. In the current
study when >5 seizures were identified and there were more than
three times from epileptic focus. Then the neurosurgeon was able to
determine the range of the precise epilepsy lesion according to
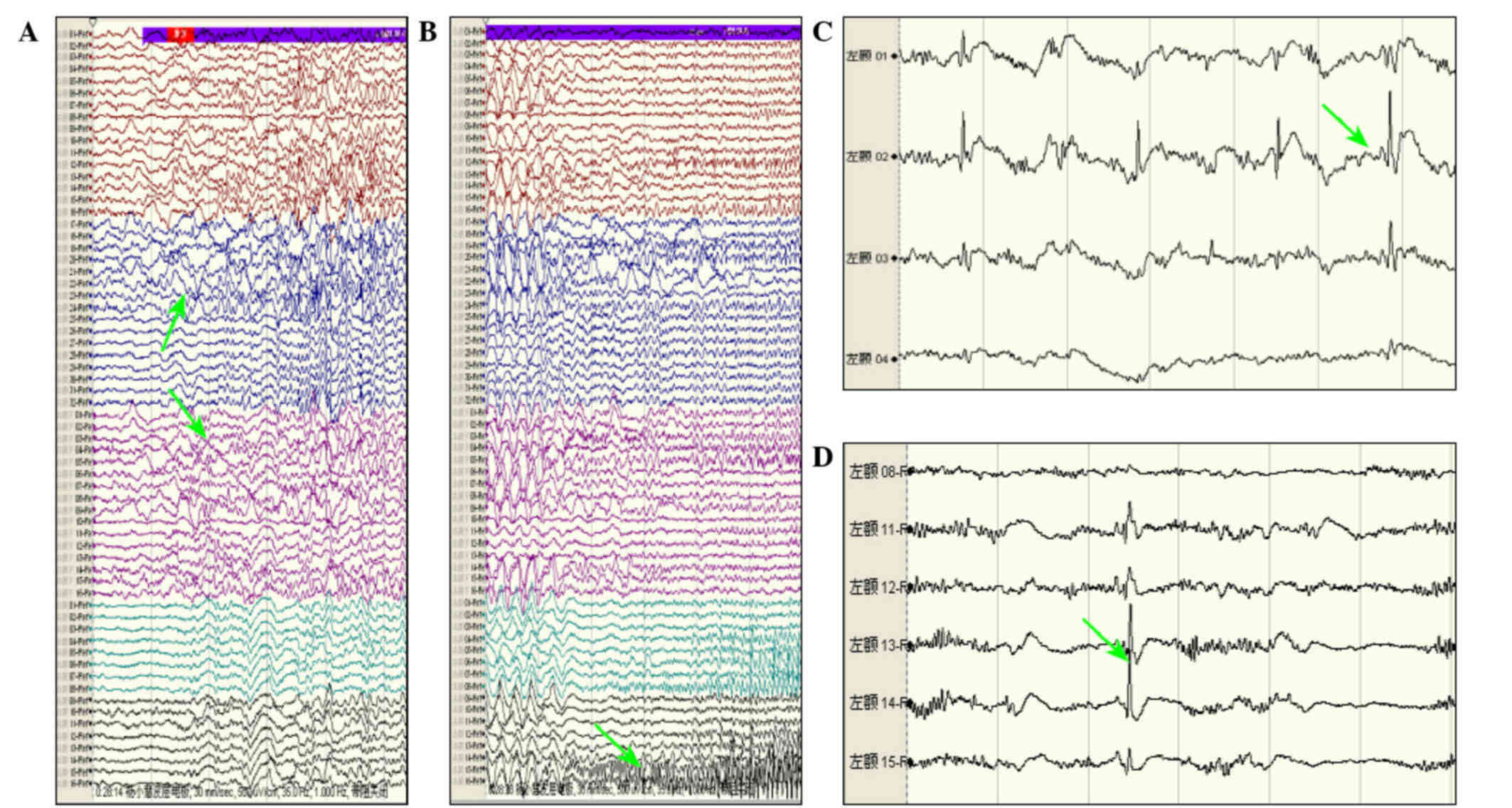
ECoG monitoring (Fig. 2A and B).
Intraoperative ECoG monitoring is also indispensable in patients
who have undergone surgery for epilepsy. The neurosurgeon should be
based on MRI lesions or ECoG accurate location of lesions to guide
cortex electrode plates which could be detected the resection of
lesions within the scope of application of each interval to >10
mm one electrode to confirm the discharge of seizures (Fig. 2C). At the same time they should
determine the scope of a wider area to the surrounding stretch
further out if there is still an epileptic focus (Fig. 2D). The discharge epileptogenic zone
should be integrated, and triggering nerve dysfunction should be
avoided. Furthermore, assessments of ECoG patterns are relevant in
order to determine the extent of the resection that can influence
the surgery outcome (P<0.001). Therefore, ECoG monitoring for
removal of epilepsy lesions prior to discharge of seizure is
significant for the prognosis for surgery.
Multivariate analysis of variables
affecting seizure recurrence
For all patients, the median overall seizure freedom
was 25.92 months at the time of the last follow-up, and 69/110
cases had experienced a seizure after the surgery. The probability
of patients remaining seizure-free was 68% at one year, 40% at
three years post-surgery and 28% at five years. Multivariate
regression analysis of the clinical characteristics and seizure
recurrence are shown in Table III.
In the multivariate analysis, the one year seizure recurrence rate
was 32% and three-year seizure recurrence rate was 60%.
Furthermore, the statistically significant variables affecting
seizure recurrence were preoperative ECoG (HR; 1.929 95% CI:
1.185–3.128, P=0.008) and the extent of resection [(HR; 2.565 95%
CI:1.331–4.943, P=0.005)], preoperative ECoG and complete resection
of lesions could significantly reduce the seizure recurrence rate.
Meanwhile, the location of resection and preoperative seizure
frequency can also influence the seizure recurrence. The seizure
recurrence rate of patients with temporal lobe epilepsy was
evidently reduced compared to those with extratemporal lobe
epilepsy and multilobe lesionectomy. Furthermore, the patients with
preoperative seizure frequency <10 per month could result in a
better prognosis. By contrast, no statistically significant
correlation with seizure recurrence and gender, age at epilepsy
onset, duration of epilepsy, age at surgery, the number of AEDs,
the history of the patient and the presurgical image or the side of
surgery was identified (P>0.05), and they were also not included
in Table III.
 | Table III.Multivariate analysis of risk factors
for seizure recurrence. |
Table III.
Multivariate analysis of risk factors
for seizure recurrence.
| Risk factor | n | HR (95% CI) | P-value |
|---|
| Preoperative
ECoG |
|
|
|
|
Yes | 56 | Reference |
|
| No | 54 | 1.925
(1.185–3.128) | 0.008 |
| Extent of
resection |
|
|
|
|
Complete resection | 96 | Reference |
|
|
Incomplete resection | 14 | 2.565
(1.331–4.943) | 0.005 |
| Location of
resection |
|
|
|
|
Temporal lobe | 78 | Reference |
|
|
Extratemporal lobe | 23 | 0.391
(0.179–0.857) | 0.019 |
|
Multilobe lesionectomy | 9 | 0.608
(0.254–1.458) | 0.265 |
| Home
seizure-frequency |
|
|
|
| Low
(<10/month) | 68 | Reference |
|
| Medium
(10–30/month) | 25 | 0.488
(0.263–0.905) | 0.023 |
| High
(>30/month) | 17 | 0.756
(0.358–1.592) | 0.461 |
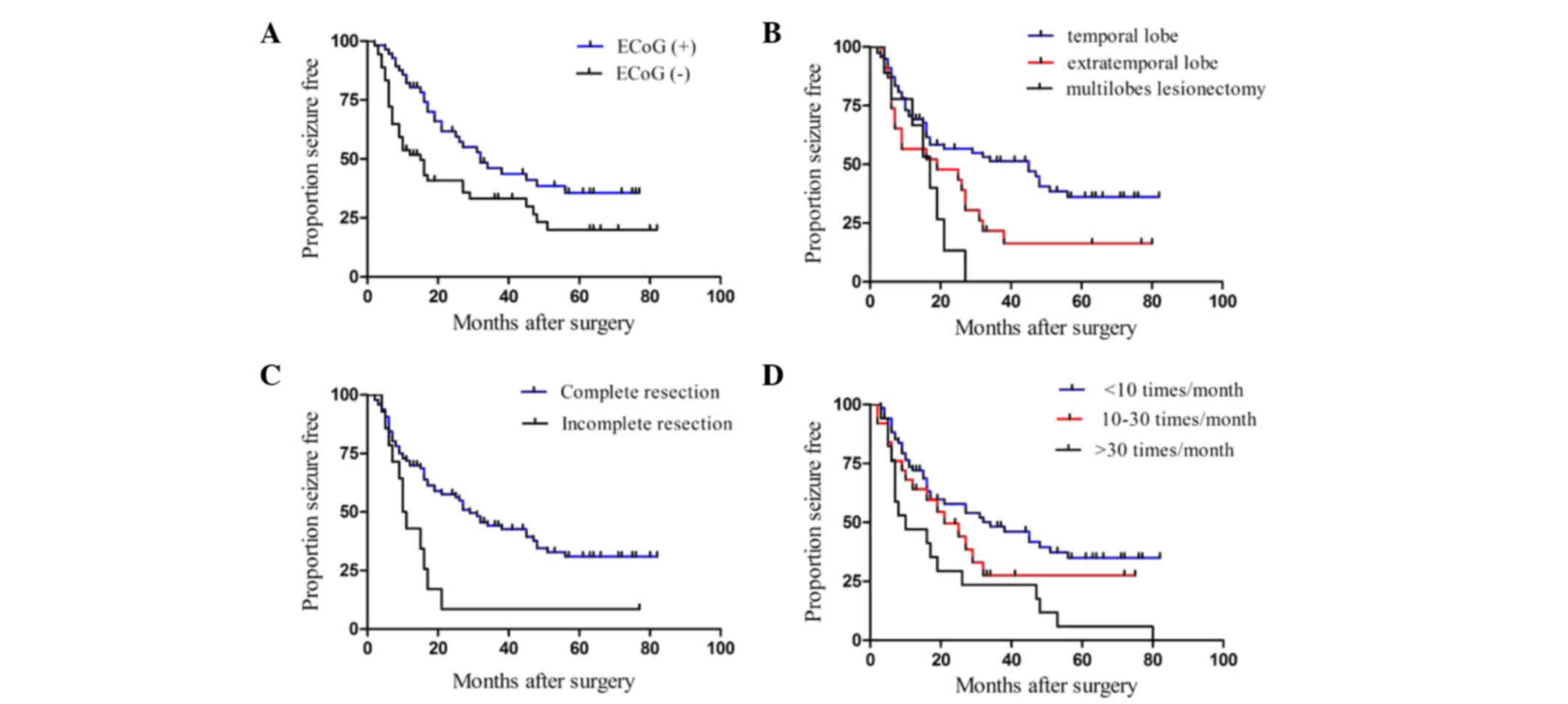
Among all patients, the one-year seizure-free rate
was 82 and 53%, and the three-year rates were 47 and 33% in
patients with or without preoperative ECoG (Fig. 3A). Furthermore, the log-rank test and
Kaplan-Meier method indicated that preoperative ECoG could
significantly enhance the seizure-free rate (P=0.009). The median
seizure-free period was 45 months in the temporal, 19 months in the
extratemporal and 17 months in the multilobe epilepsy groups, which
indicated that the prognosis of the temporal epilepsy group was
evidently better than the other two groups (P=0.012) (Fig. 3B). Meanwhile, the log-rank test and
Kaplan-Meier method indicated a significantly poor survival in
patients with incomplete resection of the lesion (P=0.005) and
higher preoperative seizure frequency (P=0.033) (Fig. 3C and D).
Quality of life in epileptic
patients
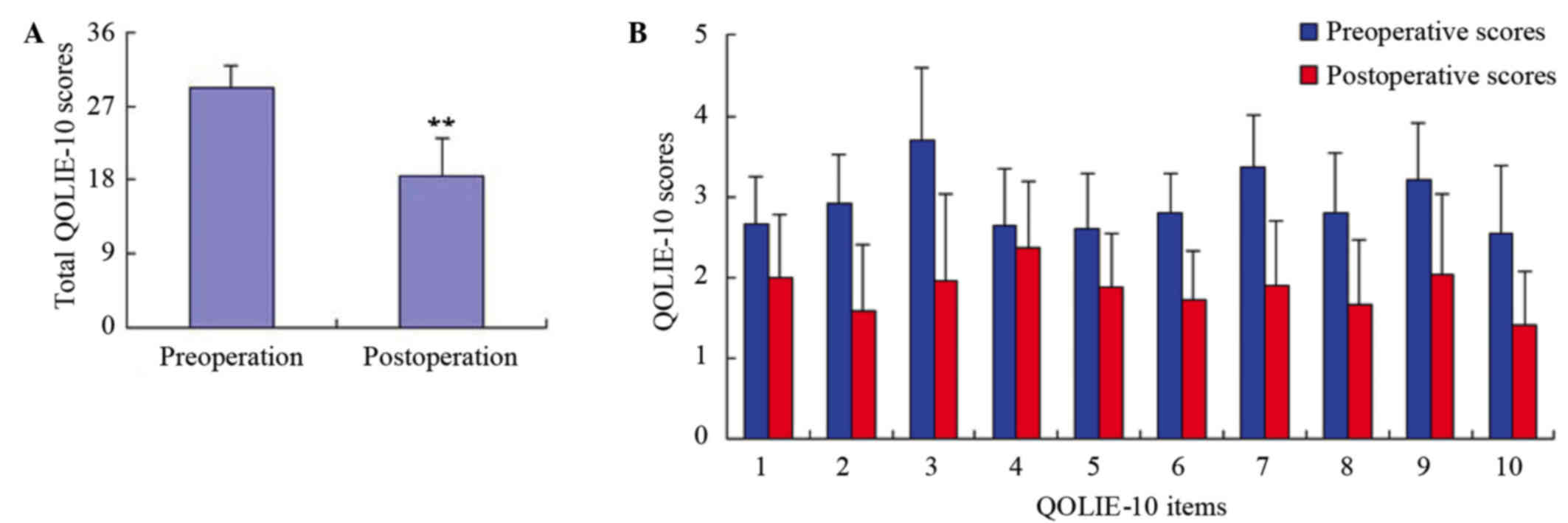
Items 1–10 of the QOLIE-10 included epilepsy-related
effects (memory, physical effects, mental effects of medication),
mental health (energy, depression, overall quality of life) and
role functioning (seizure worry, work, driving, social
limitations). The preoperative and postoperative scores of the ten
items are shown in Fig. 4.
Furthermore, the total mean scores of the 110 patients were
29.3±2.74 and 18.5±4.55 before and at one year after the surgery
for epilepsy, respectively, and the difference was shown to be
statistically significant (P<0.01).
Discussion
FCD is caused by neuronal proliferation and
differentiation, cortical functional architecture and migration
anomalies that were firstly described by Taylor et al in
1971 (1). FCD has increasingly been
diagnosed as a cause of symptomatic epilepsy, particularly for
refractory epilepsy in paediatry and as the second or third common
cause of adult patients who have undergone surgery for epilepsy
(23). It has been reported that the
incidence of epilepsy was ~46.5% (15% in adults) and (25% in
children) (24). These percentages
are significantly higher than the 1.7% observed in the general
population (24). Furthermore, the
incidence of FCD was higher but its etiology, clinical features,
imaging and pathological features were not fully understood, which
often lead to delayed diagnosis (25). Due to the severity and refractoriness
to drug therapy, surgical treatment of epilepsy associated with FCD
could be a valid therapeutic option (26). Furthermore surgical specimens allow
for a precise neuropathological diagnosis and identification of
histopathological variants (27).
Though the operation faces great change and innovation, the seizure
remission rate of the epilepsy surgery increases continuously
(28), reaching 50–70% according to
numerous clinical case reports (29,30). The
abnormal MRI signals and EEG discharges, as well as the range of
FCD lesions and its concerned functional area are all possible
influence factors that make it difficult to choose operation
strategies. Compared with other intractable epilepsy surgeries
(31), the FCD surgery complication
and fatality rates are very low; reportedly ~2% (31). Thus far, surgery treatment has
achieved satisfactory results in patients with FCD-derived
intractable epilepsy. Through retrospective analysis of epilepsy
patients in our centre, the pathology of the majority of the
patients was identified as FCD IIa and the prognosis of them were
favorable. Fauser et al (32)
conducted a retrospective study of 67 patients with FCD, and
identified the proportion of patients with Engel I and FCD IIa 12
months after the surgery to be 57.0%. However, the study by
Widdess-Walsh et al (33)
revealed that the proportion of patients with Engel I was 67%.
Hence, a total of 110 patients with a definite pathological
diagnosis of FCD IIa were retrospectively analysed, and the
proportion of patients with Engel I was found to be 65.4% which was
close to the results of other studies (34). At present, there are numerous studies
on refractory epilepsy in children, and the prognosis is
satisfactory (35). However, there
is a shortage of studies on refractory epilepsy in adults.
Therefore, the present study focused on the clinical
characteristics and prognosis of adult patients with FCD IIa and
provided the theoretical basis for the surgical treatment of
epilepsy in the future.
In the present study, clinical characteristics of
epilepsy were analysed in 110 adult patients with histologically
proven FCD IIa. The correlation between clinical indexes and Engel
classes over a one-year follow-up were emphatically analysed in
order to explore the clinical factors that influenced the
short-term prognosis of epileptic patients with FCD IIa. Out of the
110 patients, 72 (65.4%) achieved Engel class I, 20 (18.2%) Engel
class II, 11 (10%) Engel class III and 7 (6.4%) Engel class IV.
Engel Class I was defined as the better prognosis group, Engel
class II–IV as the poor prognosis group. Through statistical tests,
the present study revealed that there were no other statistically
significant differences between the Engel class and other clinical
indexes, including gender, age at epilepsy onset, duration of
epilepsy, age at surgery, seizure frequency, number of AEDs,
patient history, presurgical image or the side and location of
surgery. Furthermore, a complete resection of the lesion was the
most important clinical index that influenced the postoperative
Engel class (P=0.028). Complete resection of FCD, including the
surrounding epileptogenic areas, is the most important predictor of
seizure outcome (36,37). Furthermore, a complete surgical
resection in the patients may lead to a positive operation
efficacy, but in patients with an incomplete excision, seizure
recurrence has been related to the extension of dysplastic cortex
beyond the visible abnormality (38). Lerner et al (39) reviewed recent literature regarding
the surgical treatment of FCD, and revealed that 65–70% of the
patients can achieve a complete resection. Furthermore, the
complete remission rate of the seizure in patients with complete
resection of the lesions was ~77%, and 20% in patients with an
incomplete resection. Rowland et al (40) conducted a meta-analysis, and
demonstrated that the most significant prognostic factor for
surgical treatment of FCD was whether the lesions were resected
completely. A complete resection of FCD can influence the
short-term outcome of epilepsy, however, a multimodality approach
and teamwork are required in order to achieve this goal.
Furthermore, a clear localization of the epileptic focus is
required, and MRI and EcoG examination were important for this.
Functional MRI is a non-invasive method used for
evaluation of locating the epileptic lesions preoperatively. The
characteristics of MRI results in patients with FCD IIa typically
include a gray-white junction blurring, cortex thickening, abnormal
outer and abnormal signal of the cortex and white matter in
T2/Flair. MRI has important diagnostic value for FCD; however,
there remain cases of FCDs that are difficult to identify using
MRI. Literature reported that the diagnostic rate of FCD by MRI was
60–90% (41). Widdess-Walsh et
al (33) analysed 40 patients
with FCD-II and revealed that the positive rate of MR was 75% in
patients with FCD IIA whereas Krsek et al (29) demonstrated that the positive rate of
MR was 78% in patients with FCD IIA. Moreover, previous studies
have indicated that patients with a positive MRI had a higher
remission rate of surgical treatment (42). The present study revealed that the
positive rate of MR was 61.8% in 110 adult patients with FCD IIa,
and that there was a significant correlation between the positive
rate of MR and Engel class one year following operation (P=0.023).
To date, there are numerous reports on discharge characteristics of
scalp EEG in patients with FCD (43). Preoperative and intraoperative ECoG
was routinely performed during all surgeries in our epilepsy
surgery program. ECoG provides a unique opportunity to assess the
epileptogenicity of exposed cortical areas. Currently, 80–84% of
epilepsy centers worldwide use EcoG to guide intraoperative
surgical removal (44). ECoG is
different from scalp EEG, it is an accurate and stable location
method, particularly when the results of the MRI are negative. In
order to completely resect, ECoG monitoring was necessary. The
applications of EcoG in epilepsy operation are mainly concentrated
in the following aspects: i) Long-range cortical electrode
monitoring in preoperative assessment is used in those cases where
there has been a difficulty for negative MRI and scalp EEG
epileptogenic zone localization; ii) intraoperative ECoG could
determine the position and range of the epileptogenic focus, and
guide the surgical strategy; iii) after removing a lesion,
reiteration EcoG results could guide further treatment and
evaluation of prognosis; and iv) intraoperative cortical electrical
stimulation could locate functional areas as far as possible to
avoid nerve dysfunction. However, intraoperative EcoG demonstrates
period electrical activity of the intermittent periods.
Nevertheless, the origin of the epileptic area remains
controversial. The present study revealed that 50.9% of patients
performed a preoperative EcoG in 110 patients with FCDIIA.
Moreover, EcoG positioning was significantly related with
postoperative Engel classification at one year after surgery
(P=0.001).
The present study aimed to investigate the
prognostic factors and the risk of seizure recurrence following
surgery for epilepsy. Furthermore, the risk associated with
preoperative and operative factors was assessed. In the analysis of
the present study the occurrence of any disabling postoperative
seizure was assessed as a recurrence. In common with a number of
other studies (45,46), multivariate regression analyses with
forward elimination revealed that in epileptic patients with FCD
IIa the extent of resection, seizure frequency, preoperative ECoG
and location of resection were the most important factors that
significantly increased the risk of not becoming seizure-free
postoperatively. By contrast, the gender, age at epilepsy onset,
duration of epilepsy, age at surgery, number of AEDs, patient
history, presurgical image and the side of surgery were not
significant prognostic factors for the postoperative epilepsy
outcome. Epilepsy is a chronic disorder, characterized by
unpredictable, uncontrolled seizures that interfere with patient
lifestyles, activities and interests. The condition introduces a
number of psychosocial challenges and adaptive demands, and
threatens the quality of life. Recently, the medical community pays
increasingly more attention to quality of life improvement and the
psychological state in patients with epilepsy (47). Moreover, the evaluation of the
quality of life has gradually become an important index of clinical
therapeutic effects (48). The
QOLIE-10 scale is the current internationally recognized method for
evaluating the quality of life in patients with epilepsy. The
present study revealed that problems with the quality of life in
patients with FCD, and in the majority of cases experience panic
(49). Therefore more attention
should be paid on the mental health problems in patients with
epilepsy in order to prevent patients from giving up their lives.
In addition, the preoperative and postoperative life quality in
patients with FCD changed significantly. The postoperative quality
of life and epileptic remission rate were evidently improved
(P=0.000), which indicated that epilepsy surgery was important in
the treatment of refractory epilepsy. However, if we aimed to
achieve more ideal surgical outcomes, a series of neuroimaging
examinations before the operation would be required. In this way,
improved surgical strategies and improved remission rates of
postoperative epilepsy could be developed. Using the QOLIE-10 scale
to assess the quality of life in patients with FCD can provide an
objective basis for improving the cure rates of epilepsy
surgery.
For refractory epilepsy with FCD IIa, earlier
surgical intervention should be indicated. Complete resection is
the single most important index influencing the Engel class at one
year after surgery, and requires a multimodality approach and
teamwork in order to achieve this goal. MRI and EcoG can help
locate epileptogenic foci and improve the patients' postoperative
epileptic remission rate. A multivariate analysis was conducted
using Cox proportional hazard regression to assess the long-term
prognosis of epilepsy caused by FCD IIa, and found that
preoperative ECoG, the extent of resection, location of resection
and preoperative seizure frequency were important prognostic
factors for seizures following surgery. Furthermore, surgical
intervention and complete resection of the lesion helps improve
seizure control, physical and mental health and quality of life.
Despite the difficulties associated with a complete resection, the
majority of the patients achieve a notable reduction in seizures.
Therefore, this data may be helpful when counseling patients before
undergoing surgery.
Acknowledgements
The authors thank Mr. Hang Yin for his effort in
collecting and organizing literature and data. We also thank the
physicians who referred the patients to epilepsy surgery.
References
|
1
|
Taylor DC, Falconer MA, Bruton CJ and
Corsellis JA: Focal dysplasia of the cerebral cortex in epilepsy. J
Neurol Neurosurg Psychiatry. 34:369–387. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guerrini R, Dobyns WB and Barkovich AJ:
Abnormal development of the human cerebral cortex: Genetics,
functional consequences and treatment options. Trends Neurosci.
31:154–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fauser S, Essang C, Altenmüller DM, Staack
AM, Steinhoff BJ, Strobl K, Bast T, Schubert-Bast S, Stephani U,
Wiegand G, et al: Long-term seizure outcome in 211 patients with
focal cortical dysplasia. Epilepsia. 56:66–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prayson RA: Classification and
pathological characteristics of the cortical dysplasias. Childs
Nerv Syst. 30:1805–1812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blümcke I, Thom M, Aronica E, Armstrong
DD, Vinters HV, Palmini A, Jacques TS, Avanzini G, Barkovich AJ,
Battaglia G, et al: The clinicopathological spectrum of focal
cortical dysplasias: A consensus classification proposed by an ad
hoc task force of the ILAE diagnostic methods commission.
Epilepsia. 52:158–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raymond AA, Fish DR, Sisodiya SM,
Alsanjari N, Stevens JM and Shorvon SD: Abnormalities of gyration,
heterotopias, tuberous sclerosis, focal cortical dysplasia,
microdysgenesis, dysembryoplastic neuroepithelial tumour and
dysgenesis of the archicortex in epilepsy. Clinical, EEG and
neuroimaging features in 100 adult patients. Brain. 118:629–660.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keene DL, Jimenez CC and Ventureyra E:
Cortical microdysplasia and surgical outcome in refractory epilepsy
of childhood. Pediatr Neurosurg. 29:69–72. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen-Gadol AA, Ozduman K, Bronen RA, Kim
JH and Spencer DD: Long-term outcome after epilepsy surgery for
focal cortical dysplasia. J Neurosurg. 101:55–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kral T, Clusmann H, Blümcke I, Fimmers R,
Ostertun B, Kurthen M and Schramm J: Outcome of epilepsy surgery in
focal cortical dysplasia. J Neurol Neurosurg Psychiatry.
74:183–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asadi-Pooya AA, Nei M, Sharan A and
Sperling MR: Patient Historical Risk Factors Associated with
Seizure Outcome After Surgery for Drug-Resistant Nonlesional
Temporal Lobe Epilepsy. World Neurosurg. 91:205–209. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perry MS, Dunoyer C, Dean P, Bhatia S,
Bavariya A, Ragheb J, Miller I, Resnick T, Jayakar P and Duchowny
M: Predictors of seizure freedom after incomplete resection in
children. Neurology. 75:1448–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rowland NC, Englot DJ, Cage TA, Sughrue
ME, Barbaro NM and Chang EF: A meta-analysis of predictors of
seizure freedom in the surgical management of focal cortical
dysplasia. J Neurosurg. 116:1035–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yun CH, Lee SK, Lee SY, Kim KK, Jeong SW
and Chung CK: Prognostic factors in neocortical epilepsy surgery:
Multivariate analysis. Epilepsia. 47:574–579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wennberg R, Quesney LF, Lozano A, Olivier
A and Rasmussen T: Role of electrocorticography at surgery for
lesion-related frontal lobe epilepsy. Can J Neurol Sci. 26:33–39.
1999.PubMed/NCBI
|
|
15
|
Chang EF, Wang DD, Barkovich AJ, Tihan T,
Auguste KI, Sullivan JE, Garcia PA and Barbaro NM: Predictors of
seizure freedom after surgery for malformations of cortical
development. Ann Neurol. 70:151–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim YH, Kang HC, Kim DS, Kim SH, Shim KW,
Kim HD and Lee JS: Neuroimaging in identifying focal cortical
dysplasia and prognostic factors in pediatric and adolescent
epilepsy surgery. Epilepsia. 52:722–727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hauptman JS and Mathern GW: Surgical
treatment of epilepsy associated with cortical dysplasia: 2012
update. Epilepsia. 53:(Suppl 4). S98–S104. 2012. View Article : Google Scholar
|
|
18
|
Colon AJ, van Osch MJ, Buijs M, Grond JV,
Boon P, van Buchem MA and Hofman PA: Detection superiority of 7 T
MRI protocol in patients with epilepsy and suspected focal cortical
dysplasia. Acta Neurol Belg. 116:259–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alshafai L, Ochi A, Go C, McCoy B, Hawkins
C, Otsubo H, Snead OC, Rutka J and Widjaja E: Clinical, EEG, MRI,
MEG, and surgical outcomes of pediatric epilepsy with astrocytic
inclusions versus focal cortical dysplasia. Epilepsia.
55:1568–1575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Engel J Jr: Outcome with respect to
epileptic seizuresSurgical treatment of the epilepsies. 2nd. Raven
Press; pp. 609–621. 1993
|
|
21
|
Cramer JA, Perrine K, Devinsky O and
Meador K: A brief questionnaire screen for quality of life in
epilepsy: The QOLIE-10. Epilepsia. 37:577–582. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Devinsky O, Vickrey BG, Cramer J, Perrine
K, Hermann B, Meador K and Hays RD: Development of the quality of
life in epilepsy inventory. Epilepsia. 36:1089–1104. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Becker AJ, Blümcke I, Urbach H, Hans V and
Majores M: Molecular neuropathology of epilepsy-associated
glioneuronal malformations. J Neuropathol Exp Neurol. 65:99–108.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bast T, Ramantani G, Seitz A and Rating D:
Focal cortical dysplasia: Prevalence, clinical presentation and
epilepsy in children and adults. Acta Neurol Scand. 113:72–81.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guerrini R, Duchowny M, Jayakar P, Krsek
P, Kahane P, Tassi L, Melani F, Polster T, Andre VM, Cepeda C, et
al: Diagnostic methods and treatment options for focal cortical
dysplasia. Epilepsia. 56:1669–1686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon HE, Eom S, Kang HC, Lee JS, Kim SH,
Kim DS and Kim HD: Surgical treatment of pediatric focal cortical
dysplasia: Clinical spectrum and surgical outcome. Neurology.
87:945–951. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tassi L, Garbelli R, Colombo N, Bramerio
M, Lo Russo G, Deleo F, Milesi G and Spreafico R: Type I focal
cortical dysplasia: Surgical outcome is related to histopathology.
Epileptic Disord. 12:181–191. 2010.PubMed/NCBI
|
|
28
|
Hemb M, Velasco TR, Parnes MS, Wu JY,
Lerner JT, Matsumoto JH, Yudovin S, Shields WD, Sankar R, Salamon
N, Vinters HV and Mathern GW: Improved outcomes in pediatric
epilepsy surgery: The UCLA experience, 1986–2008. Neurology.
74:1768–1775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krsek P, Pieper T, Karlmeier A,
Hildebrandt M, Kolodziejczyk D, Winkler P, Pauli E, Blümcke I and
Holthausen H: Different presurgical characteristics and seizure
outcomes in children with focal cortcal dysplasia type I or II.
Epilepsia. 50:125–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim DW, Lee SK, Chu K, Park KI, Lee SY,
Lee CH, Chung CK, Choe G and Kim JY: Predictors of surgical outcome
and pathologic considerations in focal cortical dysplasia.
Neurology. 72:211–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirfanoglu T, Serdaroglu A, Kurt G, Erdem
A, Capraz I, Bilir E, Vural O, Ucar M, Oner AY, Onal B, et al:
Outcomes of resective surgery in children and adolescents with
focal lesional epilepsy: The experience of a tertiary epilepsy
center. Epilepsy Behav. 63:67–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fauser S, Schulze-Bonhage A, Honegger J,
Carmona H, Huppertz HJ, Pantazis G, Rona S, Bast T, Strobl K,
Steinhoff BJ, et al: Focal cortical dysplasias: Surgical outcome in
67 patients in relation to histological subtypes and dual
pathology. Brain. 127:2406–2418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Widdess-Walsh P, Kellinghaus C, Jeha L,
Kotagal P, Prayson R, Bingaman W and Najm IM: Electro-clinical and
imaging characteristic of focal cortical dysplasia: Correlation
with pathological subtypes. Epilepsy Res. 67:25–33. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Widjaja E, Otsubo H, Raybaud C, Ochi A,
Chan D, Rutka JT, Snead OC III, Halliday W, Sakuta R, Galicia E, et
al: Characteristics of MEG and MRI between Taylor's focal cortical
dysplasia (type II) and other cortical dysplasia: Surgical outcome
after complete resection of MEG spike source and MR lesion in
pediatric cortical dysplasia. Epilepsy Res. 82:147–155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sacino MF, Ho CY, Whitehead MT, Zelleke T,
Magge SN, Myseros J, Keating RF, Gaillard WD and Oluigbo CO:
Resective surgery for focal cortical dysplasia in children: A
comparative analysis of the utility of intraoperative magnetic
resonance imaging (iMRI). Childs Nerv Syst. 32:1101–1107. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hader WJ, Mackay M, Otsubo H, Chitoku S,
Weiss S, Becker L, Snead OC III and Rutka JT: Cortical dysplastic
lesions in children with intractable epilepsy: Role of complete
resection. J Neurosurg. 100:(2 Suppl Pediatrics). S110–S117.
2004.
|
|
37
|
Phi JH, Cho BK, Wang KC, Lee JY, Hwang YS,
Kim KJ, Chae JH, Kim IO, Park SH and Kim SK: Longitudinal analyses
of the surgical outcomes of pediatric epilepsy patients with focal
cortical dysplasia. J Neurosurg Pediatr. 6:49–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Edwards JC, Wyllie E, Ruggeri PM, Bingaman
W, Lüders H, Kotagal P, Dinner DS, Morris HH, Prayson RA and Comair
YG: Seizure outcome after surgery for epilepsy due to malformation
of cortical development. Neurology. 55:1110–1114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lerner JT, Salamon N, Hauptman JS, Velasco
TR, Hemb M, Wu JY, Sankar R, Shields Donald W, Engel J Jr, Fried I,
et al: Assessment and surgical outcomes for mild type I and severe
type II cortical dysplasia: A critical review and the UCLA
experience. Epilepsia. 50:1310–1335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rowland NC, Englot DJ, Cage TA, Sughrue
ME, Barbaro NM and Chang EF: A meta-analysis of predictors of
seizure freedom in the surgical management of focal cortical
dysplasia. J Neurosurg. 116:1035–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Colombo N, Tassi L, Galii C, Citterio A,
Lo Russo G, Scialfa G and Spreafico R: Focal cortical dysplasias:
MR imaging, histopathologic, and clinical correlations in
surgically treated patients with epilepsy. AJNR Am J Neuroradiol.
24:724–733. 2003.PubMed/NCBI
|
|
42
|
McIntosh AM, Averill CA, Kalnins RM,
Mitchell LA, Fabinyi GC, Jackson GD and Berkovic SF: Long-term
seizure outcome and risk factors for recurrence after extratemporal
epilepsy surgery. Epilepsia. 53:970–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Noachtar S, Bilgin O, Rémi J, Chang N,
Midi I, Vollmar C and Feddersen B: Interictal regional polyspikes
in noninvasive EEG suggest cortical dysplasia as etiology of focal
epilepsies. Epilepsia. 49:1011–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kuruvilla A and Flink R: Intraoperative
electrocorticography in epilepsy surgery: Useful or not? Seizure.
12:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Elsharkawy AE, Pannek H, Schulz R, Hoppe
M, Pahs G, Gyimesi C, Nayel M, Issa A and Ebner A: Outcome of
extratemporal epilepsy surgery experience of a single center.
Neurosurgery. 63:516–525; discussion 525–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim DW, Kim HK, Lee SK, Chu K and Chung
CK: Extent of neocortical resection and surgical outcome of
epilepsy: Intracranial EEG analysis. Epilepsia. 51:1010–1017. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Poochikian-Sarkissian S, Sidani S,
Wennberg R and Devins GM: Seizure freedom reduces illness
intrusiveness and improves quality of life in epilepsy. Can J
Neurol Sci. 35:280–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jissendi-Tchofo P, Pandit F, Vallée L,
Vinchon M, Pruvo JP, Baleriaux D and Ares Soto G: Brain regional
glucose uptake changes in isolated cerebellar cortical dysplasia:
Qualitative assessment using coregistrated FDG-PET/MRI. Cerebellum.
11:280–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ashjazadeh N, Yadollahikhales G,
Ayoobzadehshirazi A, Sadraii N and Hadi N: Comparison of the
health-related quality of life between epileptic patients with
partial and generalized seizure. Iran J Neurol. 13:94–100.
2014.PubMed/NCBI
|