Introduction
Clinical therapeutic revascularization techniques
have improved considerably in recent years; however, treatment of
extensive myocardial infarction and refractory myocardial ischemia
remains relatively difficult due to poor long-term outcomes
(1). It has been demonstrated in a
number of experimental and pre-clinical studies that gene-based
therapeutic angiogenesis is feasible (2,3).
However, the low efficiency of gene delivery to the target tissue
limits the potential of this type of therapy (4,5).
Ultrasound-targeted microbubble destruction (UTMD)
has garnered increased attention as a potential method for gene
delivery in recent years. In an ultrasound field, microbubbles
undergo either stable cavitation or inertial cavitation, which
results in oscillatory shear, high-pressure microstream and
physical stretching of endothelial cells. These bioeffects can
cause sonoporation on the surface membranes of cells, increase
blood vessel wall permeability and facilitate gene uptake (6–8). Studies
investigating the UTMD-mediated delivery of angiogenesis genes to
treat ischemic heart disease have attracted considerable attention
(9–11). However, this novel gene transfection
technique requires further development prior to its application as
a clinical treatment (12,13). Gene concentration at the site of
sonoporation may cause unsatisfactory therapeutic effects. Although
focused ultrasound destruction of microbubbles can, to a certain
extent, provide targeted gene release, microbubbles that lack a
tissue-specific ligand linkage will distribute throughout the body
via the blood circulation. Consequently, the amount of the
therapeutic gene reaching the target tissue is limited, resulting
in poor gene uptake (12,13). One possible method of increasing the
therapeutic potential of UTMD is to specifically target
microbubbles to the tissue of interest by conjugating receptor
ligands or antibodies to their surface (14).
Intercellular adhesion molecule-1 (ICAM-1), which is
expressed by inflammatory endothelial cells, participates in
leukocyte rolling and adhesion (15). Overexpression of ICAM-1 is considered
to be a marker of endothelial cell dysfunction or injury and has
been observed in a number of related diseases, including thrombosis
and myocardial infarction (15,16). The
present study attempted to conjugate an ICAM-1 antibody to cationic
microbubbles to facilitate attachment to the injured vasculature,
as well as boosting the regional microbubble population around the
injured vascular endothelial cells in the infarcted myocardium. It
was hypothesized that the use of an ICAM-1 antibody would allow
greater numbers of microbubbles to accumulate in the injured
vascular endothelial tissue and promote gene transfer through the
endothelial gap, resulting in increased gene uptake and more
efficient intravenous gene transfection.
Angiopoietin 1 (Ang-1) serves an important role in
angiogenesis and its effects following myocardial infarction last
longer than those of vascular endothelial growth factor (17,18). In
addition, Ang-1 may reduce the vascular permeability caused by
endothelial growth factors, promote vessel maturation and
ultimately improve myocardial perfusion (19,20).
Therefore, in the present study, Ang-1 was selected as the
therapeutic gene to be tested in a model of myocardial infarction
and targeted cationic microbubbles (TCMBs) loaded with an ICAM-1
monoclonal antibody were used as the carriers of the Ang-1 gene. It
was hypothesized that TCMBs would enhance gene delivery to the
ischemic area, thus increasing therapeutic angiogenesis and
improving infarcted heart function.
Materials and methods
Preparation of the TCMBs
The cationic microbubbles were composed of a
perfluoropropane gas core encapsulated by
1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany),
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide
(polyethylene glycol)-2000] (DSPE-PEG2000; Avanti Polar Lipids,
Alabaster, AL, USA), 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPA;
Sanofi Genzyme, Barr, Switzerland) and
3β-[N-(N',N'-dimethylaminoethane)-carbamoyl] cholesterol
hydrochloride (DC-Chol; Avanti Polar Lipids) [molar ratio
(39:1:40:20%)]. DC-Chol provided a positively charged surface for
the cationic microbubbles. For the biotinylated microbubbles,
DSPE-PEG2000 biotin (Avanti Polar Lipids, Inc.) was added, enabling
conjugation of the targeting ligands using biotin-streptavidin
binding chemistry. Following the generation of the biotinylated
microbubbles, streptavidin (30 µg/108 microbubbles;
Sigma-Aldrich; Merck Millipore) was added for 30 min at 4°C, as
previously described (21). The
biotinylated monoclonal ICAM-1 antibody (bs-4617R; 1:100; Beijing
Biosynthesis Biotechnology Co., Ltd., Beijing, China) was mixed
with the cationic microbubbles in a 1:100 volume ratio for 30 min
at 4°C. Static flotation was used to remove the unbound antibody
from the microbubbles by washing three times with
phosphate-buffered saline (PBS).
To confirm the conjugation of the ICAM-1 antibody to
the cationic microbubbles, 100 µl fluorescein isothiocyanate
(FITC)-labeled mouse anti-human immunoglobulin (Ig) G (ab49867;
1:100; Abcam, Cambridge, MA, USA) was added to 200 µl microbubbles
and mixed for 30 min at 4°C. The mixture was separated by low-speed
centrifugation at 4°C (400 × g for 5 min) and the upper
layer was washed three times with PBS to remove the free
FITC-conjugated IgG. The successful construction of TCMBs was
confirmed by the presence of bright green fluorescence at the
fringe of the microbubble surface, as shown using fluorescence
microscopy (Olympus Corporation, Tokyo, Japan).
To analyze the characteristics of the TCMBs,
non-targeted cationic microbubbles (CMBs) were used as a control.
The morphology of the microbubbles was examined using bright-field
and fluorescence microscopy (Olympus Corporation), the mean
diameter of the microbubbles was determined by electrozone sensing
(Multisizer™ version 3; Beckman Coulter, Inc., Brea, CA, USA)
following the manufacturer's protocol and the zeta potential of the
microbubbles was measured using a Zetasizer Nano S instrument
(Malvern Instruments, Worcestershire, UK) according to the
manufacturer's operating manual.
Conjugation of the DNA to the
microbubbles
The Ang-1 gene plasmid was constructed by ligating
the Ang-1 gene into the pcDNA3.1 vector with a cytomegalovirus
promoter to induce Ang-1 expression. A total of 20 µg Ang-1 plasmid
was mixed with 200 µl (~1×108) TCMB or CMB in 1 ml PBS.
The mixture was incubated for 15 min at room temperature and then
centrifuged at 37°C and 400 × g for 5 min to form two phases. The
upper layer contained the microbubble-bound plasmid and the lower,
clear layer contained the unbound plasmid. The subnatant was
collected and its plasmid content was analyzed using UV
spectrophotometry at 260 nm and was compared with a standard. The
standard curve was created in house using UV spectrophotometry at
260 nm to detect the Ang-1 gene plasmid with a series of different
concentration (0.01, 0.05, 0.1, 0.5, 1.0, 5.0, 10.0 and 20.0
µg/ml). The gene-carrying efficiency of the microbubbles was
defined as follows: (Total quantity of plasmid-quantity of plasmid
in the subnatant)/total quantity of plasmid.
Targeting ability of TCMBs for
inflammatory endothelial cells in vitro
Human umbilical vein endothelial cells (HUVECs) were
extracted from the endothelium of human umbilical cord veins. The
umbilical cords were acquired from the delivery room at Renmin
Hospital (Wuhan, China) and the experimental process was approved
by the Ethics Committee of Renmin Hospital. Briefly, the umbilical
vein was filled with 20 ml of 0.1% collagenase (Type II; cat. no.
17101015; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) dissolved in PBS and incubated for 15 min at 37°C. The
collagenase solution was drained from the cord and collected. The
cells in this solution were recovered via centrifugation at 37°C
and 112 × g for 5 min and transferred to culture dishes. HUVECs
were subsequently in endothelial cell medium (ECM) containing 10%
fetal bovine serum and 1% endothelial cell growth supplement
(ScienCell Research Laboratories, Inc., Carlsbad, CA, USA). The
cells were maintained for 24 h in 10-cm culture dishes at 37°C in
an atmosphere containing 5% CO2. The HUVECs were
subsequently treated with human recombinant tumor necrosis factor-α
(TNF-α; R&D systems, Inc., Minneapolis, MN, USA) to generate a
model of inflammatory endothelial cells. A total of
2×106 HUVECs were cultured in ECM supplemented with
various doses of TNF-α (0, 10, 20 and 50 ng/ml) at 37°C in an
atmosphere containing 5% CO2 for 4 h. Western blotting
was then used to detect the expression of ICAM-1. The adherent
cells were lysed in 1 ml of ice-cold tissue lysis buffer (1X
Tris-buffered saline, 1.5% Triton X-100, 0.5% deoxycholic acid,
0.1% SDS, protease inhibitor cocktail and 1 mM
phenylmethanesulfonyl fluoride; all Sigma Aldrich; Merck Millipore)
and centrifuged (12,000 × g, 20 min, 4°C), following which the
supernatants were collected. The protein concentration was
determined using a bicinchoninic acid protein assay kit (P0010;
Beyotime Institute of Biotechnology, Haimen, China). Protein
samples (30 µg/lane) were separated by 10% SDS-PAGE, transferred
onto polyvinylidene fluoride membranes and blocked with 5% nonfat
dry milk for 1 h at room temperature. Membranes were subsequently
incubated with rabbit anti-human ICAM-1 primary antibody (1:200;
bs-4617R; Beijing Biosynthesis Biotechnology Co., Ltd.) at 4°C
overnight before being incubated with horseradish
peroxidase-coupled secondary antibody (goat anti-rabbit IgG;
1:5,000; ab6721; Abcam) for 1 h at room temperature. The membranes
were washed with 20 ml TBST for 5 min 3 times and exposed to X-rays
to detect the expression bands. Image J software (version 1.4;
National Institutes of Health, Bethesda, MA, USA) was used to
quantify the bands.
The HUVECs were cultured in an inverted
parallel-flow chamber filled with extracellular matrix (Collagen I
from rat tail tendons; C3867; Sigma-Aldrich; Merck Millipore) for
microbubble attachment. When the HUVECs reached 70% confluence
(~2×106), they were activated with 50 ng/ml TNF-α at
37°C in an atmosphere containing 5% CO2 for 4 h. Resting
(unactivated) HUVECs served as a control. The TCMBs or CMBs were
passed across the HUVECs at a flow rate of 2 dynes/cm2,
as previously reported (19). The
microbubbles that adhered to the HUVECs in the presence or absence
of TNF-α stimulation were observed and counted.
Targeting ability of TCMBs to the
human umbilical cord vein ex vivo
To test the targeting ability of the TCMBs in blood
vessels, the human umbilical cord vein was used to mimic a blood
vessel. Segments of the human umbilical cord vein were washed with
a heparinized solution (20 UI/ml) and filled with ECM containing 50
ng/ml TNF-α for 6 h at 37°C to induce an inflammatory condition.
Then, 2×108 TCMB or CMB were diluted in normal saline to
a total volume of 10 ml. The diluted microbubble solutions were
then passed through the umbilical vein using an automatic infusion
pump at 2 ml/min. A 10-MHz vascular ultrasound probe (Philips
Medical Systems, Inc., Bothell, WA, USA) was used to detect the
adhesion of the microbubbles to the umbilical vein following
stimulation with or without TNF-α. A video was taken, stored and
analyzed using Qlab 6.0 software (Philips Medical Systems,
Inc.).
Acute myocardial infarction (AMI)
model
A total of 90 healthy New Zealand white rabbits
(male; 4 months old; weight, 2.5±0.2 kg) were provided by the Wuhan
Institute of Biological Products, Co., Ltd. (Wuhan, China). Rabbits
were housed at 20°C and 60% humidity with a 12 h light-dark cycle
and free to access food and water.
Rabbits were anesthetized by ear vein injection of
30 mg/kg pentobarbital sodium (P3761; Sigma-Aldrich; Merck
Millipore) and connected to an electrocardiogram (ECG). The heart
was exposed, a left thoracotomy was performed and the left
circumflex coronary artery was ligated 5 mm below the left atrial
appendage using a 6–0 polypropylene suture. The ends of the
ligature were passed through a short PE 10 tube to form a snare.
Following ligation, contraction of the regional myocardium was
decreased and the infarcted area turned pale. AMI was confirmed as
an ST-segment elevation >2 mm on the ECG. Reperfusion was
induced by releasing the snare 2 h after AMI and was verified by a
>50% drop of the ST-segment ≥15 min.
All animal experiments described in this section
conformed to the Guide for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (NIH Publication
No. 85–23, revised 1996). Approval from the Institutional Animal
Care and Use Committee of Wuhan University Health Science Center
was also obtained prior to performing experiments.
Imaging targeted TCMBs in the
infarcted heart
To test the accumulation of TCMBs in infarcted
heart, myocardial contrast echocardiography was performed as
previously reported (22). Briefly,
10 rabbits with similar lengths of infarcted myocardium (1.0–1.5
cm) were randomly selected and 1 ml of either TCMBs (n=5) or CMBs
(n=5) was slowly injected for 5 min through the ear vein 2 days
after AMI and reperfusion. The slow injection allowed for
interaction between the microbubbles and the ICAM-1 expressed in
infarcted myocardium. An S5-1 cardiac ultrasound probe (Philips
Medical Systems, Inc.) was used to observe the filling of
microbubbles into the myocardium. Ultrasound contrast images were
acquired 3 min after the microbubbles were completely infused. The
intensity of the signals originating from the retained microbubbles
in the left ventricular anterior wall (infarct area) and the
inferior wall (non-infarct area) was measured. The ratio of the
intensity of the signals (anterior wall:inferior wall) was
calculated to evaluate the adhering ability of the microbubbles to
the infarcted myocardium. These rabbits were used in further
experiments.
Experimental grouping
To ensure that infarcted areas in the experimental
animals were all similar, rabbits with an infarcted myocardium
length of >1.5 or <1.0 cm were excluded from this study. The
remaining 65 rabbits with AMI and reperfusion were randomly divided
into three groups and used for gene transfection. The targeted
cationic microbubble group (TCMB group; n=25) received an
intravenous injection of TCMBs and the Ang-1 plasmid suspension
with ultrasound exposure. The non-targeted cationic microbubble
group (CMB group; n=25) received an intravenous injection of CMBs
and the Ang-1 plasmid suspension with ultrasound exposure. The
control group (n=15) received an intravenous injection of the Ang-1
plasmid alone.
Gene transfection by
ultrasound-mediated microbubble destruction
Ultrasound-targeted microbubble destruction-mediated
gene transfection was performed 2 days after AMI. The
concentrations of the TCMB and CMB suspensions were adjusted to
~2×108 microbubbles/ml. A total of 1 ml microbubble
suspension was mixed with 1 ml Ang-1 plasmid (1 mg/ml) and the
mixture was incubated at room temperature for 15 min to ensure
sufficient contact between the plasmid and the microbubbles.
Rabbits were then injected with 2 ml plasmid-microbubble solution
via the ear vein and ultrasound irradiation was performed for gene
transfection.
The iE33 ultrasound diagnostic system with an M3S
transducer (Philips Medical Systems, Inc.) was used for ultrasound
exposure. The ‘contrast’ procedure was selected and the second
harmonic mode, with a frame rate of 80 Hz, was switched on. The
probe emission frequency and the receiving frequency were 1.7 and
3.4 MHz, respectively. An ECG trigger was performed every 4–8
cardiac cycles and the depth was set at 5 cm. Once the microbubble
infusion began, the ultrasound beam was continuously aimed from the
chest wall towards the heart of the rabbit. The ultrasound blasting
function was activated to disrupt the microbubbles when the
myocardial filling of the contrast agent reached a plateau (~30 sec
after vein injection), allowing for adhesion of the microbubbles.
The ultrasound exposure (mechanical index 1.3 and 100% overall
gain) lasted for 5 min. One flash per six cardiac cycles was used
to disrupt the microbubbles and a pulsing interval allowed
replenishment and adhesion of the microbubbles before the next
flash, as previously reported (22).
Myocardial Ang-1 expression
Rabbits were sacrificed by intravenous injection of
30 ml air following anesthesia with 30 mg/kg pentobarbital sodium.
A total of 5 rabbits were randomly selected from each group and the
hearts were harvested 3 days following transfection. The myocardial
tissue from the infarct and border region was cut into small blocks
of equal size (0.5×0.5×0.5 cm) and snap-frozen for the analysis of
Ang-1 expression.
Semi-quantitative reverse transcription-polymerase
chain reaction (RT-PCR) was used to detect myocardial Ang-1 mRNA
expression in all of the groups. The primers for Ang-1 were as
follows: Forward, 5′-TGCCATTACCAGTCAGAGG-3′ and reverse,
5′-CAAGCATCAAACCACCATC-3′; the primers for rabbit β-actin were as
follows: Forward, 5′-GTGCTTCTAGGCGGACTGTTAGA-3′ and reverse,
5′-CACGAATAAAGCCATGCCAAT-3′. Total RNA was extracted from 100 mg
myocardium using TRIzol reagent according to the manufacturer's
protocol (15596-026; Invitrogen; Thermo Fisher Scientific, Inc.).
The samples were then treated with DNase I (18068-015; Invitrogen;
Thermo Fisher Scientific, Inc.) for 15 min at room temperature, and
the RNA was further purified using an RNA cleanup kit (74204;
Qiagen, Inc., Valencia, CA, USA). cDNA was synthesized using
SuperScript III First-Strand Synthesis System RT-PCR kit
(18080-051; Invitrogen; Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol. PCR was performed with a final
reaction volume of 50 µl containing PCR amplification buffer (1X),
Taq DNA polymerase (2.5 U), dNTPs (4 mM), primers (0.4 µM) and
template DNA (4 ng). Cycling conditions were as follows: 94°C for 2
min, followed by 30 cycles of 94°C for 30 sec, 55°C for 30 sec,
72°C for 1 min, and a final extension step at 72°C for 5 min. PCR
products were electrophoresed on a 1% agarose gel and stained with
ethidium bromide. The relative expression of Ang-1 mRNA was
normalized to that of β-actin using a gel imaging analysis system
(Geliance 200; PerkinElmer, Inc., Waltham, MA, USA).
Western blot analysis was performed as described
above to determine the Ang-1 protein expression in the myocardium
from the infarcted and border regions, and the relative expression
of the Ang-1 protein was normalized to that of β-actin. The primary
antibody used was rat anti-rabbit Ang-1 polyclonal antibody (1:200;
bs-0800R; Beijing Biosynthesis Biotechnology Co., Ltd.) and the
secondary antibody was horseradish peroxidase-coupled goat anti-rat
IgG (1:2,000; ab97057; Abcam).
Cardiac function
A standard echocardiography examination was
performed in all groups the day before AMI, 2 days after AMI and 4
weeks following gene transfection. The left ventricular morphology
and motion were observed and the left ventricular end-diastolic and
end-systolic dimensions (LVEDD and LVESD, respectively), left
ventricular ejection fraction (LVEF) and left ventricular
fractional shortening (LVFS) were measured to assess changes in
left ventricular function.
Myocardial perfusion
Myocardial contrast echocardiography (MCE) was
performed 4 weeks after gene transfection. A total of 1 ml
SonoVue® microbubble solution (Bracco SpA, Milan, Italy)
was injected via the ear vein and the short-axis view at the
papillary muscle level was obtained to assess myocardial perfusion.
All acquired images were transferred to the workstation for a
frame-by-frame, off-line analysis. The myocardial blood volume was
analyzed by calculating the ratio of the intensity of the contrast
agent in the left ventricular anterior wall (infarct area) to that
in the inferior wall (non-infarct area) in the digital images. The
MCE images were analyzed using Qlab 6.0 software.
Angiogenesis
Myocardium from the infarct and border regions was
obtained 4 weeks after gene transfection and immunohistochemical
staining for von Willebrand Factor (vWF) was performed. Sections of
rabbit myocardium from the infarcted and border regions were fixed
with 4% paraformaldehyde at room temperature for 24 h and embedded
in paraffin. The sections were incubated in a citrate buffer (0.01
M; pH 6.0; P0081; Beyotime Institute of Biotechnology) at 100°C for
15 min for antigen retrieval. The sections were then blocked in
blocking buffer (5% normal goat serum; C0265; Beyotime Institute of
Biotechnology) at 37°C for 20 min and incubated with a rabbit
anti-vWF antibody (1:200; bs-10048R, Beijing Biosynthesis
Biotechnology Co., Ltd.) overnight at 4°C. Sections were
subsequently incubated with biotin conjugated secondary antibody
(goat anti-rabbit IgG; 1:200; bs-0295G-Bio, Beijing Biosynthesis
Biotechnology Co., Ltd.) for 40 min and treated with streptavidin
peroxidase for 10 min at 37°C Finally, specimens were incubated in
DAB for 5 mins at 37°C. The microvascular density (MVD) was then
determined using optical microscopy (x200) according to the Weidner
criteria (23). A pathologist was
employed to average the five visual fields with the most
capillaries in single-blind manner.
Histomorphometry
At 4 weeks after transfection, rabbit hearts were
harvested, fixed at 37°C overnight with 10% paraformaldehyde and
sectioned. The sections (thickness, 5 µm) were stained with
Masson's trichrome stain to assess the morphology of the heart,
infarct size and collagen deposition in the myocardium.
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). Differences between groups
prior to and following gene transfection were analyzed using paired
t-tests. Comparisons among multiple groups were made using a
one-way analysis of variance with Student Newman Keuls-q post hoc
analysis. All statistical analyses were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of TCMBs and DNA
conjugation
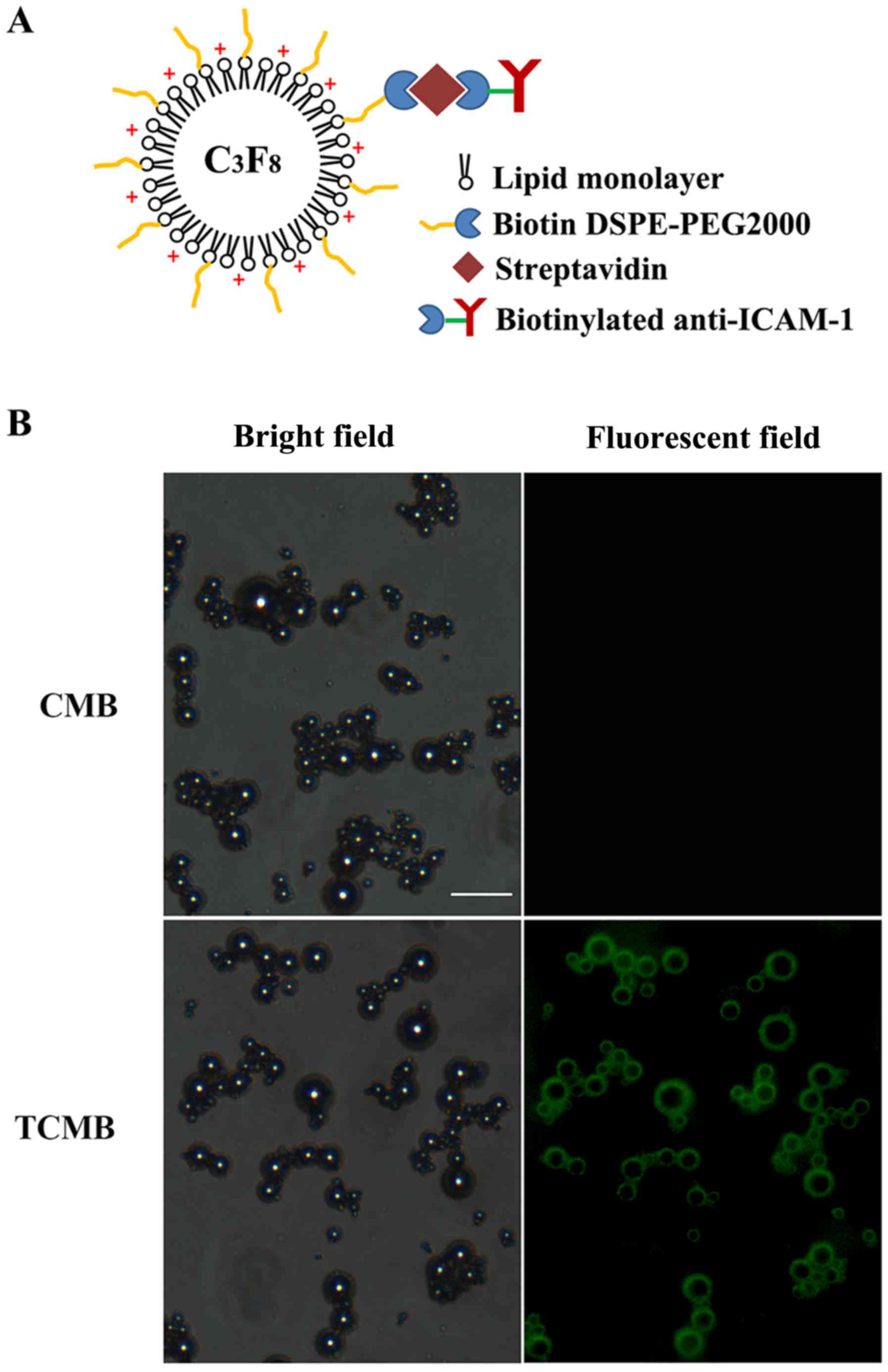
A schematic of a TCMB is presented in Fig. 1A. The microbubbles were observed to
be smooth and spherical. Fig. 1B
shows the microscopic morphology of the CMBs and TCMBs under
bright-field and fluorescence microscopy, respectively. Bright
green fluorescence was present at the periphery of each
microbubble, which indicated that the ICAM-1 antibodies were
successfully bound to the surface of the microbubbles. The average
diameters of the TCMBs and CMBs were 3.1 and 2.9 µm, respectively
and there were no significant differences between groups
(P>0.05). The zeta potentials of the TCMBs and CMBs were
+22.0±2.4 and +31.3±3.9 mV (P<0.01). This difference most likely
resulted from the conjugation of the positively charged ICAM-1
antibodies on the surface of the TCMBs. UV spectrophotometric
analysis indicated that the DNA-binding capacity of the TCMBs was
mildly reduced compared with that of the CMBs (3.3±0.7 and 4.0±0.9
µg 108/microbubble; P<0.05).
Targeting ability of TCMBs to
inflammatory endothelial cells in vitro
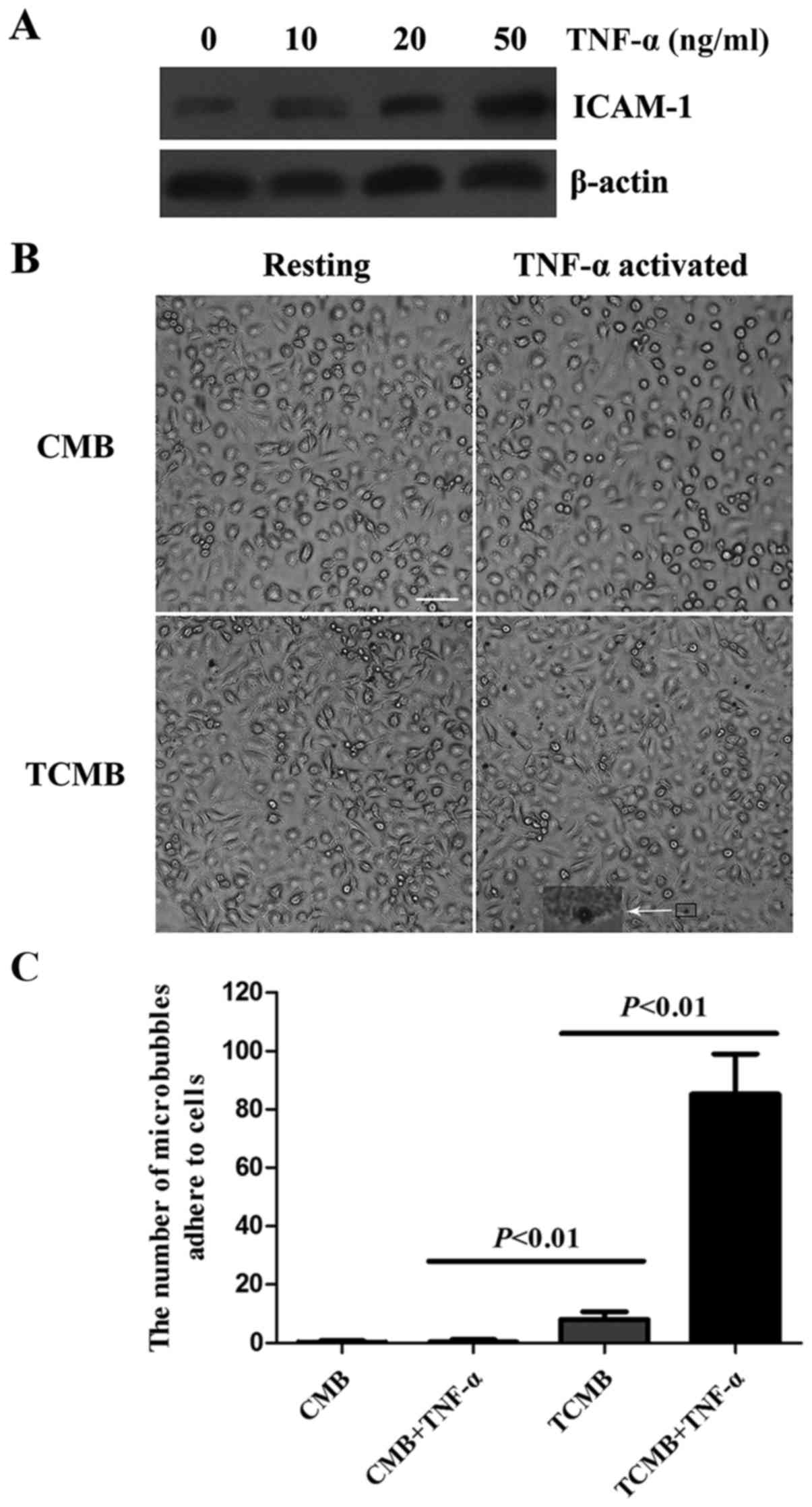
The expression of ICAM-1 in inflammatory endothelial
cells was analyzed using western blotting. The expression of ICAM-1
in HUVECs was upregulated in a dose-dependent manner following
TNF-α stimulation (Fig. 2A).
The flow chamber experiment showed that a large
number of TCMBs adhered to HUVECs following TNF-α stimulation,
whereas no significant adhesion occurred in the resting condition.
Regardless of whether the cells were stimulated with TNF-α or not,
there was no significant adhesion of CMBs to the HUVECs (Fig. 2B). However, data from microbubble
counting indicated that the number of adhered TCMBs was 18.4-fold
greater than that of CMBs following TNF-α stimulation (85±14 vs.
8±3 microbubbles per microscopic field, P<0.001; Fig. 2C).
Targeting ability of TCMBs to the
human umbilical cord vein ex vivo
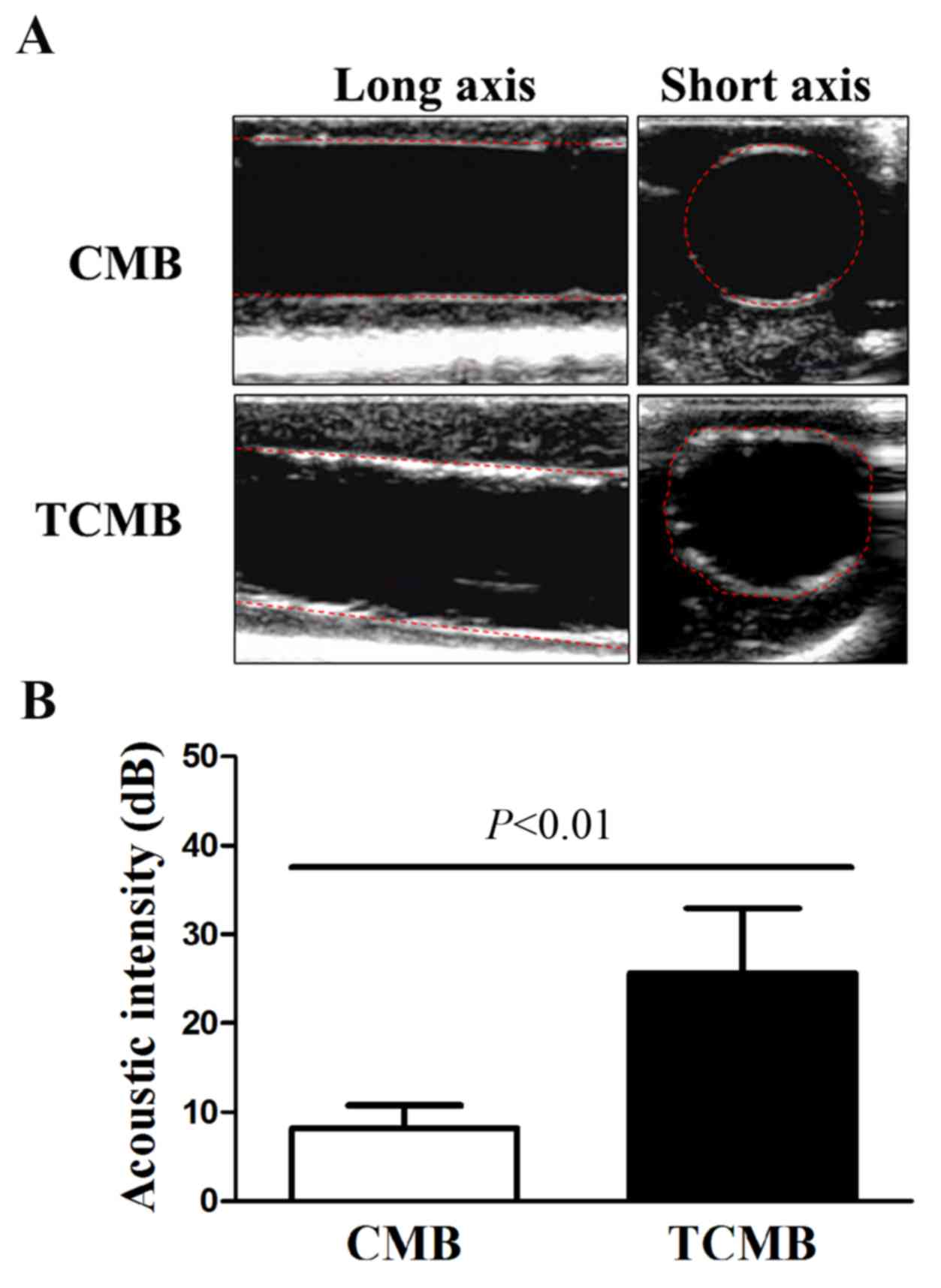
The flow chamber experiment confirmed that TCMBs
adhered to HUVEC membranes under controlled physiological
conditions. Subsequently, the umbilical cord vein was used to
simulate a vessel and further evaluate the targeted adhesion of the
TCMBs to the vascular endothelium. CMBs and TCMBs were visible in
the ultrasound field and there were no significant differences in
the visibility of the groups. The results demonstrated that TCMBs
were able to adhere to TNF-α-stimulated umbilical vein endothelium
under flow conditions but did not adhere notably in the resting
condition. No significant CMB adhesion to the umbilical vein was
observed in TNF-α-stimulated or resting conditions, which
eliminated the possibility of nonspecific adhesion. Acoustic
quantitative analysis showed that the acoustic intensity of the
TCMBs adhered to the umbilical vein endothelium was significantly
higher than that of the CMBs (25.6±7.3 vs. 8.2±2.6 dB, P<0.001;
Fig. 3).
Targeting ability of TCMBs to
infarcted myocardium in vivo
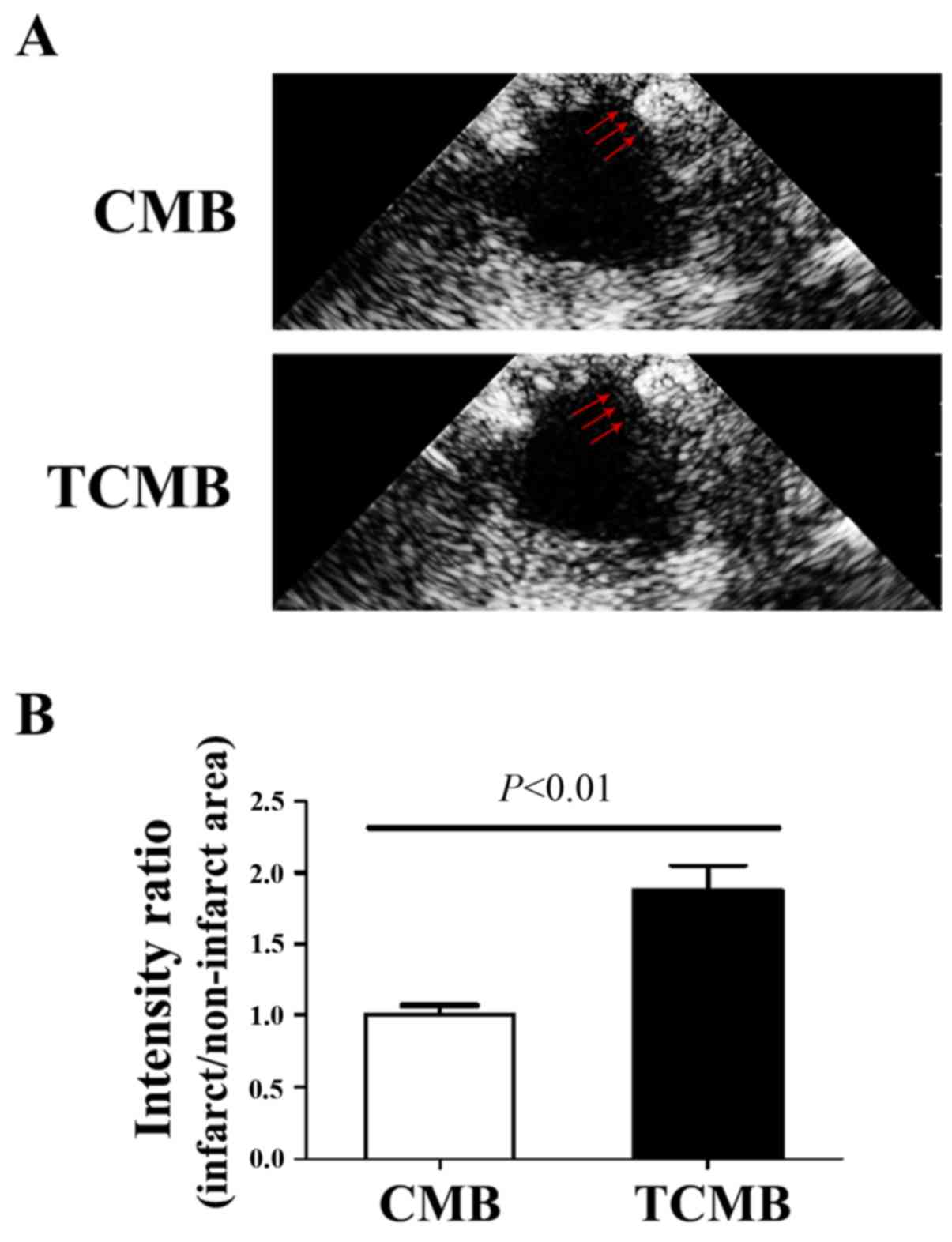
To evaluate the ability of the microbubbles to
target infarcted tissue, ultrasound imaging was performed to
determine microbubble intensity in the left ventricle 3 min
following intravenous injection. In the CMB group, no microbubble
accumulation was detected in the infarcted region. By comparison,
intense contrast enhancement was observed in the TCMB group
(Fig. 4A). The ratio of the signal
intensity (anterior wall/inferior wall) in the TCMB group was 86%
greater than that of the CMB group (P<0.01; Fig. 4B). These results demonstrate that the
TCMBs were able to adhere at the infarcted site and were
retained.
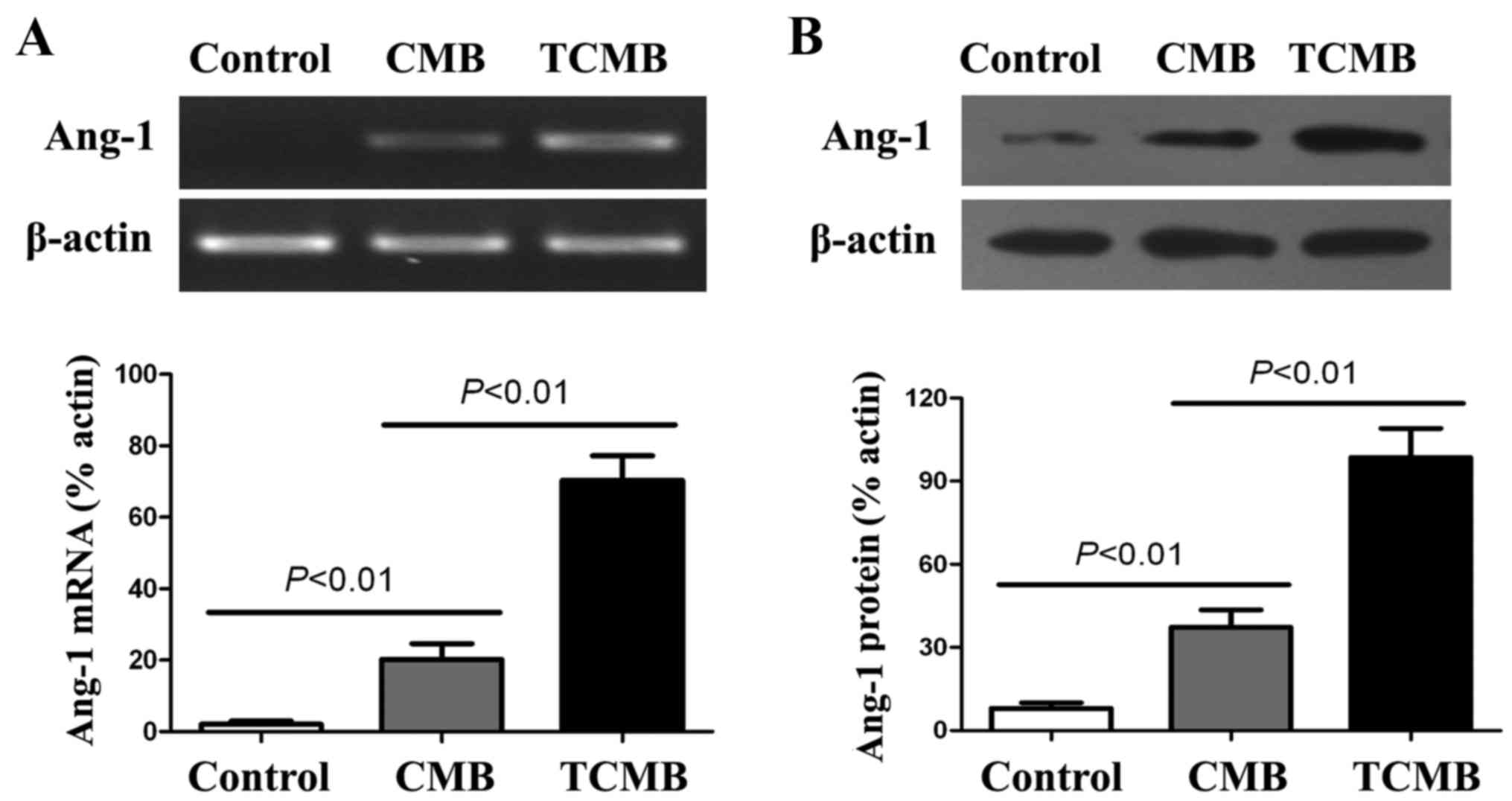
Ultrasound-mediated TCMB destruction
increases Ang-1 gene expression in ischemic myocardium
RT-PCR and western blot analyses were conducted to
evaluate the delivery of Ang-1 to the infarcted myocardium 3 days
following UTMD-mediated gene transfection. The results indicated
that the expression of Ang-1 mRNA in the TCMB group was
significantly increased compared with that in the CMB group
(P<0.01) and very low Ang-1 mRNA expression was detected in the
controls (Fig. 5A).
Results from the western blot analysis demonstrated
that the expression of Ang-1 protein in the TCMB group was
significantly higher than in the CMB group (P<0.01).
Additionally, very low Ang-1 protein expression detected in the
controls (Fig. 5B).
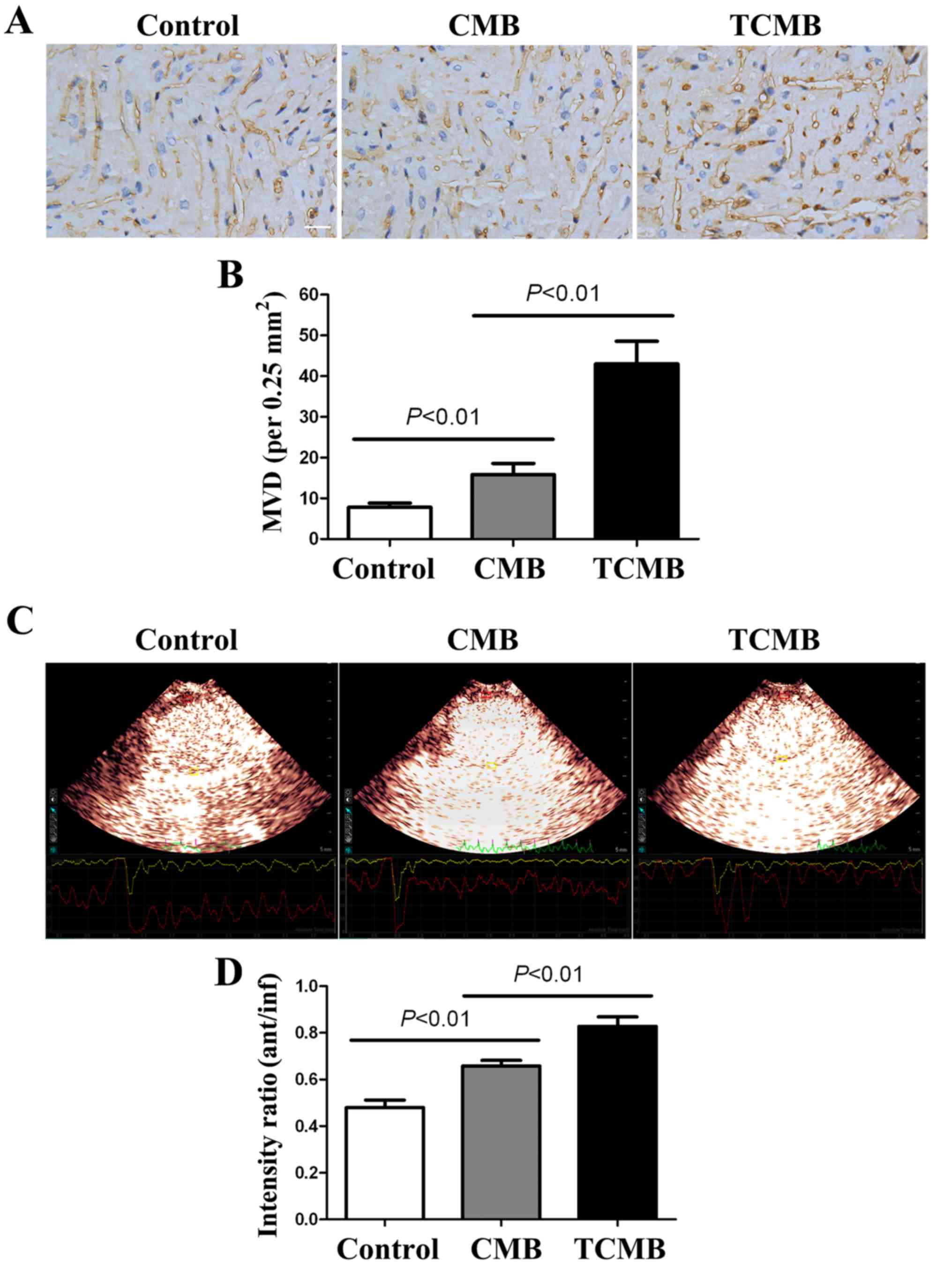
Ultrasound/ICAM-1-targeted,
microbubble-mediated Ang-1 gene transfection increases myocardial
angiogenesis and improves perfusion
At 4 weeks after gene transfection,
immunohistochemical staining for vWF was performed to determine the
microvascular density (MVD) in the infarct and border regions. The
results indicated that the MVD in the infarct and border regions
(primarily at the border region of the infarcted myocardium) of the
TCMB group was significantly increased compared with that of the
CMB and control groups (all P<0.01; Fig. 6A and B).
MCE demonstrated that the contrast agent was
markedly absent from the anterior wall; however satisfactory
filling occurred 2 days following AMI in other regions. The ratios
of the signal intensity in the anterior wall (infarct region) to
that of the inferior wall (non-infarct region) of the three groups
were low and similar to each other (data not shown). A total of 4
weeks after transfection, the perfusion of the infarcted myocardium
was improved in all groups; however, the improvement in the TCMB
group was greater than that in the CMB group, which in turn, was
greater than that in the control group (all P<0.01; Fig. 6C and D).
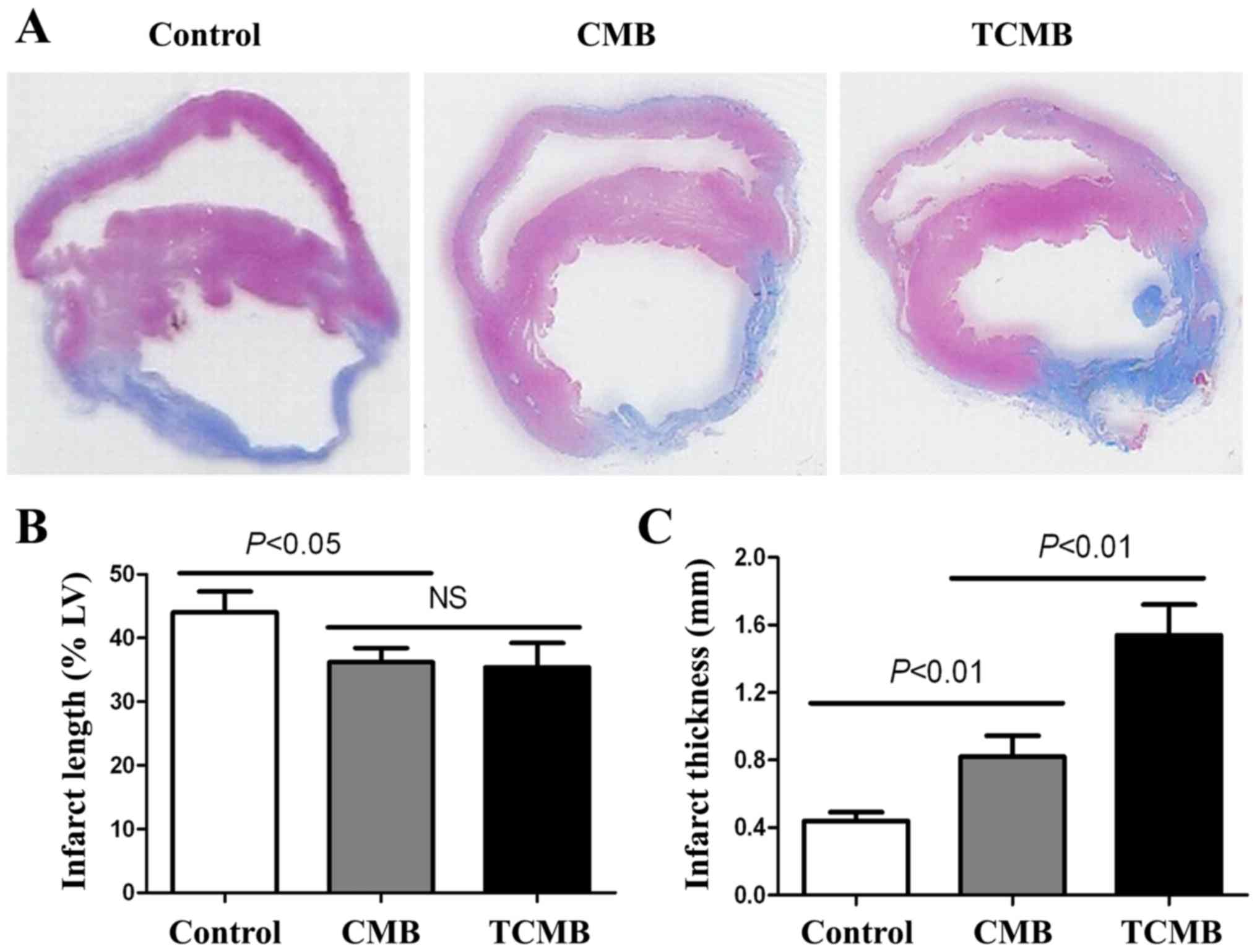
Ultrasound/ICAM-1-targeted,
microbubble-mediated Ang-1 gene transfection improves cardiac
morphology and function
Masson's trichrome staining demonstrated that the
rabbits in the control group had the largest left ventricle, the
largest range of infarction, the thinnest ventricular wall and the
greatest collagen deposition in the infarct and border myocardia.
Rabbits in the CMB group had a thicker ventricular wall and a
smaller left ventricle, a smaller range of infarction and collagen
deposition, and the TCMB group had the smallest and thickest
infarct wall compared with the control group (Fig. 7A). The TCMB group had a similar
infarct length to the CMB group; however, the infarct lengths in
both microbubble groups were significantly smaller than that of the
control group (P<0.05; Fig. 7B).
However, the TCMB group had a significantly greater infarct
thickness than the CMB and control groups (P<0.01; Fig. 7C) These data demonstrated that Ang-1
gene transfection mediated by the ultrasound destruction of TCMBs
prevented the infarcted region from thinning and extending and
improved ventricular remodeling following infarction (Fig. 7).
At 2 days after AMI, left ventricular anterior wall
motion and ejection fraction were significantly reduced in all
groups and there were no significant differences among the three
groups. Additionally, 4 weeks following Ang-1 gene transfection,
LVEDD decreased in the CMB and TCMB groups compared with the
baseline dimensions, while the LVEF and LVFS increased by various
degrees in all groups with the exception of the control group. The
greatest improvements in LVEDD, LVEF and LVFS occurred in the TCMB
group (Fig. 8).
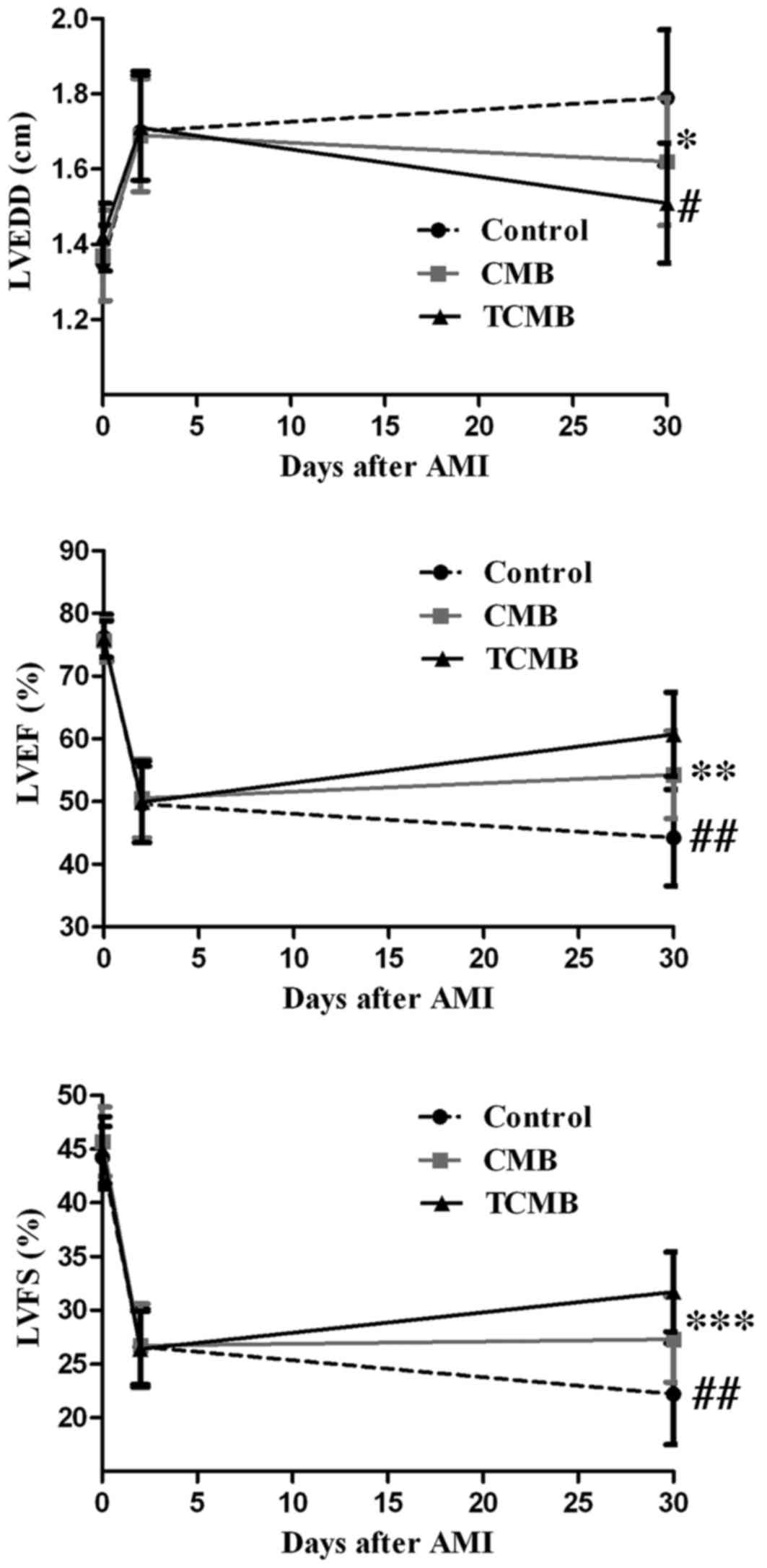 | Figure 8.Echocardiographic assessment of
cardiac function. Compared with the control group (n=10), LVEDD,
LVEF and LVFS in the CMB group (n=20) were slightly improved 4
weeks following ultrasound-targeted microbubble
destruction-mediated angiopoietin 1 gene delivery; however, a
greater improvement was observed in the TCMB group (n=20).
*P=0.001, **P=0.034, ***P=0.025 vs. control; #P=0.04,
##P=0.001 vs. CMB. LVEDD, left ventricular end-diastolic
dimension; LVEF, left ventricular ejection fraction; LVFS, left
ventricular fractional shortening; CMB, non-targeted cationic
microbubble; TCMB, targeted cationic microbubble. |
Discussion
The aim of the present study was to test the
hypothesis that cationic microbubbles conjugated to an ICAM-1
antibody by biotin-streptavidin chemical conjugation could bind to
and target the delivery of the Ang-1 gene to the infarcted heart,
thus improving cardiac function. The results of the current study
indicated that TCMBs were able to recognize injured vascular
endothelium and site-specifically deliver the Ang-1 gene to the
infarct area following myocardial infarction. Compared with the
non-targeted microbubbles, TCMBs significantly increased the
efficiency of UTMD-mediated Ang-1 gene transfection in the
myocardium of the infarcted and border regions. High expression of
Ang-1 enhanced angiogenesis, improved cardiac perfusion and
promoted myocardial repair.
UTMD is a promising strategy for gene delivery due
to its hypotoxicity, low immunogenicity and reproducibility of use
(8–11). However, the therapeutic effects of
UTMD have been somewhat limited as the efficiency of gene
transfection in vivo remains unsatisfactory (24). A number of factors, including the
ultrasound parameters, exposure equipment, the microbubble
characteristics and the types of target tissues and cells that are
transfected, affect the transfection efficiency of UTMD, and gene
concentration at the site of sonoporation is a critical factor for
gene transfection in vivo (25,26).
There are two factors that determine the gene concentration at the
site of sonoporation: The gene-binding capacity of the microbubbles
and the targeted adhesion of the microbubbles to the surface of the
cells.
The gene-binding capacity of microbubbles is a key
factor affecting the concentration of genes at the site of
sonoporation (27). Previous studies
used commercially available microbubbles, including SonoVue or
Definity microbubbles, as in vivo gene delivery vehicles
(28,29). In these studies, plasmid DNA was
mixed and incubated with microbubbles to couple the genes to the
microbubbles prior to intravenous injection. This direct coupling
strategy was simple to perform; however, the negatively charged
surfaces of the commercially available microbubbles prevented their
effective conjugation to the negatively charged nucleic acid due to
the repulsion of the electric charges. The unconjugated plasmid DNA
was cleared and degraded by endogenous nucleases prior to reaching
the target tissue (30). In the
present study, a cationic microbubble composed of DC-Chol, DSPC,
DSPE-PEG and DPPA was synthesized. DC-Chol is a well-known cationic
lipid that can provide a positive surface charge for microbubbles
(31). The other lipids were used to
form the components of the lipid shell and to increase the
stability of the microbubbles in blood. The results of the current
study demonstrated that the cationic microbubble had a significant
positive zeta potential (+31.3±3.9 mV) and the combination of the
plasmid DNA and the cationic microbubbles was as high as 4
µg/108 microbubbles in a neutral pH environment.
Furthermore, although the ICAM-1 antibody was conjugated to the
cationic microbubbles, the microbubbles showed a net positive
charge (+22.0±2.4 mV). The cationic microbubbles were able to form
stable complexes with the plasmid DNA by electrostatic attraction
and they protected the plasmid DNA from degradation by endogenous
nucleases (32). This may produce an
obvious advantage when used in vivo.
An alternative approach to increase the
concentration of genes at the site of sonoporation is to
specifically target microbubbles to the issue of interest.
Non-targeted microbubbles may disperse throughout the entire body,
resulting in unsatisfactory treatment even though the
operator-guided ultrasound destruction of the microbubbles releases
the gene with a certain degree of targeting. Previous studies have
described the successful coupling of specific ligands to
microbubbles, thereby making it possible for microbubbles to
recognize a target tissue (33,34).
Wang et al (33) constructed
a phospholipid microbubble conjugated to a single-chain antibody
specific for activated glycoprotein IIb/IIIa, due to its binding to
a ligand-induced binding site; the results demonstrated that the
targeting microbubble may be used to detect atherosclerotic
plaques. Leong-Poi et al (35) prepared αV-integrin-targeted
microbubbles by conjugating a monoclonal antibody against the αV
integrin to the microbubble surface. The targeted microbubbles
directly attached to the microvascular endothelial surface, thus
allowing assessment of the treatment as a promoter of therapeutic
angiogenesis (35). In the present
study, a cationic microbubble conjugated to an ICAM-1 antibody was
constructed. ICAM is widely distributed on the surface of many
cells, such as endothelial cells. ICAM expression is quite low
under normal conditions; however, its expression is significantly
increased following the activation of endothelial cells by
inflammatory cytokines, ischemia or hypoxia (36,37). The
upregulation of ICAM-1 expression is an important marker of
endothelial cell injury and leukocyte activation (16). Following the occurrence of ischemia,
multiple inflammatory factors induce the overexpression of ICAM-1
in microvascular endothelial cells (38,39).
Therefore, ICAM-1 is a potential marker for early detection of
inflammation/injury and may be a possible target for gene delivery.
The results of the current study demonstrated that microbubbles
conjugated to ICAM-1 antibodies were able to recognize inflammatory
vascular endothelial cells and deliver a greater concentration of
the Ang-1 gene to the ischemic myocardium through their selective
adhesion to the injured endothelium. Flow chamber studies indicated
that the coupling of Ang-1 plasmid DNA to the surface did not
interfere with the targeting of the TCMBs. Compared with the CMBs,
the number of TCMBs that adhered to the cells was 18.4-fold greater
following TNF-α stimulation in vitro. Furthermore, the
number of TCMBs that adhered to the umbilical vein endothelium was
significantly increased compared with the number of adhered CMBs.
Additionally, a large number of TCMBs were specifically retained in
and adhered to the injured vascular endothelium of the infarcted
rabbit myocardium, whereas no CMBs adhered to the infarcted
myocardium. The specific adhesion of microbubbles carrying a gene
to a site of injured vascular endothelium may improve contact
between the gene and the endothelium and increase the amount of the
gene delivered to the target tissue. The results of the current
study showed that the level of Ang-1 gene expression and the
subsequent biological effects, including changes in the morphology
and function of the infarct heart and myocardial perfusion, were
all significantly improved in the TCMB group compared with the CMB
group.
In conclusion, the data collected in the present
study data demonstrate that ICAM-1-targeted cationic microbubbles
are able to effectively carry the Ang-1 gene. They specifically
accumulate at the site of injury following myocardial infarction
and therefore may be a promising carrier for gene therapy. The use
of ultrasound and ICAM-1-targeted cationic microbubbles to promote
angiogenesis using gene therapy is a feasible, simple and effective
approach that may have clinical applications in the treatment of
refractory ischemic heart diseases.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81471674 and 81501495).
References
|
1
|
Henry TD, Satran D, Hodges JS, Johnson RK,
Poulose AK, Campbell AR, Garberich RF, Bart BA, Olson RE, Boisjolie
CR, et al: Long-term survival in patients with refractory angina.
Eur Heart J. 34:2683–2688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lassaletta AD, Chu LM and Sel FW:
Therapeutic neovascularization for coronary disease: Current state
and future prospects. Basic Res Cardiol. 106:897–909. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cochain C, Channon KM and Silvestre JS:
Angiogenesis in the infarcted myocardium. Antioxid Redox Signal.
18:1100–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katz MG, Fargnoli AS, Williams RD and
Bridges CR: The road ahead: Working towards effective clinical
translation of myocardial gene therapies. Ther Deliv. 5:39–51.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katz MG, Fargnoli AS, Pritchette LA and
Bridges CR: Gene delivery technologies for cardiac applications.
Gene Ther. 19:659–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cool SK, Geers B, Lentacker I, De Smedt SC
and Sanders NN: Enhancing nucleic acid delivery with ultrasound and
microbubbles. Methods Mol Biol. 948:195–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geis NA, Katus HA and Bekeredjian R:
Microbubbles as a vehicle for gene and drug delivery: Current
clinical implications and future perspectives. Curr Pharm Des.
18:2166–2183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J, DU LF, Chen M, Wang HH, Xing LX,
Jing LF and Li YH: Drug-loaded nano-microcapsules delivery system
mediated by ultrasound-targeted microbubble destruction: A
promising therapy method. Biomed Rep. 1:506–510. 2013.PubMed/NCBI
|
|
9
|
Smith AH, Fujii H, Kuliszewski MA and
Leong-Poi H: Contrast ultrasound and targeted microbubbles:
Diagnostic and therapeutic applications for angiogenesis. J
Cardiovasc Transl Res. 4:404–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao YY, Chen ZY, Wang YX, Lin Y, Yang F
and Zhou QL: New progress in angiogenesis therapy of cardiovascular
disease by ultrasound targeted microbubble destruction. Biomed Res
Int. 2014:8729842014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujii H, Sun Z, Li SH, Wu J, Fazel S,
Weisel RD, Rakowski H, Lindner J and Li RK: Ultrasound-targeted
gene delivery induces angiogenesis after a myocardial infarction in
mice. JACC Cardiovasc Imaging. 2:869–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castle J, Butts M, Healey A, Kent K,
Marino M and Feinstein SB: Ultrasound-mediated targeted drug
delivery: Recent success and remaining challenges. Am J Physiol
Heart Circ Physiol. 304:H350–H357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delalande A, Postema M, Mignet N, Midoux P
and Pichon C: Ultrasound and microbubble-assisted gene delivery:
Recent advances and ongoing challenges. Ther Deliv. 3:1199–1215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang H, Xiong XY, Zhang L, Wu C and Liu Y:
Adhesion of bio-functionalized ultrasound microbubbles to
endothelial cells by targeting to vascular cell adhesion molecule-1
under shear flow. Int J Nanomedicine. 6:2043–2051. 2011.PubMed/NCBI
|
|
15
|
Murciano JC, Muro S, Koniaris L,
Christofidou-Solomidou M, Harshaw DW, Albelda SM, Granger DN, Cines
DB and Muzykantov VR: ICAM-directed vascular immunotargeting of
antithrombotic agents to the endothelial luminal surface. Blood.
101:3977–3984. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan Y, Liao Y, Yang L, Wu J, Du J, Xuan W,
Ji L, Huang Q, Liu Y and Bin J: Late-phase detection of recent
myocardial ischaemia using ultrasound molecular imaging targeted to
intercellular adhesion molecule-1. Cardiovasc Res. 89:175–183.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morisada T, Kubota Y, Urano T, Suda T and
Oike Y: Angiopoietins and angiopoietin-like proteins in
angiogenesis. Endothelium. 13:71–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Yuan YL, Wang Z, Jiang B, Zhang
CS, Wang Q, Xu XH, Dong HY and Zhang ZM: Sequential, timely and
controlled expression of hVEGF165 and Ang-1 effectively improves
functional angiogenesis and cardiac function in vivo. Gene Ther.
20:893–900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paul A, Binsalamah ZM, Khan AA, Abbasia S,
Elias CB, Shum-Tim D and Prakash S: A nanobiohybrid complex of
recombinant baculovirus and Tat/DNA nanoparticles for delivery of
Ang-1 transgene in myocardial infarction therapy. Biomaterials.
32:8304–8318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fagiani E and Christofori G: Angiopoietins
in angiogenesis. Cancer Lett. 328:18–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie A, Belcik T, Qi Y, Morgan TK,
Champaneri SA, Taylor S, Davidson BP, Zhao Y, Klibanov AL,
Kuliszewski MA, et al: Ultrasound-mediated vascular gene
transfection by cavitation of endothelial-targeted cationic
microbubbles. JACC Cardiovasc Imaging. 5:1253–1262. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan P, Chen KJ, Wu J, Sun L, Sung HW,
Weisel RD and Li RK: The use of MMP2 antibody-conjugated cationic
microbubble to target the ischemic myocardium, enhance Timp3 gene
transfection and improve cardiac function. Biomaterials.
35:1063–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delalande A, Kotopoulis S, Postema M,
Midoux P and Pichon C: Sonoporation: Mechanistic insights and
ongoing challenges for gene transfer. Gene. 525:191–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panje CM, Wang DS, Pysz MA, Paulmurugan R,
Ren Y, Tranquart F, Tian L and Willmann JK: Ultrasound-mediated
gene delivery with cationic versus neutral microbubbles: Effect of
DNA and microbubble dose on in vivo transfection efficiency.
Theranostics. 2:1078–1091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo D and Saltzman WM: Enhancement of
transfection by physical concentration of DNA at the cell surface.
Nat Biotechnol. 18:893–895. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang DS, Panje C, Pysz MA, Paulmurugan R,
Rosenberg J, Gambhir SS, Schneider M and Willmann JK: Cationic
versus neutral microbubbles for ultrasound-mediated gene delivery
in cancer. Radiology. 264:721–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Liang HD, Dong B, Lu QL and
Blomley MJ: Gene transfer with microbubble ultrasound and plasmid
DNA into skeletal muscle of mice: Comparison between commercially
available microbubble contrast agents. Radiology. 237:224–229.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou J, Wang Y, Xiong Y, Wang H, Feng Y
and Chen J: Delivery of TFPI-2 using ultrasound with a microbubble
agent (SonoVue) inhibits intimal hyperplasia after balloon injury
in a rabbit carotid artery model. Ultrasound Med Biol.
36:1876–1883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lentacker I, De Geest BG, Vandenbroucke
RE, Peeters L, Demeester J, De Smedt SC and Sanders NN:
Ultrasound-responsive polymer-coated microbubbles that bind and
protect DNA. Langmuir. 22:7273–7278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ju J, Huan ML, Wan N, Hou YL, Ma XX, Jia
YY, Li C, Zhou SY and Zhang BL: Cholesterol derived cationic lipids
as potential non-viral gene delivery vectors and their serum
compatibility. Bioorg Med Chem Lett. 26:2401–2407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun L, Huang CW, Wu J, Chen KJ, Li SH,
Weisel RD, Rakowski H, Sung HW and Li RK: The use of cationic
microbubbles to improve ultrasound-targeted gene delivery to the
ischemic myocardium. Biomaterials. 34:2107–2116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Hagemeyer CE, Hohmann JD, Leitner
E, Armstrong PC, Jia F, Olschewski M, Needles A, Peter K and Ahrens
I: Novel single-chain antibody-targeted microbubbles for molecular
ultrasound imaging of thrombosis: Validation of a unique
noninvasive method for rapid and sensitive detection of thrombi and
monitoring of success or failure of thrombolysis in mice.
Circulation. 125:3117–3126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Warram JM, Sorace AG, Mahoney M, Samuel S,
Harbin B, Joshi M, Martin A, Whitworth L, Hoyt K and Zinn KR:
Biodistribution of P-selectin targeted microbubbles. J Drug Target.
22:387–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leong-Poi H, Christiansen J, Klibanov AL,
Kaul S and Lindner JR: Noninvasive assessment of angiogenesis by
ultrasound and microbubbles targeted to alpha (v)-integrinss.
Circulation. 107:455–460. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dabek J, Ligus J and Szota J:
Oligonucleotide microarray and QRT-PCR study of adhesion protein
gene expression in acute coronary syndrome patients. Inflammation.
33:398–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lawson C and Wolf S: ICAM-1 signaling in
endothelial cells. Pharmacol Rep. 61:22–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mulvihill NT and Foley JB: Inflammation in
acute coronary syndromes. Heart. 87:201–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Benson V, McMahon AC and Lowe HC: ICAM-1
in acute myocardial infarction: A potential therapeutic target.
Curr Mol Med. 7:219–227. 2007. View Article : Google Scholar : PubMed/NCBI
|