Introduction
Tuberculosis (TB) is one of the leading infectious
diseases worldwide. The latest surveillance data by the World
Health Organization (WHO) reveals that in 2006, there were 9.2
million new cases and 1.7 million deaths from TB (1). The TB pandemic has continued despite
widespread use of the only available licensed TB vaccine Bacille
Calmette-Guérin (BCG) under the WHO expanded program on
immunization and despite the use of directly observed chemotherapy
programs for those diagnosed with active disease. Although BCG
seems to provide protection against disseminated disease in
newborns and children, its efficacy against pulmonary TB is poor
especially in adults, which highlights the need for a better
vaccine regimen (2).
During the past decades, progress has been made in
explanation of BCG failure. The main potential vaccination
strategies against TB included recombinant BCG (rBCG), attenuated
strains of Mycobacterium tuberculosis (M. tb),
subunit vaccine approaches and live, and non-replicating viral
vector-based delivery systems. The most important strategy is to
stimulate more potent immune responses against M. tb. In
production of rBCG, three strategies have been mainly used. The
first is based on the restoration of deleted BCG genes (deleted in
the process of M. bovis attenuation) that encode immunogenic
antigens. Another approach is based on rBCG that produces large
amounts of strong immunogenic protective antigens, and the third is
to construct rBCG that secrete cytokines relevant in anti-TB immune
response such as interleukin (IL)-2, interferon (IFN)-γ, or IL-18
(3–5). These cytokines have been shown to
activate macrophages and kill or control the growth of the M.
tb. Although imperfect, INF-γ remains the best available
correlate of protective immunity (6,7).
Vaccines which failed to induce sufficient levels of IFN-γ are
unable to protect effectively against tuberculosis in animal models
(8).
Antigen 85B (Ag85B) is a secretory and immunogenic
protein of M. tb and BCG with mycolyl-transferase activity,
which is required for mycobacterial cell wall synthesis (9), and a study has observed rBCG30
overexpressing Ag85B resulted in better protection as compared to
its parental BCG strain (10,11).
Moreover, there was no significant safety issues in a phase I
clinical trial of rBCG30 in more than 30 healthy adult volunteers
(12,13). In the present study, the ag85b
and ifn-γ genes were inserted into Mycobacterial-E.
coli shuttle vector pMV361 in order to form novel rBCG viz
rBCG::Ag85B-IFN-γ and its efficacy was tested in C57BL/6 mice. The
Ag85B-IFN-γ fusion protein was expressed under control of the
Mycobacterial heat shock protein 60 (hsp60) promoter and the
ag85b signal sequence was utilized to secret it into the
supernatant of the culture continuously. Expression of the fusion
protein was readily detectable and IFN-γ bioactivity maintained
unaffected.
Compared with BCG, rBCG::Ag85B-IFN-γ was
substantially more active in inducing the production of IFN-γ and
tumor necrosis factor (TNF)-α from mouse splenocytes by ELISPOT and
enzyme-linked immunosorbent assay (ELISA). ELISA analysis for Ag85B
special IgG, IgG1, and IgG2c displayed that rBCG::Ag85B-IFN-γ can
induce high level antibody titer and facilitate Th1 type immune
response. Also, rBCG::Ag85B-IFN-γ improved nitric oxide (NO)
production level and enhanced antigen-specific splenocyte
proliferation.
Expression of various surface molecules on activated
macrophages is required for optimal development of protective T
cell response in tuberculosis. We characterized the expression of
surface markers in human monocytes such as THP-1 cell line
infection with rBCG::Ag85B-IFN-γ. It increased expression of CD80,
CD86 and CD40 significantly compared with BCG.
Flow cytometry analysis showed that
rBCG::Ag85B-IFN-γ activated CD4+ T cells remarkably and
improved CD8+ T cells gradually with time. Although
IFN-γ is clearly necessary, using it as a single immune parameter
may not always sufficient to bring protection (14). Multifunctional T cells secreting
IFN-γ, TNF-α, and IL-2 have been shown to correlate with protection
in Leishmania major infection in mice (15). We assess all combinations of IFN-γ,
TNF-α and IL-2 at the single-cell level by intracellular cytokine
staining and parameter flow cytometry analysis.
The immunostimulatory properties of
rBCG::Ag85B-IFN-γ increased cell mediated immune response compared
to the parent BCG strain. Considering the action of IFN-γ in
clearing the infection pathogen, rBCG::Ag85B-IFN-γ may be a
potential candidate vaccine and a superior agent for
immunotherapeutic protocols in the future.
Materials and methods
Bacterial strains and cultures
M. bovis BCG (obtained from Shanghai Institute of
Biological Products Co., Ltd., Shanghai, China), M. tb H37Rv and
rBCG::Ag85B-IFN-γ (constructed as below) were grown in Middlebrook
7H9 medium (BD Biosciences, Sparkers, MD, USA) supplemented with
0.5% glycerol, 0.05% Tween-80 and 10% ADC (BD Biosciences) or on
solid Middlebrook 7H10 Agar medium (BD Biosciences) supplemented
with 0.5% glycerol and 10% ADC. When required, the antibiotic
kanamycin was added at a concentration of 25 µg/ml. E. coli DH5-α
was grown in Luria-Bertani medium (Oxoid Ltd., Basingstoke,
Hampshire, UK) and used for cloning and extracting plasmid.
Construction of a rBCG expression
Ag85B and IFN-γ
Coding sequences for ag85b (containing signal
sequence) and mouse ifn-γ were amplified from the M. tb H37Rv
genomic DNA and mouse spleen tissue cDNA respectively by PCR using
the primers shown in Table I. The
ag85B and murine ifn-γ coding regions were cloned into the
Mycobacterial-E. coli shuttle vector pMV361, in which gene
expression is under the control of the strong M. bovis hsp60
promoter. Inserted genes were sequencing confirmed and the rBCG
substrain was produced by electroporation transfecting BCG-Danish
strain cells with the recombinant plasmid pMV361-Ag85B-INF-γ. The
transformed BCG was plated on 7H10 solid medium supplemented with
25 µg/ml kanamycin and grown at 37°C for 3 weeks. The resulting
recombinant clones containing pMV361-Ag85B-INF-γ were designated
rBCG::Ag85B-IFN-γ.
 | Table I.Primers used to engineer the
recombinant Bacille Calmette-Guérin. |
Table I.
Primers used to engineer the
recombinant Bacille Calmette-Guérin.
| Primers | Sequence |
|---|
| Ag85B | Primer F:
5′-TGTGAATTCATGACAGACGTGAGCCGAAAG-3′ |
|
| (EcoRI) |
|
| Primer R:
5′-TACGGATCCGCCGGCGCCTAACGAACTCTG-3′ |
|
| (BamH1) |
| IFN-γ | Primer F:
5′-TCTGGATCCATGAACGCTACACACTGC-3′ |
|
| (BamH1) |
|
| Primer R:
5′-ACTAAGCTTTCAGCAGCGACTCCTTTTCC-3′ |
|
|
(HindIII) |
Western blotting detection of
expressed Ag85B and INF-γ
Expression of the recombinant fusion protein by
rBCG::Ag85B-INF-γ was determined by western blotting. The
individual rBCG clone was selected and grown in Sauton medium (0.25
g MgSO4·7H2O, 0.25 g
K2HPO4, 1.0 g citric acid, 4.0 g sodium
glutamate, 30 ml glycerol, 5 mg ZnSO4 and 25 mg
ferrum-ammonium citrate in 500 ml ddH2O) containing 25
µg/ml kanamycin. After 2 weeks of growth, protein expression was
induced by heating at 45°C for 60 min. The bacterial cultures were
centrifuged at 8,000 × g for 20 min. The culture supernatants were
concentrated as previously described (16) for western blot detection. A total of
25 µl of the concentrated culture filtrates from rBCG::Ag85B-IFN-γ
were analyzed for expression of the recombinant fusion protein by
western blotting using anti-Ag85B rabbit polyclonal sera.
Determination of IFN-γ levels and
bioactivity in the bacterial culture supernatants
The ability of the bacilli to secrete murine
recombinant IFN-γ was verified by measuring the concentration of
the cytokine by ELISA in the bacterial culture supernatants
(17). The mouse INF-γ Quantikine
ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA) was used to
determine the IFN-γ activity of rBCG::Ag85B-IFN-γ following the
manufacturer's instructions. The bioactivity of IFN-γ produced by
the rBCG::Ag85B-IFN-γ strain was calculated according to the
standard curve. The experiment was performed twice.
Animal vaccination
Five-week-old female C57BL/6 mice (Slaccas
Laboratory Inc., Shanghai, China) were used in the ABSL-2 animal
facility at Second Military Medical University (Shanghai, China).
Mice received free access to food and water throughout this study.
All experiments were performed in accordance with the local Ethics
Committee guidelines. C57BL/6 mice (12 per group) were immunized
subcutaneously at the dosage 5×106 CFU of BCG or rBCG in
200 µl phosphate-buffered saline (PBS). Mice were sacrificed to
analyze the immune responses at 6 and 12 weeks after the
immunization. As an additional control, mice of the other group
were injected with PBS only. The experiment was repeated twice.
ELISPOT assay for IFN-γ from
splenocyte cultures
At 6 and 12 weeks after the vaccination, mice were
sacrificed, and their spleens removed aseptically into RPMI-1640
medium containing 10% fetal calf serum (FCS), 2 mM glutamine, 50 µM
β-mercaptoethanol, 100 µg/ml streptomycin and 100 U/ml penicillin.
Spleens were gently ground through a 70 µm cell strainer, and the
single-cell suspensions were prepared with Lympholyte-M (Cedarlane
Laboratories, Burlington, NC, USA) by density-gradient
centrifugation procedure. We used the mouse IFN-γ ELISPOT kit
(U-CyTech Biosciences, Utrecht, The Netherlands). Analyses were
conducted on the splenocytes from five mice each group. Diluted
cells were added to the wells of the ELISPOT plate at
5×105 cells per well in culture medium as described
above containing purified protein derivatives (PPD, 5 µg/ml), Ag85B
(5 µg/ml) or phytohemagglutinin (2 µg/ml, as positive control) as
stimulus. The plates were incubated at 37°C, 5% CO2,
100% humidity for 36 h and the IFN-γ secreting T cells were
evaluated. Spots were counted by use of an immunospot image
analyzer. Wells with <5 spots were not used for
calculations.
ELISA analysis for IFN-γ, TNF-α and
IL-4
Single cell suspensions were acquired and diluted in
2 ml culture medium containing the same concentration of stimulus
as described above to the 12-well plates at 1×106 cells
per well. The plates were incubated at 37°C, 5% CO2,
100% humidity for 36 h. The suspensions were collected of the
splenocyte cultures for sandwich ELISA to detect the level of the
cytokines. The cell deposits were harvested to prepare for flow
cytometry analysis. We used the mouse IFN-γ ELISA kit, TNF-α ELISA
kit and IL-4 ELISA kit (eBioscience, Inc., San Diego, CA, USA). The
concentration of the cytokines was calculated in the suspension
according to the standards curve.
NO production determination
This procedure for NO determination was based on the
Griess reaction. Culture supernatants were acquired as described
above. A total of 100 µl of the splenocyte culture supernatants or
sodium nitrite standard 100 µl were mixed with an equal volume of
Griess reagent (Sigma-Aldrich, St. Louis, MO, USA). In addition
anaerobic conditions were achieved by purging the reaction mixture
with high purity argon gas for ≥5 min. After 5 min at room
temperature, the absorbance at 550 nm (A550) was measured. The NO
concentration was calculated in the culture supernatants according
to the sodium nitrite standard.
ELISA analysis for IgG, IgG1, and
IgG2c
Sera were collected from the immunized mice to
monitor the antibody response by ELISA. Corning Costar 9018 ELISA
plates (Corning Costar, Inc., Corning, NY, USA) were coated with
Ag85B (5 µg/ml) overnight at 4°C. The plates were blocked with PBS
containing 1% bovine serum albumin (BSA; Bovogen Biologicals Pty
Ltd., East Keilor, VIC, Australia). Sera were added at serial
2-fold dilution (beginning at a 1/50 dilution). After washing, and
addition of horseradish peroxidase conjugated goat anti-mouse IgG,
IgG1 and IgG2c (SouthernBiotech, Birmingham, AL, USA) diluted at
1/5,000, 1/1,000 and 1/1,000, respectively in blocking buffer (PBS
containing 1% BSA) for 1 h, the plates displayed the staining by
o-phenylenediamine substrate. Antibody titers were expressed as
reciprocal end point titers. The mean antibody titers were the
antilog of the average logarithm for antibody titer of 5 mice.
Antigen-specific splenocyte
proliferation
Spleens of the immunized mice were removed
aseptically to acquire the lymphocytes as described above. The
isolated lymphocytes were washed in medium twice and cultured in
RPMI-1640 + 10% FCS containing 100 µg/ml streptomycin and 100 U/ml
penicillin. Cells were adjusted to 1×105 cells/ml and
0.1 ml was plated with either 20 µl PBS or 20 µl Ag85B (300 µg/ml)
in 96-well plates. Cells were cultured for 2 days in 5%
CO2, 100% humidity and 37°C for MTS assay (CellTiter
96® Aqueous Non-Radioactive Cell Proliferation Assay
kit; Promega Corp., Madison, WI, USA). The proliferation was
assessed with splenocyte stimulation index (SI) (18).
Flow cytometry analysis for
CD4+ and CD8+ subsets
The spleen tissue and single cell suspensions were
prepared as described above in cell staining buffer (BioLegend,
Inc., San Diego, CA, USA). The debris was removed by filtration of
the cell suspension through 70 µm nylon mesh strainer. Viable cells
were counted and suspended in cell staining buffer at
1×107 cells/ml. Cell suspension (100 µl) was distributed
into aseptic Eppendorf plastic tubes. Cells were blocked with PBS
containing 1% BSA. Isotype controls of fluorescein isothiocyanate
(FITC) and phycoerythrin (PE) conjugated anti-mouse IgG2b were
used. FITC 0.25 µg anti-mouse CD4 and 0.25 µg PE anti-mouse CD8
(eBioscience, Inc.) per million cells in a 100 µl total staining
volume were added, then incubated in the dark at 4°C for 20 min.
Cells were washed twice and the cell pellets were resuspend in 0.5
ml cell staining buffer for analyzes by flow cytometry
(FACSCalibur; BD Biosciences). A total of 10,000 events per test
were collected.
THP-1 cell culture and
rBCG::Ag85B-IFN-γ treatment
THP-1 cells were cultured in complete medium
RPMI-1640 supplemented with 10% FBS. The cells were seeded in
complete medium containing Phorbol-12-myristate 13-acetate (20
ng/ml) in 12-well plates at 1×106 cells/well for 18 h
and induced to macrophages. Then the monocyte derived macrophage
were treated with PBS (control group), BCG (1×107 CFU)
or rBCG::Ag85B-IFN-γ (1×107 CFU) for 36 h. Supernatants
were collected for cytokine assays, and cells were collected for
surface marker flow cytometry analysis.
Cells surface marker flow cytometry
analysis
For flow cyto-metry analysis, cells were collected,
washed and resuspended in PBS supplemented with 1% FBS, thereafter
cells were stained with fluorochrome-conjugated mAb. We divided
samples into two parts at 1×106 cells/tube. One was
stained with FITC-conjugate anti-human CD40 and PE conjugate HLA-DR
and the other was stained with FITC conjugate CD86 and PE conjugate
CD80 (Caltag Laboratories; Invitrogen Life Technologies, Carlsbad,
CA, USA). Cells were incubated in the dark at 4°C for 20 min.
Following two washes, cell pellets were resuspend in 0.5 ml cell
staining buffer for analyses by flow cytometry. A total of 10,000
events per test were collected. Data were analyzed using CellQuest
software (version 7.5.3.; BD Biosciences) or FACS Express 3
software (DeNovo Software, Ontario, ON, Canada).
Intracellar cytokine staining
analysis
The peripheral blood from the vaccinated mice were
used to acquire peripheral blood mononuclear cell (PBMC)
single-cell suspensions using Histopaque-1077 kit (Sigma-Aldrich)
based on density centrifugation. The cells were diluted in 2 ml
culture medium containing the same concentration of stimulus as
described above to the 12-well plate at 1×106 cells per
well. The plate was incubated at 37°C, 5% CO2, 100%
humidity for 36 h. Brefeldin A solution (eBioscience, Inc.) of 1 µl
was added into the cell culture for 4 h to inhibit the cytokine
transport. Cells were harvested and 100 µl of Reagent A was added
(fixation medium, fix and perm cell permeablization reagent; Caltag
Laboratories; Invitrogen Life Technologies) and incubated for 15
min at room temperature. Cells were washed, and the cell pellets
resuspended, then adding 100 µl of Reagent B (permeabilization
medium) and the 1 µl FITC conjugate anti-mouse IL-2, 1.25 µl PE
conjugate IFN-γ and 20 µl PerCP-Cy5.5 conjugate TNF-α (eBioscience,
Inc.) per million cells. Vortexed and incubated for 20 min in the
dark at 4°C. Cells were wash and the cell pellets resuspend in 0.5
ml of cell staining buffer for analyzes by flow cytometry.
Data analysis
Statistical significance was determined using
one-way ANOVA with Kruskal-Wallis tests, Tukey and Dunnett tests of
GraphPad Prism 5.0 for Windows (GraphPad Software, Inc., San Diego,
USA). PBS group was regard as negative control. All the other
groups were compared with BCG group. P<0.05 was considered to
indicate a statistically significant difference.
Results
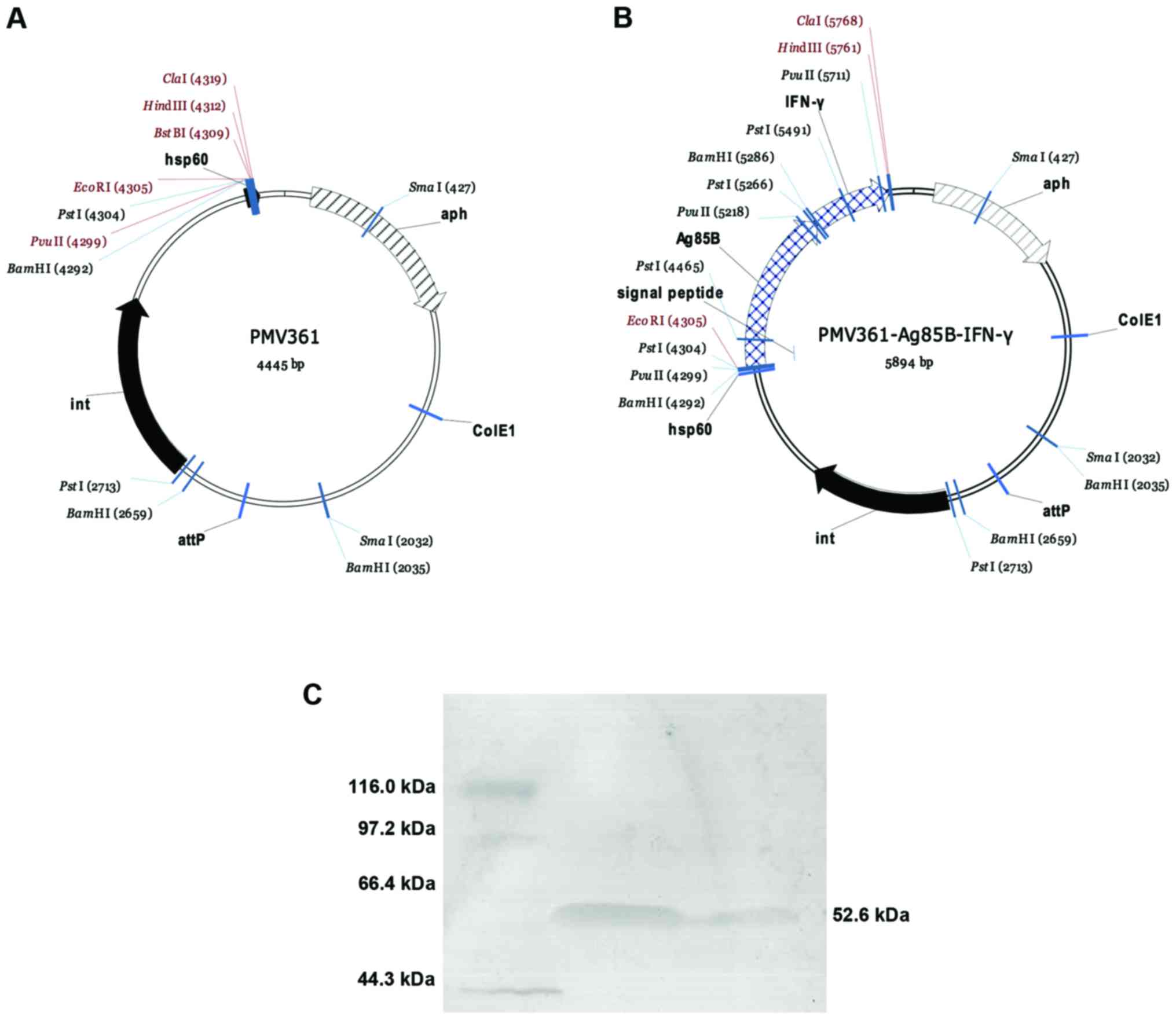
Construction of rBCG secreting Ag85B
and IFN-γ
Novel rBCG capable of expressing Ag85B and IFN-γ, on
a shuttle vector pMV361 is shown in (Fig. 1A). Further, rBCG::Ag85B-IFN-γ
expressed and secreted a relatively high level of fusion protein as
shown in Fig. 1B. The bioactivity of
IFN-γ produced by the rBCG::Ag85B-IFN-γ strain was 21.85±7.06 pg/ml
(equal to 2.2×106 U/mg).
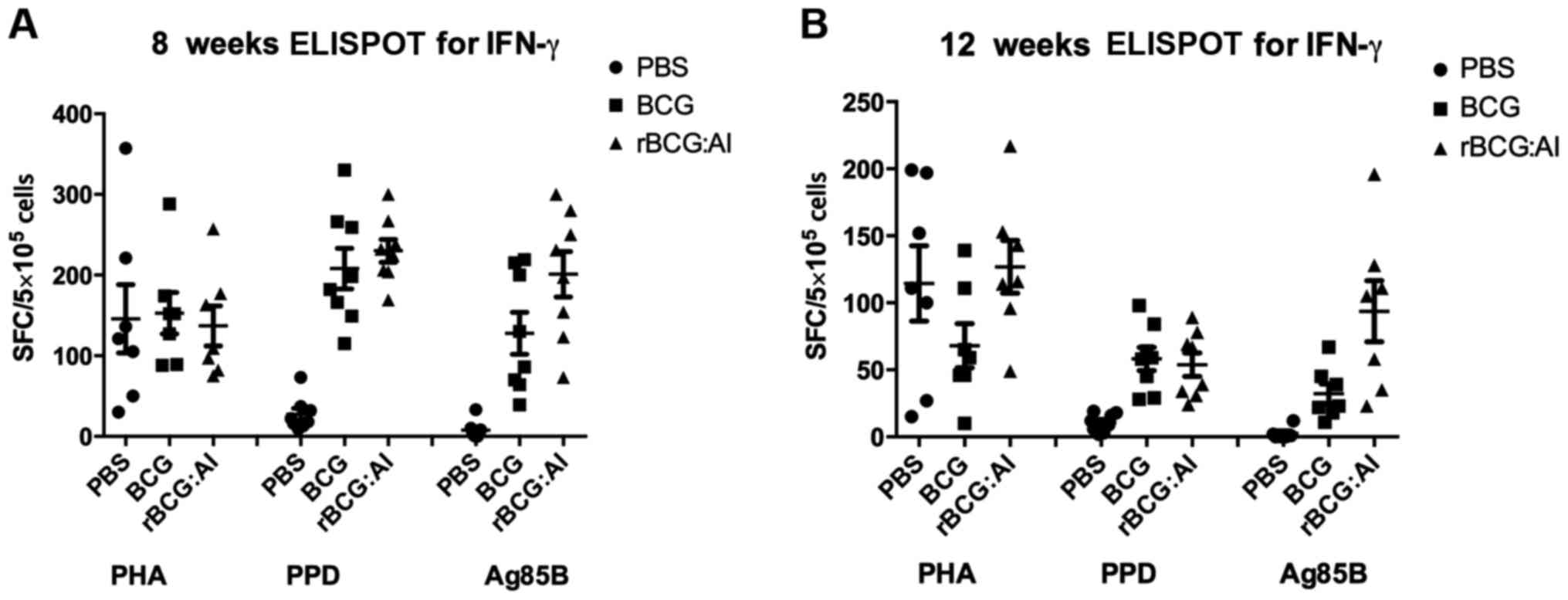
Cytokine response
Cell-mediated immune responses were evaluated by
IFN-γ ELISPOT assay and sandwich ELISA analysis for IFN-γ, TNF-α
and IL-4. The amount of IFN-γ secreted is shown in Fig. 2. It was noted that in response to
purified Ag85B, IFN-γ secretion at 6 weeks was higher than that at
12 weeks. The IFN-γ level of rBCG::Ag85B-IFN-γ was 1.6- and 3-fold
that of BCG at 6 and 12 weeks, respectively.
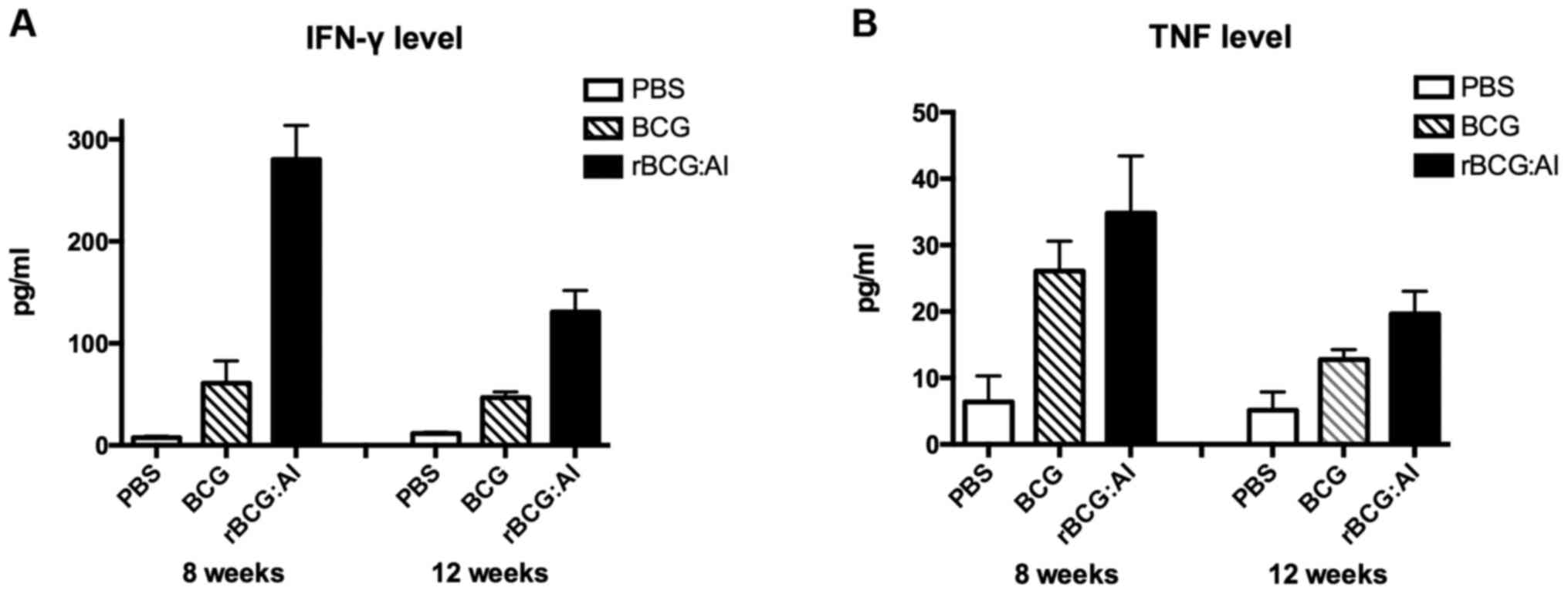
Moreover, the amount of IFN-γ secreted exhibited a
decline at 12 weeks in all the groups. The effect of
rBCG::Ag85B-IFN-γ on the Th1 or Th2 immune response is shown in
Fig. 3. Mice immunized with
rBCG::Ag85B-IFN-γ showed a significant increase in release of IFN-γ
in response to stimulation with Ag85B compared with BCG at 6 and 12
weeks. Mice immunized with rBCG::Ag85B-IFN-γ showed no difference
in releasing TNF-α in response to Ag85B compared with BCG at 6
weeks, but at 12 weeks they showed significantly higher level than
those immunized with BCG.
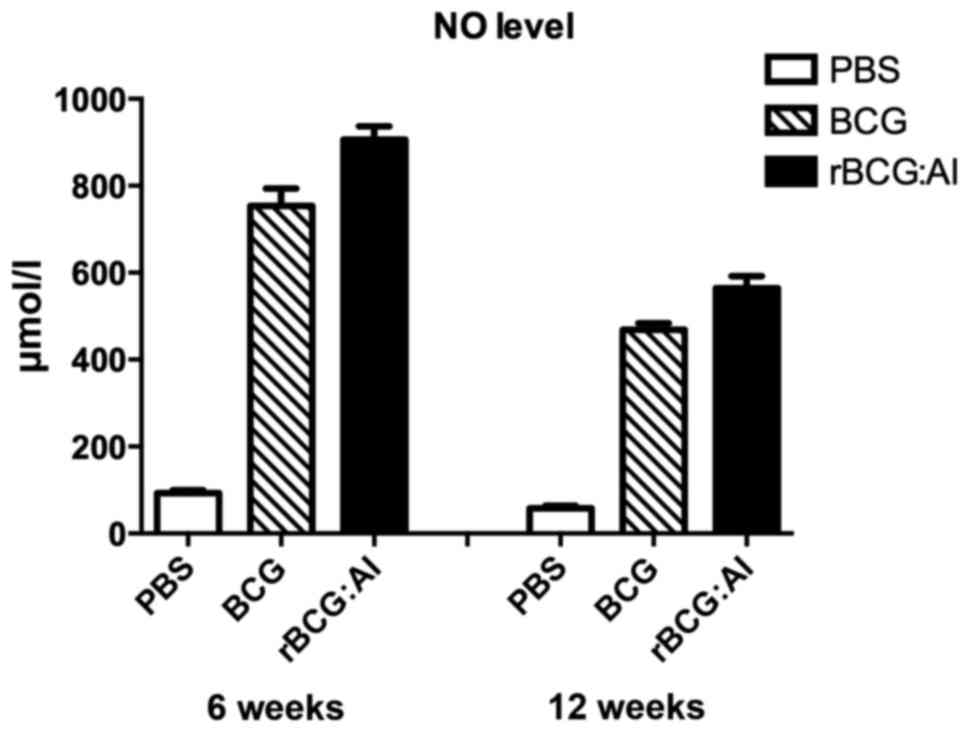
NO production levels
The NO levels of the culture supernatant of the mice
vaccinated with rBCG::Ag85B-IFN-γ or BCG (>450 µmol/l) showed
higher levels than the mice vaccinated with PBS (<100 µmol/l) at
6 and 12 weeks. The NO level of the rBCG::Ag85B-IFN-γ group was
significant higher than BCG group at 6 and 12 weeks (Fig. 4).
Humoral immune responses
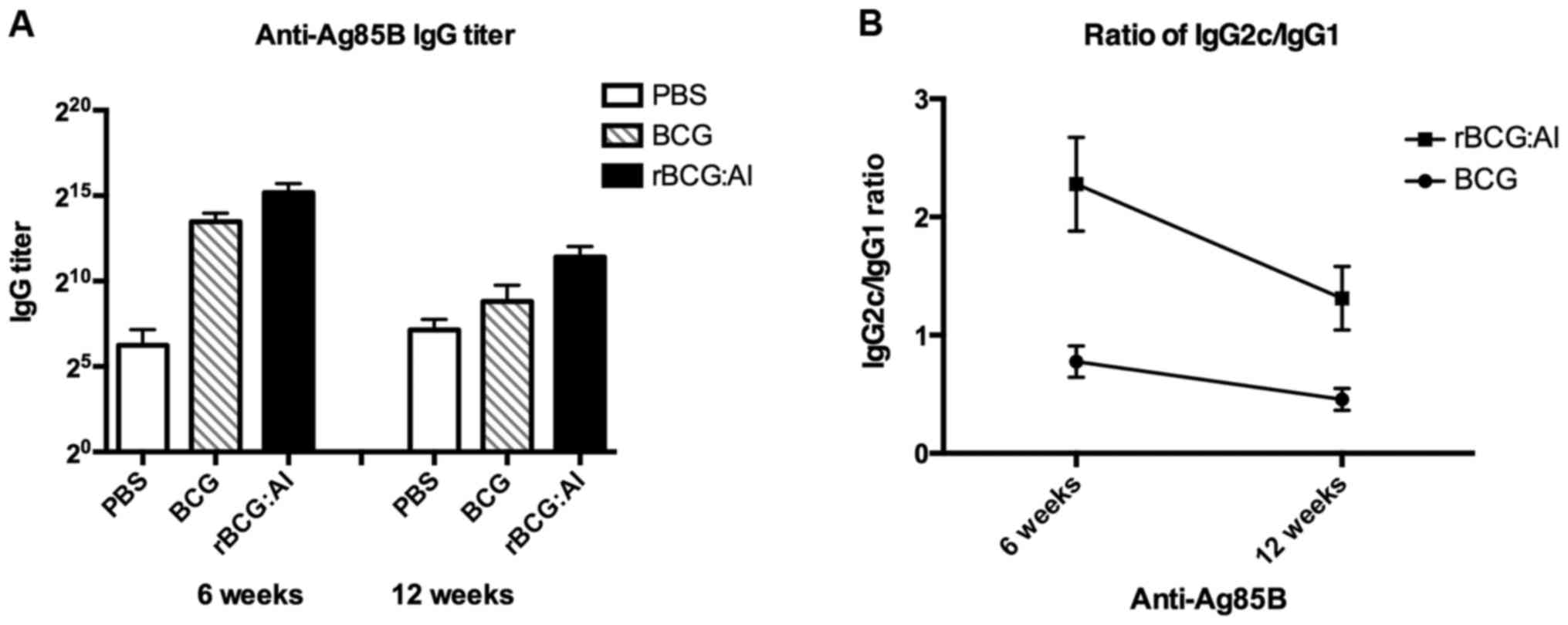
Fig. 5A and B
illustrate the levels of antibody response in the sera of mice
against purified Ag85B. Compared with the PBS control group, mice
vaccinated either with BCG or rBCG::Ag85B-IFN-γ strains induced
higher levels of antibody against Ag85B. However, the mean IgG
titers were as high as 1:33,900 and 1:2,460 in mice immunized with
rBCG::Ag85B-IFN-γ at 6 and 12 weeks, which were significant higher
than those immunized with BCG (1:10,600 and 1:300, p<0.01). The
antibody titers of the PBS control group were only ~1:50.
Fig. 5C illustrates
the antibody levels of IgG1 and IgG2c isotype against Ag85B. The
ratios of IgG2c/IgG1 were calculated to determine the induction of
Th1 or Th2 responses in animals (19). As a result in response to Ag85B, the
ratio of mice immunized with rBCG::Ag85B-IFN-γ was much higher than
that of BCG at 6 weeks and the ratio was higher than BCG at 12
weeks also. The results collectively revealed that the capability
of the induction of Th1 protection immune response of
rBCG::Ag85B-IFN-γ was higher than BCG in the experiments.
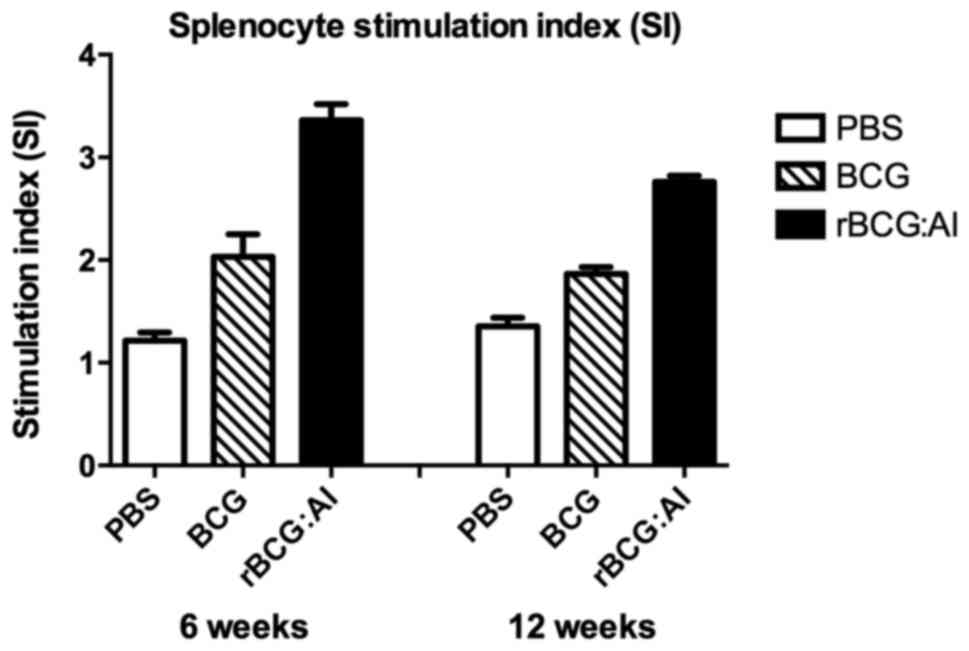
Antigen-specific splenocyte
proliferation
The antigen-specific proliferation of the
splenocytes isolated from the mice immunized with rBCG::Ag85B-IFN-γ
was significantly higher than that of BCG or PBS as determined by
SI also at 6 or 12 weeks (Fig.
6).
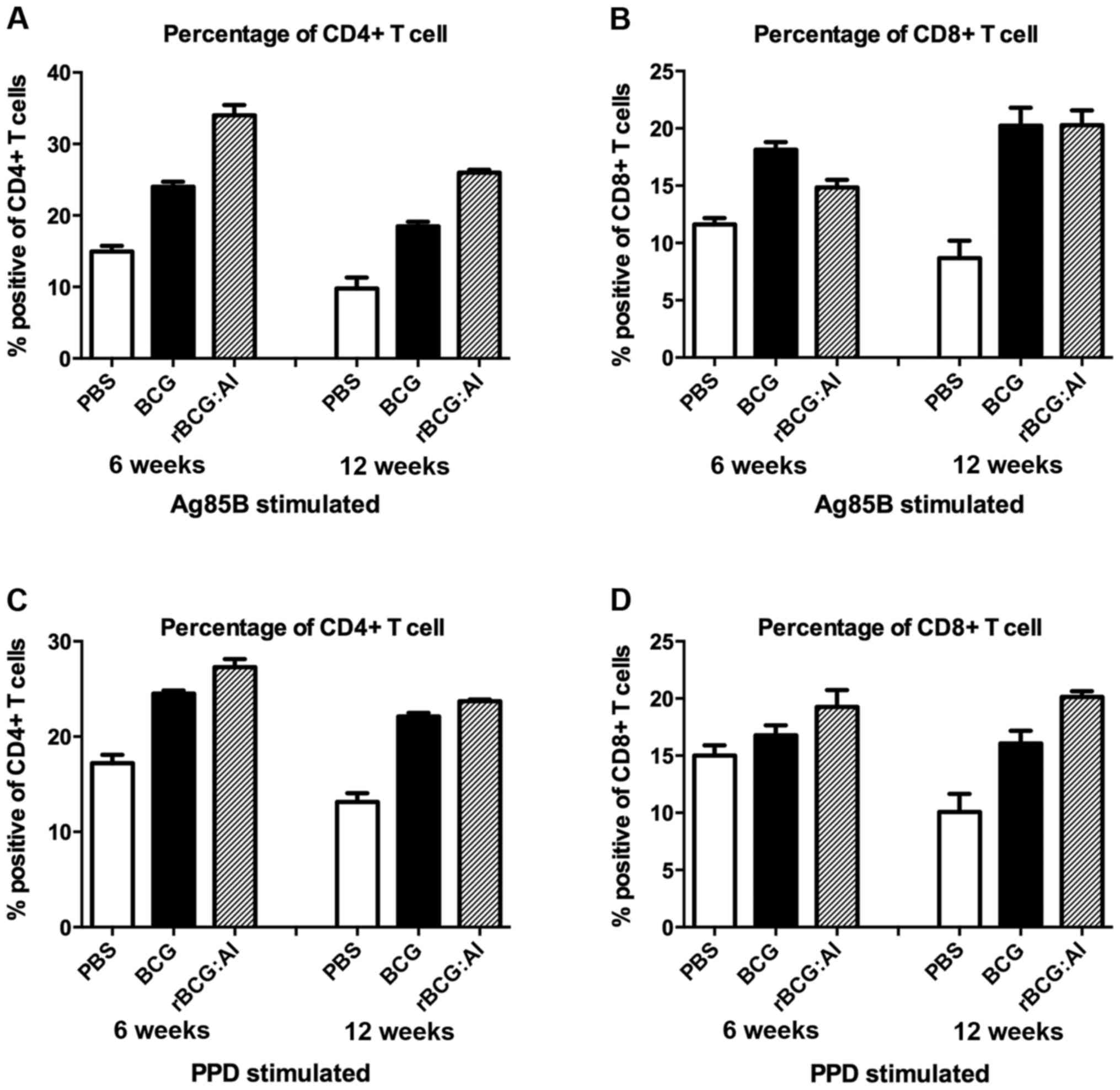
CD4+ and CD8+ T
cell subset analysis
After vaccination with different vaccines, the
lymphocyte subsets were examined for differences in their
percentages by flow cytometry. As shown in Fig. 7, the levels of the CD4+
and CD8+ T cell populations were significantly higher in
the rBCG::Ag85B-IFN-γ or BCG group than that in the PBS group. The
percentage of CD4+ T cells of rBCG::Ag85B-IFN-γ was
significant higher than that of BCG at 6 and 12 weeks whether
stimulated with Ag85B or PPD.
The percentage of CD8+ T cells of
rBCG::Ag85B-IFN-γ was significant lower than that of BCG at 6 weeks
when stimulated with Ag85B, but at 12 weeks, they showed no
difference. The percentage of CD8+ T cells in the mice
vaccinated with rBCG::Ag85B-IFN-γ showed no difference with BCG at
6 weeks when stimulated with PPD, but at 12 weeks they were higher
than that of BCG. Moreover, the CD4+/CD8+
ratio in mice immunized with rBCG::Ag85B-IFN-γ was significant
higher than that of BCG. Therefore, the rBCG::Ag85B-IFN-γ enhanced
CD4+ T cells were significantly higher and hence showed
increased CD4+/CD8+ ratio.
Cells surface marker expression
level
rBCG::Ag85B-IFN-γ enhanced expression of CD80, CD86,
CD40 and HLA-DR. Changes in expression were found to be more
significant for CD80, CD86 and CD40. The cells infected by
rBCG::Ag85B-IFN-γ produced a 12–38% increase in the percentage of
positive cells of these markers compared to infected with BCG and
demonstrated small increases in expression of HLA-DR. ALs
especially showed a trend of increasing CD80 expression when
compared with BCG.
Multifunctional T cell analysis
Using multiparameter flow cytometry, we compared the
quality of the immune response in different vaccination groups to
Ag85B. PBMC were analyzed for their intracellular expression of
IFN-γ, TNF-α and IL-2, allowing us to follow the frequency of cells
that produced various combinations of IFN-γ, TNF-α and IL-2
positive for either a single or up to three cytokines
simultaneously. The results showed half of the responses in the
rBCG::Ag85B-IFN-γ group was 3+ and 2+ T
cells, whereas, the response after BCG vaccination was dominated by
1+ and 2+ T cells.
Discussion
rBCG has several advantages over other novel
vaccines by reasons of low cost, easy production and convenient
storage. However, BCG could not be easily replaced by novel vaccine
candidates despite the controversies over efficiency. rBCG
expressing protective antigens represents a very promising
possibility for use as an efficient vaccine against TB. It could be
envisaged that continuous overexpression of selected candidate
epitopes as the case for a live vaccine like BCG may be pivotal for
vaccine efficacy (7). The results of
phase 1 trial of rBCG30 provide proof of principal that rBCG30
could safely enhance human TB immunity and support further
development of rBCG overexpressing Ag85B for TB vaccination
(13). Moreover, cytokines are
considered as potent immune responses and the potent IFN-γ
producers are crucial for protection against M. tb (17,20).
IFN-γ, which synergizes with TNF-α, is central to the activation of
macrophages and the isolation of M. tb inside the granuloma
(2). A previous study indicated that
BCG strains secreting cytokines, in particular GM-CSF, IL-2 and
IFN-γ, could modify and potentiate the immune response to BCG
antigens (3). The local expression
of IFN-γ by the rBCG results in more efficient bacterial clearance
which is accompanied by a reduction in tissue pathology (21). Similarly, mouse IFN-γ was coexpressed
with Ag85B in the present study. Further it has been proved that
the IFN-γ produced by the rBCG::Ag85B-IFN-γ strain was bioactive
and was probably negatively affected by fusion Ag85B sustained as
an efficient antigen. It is believed that the stronger cell
mediated immune response was induced by rBCG::Ag85B-IFN-γ and may
be due to either IFN-γ in the fusion construct promoting the
development of immunity or that Ag85B may act as an efficient
antigen.
The dominant immunological consequence of infection
with M. tb or vaccination with rBCG is priming of
Th1-oriented CD4+ T cells which in turn, potentiates the
antimicrobial function of macrophages through release of IFN-γ.
Interestingly, CD4+ T cells prominently produce IFN-γ
during the first few weeks of infection, while CD8+ T
cell production of IFN-γ occurs later. Thus, CD4+ T
cells play a greater role in early defense against M. tb
infection, and both CD4+ and CD8+ T cells
control latent mycobacteria (22).
Both the lymphocyte proliferation and IFN-γ secretion in response
to Ag85B, including the percentage CD4+ T cells of
rBCG::Ag85B-IFN-γ were significant higher than those of BCG.
IFN-γ production may act in part by inducing the
production of inducible NO synthase. Inhibitors of the NO
production aggravate tuberculosis infection (23). Activated macrophages could act
directly as effector cells by producing microbicidal molecules such
as reactive oxygen intermediates, NO and lysosomal enzymes
(24). rBCG::Ag85B-IFN-γ induced
higher NO levels than BCG in the splenocyte culture and suggested
that it activated macrophages efficiently. The levels of IgG1 and
IgG2c reflect the stimulation of Th2 and Th1 cells, respectively.
Also in the present study, rBCG::Ag85B-IFN-γ increased the Th1
responses, and weaken the Th2 responses when memory was suppressed
by regulatory mechanisms.
In addition to production of cytokines, macrophages
also influence T cell response. Expression of various surface
molecules on activated macrophages, including MHC-II (HLA-DR),
CD80, CD86 and CD40, is required for optimal development of
protective T cell response in tuberculosis (25). The use of costimulation-based
immunomodulators may have significant repercussions on the
induction of host protective immunity against tuberculosis
(26). In light of previous work, we
investigated how rBCG::Ag85B-IFN-γ modulated the activities of
macrophages by characterizing the expression of surface markers
during infection. THP-1 cell derived macrophages increased the four
surface marker expression significantly when infected with
rBCG::Ag85B-IFN-γ. HLA-DR is a major antigen presenting molecule
and its expression is impaired upon infection by M. tb
(27). High levels of expression of
MHC-II along with CD40, CD80, and CD86 contribute to the efficiency
of antigen presenting cells for stimulating T cells (28). rBCG::Ag85B-IFN-γ improved the
capabilities of multifunctional T cell population compared with BCG
and enhanced IL-2 level (IL-2+, IL-2+
TNF-α+ and IL-2+ TNF-α+ IFN-γ)
significantly. IL-2-expressing cells were significantly more likely
to have a central memory phenotype. Furthermore, the induction of
memory T cells with multifunctional properties appears to provide a
good correlate for protection against tuberculosis (29).
In conclusion, the present study indicated that
rBCG::Ag85B-IFN-γ strain could enhance the cell mediated immune
response. Moreover, it could be a good candidate vaccine against TB
and may be a superior agent for immunotherapeutic vaccine in the
future. However, the safety as well as stability concerns of the
rBCG::Ag85B-IFN-γ should be considered.
Acknowledgements
This study was supported by the Science Research
Project of Eleventh Five-Year Plan grant (nos. 2008ZX-103-013 and
2008ZX-103-011) and the National High Technology Research and
Development Program of China (863 Program, grant no.
2006AA02Z445).
References
|
1
|
World Health Organization (WHO): Global
tuberculosis control: Surveillance, planning, financing: WHO report
2008. WHO; Geneva: 2008
|
|
2
|
Skeiky YA and Sadoff JC: Advances in
tuberculosis vaccine strategies. Nat Rev Microbiol. 4:469–476.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murray PJ, Aldovini A and Young RA:
Manipulation and potentiation of antimycobacterial immunity using
recombinant bacille Calmette-Guérin strains that secrete cytokines.
Proc Natl Acad Sci USA. 93:934–939. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Donnell MA, Aldovini A, Duda RB, Yang H,
Szilvasi A, Young RA and DeWolf WC: Recombinant Mycobacterium
bovis BCG secreting functional interleukin-2 enhances gamma
interferon production by splenocytes. Infect Immun. 62:2508–2514.
1994.PubMed/NCBI
|
|
5
|
Luo Y, Yamada H, Chen X, Ryan AA, Evanoff
DP, Triccas JA and O'Donnell MA: Recombinant Mycobacterium
bovis bacillus Calmette-Guérin (BCG) expressing mouse IL-18
augments Th1 immunity and macrophage cytotoxicity. Clin Exp
Immunol. 137:24–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flynn JL and Chan J: Immunology of
tuberculosis. Annu Rev Immunol. 19:93–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eddine Nasser A and Kaufmann SH: Improved
protection by recombinant BCG. Microbes Infect. 7:939–946. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agger EM and Andersen P: Tuberculosis
subunit vaccine development: On the role of interferon-γ. Vaccine.
19:2298–2302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D'Souza S, Rosseels V, Romano M, Tanghe A,
Denis O, Jurion F, Castiglione N, Vanonckelen A, Palfliet K and
Huygen K: Mapping of murine Th1 helper T-Cell epitopes of mycolyl
transferases Ag85A, Ag85B, and Ag85C from Mycobacterium
tuberculosis. Infect Immun. 71:483–493. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horwitz MA and Harth G: A new vaccine
against tuberculosis affords greater survival after challenge than
the current vaccine in the guinea pig model of pulmonary
tuberculosis. Infect Immun. 71:1672–1679. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horwitz MA, Harth G, Dillon BJ and
Maslesa-Galic' S: Recombinant bacillus calmette-guerin (BCG)
vaccines expressing the Mycobacterium tuberculosis 30-kDa
major secretory protein induce greater protective immunity against
tuberculosis than conventional BCG vaccines in a highly susceptible
animal model. Proc Natl Acad Sci USA. 97:13853–13858. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horwitz MA: Recombinant BCG expressing
Mycobacterium tuberculosis major extracellular proteins.
Microbes Infect. 7:947–954. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoft DF, Blazevic A, Abate G, Hanekom WA,
Kaplan G, Soler JH, Weichold F, Geiter L, Sadoff JC and Horwitz MA:
A new recombinant bacille Calmette-Guérin vaccine safely induces
significantly enhanced tuberculosis-specific immunity in human
volunteers. J Infect Dis. 198:1491–1501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Y, Liu W, Shen H, Yan J, Qu D and Wang
H: Recombinant Mycobacterium bovis BCG expressing the
chimeric protein of antigen 85B and ESAT-6 enhances the Th1
cell-mediated response. Clin Vaccine Immunol. 16:1121–1126. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Forbes EK, Sander C, Ronan EO, McShane H,
Hill AV, Beverley PC and Tchilian EZ: Multifunctional, high-level
cytokine-producing Th1 cells in the lung, but not spleen, correlate
with protection against Mycobacterium tuberculosis aerosol
challenge in mice. J Immunol. 181:4955–4964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Zhu B, Wang Q, Chen J, Qie Y, Wang J
and Wang H, Wang B and Wang H: Recombinant BCG coexpressing Ag85B,
ESAT-6 and mouse-IFN-γ confers effective protection against
Mycobacterium tuberculosis in C57BL/6 mice. FEMS Immunol Med
Microbiol. 51:480–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moreira AL, Tsenova L, Murray PJ, Freeman
S, Bergtold A, Chiriboga L and Kaplan G: Aerosol infection of mice
with recombinant BCG secreting murine IFN-γ partially reconstitutes
local protective immunity. Microb Pathog. 29:175–185. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi Z, Fu Y, Yang C, Li J, Luo X, Chen Q,
Zeng W, Jiang S, Jiang Y, He Y, et al: Recombinant M.
smegmatis vaccine targeted delivering IL-12/GLS into
macrophages can induce specific cellular immunity against M.
tuberculosis in BALB/c mice. Vaccine. 25:638–648. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martin RM and Lew AM: Is IgG2a a good Th1
marker in mice? Immunol Today. 19:491998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo Y, Chen X, Han R and O'Donnell MA:
Recombinant bacille Calmette-Guérin (BCG) expressing human
interferon-alpha 2B demonstrates enhanced immunogenicity. Clin Exp
Immunol. 123:264–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wangoo A, Brown IN, Marshall BG, Cook HT,
Young DB and Shaw RJ: Bacille Calmette-Guérin (BCG)-associated
inflammation and fibrosis: Modulation by recombinant BCG expressing
interferon-γ (IFN-γ). Clin Exp Immunol. 119:92–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong P and Pamer EG: CD8 T cell responses
to infectious pathogens. Annu Rev Immunol. 21:29–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cooper AM, Adams LB, Dalton DK, Appelberg
R and Ehlers S: IFN-γ and NO in mycobacterial disease: New jobs for
old hands. Trends Microbiol. 10:221–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rook GA, Seah G and Ustianowski A: M.
tuberculosis: Immunology and vaccination. Eur Respir J.
17:537–557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng Y, Yang X, Liu Z, Liu Y, Su B, Ding
Y, Qin L, Yang H, Zheng R and Hu Z: Continuous treatment with
recombinant Mycobacterium tuberculosis CFP-10-ESAT-6 protein
activated human monocyte while deactivated LPS-stimulated
macrophage. Biochem Biophys Res Commun. 365:534–540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhatt K, Uzelac A, Mathur S, McBride A,
Potian J and Salgame P: B7 costimulation is critical for host
control of chronic Mycobacterium tuberculosis infection. J
Immunol. 182:3793–3800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kan-Sutton C, Jagannath C and Hunter RL
Jr: Trehalose 6,6′-dimycolate on the surface of Mycobacterium
tuberculosis modulates surface marker expression for antigen
presentation and costimulation in murine macrophages. Microbes
Infect. 11:40–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seder RA, Darrah PA and Roederer M: T-cell
quality in memory and protection: Implications for vaccine design.
Nat Rev Immunol. 8:247–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lindenstrøm T, Agger EM, Korsholm KS,
Darrah PA, Aagaard C, Seder RA, Rosenkrands I and Andersen P:
Tuberculosis subunit vaccination provides long-term protective
immunity characterized by multifunctional CD4 memory T cells. J
Immunol. 182:8047–8055. 2009. View Article : Google Scholar : PubMed/NCBI
|