Introduction
Osteosarcoma (OS) is the most common primary bone
tumor in children and adolescents and has a high propensity for
local invasion and distant metastasis (1). With the current multidisciplinary
treatments, there have been no significant improvements in the
overall prognosis of OS patients within the last two decades and,
currently, 60–70% of patients with localized disease survive
(2). Previous studies have estimated
the 5-year survival rate of patients with metastatic diseases to be
<20% (3,4). The leading causes of mortality in
patients with OS is lung metastases and, therefore, the
identification of the molecular mechanisms of OS metastasis is
important in order to improve the prognosis of OS.
Fatty acid synthase (FASN) is an enzyme involved in
mammalian endogenous lipogenesis that functions to catalyze the
synthesis of long-chain fatty acids. Research has demonstrated that
FASN is overexpressed in various human tumors and FASN has been
identified as a crucial factor for sustaining a number of
biological features of cancer cells (5–9).
Inhibition of FASN may suppress cancer cell proliferation and
metastasis in vitro and in vivo (10–14).
Previous studies have demonstrated that FASN is overexpressed in OS
cells and tissues and knockdown of FASN may suppresses the invasion
and migratory ability of OS cells by downregulating the human
epidermal growth factor receptor 2 (HER2)/phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt)/nuclear factor (NF)-κB signaling
pathway in vitro (15,16);
however, the tumor microenvironment has an important influence on
tumor progression.
In the present study, the effect of inhibiting FASN
on the growth and metastasis of OS cells in nude mice and the
potential molecular mechanisms of such effects were investigated.
The aim was to demonstrate that the knockdown of FASN may suppress
OS cell growth and metastasis, at least partly, by inhibiting the
HER2/PI3K/Akt/NF-κB signaling pathway in vivo.
Materials and methods
Ethics statement
Animal experiments were approved by the Animal Care
Committee of The First Affiliated Hospital of Nanchang University
(Nanchang, China) and were conducted according to the institutional
guidelines for animal care.
Cell culture
Cells from the human OS cell line, 143B, were
purchased from the Shanghai Cell Bank, Chinese Academy of Sciences
(Shanghai, China), and were cultured in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum (both Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and incubated at 37°C in
5% CO2.
Lentivirus vector construction of
small hairpin (sh) FASN
shRNA targeting FASN mRNA were designed online
(invitrogen.com/rnai) according to the
human mRNA sequence encoding the FASN gene (NM_004104.4) and a
plasmid with the highest silencing rate was selected from a
previous study for use (16).
Recombinant lentivirus vector was constructed according to the
manufacturer's instructions (Invitrogen; Thermo Fisher Scientific,
Inc.) for the generation of FASN-shRNA lentivirus (Lv-shFASN) and
negative control lentivirus (Lv-negative).
Tumor xenograft model
A total of 50 male athymic nude BALB/c mice (weight,
15±2 g; age, 6±2 weeks) were provided by the Department of
Laboratory Animal Science of Nanchang University (Nanchang, China).
Mice were maintained in a 12-h light/dark cycle at a temperature of
20–26°C and humidity of 40–70%, with access to food and water. For
subcutaneous implantation, 143B cells were suspended in
phosphate-buffered saline and 200 µl of cell suspension
(1×107 cells) was injected subcutaneously under the
dorsal skin of nude mice. Mice were intraperitoneally injected with
1% pentobarbital sodium (40 mg/kg body weight) and sacrificed by
cervical dislocation 4 weeks subsequent to successful tumor
establishment. Tumors were removed and cut into 1-mm3
blocks and two blocks of tumor tissue were implanted into the
proximal tibia bone marrow cavity of mice. Following 7 days, mice
were randomly divided into two groups (n=12 mice/group) for
Lv-shFASN and Lv-negative treatment. Lv-shFASN treatment was
administered by injecting 0.2 ml (108 copies/ml) of
Lv-shFASN into tumors three times per week. The Lv-negative
treatment group was injected with Lv-negative in the same manner as
for the Lv-shFASN group. Xenograft tumor sizes were observed
dynamically by measuring the outer skin each week. Mice were
sacrificed, using the same method described above, at 5 weeks
subsequent to the final injection. The volume and weight of the
tumors and the incidence of spontaneous metastasis to the lungs
were evaluated.
Hematoxylin and eosin (H&E)
staining
Paraffin embedding, slicing (4-µm thick), and
H&E staining were performed according to the manufacturer's
instructions (Sysmex Corp., Kobe, Japan).
Western blot analysis
Total protein from OS tumor xenograft cells was
extracted using radioimmunoprecipitation assay lysis buffer
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 60 µg/ml
phenylmethylsulfonyl fluoride, according to the manufacturer's
instructions. Protein concentration was determined using the
Bradford assay. Proteins were separated by 10% SDS-PAGE (10
µg/lane) and transferred to polyvinylidene difluoride membranes.
Non-specific binding sites were blocked by immersing the membrane
in 5% non-fat milk (Gibco; Thermo Fisher Scientific, Inc.) for 1 h
at room temperature on a shaking platform. Membranes were incubated
with the following primary antibodies overnight at 4°C: Mouse
anti-FASN (1:500; sc-55580) and mouse anti-β-actin antibodies
(1:2,000; sc-47778; both Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). The membrane was washed three times, 5 min each, with 20
ml of TBS-Tween-20 (Thermo Fisher Scientific, Inc.). Following
this, the membrane was incubated with goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:5,000; 610-103-043;
Rockland Immunochemicals, Inc., Pottstown, PA, USA) for 1.5 h at
room temperature. The membrane was washed again three times, 5 min
each, with 20 ml of TBS-Tween-20. Immunoreactive bands were
visualized using an enhanced chemiluminescence reagent (Thermo
Fisher Scientific, Inc.). The intensity of western blot bands was
measured using ImageJ v.1.48 software (National Institutes of
Health, Bethesda, MA, USA). This procedure for western blot
analysis was conducted according to a previous study (16).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from 143B cells was extracted using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Genomic DNA was removed by DNase
treatment (Takara Biotechnology Co., Ltd., Dalian, China). mRNA
expression levels of NF-YA and FASN were evaluated by RT-qPCR using
a thermal cycler (Applied Biosystems 2720; Applied Biosystems;
Thermo Fisher Scientific, Inc.), with β-actin as the internal
reference gene. The primer sequences used were as follows: β-actin
(295 bp), forward 5′-TCACCCACACTGTGCCATCATCGA-3′ and reverse
5′-CAGCGGAACCGCTCATTGCCAATGG-3′; NF-YA (238 bp), forward
5′-TTGTTGGTCAGGGTTTACAGC-3′ and reverse 5′-ACGCTCCACGATGTCACTAA-3′;
and FASN (262 bp), forward 5′-GTCGGAGAACTTGCAGGAGT-3′ and reverse
5′-TCCTCGGAGTGAATCTGGGT-3′. Total RNA concentration was determined
by spectrophotometry at 260 nm and the purity was determined by
calculating the 260/280 ratio with a BioPhotometer (Eppendorf,
Hamburg, Germany). A two-step reverse transcription kit (Promega
Corp., Madison, WI, USA) was used to obtain cDNA, according to the
manufacturer's instructions, which was then used as the template
for qPCR. TaqMan Real-Time PCR master mix (Thermo Fisher
Scientific, Inc.) was used for amplification under the following
conditions: Pre-denaturation at 95°C for 1 min, followed by 40
cycles of 95°C for 15 sec, 58°C for 20 sec and 72°C for 20 sec.
Dissolution curves were obtained between 72–95°C, with 1°C
increases every 20 sec. Data was normalized to β-actin, according
to the 2−ΔΔCq method (17). This procedure for RT-qPCR was
conducted according to a previous study (16). Six independent experiments were
performed over multiple days.
Statistical analysis
Data analysis was conducted using SPSS v.l3.0
software (SPSS, Inc., Chicago, IL, USA). Count data were analyzed
using non-parametric Wilcoxon rank sum test analysis. Measurement
data were expressed as the mean ± standard deviation. Statistical
significance for the difference between groups was assessed using
an independent-samples t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Lv-shFASN inhibits FASN expression in
mice with intratibial 143B OS xenografts
In order to evaluate whether Lv-shFASN was able to
inhibit FASN expression in mice with intratibial 143B OS
xenografts, Lv-shFASN and Lv-negative were injected into xenograft
tumors. FASN mRNA and protein expression levels in the tumors were
detected using RT-qPCR and western blot analysis, respectively.
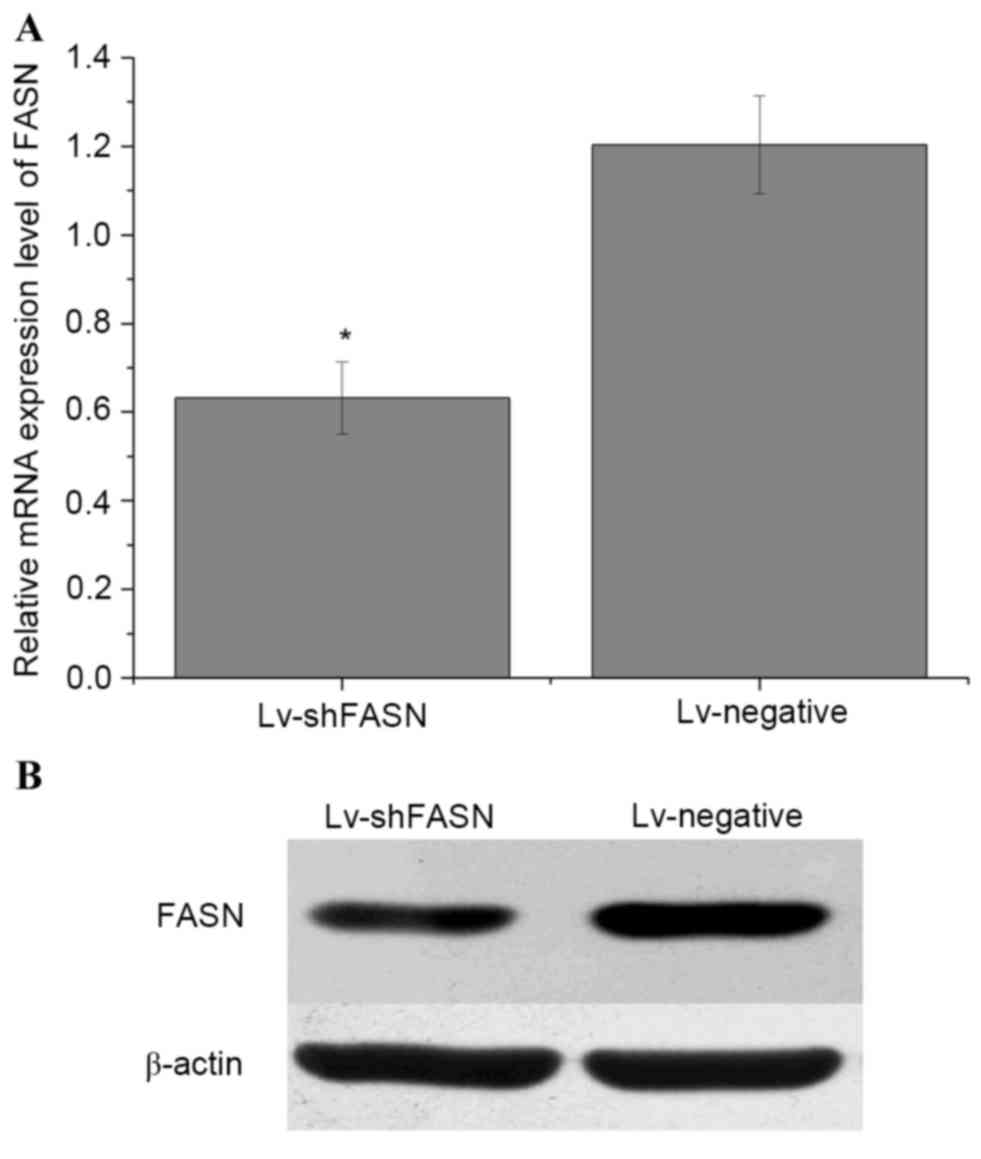
Results demonstrated that the mRNA (Fig.
1A) and protein (Fig. 1B)
expression levels of FASN in the tumors of the Lv-shFASN treatment
group were significantly lower (P<0.05) than those in the
Lv-negative group. These results indicate that Lv-shFASN was able
to inhibit FASN expression in xenograft tumors in nude mice.
Knockdown of FASN inhibits OS cell
growth in nude mice
In order to evaluate the effect of the knockdown of
FASN on OS cell growth in nude mice, Lv-shFASN was injected into
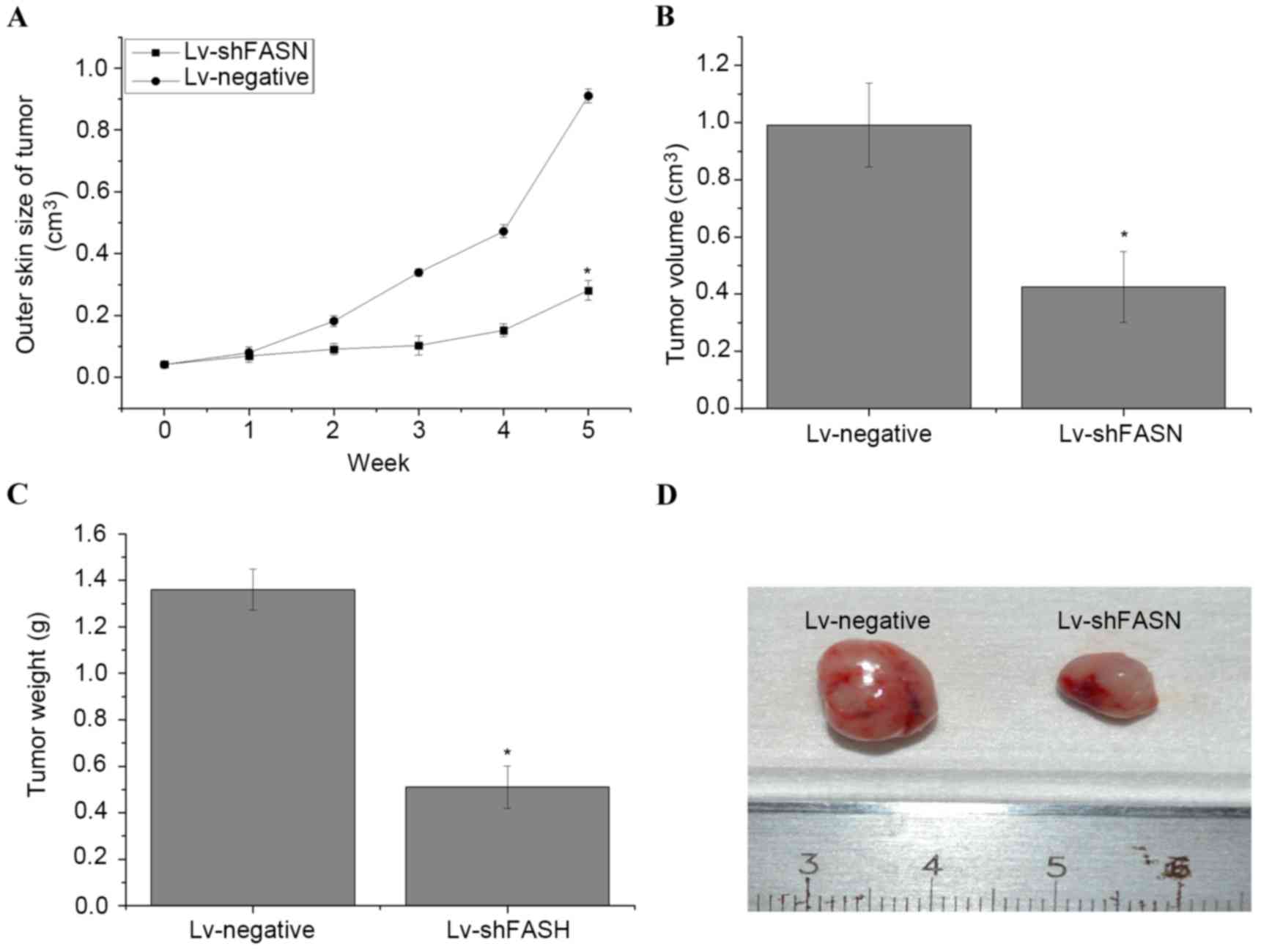
xenograft tumors. Results demonstrated that tumor growth in mice
injected with Lv-shFASN was significantly reduced compared with
tumor growth in the Lv-negative group (P<0.001 on week 5;
Fig. 2A). Significant reductions in
tumor volume (Fig. 2B) and weight
(Fig. 2C) were observed in the
xenograft tumors of the mice in the Lv-shFASN group compared with
those in the Lv-negative group (P<0.05). These results suggest
that the knockdown of FASN inhibits OS cell growth in mice with
intratibial 143B OS xenografts.
Knockdown of FASN inhibits OS cell
metastasis in nude mice
In order to investigate the effect of the knockdown
of FASN on OS cell metastasis in nude mice, Lv-shFASN was injected
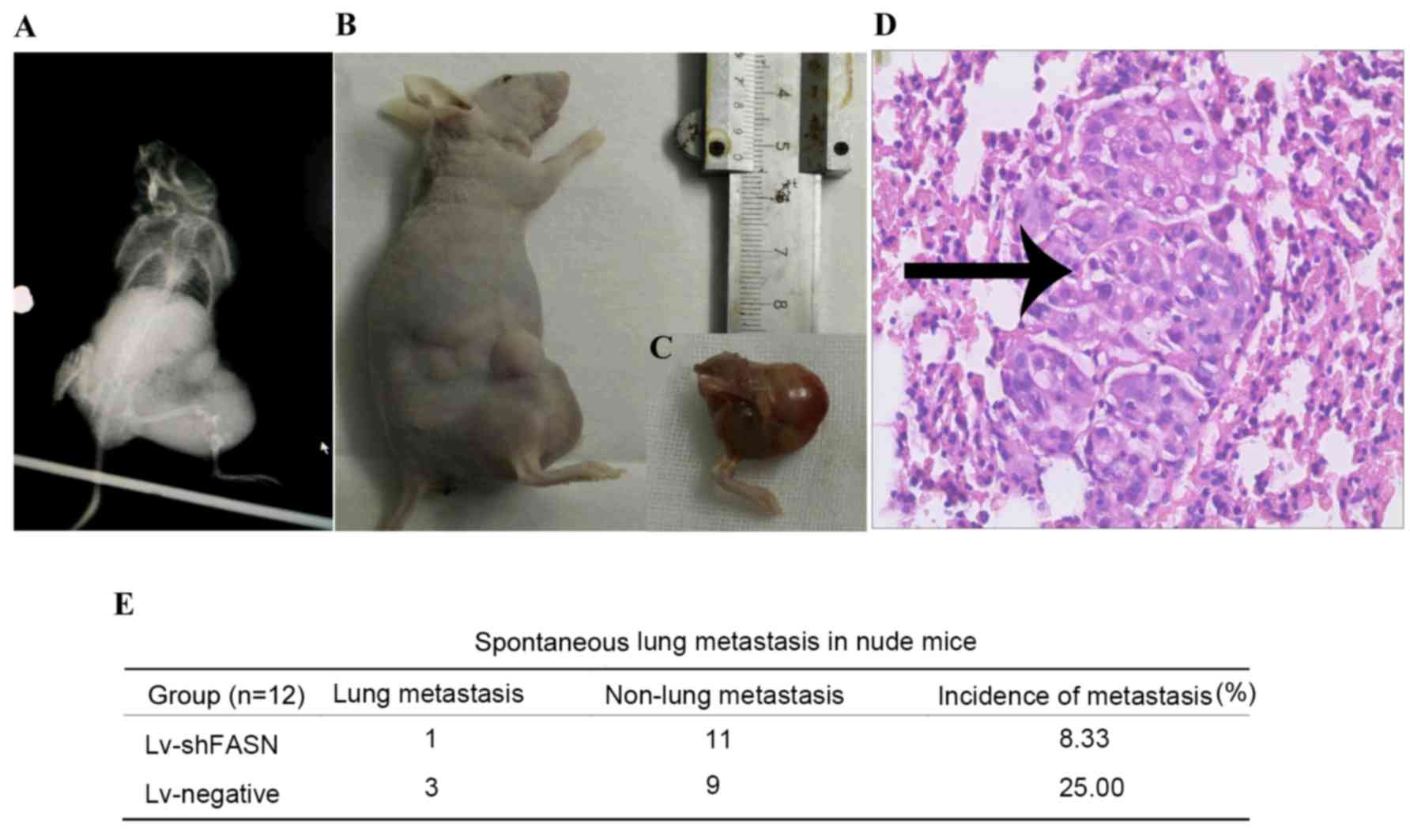
into xenograft tumors. The incidence of spontaneous lung metastasis
was evaluated using H&E staining. Results demonstrated that the
incidence of spontaneous metastasis was significantly reduced in
Lv-shFASN-treated mice (8.33%) compared with the incidence of
metastasis in mice in the Lv-negative group (25.00%; P<0.001).
These results suggest that the knockdown of FASN inhibits OS cell
metastasis in mice with intratibial 143B osteosarcoma xenografts
(Fig. 3).
Silencing of FASN inhibits the
activation of HER2/PI3K/Akt signaling in OS xenografts
In order to investigate the potential molecular
mechanisms of the knockdown of FASN on the inhibition of OS cell
growth and metastasis, the expression levels of phosphorylated
(p)-HER2, HER2, PI3K, p-Akt and Akt protein in OS xenograft tumors
was detected using western blot analysis. Results demonstrated that
p-HER2, HER2, PI3K, p-Akt and Akt protein expression levels in
xenograft tumors treated with Lv-shFASN were markedly lower than
those in mice injected with Lv-negative (Fig. 4). These results demonstrate that the
inhibition of FASN downregulates the activation of the
HER2/PI3K/Akt signaling pathway in OS xenografts.
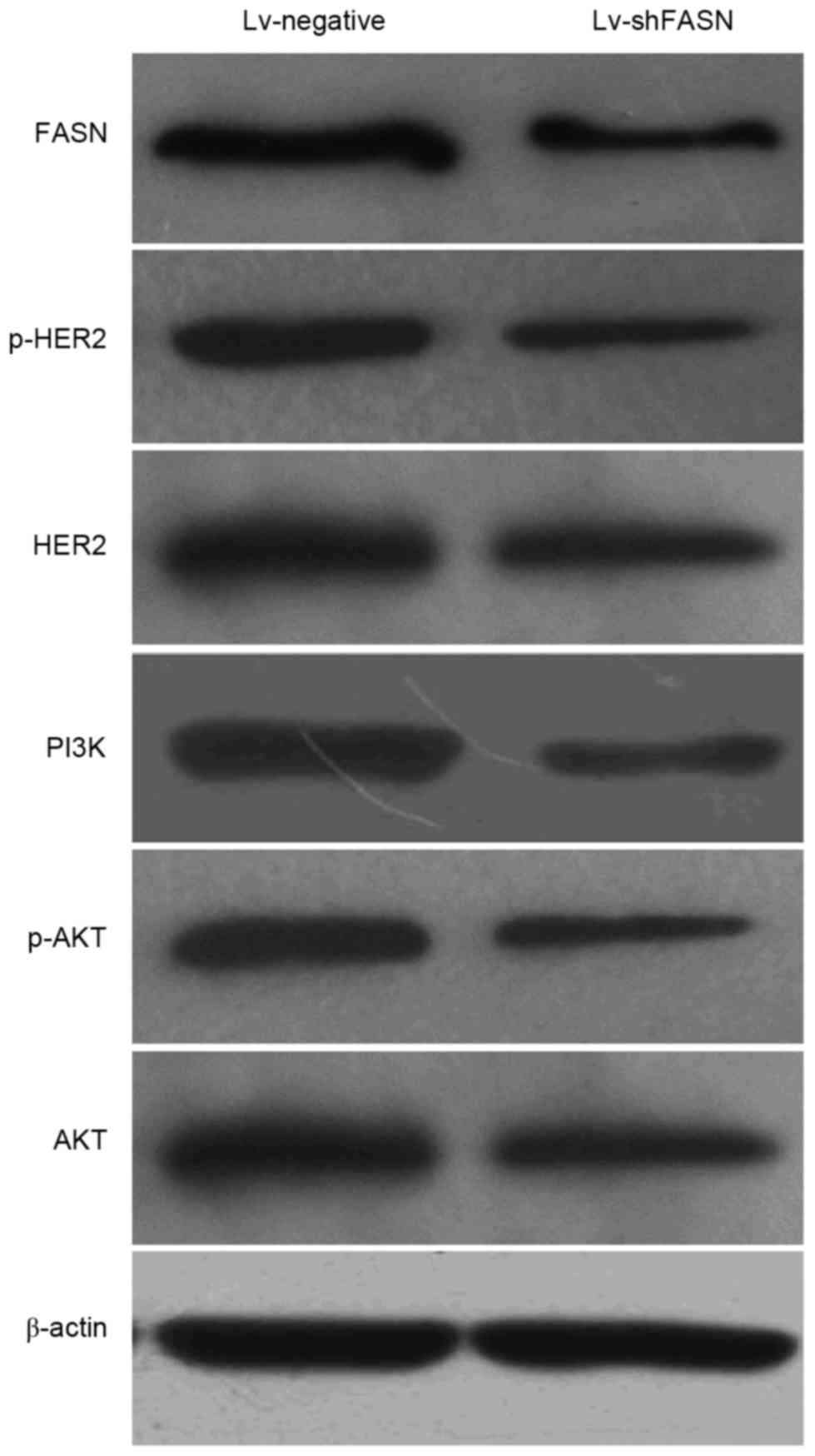 | Figure 4.Western blot analysis demonstrated
that the protein expression levels of p-HER2, HER2, PI3K, p-Akt and
Akt were markedly reduced when OS xenograft tumors were injected
with Lv-shFASN compared with injection with Lv-negative. HER2,
human epidermal growth factor receptor 2; PI3K, phosphoinositide
3-kinase; Akt, protein kinase B; p, phosphorylated; Lv, Lentivirus
vector; sh, small hairpin; FASN, fatty acid synthase; OS,
osteosarcoma; Lv-negative, control group. |
Discussion
Despite the introduction of multi-agent chemotherapy
in the 1970s and refinements in surgical techniques that increase
the long-term survival rate of patients with localized OS, little
has changed for patients with metastatic disease (18). Therefore, a more detailed
understanding of the pathophysiological mechanisms in OS metastasis
is necessary for the development of novel treatment strategies to
improve the prognosis for OS patients with metastatic disease.
Although several evolutionary signaling pathways have been
demonstrated to be associated with OS pathogenesis, including the
Wnt, Notch, mechanistic target of rapamycin and PI3K/Akt signaling
pathways (19–22), the molecular mechanisms of OS
metastases have not been fully elucidated.
The FASN gene (also known as OA-169) has recently
been identified and is considered to be crucial for endogenous
lipogenesis in mammals, and is responsible for catalyzing the
synthesis of long-chain fatty acids (23). In the majority of normal cells, FASN
expression is not observed due to the presence of abundant amounts
of dietary lipids (24); however,
FASN expression levels are increased in various types of human
malignant cancer, including colon (25), ovarian (26), prostate (27) and breast (28) cancer. A study by Agostini et
al (29) demonstrated that
orlistat, a small molecular inhibitor of FASN, reduces the growth
and metastasis of orthotopic tongue oral squamous cell carcinomas.
A study by Seguin et al (30)
demonstrated that orlistat reduces experimental metastases and
angiogenesis in B16-F10 melanomas. Furthermore, previous in
vitro studies have demonstrated that FASN may contribute to OS
cell migration and invasion (31,32). In
the present study, it was demonstrated that the knockdown of FASN
was able to inhibit OS cell growth and metastasis to the lungs in
nude mice. Although previous studies have indicated that FASN may
contribute to the metastasis of OS cells, the potential molecular
mechanisms of how elevated FASN expression promotes OS cells growth
and metastasis have remained unclear.
PI3K and Akt have a crucial role in the metastasis
of tumor cells through the activation of NF-κβ/matrix
metalloproteinase signaling, which promotes extracellular matrix
degradation (33). A study by Tomek
et al (34) indicated that
the inhibition of FASN induces ubiquitination and degradation of
PI3K signaling proteins in ovarian cancer. A previous study
demonstrated that small interfering RNA silencing of FASN decreased
the PI3K/Akt/NF-κB signaling pathway in vitro (32); however, the mechanism (s) responsible
for the inhibition of Akt activity due to the downregulation of
FASN have not been investigated. A study by Puig et al
(35) revealed that inhibition of
FASN resulted in a marked decrease in the active forms of the HER2
protein. FASN-mediated lipogenesis produces phospholipids that are
incorporated into cell membranes and partitioned into lipid rafts,
which accommodate HER proteins and form signaling platforms. FASN
inhibition destabilizes these lipid rafts, triggering the
degradation of HER proteins and impeding the membrane recruitment
of downstream mediators of Akt and resulting in the downregulation
of p-Akt (36). A study by Li et
al (37) suggested that the
downregulation of FASN effectively inhibits the activity of the
HER2-PI3K/Akt axis and alters the malignant phenotype in colorectal
cancer cells. Furthermore, a previous study demonstrated that the
inhibition of FASN in OS cells resulted in the downregulation of
HER2 and p-HER2 protein and inhibited the activation of PI3K/Akt
signaling in vitro (16). In
the present study, it was observed that the inhibition of FASN
decreased the activation of the HER2/PI3k/Akt signaling pathway in
OS xenografts.
In conclusion, the present study demonstrated that
the inhibition of FASN suppresses OS growth and metastasis, at
least partly, by inhibiting the HER2/PI3K/Akt signaling pathway in
nude mice. Targeting the FASN/HER2/PI3K/Akt signaling pathway,
therefore, may be a potential therapeutic strategy for OS
management; however, further research is required in order to
identify exactly how FASN inhibition may be used as a novel
therapeutic strategy.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (grant no. 81260400)
and the Natural Science Foundation of Jiangxi Province (grant no.
20114BAB205093).
References
|
1
|
Salah S, Ahmad R, Sultan I, Yaser S and
Shehadeh A: Osteosarcoma with metastasis at initial diagnosis:
Current outcomes and prognostic factors in the context of a
comprehensive cancer center. Mol Clin Oncol. 2:811–816.
2014.PubMed/NCBI
|
|
2
|
Jawad MU, Cheung MC, Clarke J, Koniaris LG
and Scully SP: Osteosarcoma: Improvement in survival limited to
high-grade patients only. J Cancer Res Clin Oncol. 137:597–607.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mialou V, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles
AS and Hartmann O: Metastatic osteosarcoma at diagnosis: Prognostic
factors and long-term outcome-the French pediatric experience.
Cancer. 104:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss
J, Szendroi M, Csoka M and Kovacs G: Good prognosis of localized
osteosarcoma in young patients treated with limb-salvage surgery
and chemotherapy. Pediatr Blood Cancer. 57:415–422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hess D and Igal RA: Genistein
downregulates de novo lipid synthesis and impairs cell
proliferation in human lung cancer cells. Exp Biol Med (Maywood).
236:707–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alo PL, Amini M, Piro F, Pizzuti L,
Sebastiani V, Botti C, Murari R, Zotti G and Di Tondo U:
Immunohistochemical expression and prognostic significance of fatty
acid synthase in pancreatic carcinoma. Anticancer Res.
27:2523–2527. 2007.PubMed/NCBI
|
|
7
|
Walter K, Hong SM, Nyhan S, Canto M,
Fedarko N, Klein A, Griffith M, Omura N, Medghalchi S, Kuhajda F
and Goggins M: Serum fatty acid synthase as a marker of pancreatic
neoplasia. Cancer Epidemiol Biomarkers Prev. 18:2380–2385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Migita T, Ruiz S, Fornari A, Fiorentino M,
Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E,
et al: Fatty acid synthase: A metabolic enzyme and candidate
oncogene in prostate cancer. J Natl Cancer Inst. 101:519–532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva SD, Cunha IW, Younes RN, Soares FA,
Kowalski LP and Graner E: ErbB receptors and fatty acid synthase
expression in aggressive head and neck squamous cell carcinomas.
Oral Dis. 16:774–780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saati GE and Archer MC: Inhibition of
Fatty Acid synthase and sp1 expression 3-3′-diindolylmethane in
human breast cancer cells. Nutr Cancer. 63:790–794. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Notarnicola M, Pisanti S, Tutino V, Bocale
D, Rotelli MT, Gentile A, Memeo V, Bifulco M, Perri E and Caruso
MG: Effects of olive oil polyphenols on fatty acid synthase gene
expression and activity in human colorectal cancer cells. Genes
Nutr. 6:63–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Notarnicola M, Messa C, Refolo MG, Tutino
V, Miccolis A and Caruso MG: Polyunsaturated fatty acids reduce
fatty acid synthase and hydroxy-methyl-glutaryl CoA-reductase gene
expression and promote apoptosis in HepG2 cell line. Lipids Health
Dis. 10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zecchin KG, Rossato FA, Raposo HF, Melo
DR, Alberici LC, Oliverira HC, Castilho RF, Coletta RD, Vercesi AE
and Graner E: Inhibition of fatty acid synthase in melanoma cells
activates the intrinsic pathway of apoptosis. Lab Invest.
91:232–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murata S, Yanagisawa K, Fukunaga K, Oda T,
Kobayashi A, Sasaki R and Ohkohchi N: Fatty acid synthase inhibitor
cerulenin suppresses liver metastasis of colon cancer in mice.
Cancer Sci. 101:1861–1865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZL, Wang G, Peng AF, Luo QF, Zhou Y
and Huang SH: Fatty acid synthase expression in osteosarcoma and
its correlation with pulmonary metastasis. Oncol Lett. 4:878–882.
2012.PubMed/NCBI
|
|
16
|
Wang TF, Wang H, Peng AF, Luo QF, Liu ZL,
Zhou RP, Gao S, Zhou Y and Chen WZ: Inhibition of fatty acid
synthase suppresses U-2 OS cell invasion and migration via
downregulating the activity of HER2/PI3K/AKT signaling pathway in
vitro. Biochem Biophys Res Commun. 440:229–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment - Where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang R, Zheng J, Zhang DS, Yang YH and
Zhao ZF: Wnt1-induced MAFK expression promotes osteosarcoma cell
proliferation. Genet Mol Res. 14:7315–7325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao S, Kurenbekova L, Gao Y, Roos A,
Creighton CJ, Rao P, Hicks J, Man TK, Lau C, Brown AM, et al: NKD2,
a negative regulator of Wnt signaling, suppresses tumor growth and
metastasis in osteosarcoma. Oncogene. 34:5069–5079. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gupte A, Baker EK, Wan SS, Stewart E, Loh
A, Shelat AA, Gould CM, Chalk AM, Taylor S, Lackovic K, et al:
Systematic Screening Identifies Dual PI3K and mTOR Inhibition as a
conserved therapeutic vulnerability in osteosarcoma. Clin Cancer
Res. 21:3216–3229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tao J, Jiang MM, Jiang L, Salvo JS, Zeng
HC, Dawson B, Bertin TK, Rao PH, Chen R, Donehower LA, et al: Notch
activation as a driver of osteogenic sarcoma. Cancer Cell.
26:390–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uemoto Y, Abe T, Tameoka N, Hasebe H,
Inoue K, Nakajima H, Shoji N, Kobayashi M and Kobayashi E:
Whole-genome association study for fatty acid composition of oleic
acid in Japanese Black cattle. Anim Genet. 42:141–148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuhajda FP: Fatty acid synthase and
cancer: New application of an old pathway. Cancer Res.
66:5977–5980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaytseva YY, Harris JW, Mitov MI, Kim JT,
Butterfield DA, Lee EY, Weiss HL, Gao T and Evers BM: Increased
expression of fatty acid synthase provides a survival advantage to
colorectal cancer cells via upregulation of cellular respiration.
Oncotarget. 6:18891–19904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bauerschlag DO, Maass N, Leonhardt P,
Verburg FA, Pecks U, Zeppernick F, Morgenroth A, Mottaghy FM, Tolba
R, Meinhold-Heerlein I and Bräutigam K: Fatty acid synthase
overexpression: Target for therapy and reversal of chemoresistance
in ovarian cancer. J Transl Med. 13:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamada S, Horiguchi A, Kuroda K, Ito K,
Asano T, Miyai K and Iwaya K: Elevated fatty acid synthase
expression in prostate needle biopsy cores predicts upgraded
Gleason score in radical prostatectomy specimens. Prostate.
74:90–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porta R, Blancafort A, Casòliva G, Casas
M, Dorca J, Buxo M, Viñas G, Oliveras G and Puig T: Fatty acid
synthase expression is strongly related to menopause in early-stage
breast cancer patients. Menopause. 21:188–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agostini M, Almeida LY, Bastos DC, Ortega
RM, Moreira FS, Seguin F, Zecchin KG, Raposo HF, Oliveira HC,
Amoêdo ND, et al: The fatty acid synthase inhibitor orlistat
reduces the growth and metastasis of orthotopic tongueoral squamous
cell carcinomas. Mol Cancer Ther. 13:585–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seguin F, Carvalho MA, Bastos DC, Agostini
M, Zecchin KG, Alvarez-Flores MP, Chudzinski-Tavassi AM, Coletta RD
and Graner E: The fatty acid synthase inhibitor orlistat reduces
experimental metastases and angiogenesis in B16-F10 melanomas. Br J
Cancer. 107:977–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu ZL, Mao JH, Peng AF, Yin QS, Zhou Y,
Long XH and Huang SH: Inhibition of fatty acid synthase suppresses
osteosarcoma cell invasion and migration via downregulation of the
PI3K/Akt signaling pathway in vitro. Mol Med Rep. 7:608–612.
2013.PubMed/NCBI
|
|
32
|
Liu ZL, Zhou Y, Luo QF, Hu M, Wang G,
Huang SH and Shu Y: Inhibition of fatty acid synthase supresses
osteosarcoma cell invasion and migration. Indian J Pathol
Microbiol. 55:163–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tomek K, Wagner R, Varga F, Singer CF,
Karlic H and Grunt TW: Blockade of fatty acid synthase induces
ubiquitination and degradation of phosphoinositide-3-kinase
signaling proteins in ovarian cancer. Mol Cancer Res. 9:1767–1779.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Puig T, Turrado C, Benhamú B, Aguilar H,
Relat J, Ortega-Gutiérrez S, Casals G, Marrero PF, Urruticoechea A,
Haro D, et al: Novel inhibitors of fatty acid synthase with
anticancer activity. Clin Cancer Res. 15:7608–7615. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang B, Li EH, Lu YY, Jiang Q, Cui D,
Jing YF and Xia SJ: Inhibition of fatty-acid synthase suppresses
P-AKT and induces apoptosis in bladder cancer. Urology. 80:484.
e9–15. 2012. View Article : Google Scholar
|
|
37
|
Li N, Lu H, Chen C, Bu X and Huang P: Loss
of fatty acid synthase inhibits the ‘HER2-PI3K/Akt axis’ activity
and malignant phenotype of Caco-2 cells. Lipids Health Dis.
12:832013. View Article : Google Scholar : PubMed/NCBI
|