Introduction
Choroidal melanoma is a serious primary intraocular
malignant tumor that occurs in adults (1). The incidence of malignant melanoma has
been demonstrated to be increasing worldwide (2). Choroidal melanoma is fatal in ~50% of
patients due to its latency and metastatic potential, and the
principal target organ for metastasis is the liver (3,4). Early
detection and therapy is important for improving the prognosis of
patients (5); however, the
underlying molecular mechanisms of disease development remain to be
elucidated.
Gambogenic acid (GNA;
C38H46O9; molecular weight, 646.32
kDa) has recently been recognized as a type of antitumor drug
(6). GNA is a polyprenylated
xanthone, which is isolated from the gum resin gamboge and has a
structure similar to gambogic acid (GA) (7). It has been reported to have an
inhibitory effect on many types of cancer cells via inhibition of
proliferation, metastasis, and apoptosis induction, which suggests
that it may be a potential pharmacological agent in cancer therapy
(8). Previous studies have
demonstrated that GNA has lower toxicity and a wider spectrum of
potent antitumor activity than GA (9,10).
Additionally, GNA is able to inhibit cell proliferation in human
hepatoma HepG2 cells via inducing cell apoptosis, and may be a
possible pharmacological treatment strategy in HepG2 cells
(6). GNA is able to induce G1 cell
arrest in lung cancer cells via glycogen synthase kinase
3β-dependent cyclin D1 degradation and may have therapeutic
potential in the treatment of lung cancer (11). GNA has also been demonstrated to
serve a role in the apoptosis of U251 glioblastoma cells through
inactivation of the protein kinase B (Akt) pathway (12). It has recently been demonstrated that
GNA is associated with the induction of apoptosis in the B16
melanoma cell line (13). Despite
these findings the regulatory mechanism by which GNA exerts its
antitumor effects on choroidal melanoma progression has not yet
been fully elucidated.
The present study aimed to explore the effects of
GNA on the malignant behaviors of choroidal melanoma cells
including cell growth, cell cycle, cell migration and invasion, and
its regulatory mechanism. The findings of the present study may
serve as a theoretical basis for the development of an effective
therapy for the treatment of choroidal melanoma.
Materials and methods
Cell culture
The human choroidal melanoma cell line OCM-1 was
purchased from the American Type Cell Culture Collection (Manassas,
VA, USA) and cultured at 37°C for 24 h in RPMI-1640 medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 100 µg/ml streptomycin, 100 U/ml penicillin, and 10% fetal
bovine serum (FBS; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) at 37°C in a humidified atmosphere containing 5%
CO2.
Cell viability assay
Cell viability was assessed via MTT assay performed
as previously described with some modifications (14). Briefly, 1×104 OCM-1 cells
were seeded in triplicate into 96-well plates. Cells were incubated
at 37°C for 24 h in RPMI-1640 medium supplemented with different
concentrations of GNA (0, 0.75, 1.5, 3, 4.5, and 6 µM;
Sigma-Aldrich; Merck Millipore). A total of 50 µl MTT solution
(Roche Applied Science, Penzberg, Germany) was added 24, 48 or 72 h
post-treatment and cells were incubated at 37°C for a further 4 h.
A total of 150 µl dimethylsulfoxide was subsequently added to
solubilize the formazan crystals. The optical density (OD) of each
well was determined using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The cell viability ratio
was calculated according to following formula: Cell viability rate
(%) = OD of the experimental samples / OD of the control ×100.
Colony formation assay
OCM-1 cells (2×103) were trypsinized and
seeded in triplicate into six-well plates. Cells were cultured at
37°C for 72 h in RPMI-1640 medium containing GNA of different
concentrations (0, 0.75, 1.5, 3, 4.5, and 6 µM), which was
replenished every two days. The resulting colonies were stained
with 0.05% crystal violet and the number of colonies was counted
using a microscope (Olympus Corporation, Tokyo, Japan).
Wound healing assay
A wound-healing assay was used to detect cell
migration. Briefly, cells were treated with different
concentrations of GNA (0, 0.75, 1.5, 3 µM) and seeded in triplicate
in six-well plates at 37°C for 24 h. When cells reached 90%
confluence, a scrape wound was scratched on the cell layer using a
sterile pipette tip. Cell migration ability was assessed by
calculating the percentage of wound closure. The speed of wound
closure was defined as the distance between wound edges from 0 to
72 h. Each experiment was conducted in triplicate.
Transwell invasion assay
The Transwell was coated with 0.1% gelatin
(Sigma-Aldrich; Merck Millipore) for 30 min at 37°C. Following
three rounds of washing with PBS (pH 7.4), 100 µl OCM-1 cells
(4×104 cells/well) was seeded into the top chambers of
the Transwell, whereas the bottom chambers were filled with
RPMI-1640 medium with 20% FBS. The top and bottom chambers
contained the same series of concentrations of GNA (0, 0.75, 1.5, 3
µM). OCM-1 cells were incubated for 24 h at 37°C and cells on the
bottom side of the membrane, which were considered to be invading
cells, were subsequently fixed with 4% paraformaldehyde at 37°C for
20 min. Cells were washed with PBS three times and stained with
0.05% crystal violet for 10 min. Fields were selected at random,
observed using an inverted light microscope (Olympus Corporation)
and quantified by manual counting.
Cell cycle assay
OCM-1 cells (1×105) were seeded in
six-well plates and treated with different concentrations of GNA
(0, 0.75, 1.5, and 3 µM). Briefly, cells were harvested at 24 and
48 h post-treatment with GNA. Thereafter, the cells were washed
with PBS, fixed with 70% ethanol at −20°C for 24 h and centrifuged
at 300 × g for 5 min. Then the cells were washed with PBS/1% bovine
serum albumin (Sigma-Aldrich; Merck Millipore) and stained with 30
µg/ml propidium iodide containing 0.25 mg/ml RNase A for 30 min in
the dark. The cell cycle assay was performed using the Propidium
Iodide Nucleic Acid Stain kit (P3566; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in accordance with the
manufacturer's protocol. Cell cycle distribution was analyzed using
the FACSCalibur using Cell FIT software (Easy curve Fit 2.0) (both
from BD Biosciences, San Jose, CA, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
OCM-1 cells (1×105) were grown in
six-well plates, which were incubated with GNA (0, 0.75, 1.5, 3 µM)
with or without LY294002 (15 mΜ; Sigma-Aldrich; Merck Millipore)
for 24 h. LY294002, a specific inhibitor of the phosphoinositide
3-kinase (PI3K)/Akt signaling pathway, is able to significantly
repress Akt phosphorylation (15).
Total RNA was isolated from cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and treated with DNase I. Purified
RNA at density of 0.5 µg/µl with nuclease-free water was used for
cDNA synthesis with the PrimeScript First Strand cDNA Synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturers protocol. Relative mRNA expression levels of cell
cycle-associated proteins [cyclin D1, cyclin E, cyclin-dependent
kinase (CDK) 2 and P21] and epithelial-mesenchymal transition
(EMT)-associated molecules [epithelial (E)-cadherin, α-smooth
muscle actin (SMA) and vimentin] were then determined via RT-qPCR
assays using a SYBR-Green qPCR master mix kit (Toyobo Co., Ltd.,
Osaka, Japan) according to the manufacturer's protocol. The total
reaction system of 20 µl volume was as follows: 1 µl cDNA, 10 µl
SYBR Premix Ex Taq, 1 µl each of the primers (10 µM), and 7 µl
ddH2O. The primer sequences used for RT-qPCR
amplification are presented in Table
I. The amplification conditions were as follows: Denaturation
at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec,
annealing at 60°C for 30 sec, in which fluorescence signal was
collected, and extension at 72°C for 30 sec. The expression of
GAPDH mRNA was used as internal control. Relative expression values
from three independent experiments were analyzed by an ABI PRISM
7300 and calculated using the 2−ΔΔCq method (16).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| Cyclin D | F:
GCCTCTAAGATGAAGGAGACCAT |
|
| R:
CATTTTGGAGAGGAAGTGTTCAAT |
| Cyclin E | F:
GGATGTTGACTGCCTTGA |
|
| R:
CACCACTGATACCCTGAAA |
| CDK2 | F:
GCGAATTCCCCAGCCCTAATCTCA |
|
| R:
GCCTCGAGAACCCTCTTCAGCAATA |
| P21 | F:
ATGTCCAATCCTGGTGATGT |
|
| R:
TGCAGCAGGGCAGAGGAAGT |
| α-SMA | F:
CCAGAGCAAGAGAGGGATCCT |
|
| R:
TGTCGTCCCAGTTGGTGATG |
| E-cadherin | F:
CCAGTATCGTCCCCGTCCT |
|
| R:
CGGCTGCCTTCAGGTTTT |
| Vimentin | F:
CGACAAGGTGCGCTTCCT |
|
| R:
CCTGGCCCTTGAGCTGC |
| GAPDH | F:
TTCACCACCATGGAGAAGGC |
|
| R:
GGCATGGACTGTGGTCATGAG |
Western blot analysis
Cells treated with different concentrations of GNA
(0, 0.75, 1.5, 3 µM) with or without LY294002 (15 µM) were lysed in
ice-cold radioimmunoprecipitation assay buffer (Sigma-Aldrich;
Merck Millipore) and the lysate was collected by centrifugation at
4,000 × g at 4°C for 5 min. Protein concentration was determined
using the bicinchoninic acid method. A total of 30 µg lysate was
separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (GE Healthcare Life Sciences). Membranes were
subsequently blocked with 5% skimmed milk for 1 h at room
temperature and probed with primary antibodies for PI3K (ab86714;
1:100), Akt (ab8805; 1:100), cyclin D1 (ab134175; 1:100), cyclin E
(ab3927; 1:100), CDK2 (ab321147; 1:100) and P21 (ab109520; 1:100;
all Sigma-Aldrich; Merck Millipore) at 4°C overnight. Membranes
were washed with Tris-buffered saline containing 0.1% Tween-20 and
subsequently incubated at room temperature with anti-horseradish
peroxidase-conjugated secondary antibodies against EPR3312
(ab197034; 1:1,000; Sigma-Aldrich; Merck Millipore) for 1 h.
Protein blots were washed and visualized using an enhanced
chemiluminescence detection (ECL) kit (Merck Millipore).
Statistical analysis
All values are expressed as the mean ± standard
deviation calculated from three independent experiments. Student's
t-test or one-way analysis of variance was performed using SPSS
v17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
GNA inhibits the viability of OCM-1
cells in a dose-dependent manner
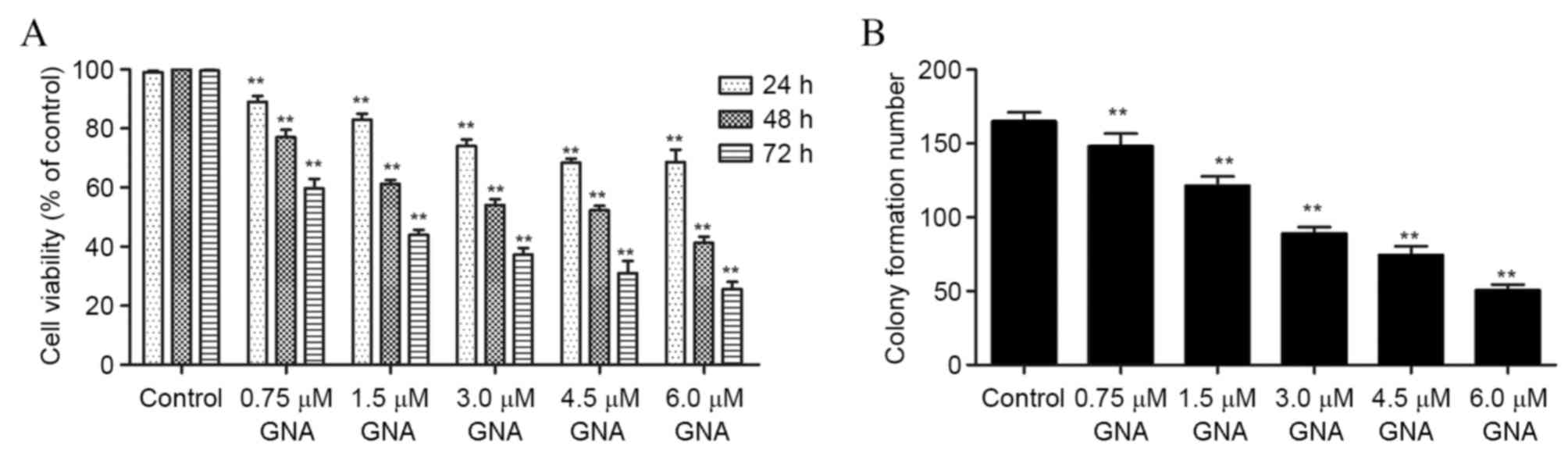
The results of the MTT assay demonstrated that the
viability of OCM-1 cells was significantly inhibited with the
increase in time and dosage of GNA treatment (P<0.01; Fig. 1A). Additionally, treatment with GNA
resulted in a significant dose-dependent decrease in the colony
formation ability of OCM-1 cells (P<0.01; Fig. 1B).
GNA induces G0/G1 arrest in a
dose-dependent manner
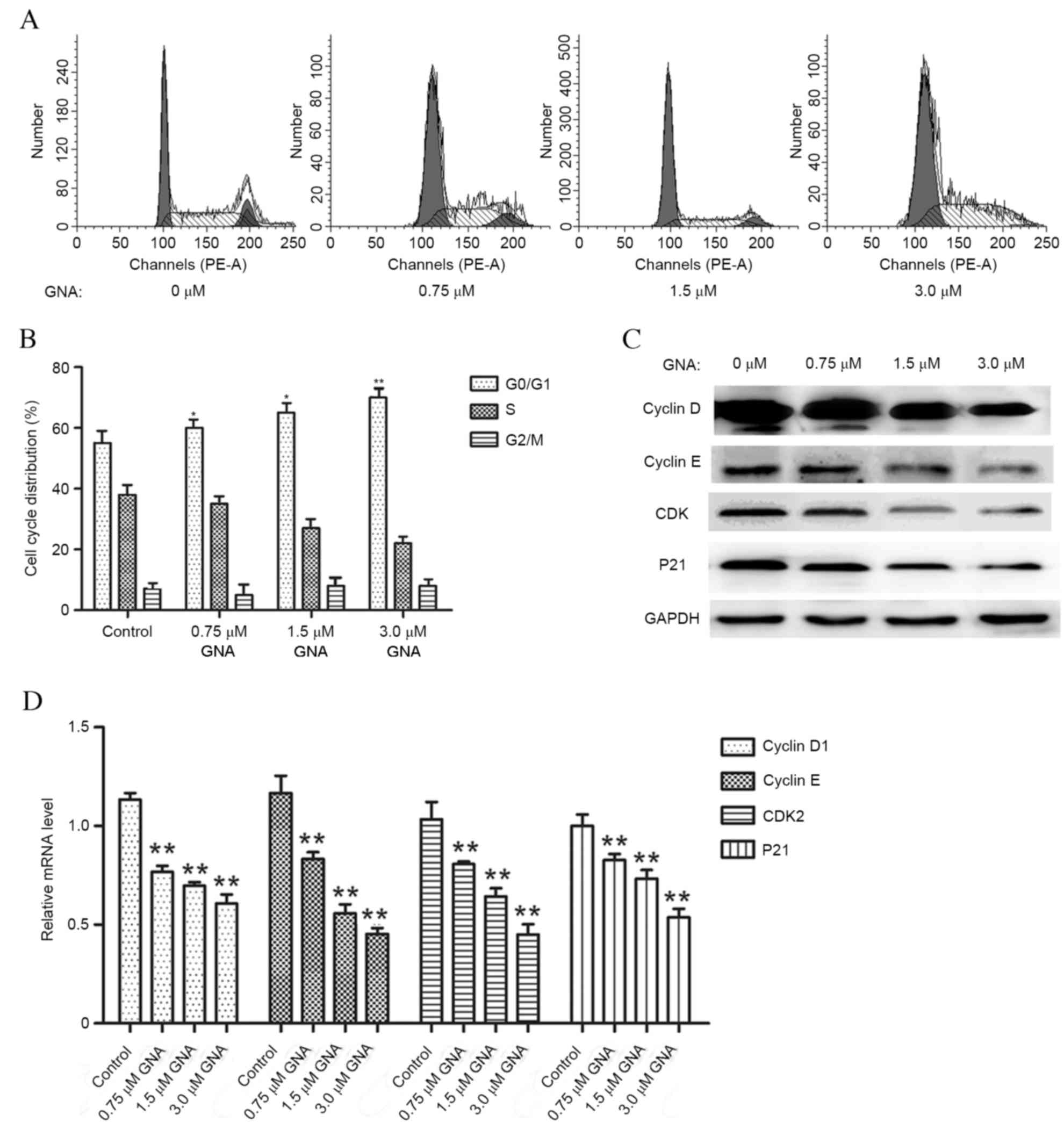
Flow cytometry was used to investigate the effects
of GNA on cell cycle (Fig. 2A). The
results demonstrate that GNA gradually increased the proportion of
OCM-1 cells in the G0/G1 phase in a dose-dependent manner. GNA
concentration increased in a dose-dependent manner up to 1.5 µM
(P<0.05) and increased further to 3.0 µM (P<0.01; Fig. 2B). Additionally, following GNA
treatment, the cell cycle-associated molecules were found to have
markedly decreased protein expressions (Fig. 2C) and the mRNA levels of these
molecules significantly decreased in a dose-dependent manner
compared with the control (Fig. 2D;
P<0.01).
GNA may inhibit cell growth via
inhibition of the PI3K/AKT pathway
To further explore the underlying mechanism for
GNA-induced cell growth inhibition, LY294002, a specific inhibitor
of the PI3K/Akt signaling pathway was used to treat cells. The
results demonstrated that treatment with GNA or LY294002 resulted
in a marked decrease in the protein expression of p-AKT/AKT
(Fig. 3A). Furthermore, the
combination of GNA and LY294002 treatment resulted in a marked
decrease in p-AKT/AKT expression compared with the cells treated
with GNA or LY294002 alone. Additionally, similar results were
obtained regarding the expression of cell cycle-associated
molecules cyclin D1, cyclin E, CDK2 and P21 (P<0.01; Fig. 3B), and cell viability (P<0.01;
Fig. 3C).
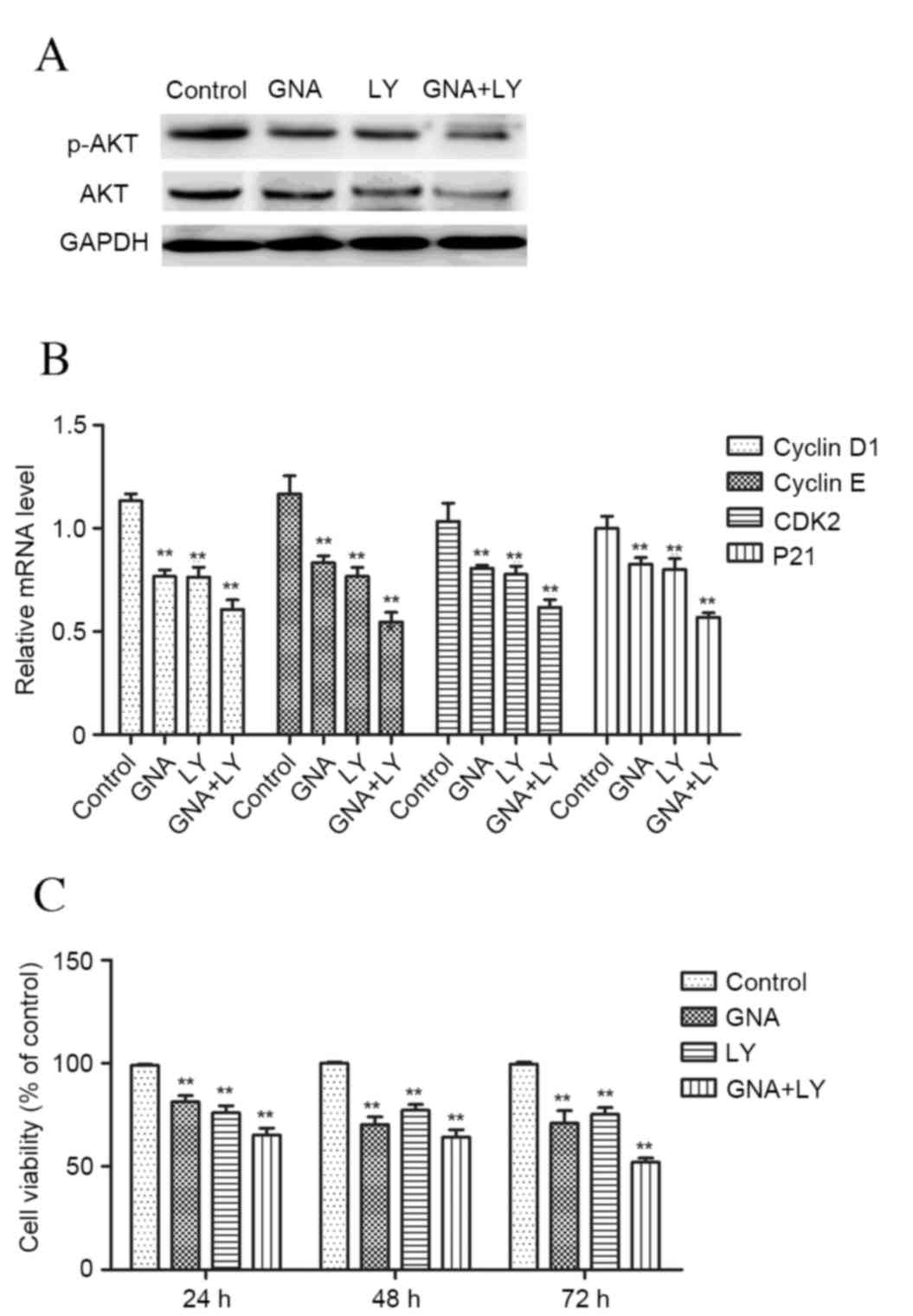 | Figure 3.Effects of GNA and LY294002, a
specific inhibitor of the PI3K/Akt signaling pathway, on cell
growth. (A) The protein expression of p-AKT/AKT. (B) The expression
of cell cycle-associated molecules (cyclin D1, cyclin E, CDK2 and
P21) (C) MTT assay of cell viability. Data are presented as the
mean ± standard deviation. **P<0.01 vs. control group. GNA,
gambogenic acid; LY, LY294002; p, phosphorylated; PI3K,
phosphoinositide 3-kinase; AKT, protein kinase B; CDK, cyclin
dependent kinase. |
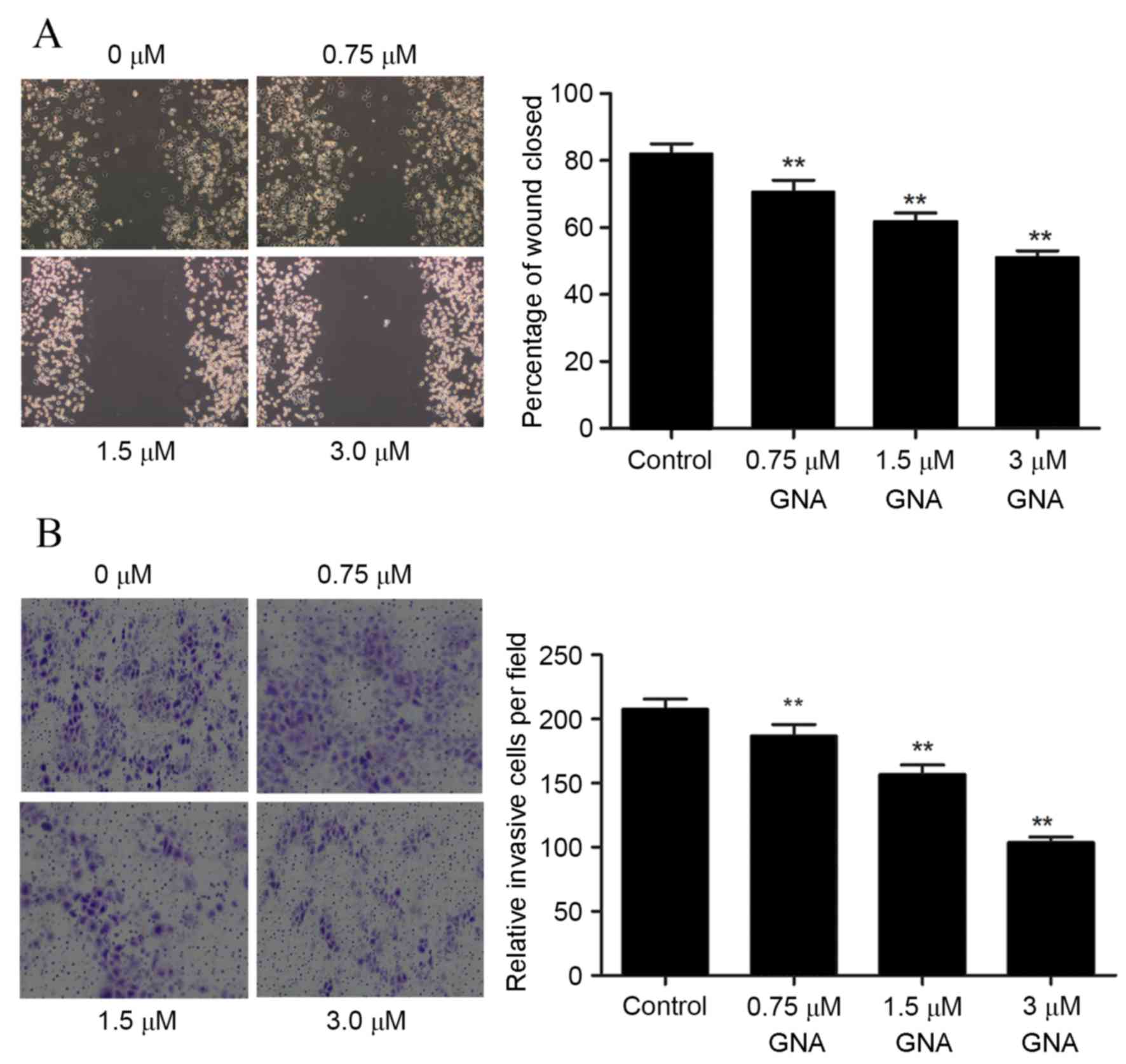
GNA reduces the migration and invasion
of OCM-1 cells
A wound healing assay was used to investigate cell
migration following treatment with different concentrations of GNA,
and the results demonstrated that treatment with GNA significantly
reduced the migration ability of OCM-1 cells at all concentrations
(P<0.05; Fig. 4A). Furthermore,
the invasive ability of cells was assessed using Transwell assay,
and the results revealed that the number of invasive cells was
significantly reduced with GNA treatment (P<0.05; Fig. 4B). In order to further verify the
association between GNA and the PI3K/AKT pathway, the expression of
PI3K/AKT pathway-related protein was detected using western blot
analysis. Cells were divided into four groups: Control, GNA (3 µM),
LY294002 (15 µM) and GNA (3 µM) + LY294002 (15 µM). The protein and
mRNA expressions of the EMT-associated proteins E-cadherin, α-SMA
and vimentin were examined via RT-qPCR and western blotting,
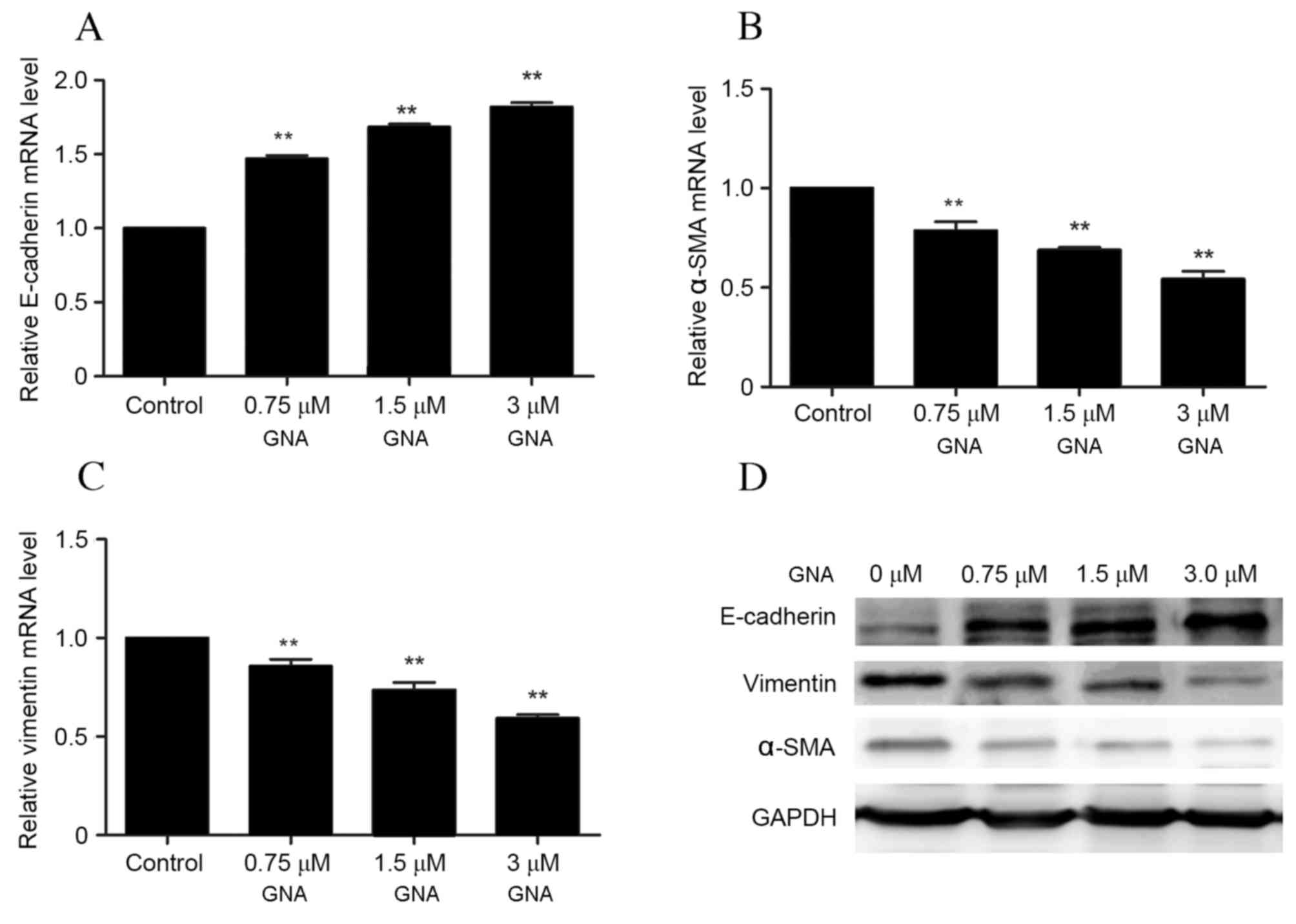
respectively. A significant dose-dependent increase in the mRNA
levels of E-cadherin was observed with GNA treatment compared with
the control group (P<0.01; Fig.
5A), whereas the levels of α-SMA and vimentin mRNA
significantly decreased with GNA treatment in a dose-dependent
manner (P<0.01; Fig. 5B and C).
Similarly, western blot analysis demonstrated that levels of
E-cadherin protein were markedly increased with increasing dosages
of GNA, whereas protein levels of α-SMA and vimentin levels were
markedly downregulated in a dose-dependent manner (Fig. 5D).
Discussion
Choroidal melanoma is a serious metastatic malignant
melanoma with poor prognosis (1). In
recent years, increasing numbers of studies have been conducted to
identify effective chemotherapy regimens with fewer side effects
(2,4). In the present study, the effects of GNA
on the malignant behaviors of choroidal melanoma cells and the
possible underlying mechanisms were investigated. It was
demonstrated that GNA was able to inhibit cell viability and induce
cell cycle arrest at the G0/G1 phase in a dose-dependent manner via
repressing the expression of cyclin D1, cyclin E, CDK2 and P21.
Furthermore, GNA treatment suppressed cell migration and invasion
in a dose-dependent manner. The results of the present study
suggest that combined treatment with LY294002, which is a specific
inhibitor of the PI3K/AKT pathway, and GNA exerts an additive
effect on the growth of OCM-1 cells, which implies that the
PI3K/AKT pathway may be an essential mechanism associated with
GNA-induced growth inhibition.
GNA has previously been demonstrated to inhibit the
proliferation of A549 cells via inducing cell cycle arrest
(10). GNA is also able to inhibit
cell proliferation, induce cell cycle arrest at G1 phase and
suppress colony-forming activity of lung cancer cells (11). Consistent with these previous
findings, the present study also demonstrated that GNA was able to
inhibit OCM-1 cell growth and induce cell cycle arrest at the G0/G1
phase via repressing the expression of cyclin D1, cyclin E, CDK2
and P21. To further explore the possible mechanisms associated with
GNA-induced growth inhibition and cell cycle arrest, the PI3K/AKT
pathway specific inhibitor LY294002 was employed. The PI3K/AKT
signaling pathway is crucial to the control of cell growth and
survival (17). It has previously
been demonstrated that the PI3K pathway is constitutively active in
melanoma via increased expression of AKT and active AKT in the
PI3K/ATK signaling pathway is able to increase cell proliferation
and survival (18,19). Additionally, the PI3K/AKT pathway is
also associated with cell cycle progression (20). AKT is able to induce pancreatic
β-cell proliferation via regulating cell cycle components, such as
cyclin D1, p21 and CDK4 (21).
Furthermore, the PI3K/AKT pathway has previously been reported to
serve an important role in melanoma initiation and therapeutic
resistance (22). The results of the
present study demonstrated that treatment with either GNA or
LY294002 resulted in a marked decrease in p-AKT/AKT expression,
whereas the combination of GNA and LY294002 was able to effectively
inhibit the expression of p-AKT/AKT. Similar results were observed
in the MTT assay, with the combined treatment resulting in a
greater inhibition of the cell viability. It may therefore be
suggested that treatment with GNA may inhibit cell growth and
induce G0/G1 arrest in choroidal melanoma via involvement of the
PI3K/AKT pathway, and that co-treatment with GNA and LY294002 may
be an efficient therapeutic approach for the treatment of choroidal
melanoma.
The metastatic activity of cancer cells is an
important aspect of cancer progression. In the present study, it
was demonstrated that the administration of GNA at different
concentrations was able to reduce the migration and invasive
ability of OCM-1 cells, which indicates that GNA may have an
inhibitory role in tumor metastasis. Furthermore, the expressions
of EMT-associated proteins (E-cadherin, α-SMA and vimentin) were
examined. Studies revealed that EMT is a major contributor to the
biological process of metastasis in a variety of cancers (23,24),
including malignant melanoma (25).
The upregulation of the EMT regulatory factor snail family
transcriptional repressor 2, which is a transcriptional repressor
of E-cadherin, functions as a precursor to the metastasis and
invasion of melanoma (26),
suggesting that decreased expression of E-cadherin may promote cell
metastasis and invasion in malignant melanoma. Canel et al
(27) demonstrated that the loss of
E-cadherin function was correlated with tumor invasion and
metastasis. Furthermore, it has previously been demonstrated that
vimentin is overexpressed in malignant melanoma and its
overexpression is correlated with accelerated tumor invasion and
poor prognosis, suggesting that it may be a potential target for
cancer therapy (28). Li et
al (29) also demonstrated that
vimentin may function as an indicator for melanoma hematogenous
metastasis and poor prognosis. In the present study, the expression
of E-cadherin markedly increased with the increase of the dose of
GNA, whereas the expression levels of α-SMA and vimentin decreased
in a dose-dependent manner. These results are in accordance with
previous studies and suggest that GNA exerts an inhibitory effect
on the metastasis of choroidal melanoma.
In conclusion, the findings of the present study
indicate that GNA may inhibit cell growth and induce G0/G1 arrest.
Furthermore, GNA may inhibit cell metastasis via regulating
EMT-associated molecules. The PI3K/Akt signaling pathway may be a
key mechanism involved in the development and progression of
choroidal melanoma. GNA may serve as a potential therapeutic
reagent for the treatment of this disease.
References
|
1
|
Shukla S, Acharya S and Dulani M: Choroid
melanoma-A rare case report. J Clin Diagn Res. 9:ED09–ED10.
2015.
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Damato B, Eleuteri A, Taktak AF and
Coupland SE: Estimating prognosis for survival after treatment of
choroidal melanoma. Prog Retin Eye Res. 30:285–295. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aubin JM, Rekman J, Vandenbroucke-Menu F,
Lapointe R, Fairfull-Smith RJ, Mimeault R, Balaa FK and Martel G:
Systematic review and meta-analysis of liver resection for
metastatic melanoma. Br J Surg. 100:1138–1147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asadi S, Vaez-Zadeh M, Masoudi SF, Rahmani
F, Knaup C and Meigooni AS: Gold nanoparticle-based brachytherapy
enhancement in choroidal melanoma using a full Monte Carlo model of
the human eye. J Appl Clin Med Phys. 16:55682015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan F, Wang M, Li J, Cheng H, Su J, Wang
X, Wu H, Xia L, Li X, Chang HC and Li Q: Gambogenic acid induced
mitochondrial-dependent apoptosis and referred to phospho-Erk1/2
and phospho-p38 MAPK in human hepatoma HepG2 cells. Environ Toxicol
Pharmacol. 33:181–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu GB, Yang XX and Huang QS: Isolation and
structure of neo-gambogic acid from Gamboge (Garcinia hanburryi).
Yao Xue Xue Bao. 19:636–639. 1984.In Chinese. PubMed/NCBI
|
|
8
|
Wang X and Chen W: Gambogic acid is a
novel anti-cancer agent that inhibits cell proliferation,
angiogenesis and metastasis. Anticancer Agents Med Chem.
12:994–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qu BX: The experimental studies on
antineoplastic action of gambogic II. Chinese J Clin Oncol.
1:0211991.
|
|
10
|
Li Q, Cheng H, Zhu G, Yang L, Zhou A, Wang
X, Fang N, Xia L, Su J, Wang M, et al: Gambogenic acid inhibits
proliferation of A549 cells through apoptosis-inducing and cell
cycle arresting. Biol Pharm Bull. 33:415–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu XJ, Han QB, Wen ZS, Ma L, Gao J and
Zhou GB: Gambogenic acid induces G1 arrest via GSK3β-dependent
cyclin D1 degradation and triggers autophagy in lung cancer cells.
Cancer Lett. 322:185–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen HB, Zhou LZ, Mei L, Shi XJ, Wang XS,
Li QL and Huang L: Gambogenic acid-induced time- and dose-dependent
growth inhibition and apoptosis involving Akt pathway inactivation
in U251 glioblastoma cells. J Nat Med. 66:62–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Wang M, Cheng H, Su JJ and Li QL:
Effect of gambogenic acid on apoptosis of melanoma cell line B16. J
Anhui Traditional Chinese Med College. 1:0202013. View Article : Google Scholar
|
|
14
|
Nogami H, Hiraoka Y and Aiso S: Estradiol
and corticosterone stimulate the proliferation of a GH cell line,
MtT/S: Proliferation of growth hormone cells. Growth Horm IGF Res.
29:33–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lejeune FJ, Rimoldi D and Speiser D: New
approaches in metastatic melanoma: Biological and molecular
targeted therapies. Expert Rev Anticancer Ther. 7:701–713. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robertson GP: Functional and therapeutic
significance of Akt deregulation in malignant melanoma. Cancer
Metastasis Rev. 24:273–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fatrai S, Elghazi L, Balcazar N,
Cras-Méneur C, Krits I, Kiyokawa H and Bernal-Mizrachi E: Akt
induces beta-cell proliferation by regulating cyclin D1, cyclin D2,
and p21 levels and cyclin-dependent kinase-4 activity. Diabetes.
55:318–325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davies MA: The role of the PI3K-AKT
pathway in melanoma. Cancer J. 18:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caramel J, Papadogeorgakis E, Hill L,
Browne GJ, Richard G, Wierinckx A, Saldanha G, Osborne J,
Hutchinson P, Tse G, et al: A switch in the expression of embryonic
EMT-inducers drives the development of malignant melanoma. Cancer
Cell. 24:466–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fenouille N, Tichet M, Dufies M, Pottier
A, Mogha A, Soo JK, Rocchi S, Mallavialle A, Galibert MD, Khammari
A, et al: The epithelial-mesenchymal transition (EMT) regulatory
factor SLUG (SNAI2) is a downstream target of SPARC and AKT in
promoting melanoma cell invasion. PLoS One. 7:e403782012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. Journal of cell science. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cellular
and Molecular Life Science. 68:3033–3046. 2011. View Article : Google Scholar
|
|
29
|
Li M, Zhang B, Sun B, Wang X, Ban X, Sun
T, Liu Z and Zhao X: A novel function for vimentin: The potential
biomarker for predicting melanoma hematogenous metastasis. J Exp
Clin Cancer Res. 29:1092010. View Article : Google Scholar : PubMed/NCBI
|