Introduction
Kidney cancer is the one of the leading causes of
cancer-related mortality worldwide. Renal clear cell carcinoma
(RCC) accounts for ~85% of kidney cancer diagnoses, particularly in
the United States (1,2).
Protein kinase C (PKC) represents a family of
serine/threonine kinases that are classified into three major
groups: Classical (α, βI, βII and γ), novel (δ, ε, η and θ) and
non-classical (µ, ζ and ι). These kinases may be involved in
growth, differentiation, apoptosis, tumor promotion and migration
(3–5). As a result, all of these PKC isozymes
have distinct functions and sometimes they even have opposing roles
in cancer (6,7). For instance, in glioma cells, PKCα
enhances cell proliferation, indicating that it may have a role in
tumor promotion. However, overexpression of PKCδ inhibits cell
proliferation and may be associated with cell apoptosis (8). Previous results have suggested that
PKCα may be closely associated to cancer progression (5). Notably, a high level of PKCα expression
was strongly associated with a high migratory activity of colon
cancer cells, and a translocation of the activated PKCα at the
plasma membrane was observed (9). By
contrast, cell apoptosis was induced in LNCaP cells of prostate
carcinoma upon activation of PKCα, indicating tumor suppressive
properties (10). Previous research
from our laboratory revealed that high expression and activation of
PKCα is associated with tumor progression in superficial bladder
carcinoma, and abnormal activation of PKCα may result in endogenic
resistance to chemotherapy drugs, such as adriamycin (11). Furthermore, it has been suggested
that the unusual translocation of PKCα between the plasma membrane
and cytosol could be involved in the progression of kidney
carcinoma (12). However, the
mechanism of PKCα in kidney cancer and its efficiency thus far
remained unclear. Whether PKCα functioned as a tumor promoter or
suppressor in kidney cancer was also unclear. Therefore, the
present study investigated the expression of PKCα in both clinical
specimens and kidney cancer cell lines, and used gene silencing
technology in order to identify the function of PKCα in kidney
cancer.
Materials and methods
Cell culture
All cell lines were purchased from Cell Bank of
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China). In total, four different types of
kidney cell lines selected: ACHN (Obtained from carcinomatous
pleural effusion of kidney cancer), 786-O (renal adenocarcinoma),
caki-1 (skin metastasis of kidney cancer) and HKC (immortalized
renal tubular epithelial cells). ACHN and 786-O were cultured in
RPMI-1640 (Lonza, Verviers Sprl, Verviers, Belgium) basal medium.
Caki-1 was grown in McCOY's 5A medium (Gibco; Thermo Fischer
Scientific, Inc., Grand Island, NY, USA), and F12 basal medium
(Gibco; Thermo Fischer Scientific, Inc.) was selected for the HKC
cells. Cells were also supplemented with 10% fetal bovine serum
(FBS; EuroClone SpA, West York, UK) with antibiotics (100 U/ml
penicillin and 100 mg/ml streptomycin; both Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) in a humidified 5%
CO2 incubator at 37°C.
PKCα inhibitors and antibodies
The PKCα inhibitor GO6976 and calphostin C were
obtained from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany)
and dissolved in dimethylsulfoxide (Sigma-Aldrich; Merck
Millipore). Antibodies against PKCα (sc-8393) and poly-ADP-ribose
polymerase 1 (PARP-1; sc-25780) were purchased from Santa Cruz
Biotechnology, Inc.). Anti-caspase 3 was purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA), anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH; WH0002597M1) was obtained from
Sigma-Aldrich (Merck Millipore) and anti-caspase-9 (WL01551) was
supplied by Wanleibio Co., Ltd. (Shenyang, China).
Small-interfering RNA (siRNA), plasmid
and cell transfection
The sequences of PKCα siRNA were as follows:
Forward, 5′-GUGCCAUGAAUUUGUUACUTT-3′ and reverse,
5′-AGUAACAAAUUCAUGGCACTT-3′. Inactivation of PKCα in ACHN cells was
achieved when ACHN cells stably expressed the PKCα-dominant
negative (DN; ACHN-DN) PKCα. PKCα-siRNA and ACHN-DN plasmid (2.5 µg
of plasmid DNA) transfections were both performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA). Lipofectamine®
2000 was used for 12 µl in a 2-ml transfection system. After 24 h
of transfection, the cells were replaced by RPMI-1640 basal medium
for 24 h and were then processed in two parts. In the first part
the cells were harvested following siRNA transfection and RPMI-1640
basal medium treatment for 24 h; In the second part the cells were
continuously treated with G418 (600 µg/ml) for selecting stable
clones for a period of 14 days. The medium was changed every 48 h
and colonies of G418-resistant cells were selected.
Samples
In total, 20 patients (aged 31–79 years; 10 men and
10 women) who were diagnosed with primary kidney carcinoma were
selected from the Department of Urology in the First Hospital of
China Medical University (Shenyang, China) between December 2011
and July 2013. Seven cases had left RCC and thirteen cases had
right RCC. The diagnosis was confirmed by pathological examination,
and the histological subtype was identified as RCC. In addition,
none of the patients accepted chemotherapy or radiation therapy
treatments. Samples of the normal control kidney tissue were
collected from each patient with a distance of >3 cm from the
tumor. A part of tumor tissues and the corresponding normal tissues
were quickly frozen in −80°C for protein extraction and the
remainder was perfused with phosphate-buffered saline (PBS) and
fixed with 4% formalin overnight for paraffin embedment and
immunohistochemical staining.
Quantitative polymerase chain reaction
(qPCR) analysis
RNAs were extracted with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions, and were then quantified using a NanoDrop ND-100
spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA,
USA). Reagents were purchased from Takara Bio, Inc. (Otsu, Japan)
and were used to detect the expression of PKCα. The reaction was 20
µl in total and a LightCycler 480 qPCR system (Roche Diagnostics,
Basel, Switzerland) was used for the reaction. The conditions were
the following: 50°C for 2 min, 95°C for 5 min, 45 cycles of 95°C
for 40 sec and 55°C for 30 sec. Data were analyzed using the
2−ΔΔct method and mRNA expression was normalized against
β-actin RNA. The primer sequences for β-actin were as follows:
Sense, 5′-CTCCATCCTGGCCTCGCTGT-3′ and anti-sense,
5′-GCTGTCACCTTCACCGTTCC-3′. In addition, the PKCα mRNA expression
was detected using the following primers: Sense,
5′-GGAACCACAAGCAGTATT-3′ and anti-sense, 5′-GTCCTTCTGAATCCAACAT-3′.
The PKCβ sequences were: Sense, 5′-AAATGCTCCCTCAACCCT-3′ and
anti-sense, 5′-TCAAATCCCAATCCCAAA-3′ and the PKCγ sequences were:
sense, 5′-GCGGCTGGAACGATTGGA-3′ and anti-sense,
5′-TGGCGGCGGGTGAGATTAC-3′.
Western blotting
The purchased cells were lysed in the culture flask
using RIPA buffer containing the protease inhibitor
phenylmethanesulfonyl fluoride, then centrifuged at 13,200 ×
g for 30 min. Total cellular proteins were extracted from
kidney tissues using a protein extraction buffer RIPA containing
protease inhibitors. Equal quantities of protein (50 µg cells and
100 µg tissues) extracts were subjected to 10 % SDS-PAGE
electrophoresis at 220 V and the resolved proteins were transferred
onto polyvinylidene fluoride membranes. The membranes were then
blocked for 60 min in a 37°C temperature-controlled shaking table,
and subsequently incubated with the following primary antibodies:
Polyclonal mouse anti-PKCα (1:1,000), polyclonal rabbit anti-PARP-1
(1:1,000), polyclonal rabbit anti-caspase-3 (1:1,000), polyclonal
rabbit anti-caspase-9 (1:1,000) and anti-GAPDH (1:2,000) overnight
at 4°C. The following day, Tris-buffered saline and Tween-20 was
used to remove unbound antibodies and then incorporated with the
secondary antibody diluted at 1:2,000 for 60 min in a 37°C
temperature-controlled shaking table. The bands were then
visualized by chemiluminescence using the EC3 Imaging System (UVP
LLC, Cambridge, UK).
Immunohistochemical staining
Fresh kidney tissue samples obtained from the
patients (~1.5×1.5×0.2 cm) were perfused with PBS and fixed with 4%
formalin overnight. The next day, tissues were embedded in paraffin
and then 4-µm sections were placed on glass slides. Afterwards,
antigen retrieval was performed for 2.5 min. Next, an
immunohistochemical kit (Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd., Beijing, China) was used, then the slides were incubated
with primary antibody (monoclonal mouse anti-PKCα; 1:100; sc-8393;
Santa Cruz Biotechnology, Inc.) overnight at 4°C. Subsequently,
3,3′-diaminobenzidine staining was applied for 3 min then rinsed
out immediately. In addition, hematoxylin staining solution was
used for nuclear counterstaining, and the dyes were rinsed well
under running water for >3 h. Finally, the slides were mounted
and analyzed using an upright metallurgical microscope (IX71S8F-3;
Olympus Corporation, Tokyo, Japan).
Flow cytometry analysis
After 24 h with the PKCα inhibitors, the cells were
washed with PBS and detached with 0.25% trypsin. Subsequently, the
cells were centrifuged at 1,200 × g for 5 min and the
supernatant solutions discarded. Next, cells were washed twice with
PBS, and the mixture was centrifuged at 1,200 × g for 5 min.
Afterwards, the cell mass was resuspended in 400 µl PBS and
detected with a Annexin V-fluorescein isothiocyanate/propidium
iodide kit (Beyotime Institute of Biotechnology, Haimen, China)
using a flow cytometer (FACSCalibur Flow Cytometer; BD Pharmingen,
San Diego, CA, USA) and following the manufacturer's instructions.
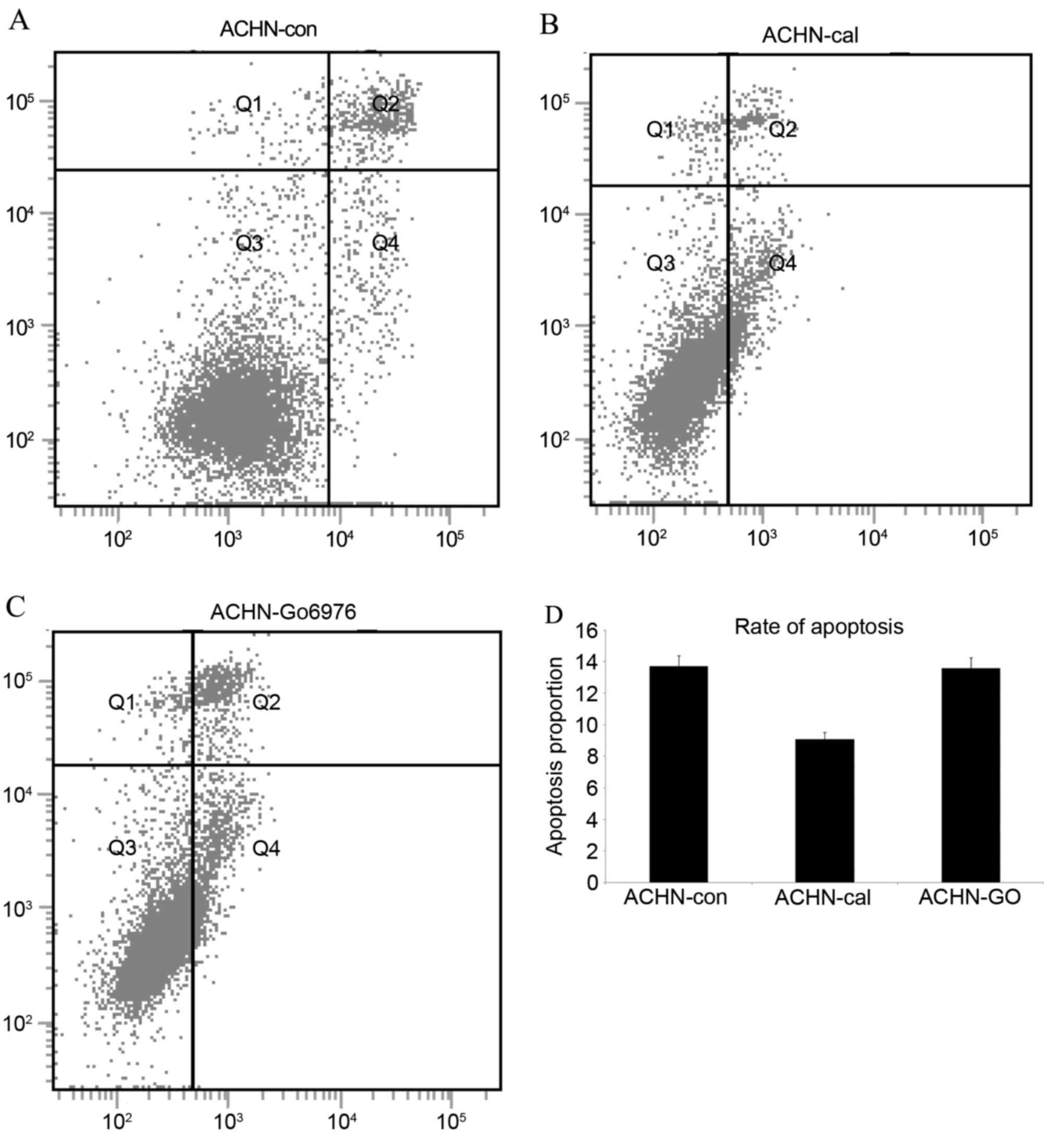
Q2 represented late stage apoptotic and Q4 represented early
apoptotic cells. In addition, Q2+Q4 was used to detect
apoptosis.
Scratch wound healing assay
The migration capacities of ACHN cells and the
PKCα-DN-expressing cell line ACHN-DN were investigated. The two
types of cells were plated at a density of 1×105
cells/well in 24-well plates. Cells were then incubated in
RPMI-1640 medium containing 10% FBS for 24 h to 80% confluence,
then a scratch was performed using a 200 µl pipette tip, and the
cells were grown for 24 h in serum-free medium. The scratch spaces
were then analyzed using an inverted microscope.
Statistical analysis
Statistical analysis was performed using SPSS for
windows 13.0 statistical analysis software (SPSS, Inc., Chicago,
IL, USA). In addition, the independent and the paired t-test were
used. P<0.05 was used to indicate a statistically significant
difference.
Results
PKCα expression was frequently
decreased in human kidney cancer tissues
In total, 18 pairs of cancer tissue and their
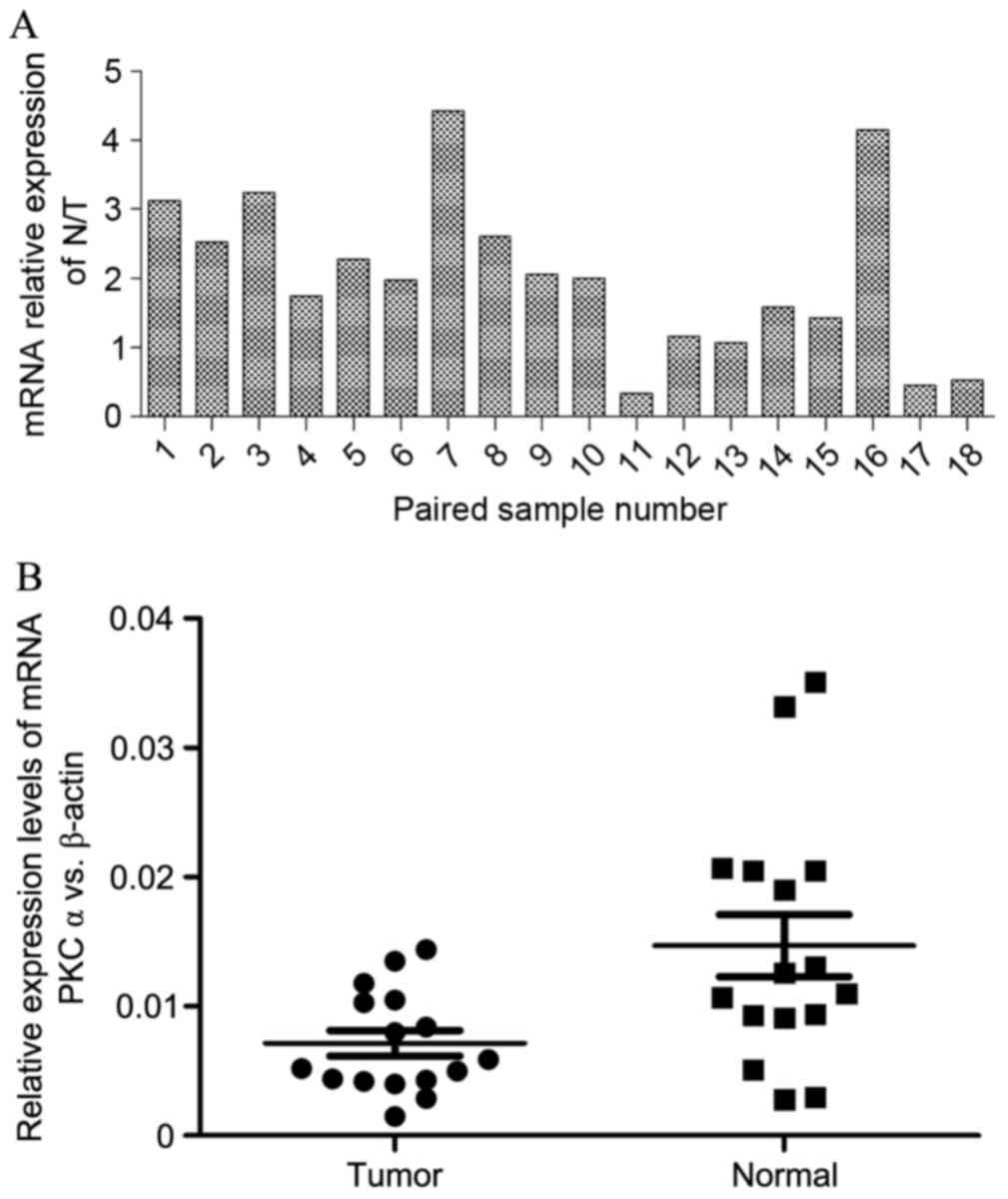
corresponding normal samples were selected. The results of Fig. 1A indicated that PKCα was
significantly downregulated in the majority of kidney carcinoma (15
out of 16) using qPCR, where numbers 17 and 18 were considered to
be outliers based on statistical algorithms. Afterwards, the pairs
were disrupted to compare normal tissues and tumor tissues, and a
high expression rate of PKCα in kidney normal tissues was
identified (Fig. 1B). In addition,
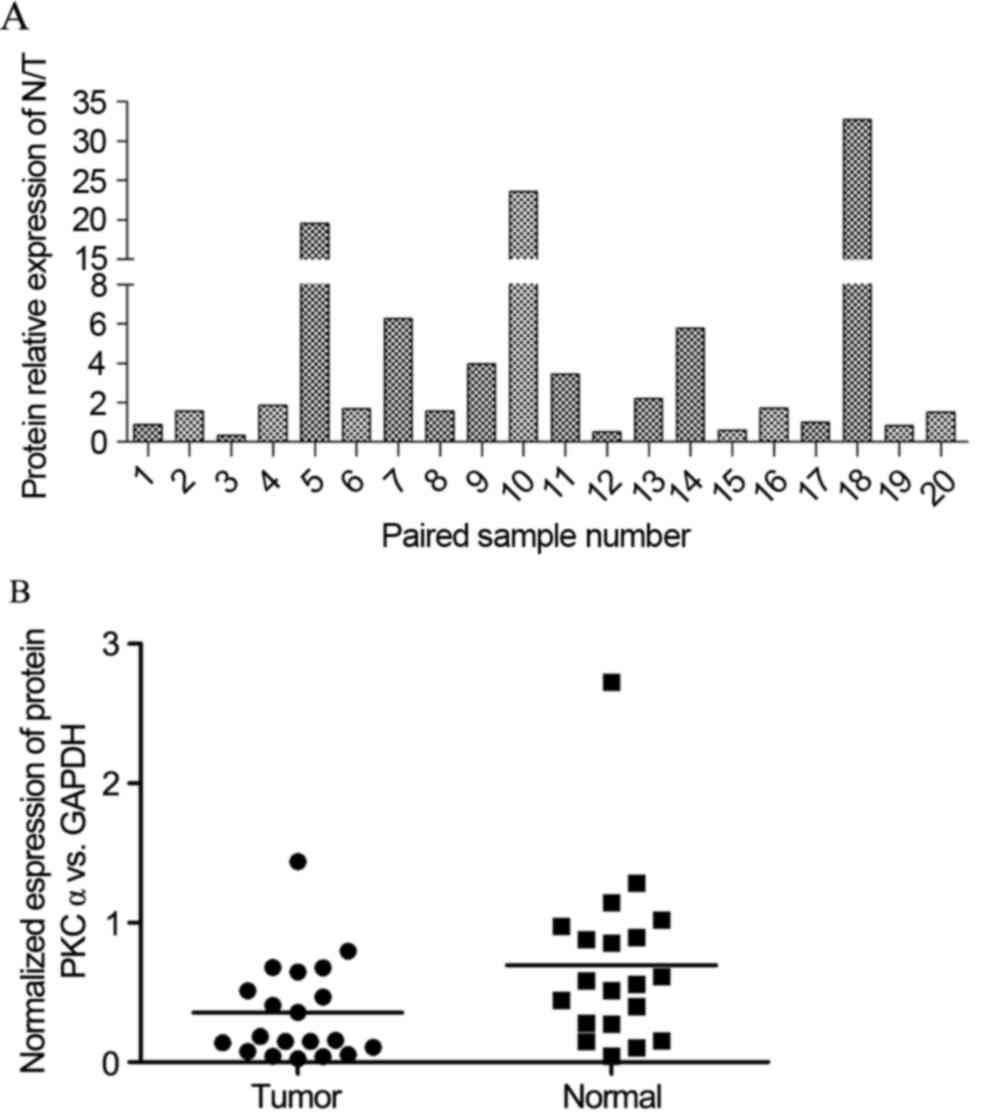
the results of western blotting revealed that the levels of PKCα
were evidently decreased in ~80% kidney tumors (16 in 20) (Fig. 2A). However, kidney cancer tissues
still show a lower expression than before, after the pairs were
separated (Fig. 2B).
qPCR results demonstrated that the
expression of PKCα was upregulated in metastatic cancer cell
lines
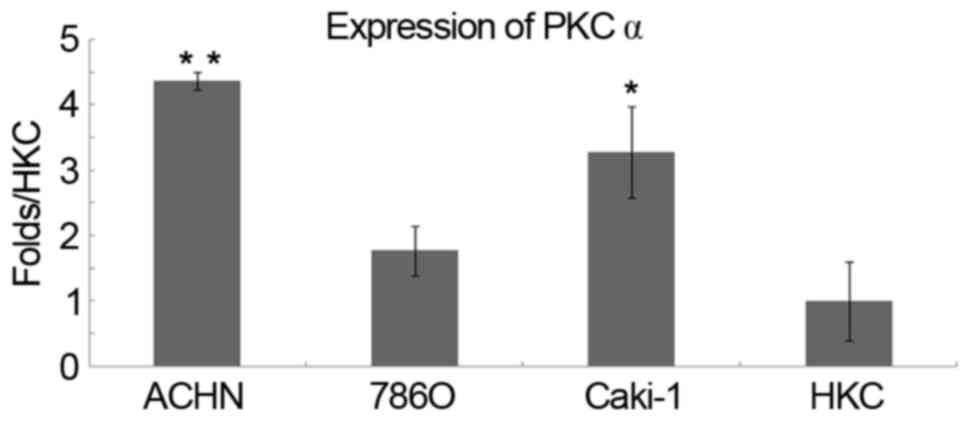
The expression of PKCα was examined by qPCR in renal
tubular epithelial HKC cells, in two types of metastatic cancer
cell lines and in the human RCC cell line, 786-O. The results
indicated that PKCα was significantly upregulated in ACHN (P=0.004)
and Caki-1 (P=0.033) cells compared with the HKC cells (Fig. 3). However, proteins associated with
metastasis require further studies in the two cell lines.
Drugs, siRNA and plasmids were used to
decrease PKCα, however, there was no increase in apoptosis
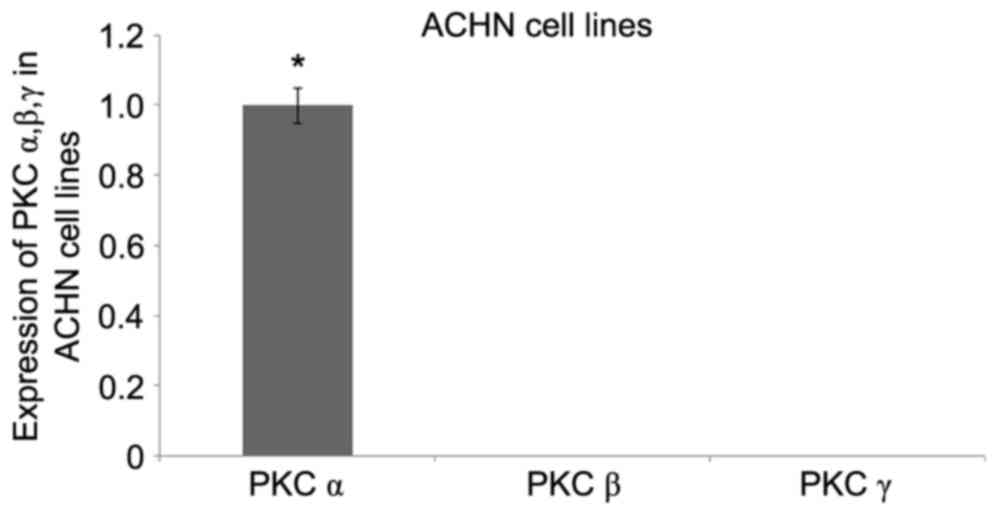
Initially, the expression pattern of the PKC
classical types family (PKCα, β and γ) was analyzed in ACHN cells.
qPCR analysis revealed that PKCα expression was prominent in the
ACHN cell line, whereas the expression levels of PKCβ and PKCγ was
too faint to be detected (Fig. 4),
indicating that PKCα may mediate specific and vital functions in
kidney cancer cells. Calphostin C and GO6976 were inhibitors of the
PKC classical types. Since PKCα occupied the main status, the
effect of the other two PKC types was considered to be minimal. In
addition, the two drugs simply inhibited PKCα. The effects of
calphostin C (100 nM) and GO6976 (500 nM) were investigated by flow
cytometry and western blotting, and the results demonstrated that
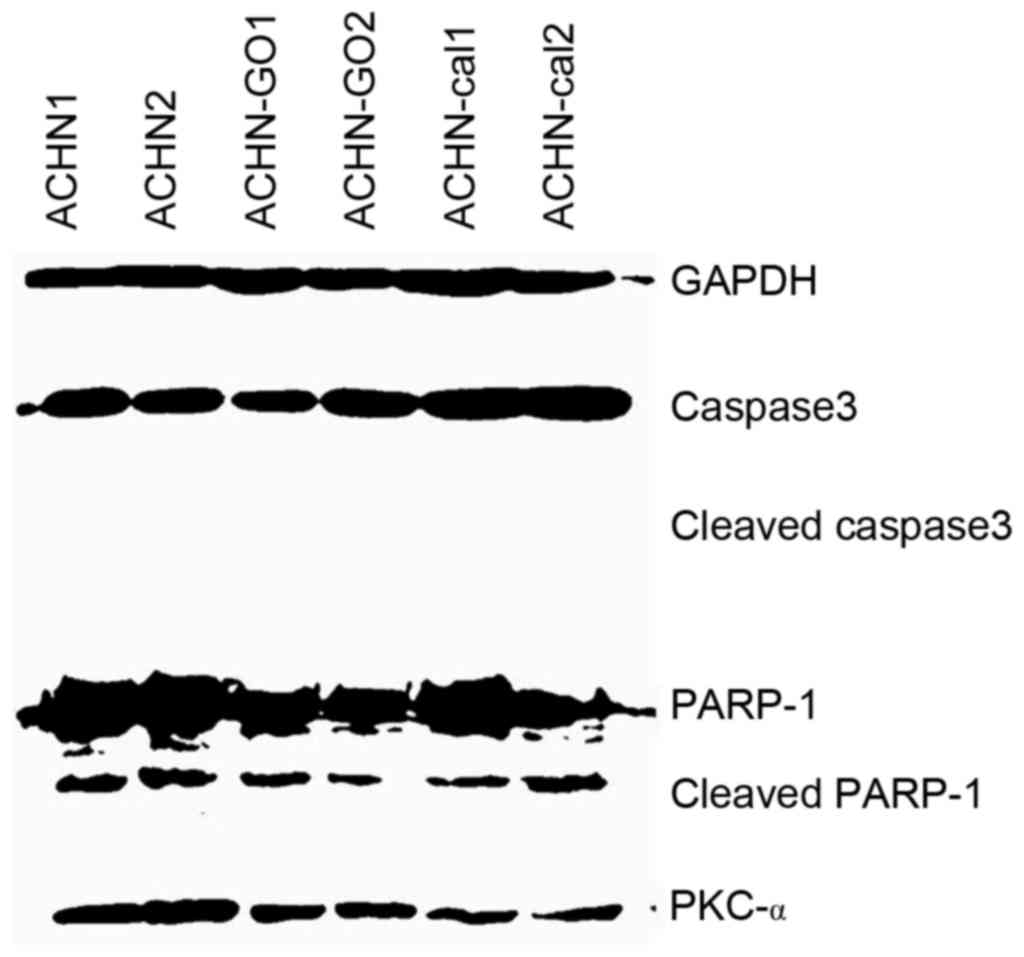
PKCα expression was reduced. Furthermore, three types of
apoptosis-regulated proteins were also detected (caspase-3, −9 and
PARP-1). PKC inhibitors led to a no significant increase in the
apoptosis rates of ACHN cells (Fig.
5), and there were no increasing cleaved bands of the
apoptosis-regulated proteins (Fig.
6). Based on the lack of increase of apoptosis-regulated
protein expression following treatment with the PKC inhibitors in
kidney cancer cells, it was speculated that PKCα may be not be an
inhibitor of apoptosis. In order to verify this assumption,
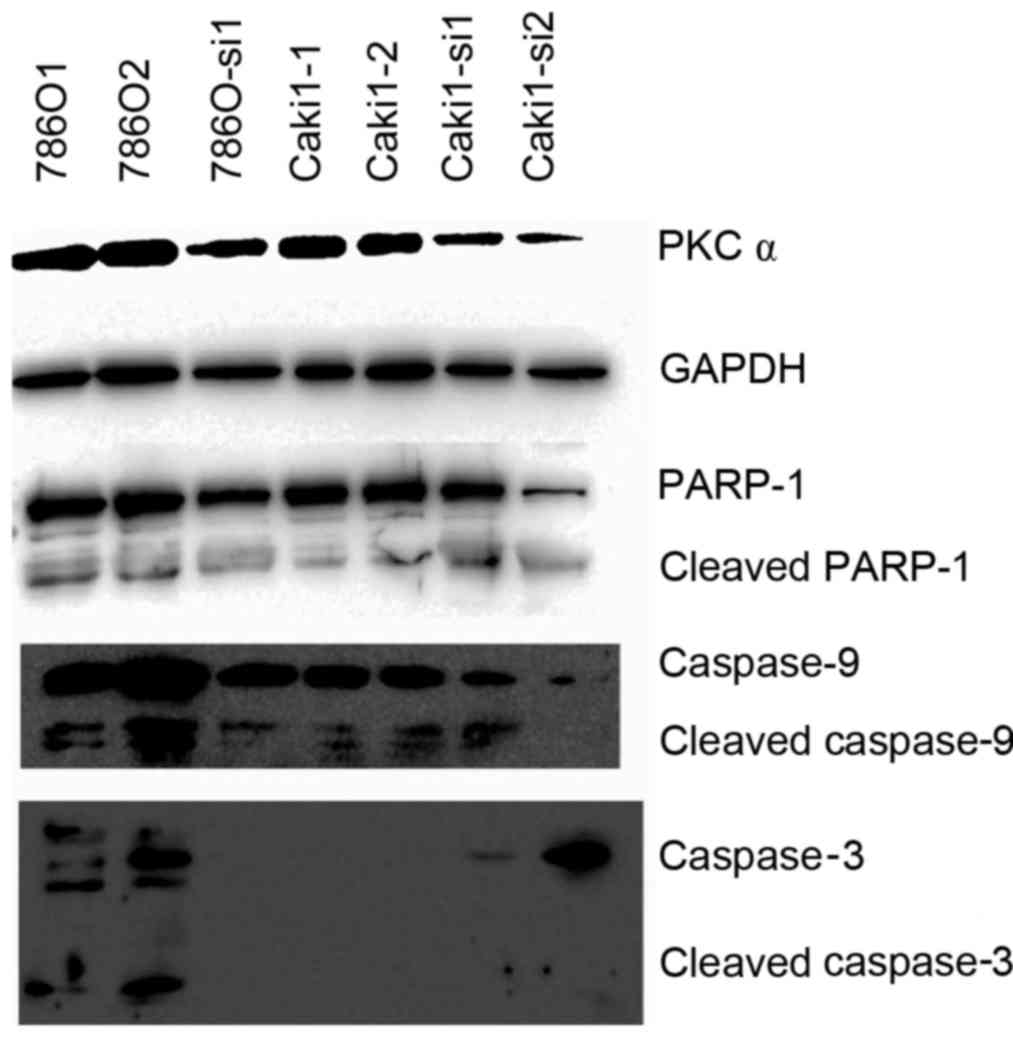
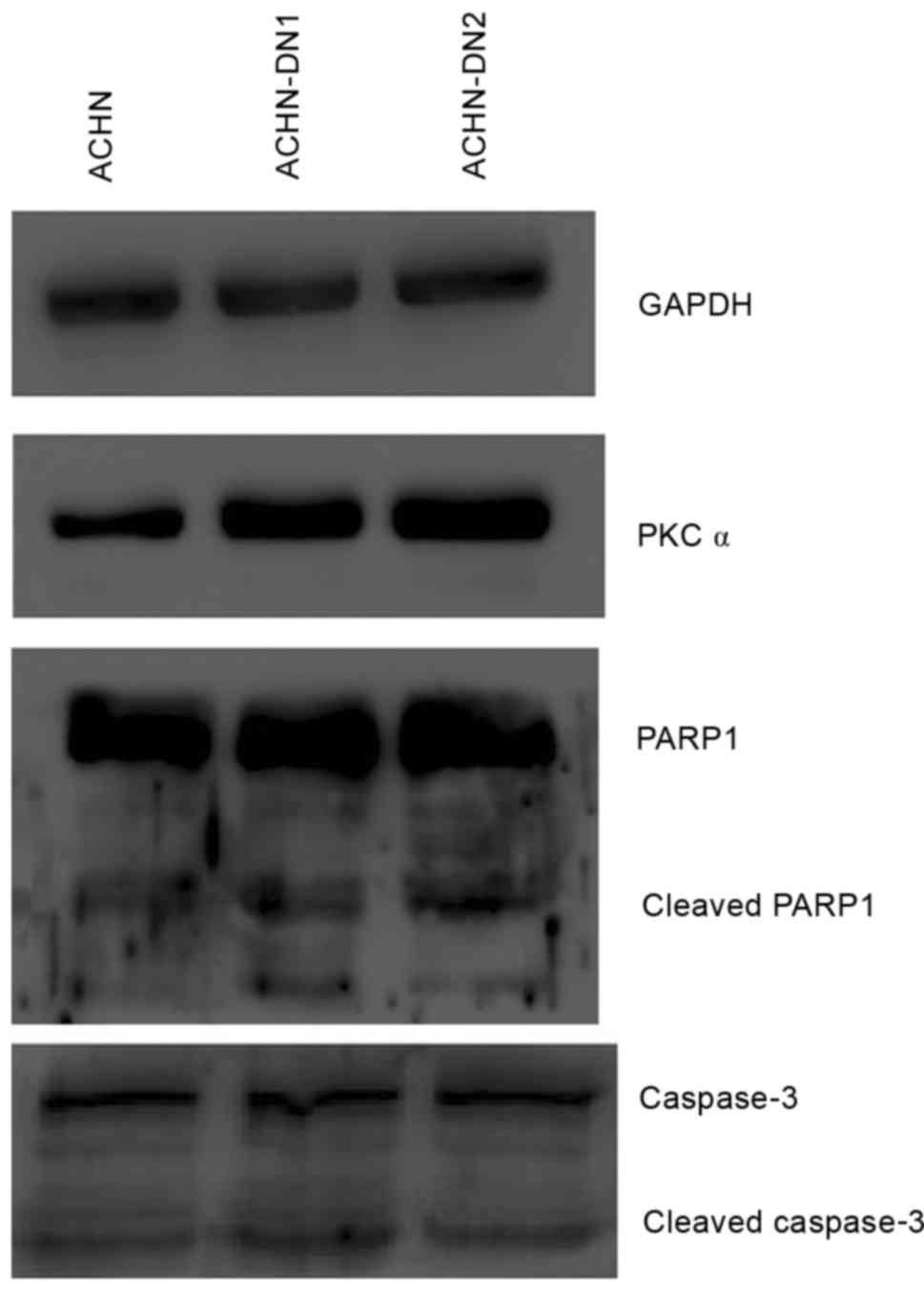
Lipofectamine 2000 was used to transfect siRNA in order to
knockdown PKCα in ACHN and Caki-1 cells. In addition, it was used
to get the kinase inactivation plasmid (ACHN-DN) to inactivate
PKCα. A successful knockdown of PKCα was confirmed by western
blotting. In addition, no significant effects were identified on
basal apoptosis-regulated protein expression (Fig. 7). ACHN-DN inactivated PKCα but
increased its expression. Meanwhile, the condition of
apoptosis-regulated proteins remained the same (Fig. 8). These results suggested that PKCα
have not yet been found to have a major impact on suppressing
apoptosis in kidney carcinoma.
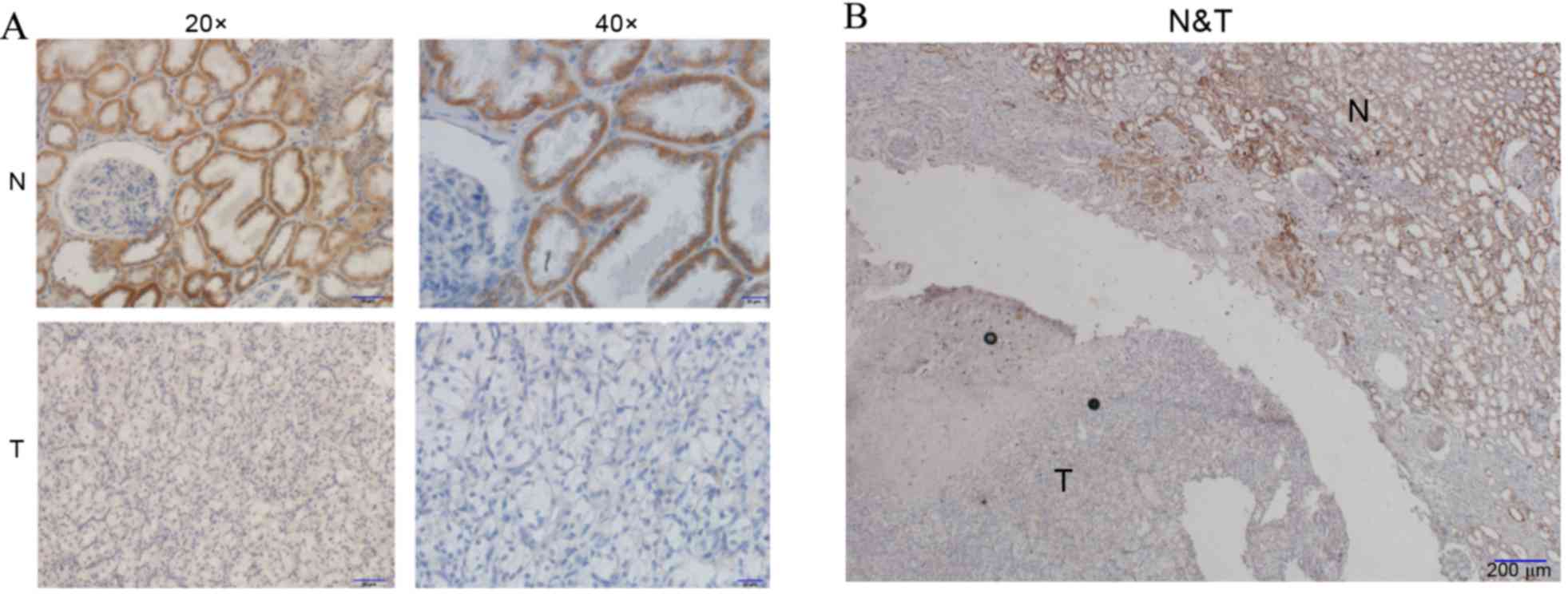
PKCα protein stained strongly in
cytoplasm of normal kidney tubular epithelial cells but not in
glomeruli
On account of its higher and more prevalent
expression in the kidney, PKCα expression was also investigated by
immunohistochemical analysis of RCC and normal sections. It was
demonstrated that PKCα protein was expressed in the normal kidney
proximal tubular epithelial and distal convoluted tubule cells, and
it stained the cytoplasm strongly, whilst being absent or
negligible in the glomeruli. In addition, PKCα staining in RCC
tissue sections was shown to have a very weak brown staining
throughout the cytoplasm and sometimes even negatively stained.
Finally, almost all 24 pairs of tissues exhibited similar staining
characteristics, where strong staining of PKCα protein was
indicated in the cytoplasm of normal kidney tissue and weak
staining was observed in RCC tissues (Fig. 9).
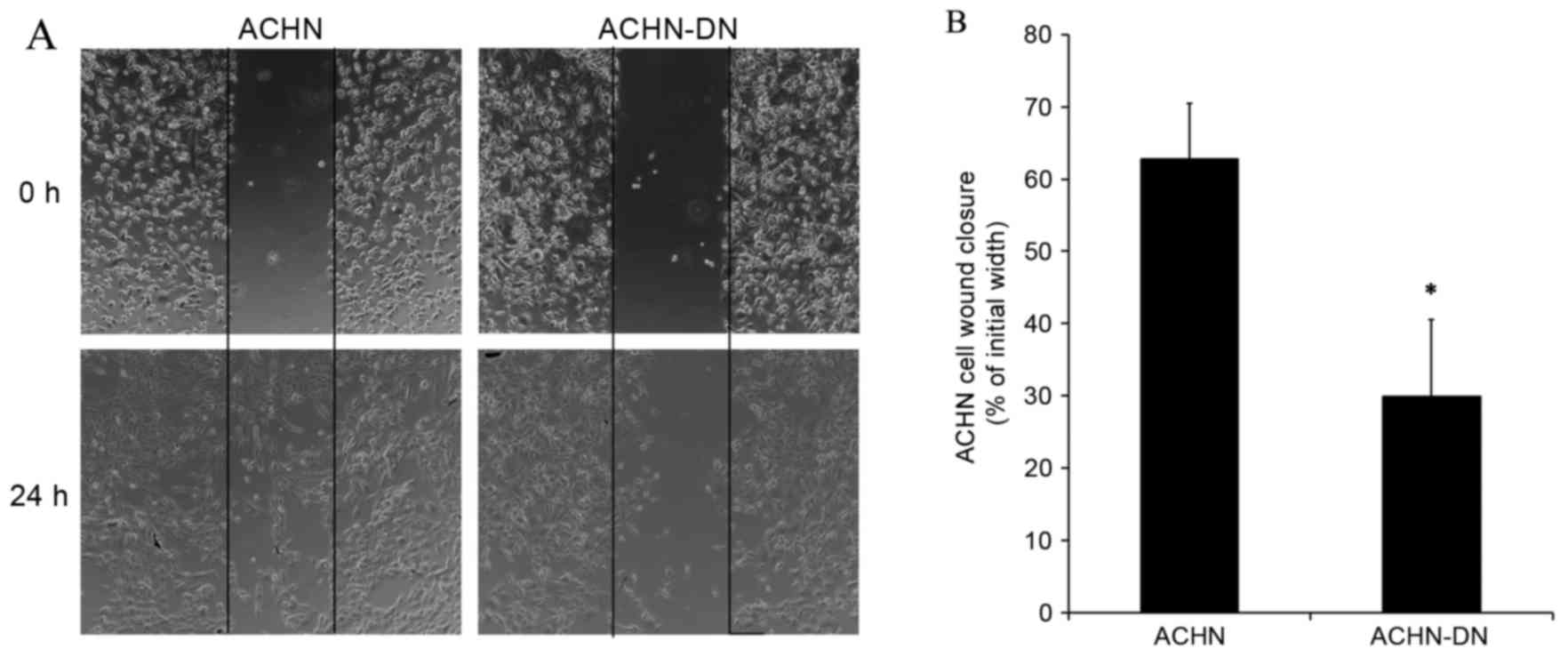
Decrease of migration ability
following transfection with PKCα-DN
In order to analyze a possible effect of PKCα on
kidney cancer cell migration, wound-healing assay was applied. It
was revealed that transfection with PKCα-DN significantly inhibited
the migration of ACHN cells (Fig.
10). In addition, an altered morphology of ACHN cells was noted
following transfection. ACHN cells acquired a more angular shape
compared to the untransfected control cells, and the healing rate
was subject to statistical analysis.
Discussion
The present study revealed that the PKC family had
different biological effects in different types of tumors. The
upregulation or downregulation of PKC and their isoenzyme or their
biological effect had previously been studied in human tumor
samples (13–17). Downregulation of PKC isozymes was
associated with the occurrence and progression of several human
cancer types. Previous studies had verified that three types of PKC
isozymes, α, β and δ, were predominant in the normal bladder
epithelium. Furthermore, PKCβ and δ were downregulated in
transitional cell carcinomas; however, PKCα increased (18,19).
In the present study, two inhibitors (calphostin C
and GO6976) were used to decrease PKCα. As earlier work confirmed,
Ca2+ spiking can be prevented by the PKC inhibitor
calphostin C (20) due to
conventional PKCs (α, βI, βII and γ) being activated by
Ca2+. Therefore, calphostin C mainly inhibited PKCα, βI,
βII and γ. In addition, GO6976 had previously been demonstrated to
inhibit PKC isoenzymes α and βI (21–23). In
Fig. 4, it was indicated that PKCβ
and γ had low expression in the kidney cancer cell line, therefore
GO6976 and calphostin C mainly inhibited PKCα. Thus,
apoptosis-related proteins were selected to evaluate the impact on
apoptosis following the addition of the two inhibitors. It was
revealed that the expression of apoptosis-related proteins,
caspase-3, −9 and PARP-1, had not changed significantly, and no
hydrolysis bands had evidently increased. Although the focus was on
siRNA and the PKCα inactive plasmid, there was no increase observed
in cell apoptosis. Therefore, PKCα was not considered to have a
major impact on suppressing apoptosis in kidney carcinoma.
Based on the results on the detection of tumor
tissues, PKCα was found to have a high expression in normal kidney
but decreased in tumor tissues. In other words, PKCα may decrease
when normal kidney tissue becomes cancerous. Notably, when we
compared one patient's tumor tissues with normal ones, PKCα
presented a lower expression in tumors. However, when we disrupted
the pairs the result demonstrated no statistical significance.
These results indicated that the expression of PKCα varied from
person to person; therefore, the most accurate method was to
compare tumor tissue pairs with the normal control ones.
Fig. 3 indicated that
in two kidney cancer transferred cell lines, ACHN and Caki-1, PKCα
was markedly expressed. However, more research is required in order
to understand the mechanism and metastasis-related proteins in the
two cell lines, and whether they are correlated with PKCα. Previous
research affirmed that in retinal pigment epithelium cells, the
wound healed more slowly in the siRNA-PKCα compared to the
non-siRNA group at the three time points (24). This result suggests that PKCα may be
important in cell migration. In the results of the present study,
following inactivation of PKCα, cell migration was inhibited which
suggested that PKCα may also be important in the ability to move in
kidney cancer cell lines.
Immunohistochemical staining revealed that the
expression of PKCα protein was detected in the normal kidney
proximal tubular epithelial and distal convoluted tubule cells,
which were predominantly located in the cytoplasm, and exhibited
very strong staining. Meanwhile, in tumor sections, PKCα stained
very weakly or not at all. This observation was consistent with the
results of western blotting. In conclusion, it was shown that the
inhibition of PKCα may not be due to a significant gain in
expression of apoptotic proteins, and that PKCα does not function
by suppressing apoptosis in kidney cancer cells. In addition, PKCα
was decreased in tumor tissues of the kidney. Therefore,
downregulation of PKCα may be an early event in the development of
kidney carcinoma, but the mechanisms by which PKCα functioned
remained elusive. In conclusion, the results of the present study
demonstrated that inhibition of PKCα may not contribute to
apoptosis progression in kidney carcinoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172438) and the
Doctoral Fund of the Ministry of Education of China (grant no.
20112104110006).
Glossary
Abbreviations
Abbreviations:
|
PKC
|
protein kinase C
|
|
RCC
|
renal clear cell carcinoma
|
|
ADM
|
adriamycin
|
|
PARP
|
poly-ADP-ribose polymerase
|
|
DMSO
|
dimethylsulfoxide
|
|
PBS
|
phosphate-buffered saline
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
References
|
1
|
Kent EE, Ambs A, Mitchell SA, Clauser SB,
Smith AW and Hays RD: Health-related quality of life in older adult
survivors of selected cancers: Data from the SEER-MHOS linkage.
Cancer. 121:758–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow WH, Devesa SS, Warren JL and Fraumeni
JF Jr.: Rising incidence of renal cell cancer in the United States.
JAMA. 281:1628–1631. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Webster CR, Johnston A and Anwer MS:
Protein kinase Cδ protects against bile acid apoptosis by
suppressing pro-apoptotic JNK and BIM pathways in human and rat
hepatocytes. Am J Physiol Gastrointest Liver Physiol.
307:G1207–G1215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishizuka Y: Intracellular signaling by
hydrolysis of phospholipids and activation of protein kinase C.
Science. 258:607–614. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aaltonen V and Peltonen J: Protein Kinase
C FamilyEncyclopedia of Cancer. Schwab M: Springer; Berlin: pp.
2469–2472. 2009
|
|
6
|
Fields AP and Murray NR: Protein kinase C
isozymes as therapeutic targets for treatment of human cancers. Adv
Enzyme Regul. 48:166–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang LL, Cao FF, Wang Y, Meng FL, Zhang
Y, Zhong DS and Zhou QH: The protein kinase C (PKC) inhibitors
combined with chemotherapy in the treatment of advanced non-small
cell lung cancer: Meta-analysis of randomized controlled trials.
Clin Transl Oncol. 17:371–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mandil R, Ashkenazi E, Blass M, Kronfeld
I, Kazimirsky G, Rosenthal G, Umansky F, Lorenzo PS, Blumberg PM
and Brodie C: Protein kinase Calpha and protein kinase Cdelta play
opposite roles in the proliferation and apoptosis of glioma cells.
Cancer Res. 61:4612–4619. 2001.PubMed/NCBI
|
|
9
|
Masur K, Lang K, Niggemann B, Zanker KS
and Entschladen F: High PKC alpha and low E-cadherin expression
contribute to high migratory activity of colon carcinoma cells. Mol
Biol Cell. 12:1973–1982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka Y, Gavrielides MV, Mitsuuchi Y,
Fujii T and Kazanietz MG: Protein kinase C promotes apoptosis in
LNCaP prostate cancer cells through activation of p38 MAPK and
inhibition of the Akt survival pathway. J Biol Chem.
278:33753–33762. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong C, Zhu Y, Liu D, Yu M, Li S, Li Z,
Sun Z and Liu G: Role of protein kinase C-alpha in superficial
bladder carcinoma recurrence. Urology. 65:1228–1232. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chuize K, Yuyan Z, Zhe Z, Tao L, Meng Y
and Qi Y: Protein kinase C-alpha is expressed and activated during
the development of renal cell carcinoma. Urology. 76:514.e1–e5.
2010. View Article : Google Scholar
|
|
13
|
Zhang HT, Zhang D, Zha ZG and Hu CD:
Transcriptional activation of PRMT5 by NF-Y is required for cell
growth and negatively regulated by the PKC/c-Fos signaling in
prostate cancer cells. Biochim Biophys Acta. 1839:1330–1340. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molè D, Gentilin E, Gagliano T, Tagliati
F, Bondanelli M, Pelizzo MR, Rossi M, Filieri C, Pansini G, Uberti
EC degli and Zatelli MC: Protein kinase C: A putative new target
for the control of human medullary thyroid carcinoma cell
proliferation in vitro. Endocrinology. 153:2088–2098. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bergelin N, Löf C, Balthasar S, Kalhori V
and Törnquist K: S1P1 and VEGFR-2 form a signaling complex with
extracellularly regulated kinase 1/2 and protein kinase C-alpha
regulating ML-1 thyroid carcinoma cell migration. Endocrinology.
151:2994–3005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu YH, Yao J, Chan LC, Wu TJ, Hsu JL,
Fang YF, Wei Y, Wu Y, Huang WC, Liu CL, et al: Definition of PKC-α,
CDK6, and MET as therapeutic targets in triple-negative breast
cancer. Cancer Res. 74:4822–4835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Y, Zhou W, Xue L, Zhang W and Zhan Q:
Nicotine activates YAP1 through nAChRs mediated signaling in
esophageal squamous cell cancer (ESCC). PLoS One. 9:e908362014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langzam L, Koren R, Gal R, Kugel V, Paz A,
Farkas A and Sampson SR: Patterns of protein kinase C isoenzyme
expression in transitional cell carcinoma of bladder. Relation to
degree of malignancy. Am J Clin Pathol. 116:377–385. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varga A, Czifra G, Tállai B, Németh T,
Kovács I, Kovács L and Bíró T: Tumor grade-dependent alterations in
the protein kinase C isoform pattern in urinary bladder carcinomas.
Eur Urol. 46:462–465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan J and Byron KL: Ca2+ signalling in rat
vascular smooth muscle cells: A role for protein kinase C at
physiological vasoconstrictor concentrations of vasopressin. J
Physiol. 524:821–831. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bailey TA, Luan H, Tom E, Bielecki TA,
Mohapatra B, Ahmad G, George M, Kelly DL, Natarajan A, Raja SM, et
al: A kinase inhibitor screen reveals protein kinase C-dependent
endocytic recycling of ErbB2 in breast cancer cells. J Biol Chem.
289:30443–30458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aaltonen V, Koivunen J, Laato M and
Peltonen J: PKC inhibitor Go6976 induces mitosis and enhances
doxorubicin-paclitaxel cytotoxicity in urinary bladder carcinoma
cells. Cancer Lett. 253:97–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du HF, Ou LP, Yang X, Song XD, Fan YR, Tan
B, Luo CL and Wu XH: A new PKCα/β/TBX3/E-cadherin pathway is
involved in PLCε-regulated invasion and migration in human bladder
cancer cells. Cell Signal. 26:580–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu S, Jiang Z, Huang Z, Chen X, Qian X,
Gao Q and Zheng H: Migration of retinal pigment epithelium cells is
regulated by protein kinase Cα in vitro. Invest Ophthalmol Vis Sci.
54:7082–7090. 2013. View Article : Google Scholar : PubMed/NCBI
|