Introduction
Primary angiitis of the central nervous system
(PACNS) is a rare idiopathic disorder that results in multifocal
inflammation of the small and medium-sized blood vessels in the
brain, which causes focal or diffuse neurological symptoms
(1,2). PACNS may occur at any age, but is
predominantly observed in children aged between 4 and 6 years old,
with no clear gender predilection (3). PACNS is a rare disease, with incidences
of 1–2.4 per million per year reported in Europe and America
(4). Although PACNS is extremely
rare, the number of reported cases has gradually increased since
the late 1970s and after the diagnostic criteria had been devised
(5). Viral infections increase the
possibility of PACNS, particularly in immunocompromised patients
(5). It is important to diagnose it
promptly, since results on a small case series have suggested a
favourable response to corticosteroids and immunosuppressive
therapy (6). However, the diagnosis
is often difficult due to its rarity and nonspecific clinical
presentation (7). The range of
differential diagnosis is broad, including infection, connective
tissue diseases and systemic vasculitis, which require careful
exclusion (8). Magnetic resonance
imaging (MRI) is the primary neuroimaging modality for patients
with suspected PACNS (9). Most PACNS
patients have abnormal findings on MRI. Therapy comprises of
immunosuppressants, including corticosteroids alone or, preferably,
in combination with cyclophosphamide (10).
The present study performed a retrospective analysis
of 19 cases of PACNS from Suqian City People's Hospital (Suqian,
China) to characterise the MRI features of PACNS and to evaluate
the diagnostic value and clinical significance of MRI.
Materials and methods
Patients
The Institutional Review Boards of Nanjing First
Hospital (Nanjing, China) as well as the Nanjing Medical University
local Ethics Committees approved this investigation. Written
consent was obtained from all patients. A total of 19 PACNS
patients consecutively hospitalized at Suqian City People's
Hospital from February 2009 to January 2015 (7 males and 12
females; mean age, 42 years; age range, 34–52 years) were included.
Regarding the mode of disease onset, 9 cases presented with sudden
or progressive limb weakness, 14 with cephalalgia, 6 with apathy
and cognitive disorder, 5 with physical convulsion and 7 with
distortion of commissure. These patients were diagnosed by clinical
criteria, which included i) Symptoms of cephalalgia and multifocal
nervous system disorder, lasting for >6 months, or severe
initial symptoms; ii) angiographic findings of involvement of
multiple segmental arterial structures; iii) exclusion of systemic
inflammation or infectious diseases; and iv) vascular inflammatory
lesions identified on biopsy of pia mater and brain parenchyma
(Table I).
 | Table I.Clinical features, treatment and
clinical course. |
Table I.
Clinical features, treatment and
clinical course.
| Case number | Age (years)/sex | Symptoms | Location of
lesions | Magnetic resonance
imaging findings (contrast-enhanced pattern) | Treatment | Follow-up period | Clinical course |
|---|
| 1 | 48/M | Limb weakness,
cognitive abnormalities | Bilateral frontal,
temporal and parietal lobes | Patchy,
cord-like | Steroids, CTX | 6 months | Stable |
| 2 | 42/M | Headache, limb
weakness, seizures | Bilateral frontal,
temporal and parietal lobes | Patchy | Steroids | 12 months | Stable |
| 3 | 37/F | Headache, limb
weakness, distortion of commissure | Right temporal
lobe | Patchy | Steroids, CTX | 3 months | Stable |
| 4 | 50/F | Headache, cognitive
abnormalities | Bilateral frontal,
temporal and parietal lobes | Patchy, goral | Steroids | 24 months | Stable |
| 5 | 42/M | Headache, distortion
of commissure | Brainstem | Goral | Steroids, CTX | 2 months | Relapse |
| 6 | 34/F | Limb weakness,
seizures | Left parietal
lobe | Patchy | Steroids | 36 months | Stable |
| 7 | 52/F | Headache, distortion
of commissure | Bilateral frontal,
temporal and parietal lobes | Patchy | Steroids | 3 months | Stable |
| 8 | 37/F | Headache, cognitive
abnormalities, seizures | Right temporal
lobe | Goral | Steroids, CTX | 14 months | Stable |
| 9 | 39/M | Headache, distortion
of commissure | Bilateral frontal,
temporal and parietal lobes | Patchy, cord-like,
goral | Steroids, CTX | 24 months | Stable |
| 10 | 51/F | Limb weakness | Bilateral basal
ganglia | Patchy, cord-like,
goral | Steroids | 7 months | Stable |
| 11 | 39/F | Headache, distortion
of commissure | Bilateral frontal,
temporal and parietal lobes | Patchy | Steroids, CTX | 12 months | Stable |
| 12 | 45/F | Headache, limb
weakness | Left frontal
lobe | Patchy, goral | Steroids, CTX | 24 months | Stable |
| 13 | 46/M | Headache, cognitive
abnormalities, seizures | Bilateral basal
ganglia | Patchy | Steroids | 18 months | Stable |
| 14 | 39/M | Headache, limb
weakness | Bilateral frontal,
temporal and parietal lobes | Goral | Steroids | 12 months | Stable |
| 15 | 41/F | Headache,
distortion of commissure | Bilateral frontal,
temporal and parietal lobes | Cord-like, | Steroids | 24 months | Stable |
| 16 | 42/F | Limb weakness,
cognitive abnormalities | Left cerebellar
hemisphere | Patchy | Steroids | 8 months | Stable |
| 17 | 36/F | Headache, limb
weakness | Bilateral basal
ganglia | Goral | Steroids | 12 months | Stable |
| 18 | 35/F | Headache,
distortion of commissure | Left frontal
lobe | Patchy | Steroids | 6 months | Stable |
| 19 | 40/M | Cognitive
abnormalities, seizures | Bilateral frontal,
temporal and parietal lobes | Patchy, cord-like,
goral contrast-enhanced | Steroids, CTX | 3 months | Relapse |
After diagnosis, the patients underwent treatment
with steroids or steroids plus immunosuppressive agents, which
resulted in improvement or disappearance of symptoms and partial or
complete disappearance of the lesions.
Examination procedure
All patients underwent an MRI examination on a 1.5 T
scanner (Avanto®; Siemens, Erlangen, Germany). The MRI
scan protocol included the following: T1-weighted imaging [T1WI;
response time (TR), 448 msec; echo time (TE), 8.7 msec; field of
view, 230 mm], T2WI (TR, 4,500 msec; TE, 91 msec), fluid-attenuated
inversion recovery (FLAIR; TR, 8,000 msec; TE, 106 msec),
diffusion-weighted imaging (DWI; b=1,000 sec/mm2),
apparent diffusion coefficient (ADC) mapping (TR, 3,400 msec; TE,
102 msec) and susceptibility-weighted imaging (SWI; TR, 49 msec;
TE, 40 msec). All enhanced scanning procedures used T1WI, 5-mm
section thickness and a 1.5-mm gap with gadopentate dimeglumine
(0.1 mmol/kg) as the contrast medium.
Results
Cases
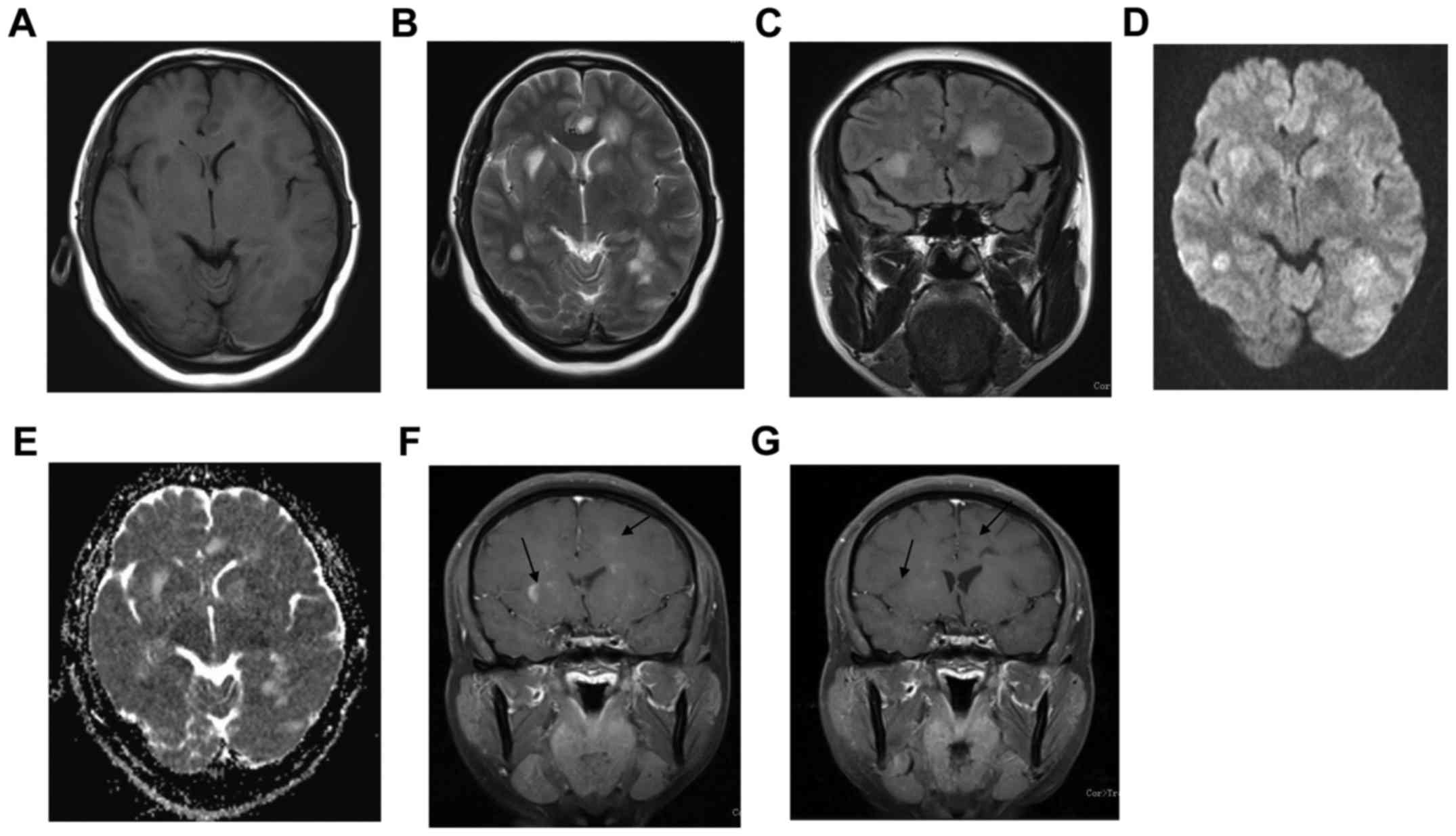
MRI findings showed 7 cases with unilateral lesions
and 12 cases with bilateral lesions involving grey and white
matter, often with indistinct margins (Figs. 1 and 2; Table I).
Oedema was seen bordering six lesions. Nine cases showed bilateral
asymmetrical involvement of frontal, temporal and parietal lobes,
and three cases showed bilateral asymmetrical basal ganglia
involvement. One case of brainstem, one case of left cerebellar
hemisphere, two cases of right temporal lobe, two cases of frontal
lobe and one case of left parietal lobe involvement were also
identified, the involvement or location of lesions were shown in
Table I.
All lesions were slightly hypointense on T1WI,
slightly hyperintense on T2WI, markedly hyperintense on FLAIR, iso-
or slightly hyperintense on DWI and hyperintense on ADC mapping. On
SWI, distortion and thickening of vessels was identified (Fig. 2F). After contrast injection, all
lesions showed focal, cord-like or goral enhancement (Figs. 1 and 2).
The first case was a 45-year-old female who
experienced initially headache and fatigue in early 2009. She
developed bilateral limb weakness. Neurological examination
revealed dysarthria, ataxia and quardriparesis. Brain MRI showed
the left frontal and parietal lobes as well as bilateral basal
ganglia regions appeared hypointense on T1WI and slightly
hyperintense on T2WI. The lesion appeared slightly hyperintense on
DWI and ADC. On contrast-enhanced T1WI, left frontal and bilateral
basal ganglia regions showed patchy enhancement (Fig. 1).
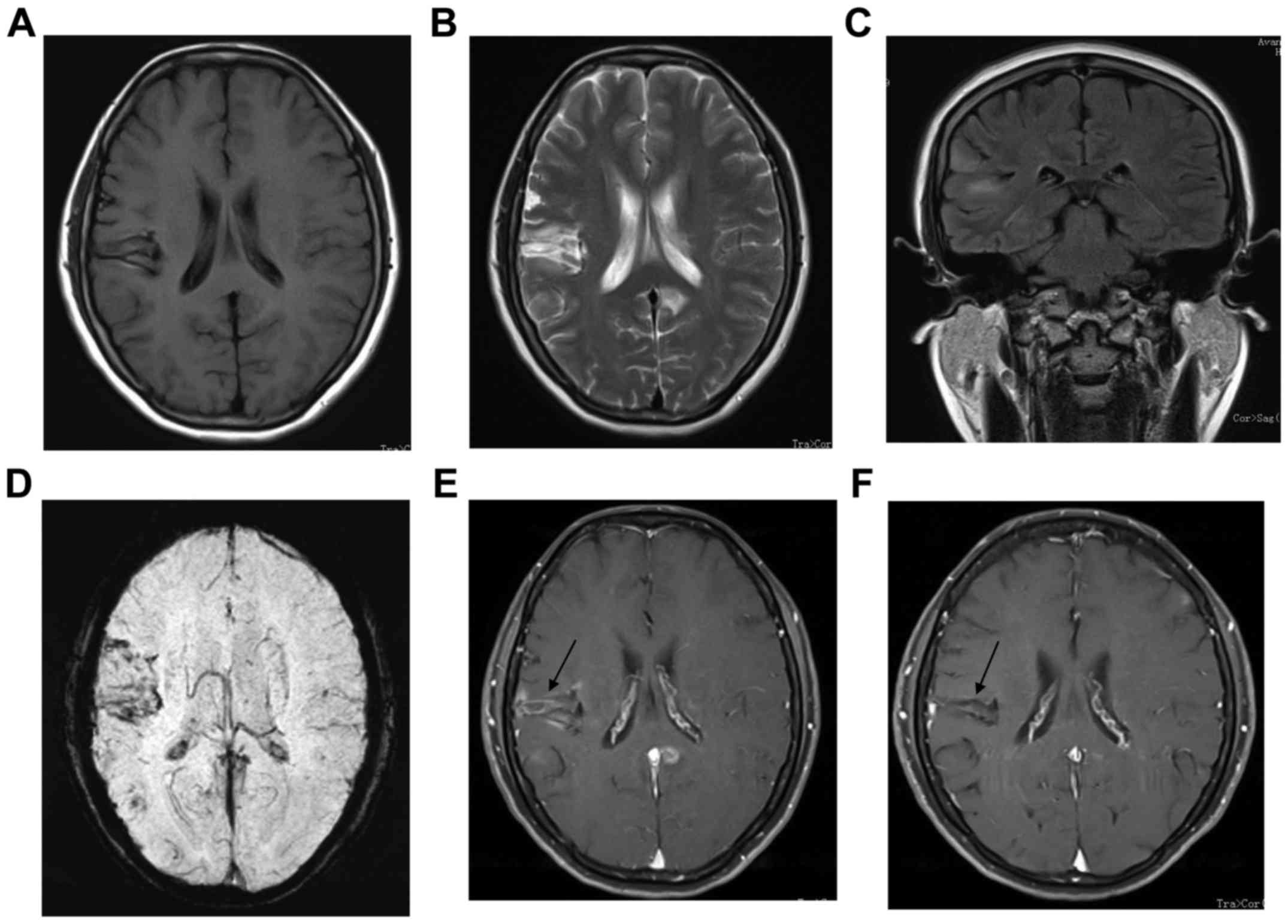
The second case was a 37-year-old female who
presented with sustained treatment-responsive headache that had
been begun February 2009. She was admitted to hospital with severe
diffuse headache, limb weakness and distortion of commissure a few
months later. There was no history of drug abuse. Routine
laboratory evaluation, serum immunoprotein electrophoresis,
ryoglobulins, coagulation studies, urine analysis, chest x-ray and
echocardiography revealed no abnormalities. Brain MRI indicated
patchy lesions with isointense T1 and hyperintense T2 signal in
right temporal lobe (Fig. 2).
Treatment and outcome
Among the 19 PACNS patients, less severely ill
patients were treated with prednisone (1–2 mg/kg/day), while eight
patients with a severe condition were administered intravenous
pulse therapy of 1 g methylprednisolone for three days plus
intravenous cyclophosphamide (CTX; 10 mg/kg) every two weeks for
three months. In a total of 17 patients, clinical symptoms and MRI
appearance had apparently improved (Figs. 1G and 2E), and only two patients relapsed.
Discussion
In 1959, Cravioto and Feigin (11) were the first to recognize the entity
of PACNS after examination of brains on autopsy. PACNS attracted
more attention when instances of successful treatment were reported
in the 1980s (12). It was then
referred to as ‘granulomatous angiitis of the CNS’, with the
lesions mainly consisting of granulomas. In 1983, the term
‘isolated angiitis of the central nervous system’ (IACNS) was
adopted for the disease. However, as certain patients presented
with extracranial involvement, the term IACNS had limitations, and
in recent years, the term PACNS has therefore been used in common
practice (13).
According to Kelley (14), PACNS may be due to direct damage to
blood vessels from infection or an immunoreaction mediated by
immune complex deposits from auto-antibodies. PACNS may occur at
any age. The mean age of the cohort of the present study was 42
years. The mode of onset may be acute or insidious. The prodrome
usually lasts for half a year or longer, featuring chronic
processes and easy recurrence. PACNS has varied clinical symptoms
with headache being most common one (15), which occurred in 14 cases of the
present study. Symptoms may correspond to focal or diffuse CNS
involvement. Focal symptoms include hemiparesis and seizures;
diffuse symptoms are headache, apathy, cognitive abnormalities and
psychosis (16).
The histopathologic features of PACNS include
inflammatory infiltrates composed mainly of lymphocytes accompanied
by histiocytes and plasma cells, particularly involving small
leptomeningeal and intracerebral arteries (17,18).
Small vessels can be distinguished by bleeding of small vessels in
brain parenchyma. At the acute stage, the vascular wall is
infiltrated by lymphocytes, plasmatocytes, monocytes and giant
cells. In the chronic stage, achroacytes and multinucleate giant
cells with focal fibrosis of the vascular wall are observed.
Langerhans cells are found in granulomatous arterial angiitis. At
the stable stage, scar tissues develop (19).
A confirmed diagnosis of PACNS generally requires
digital subtraction angiography (DSA) or biopsy, but none of them
shows a high diagnostic rate (20,21).
This is due to clinically suspected PACNS having a low
detectability on DSA and biopsy results being subject to sampling
errors and showing a variation depending on the course of the
disease (22). The high proportion
of negative biopsies in patients with clinical and radiographic
features of PACNS may be explained as follows: i) The
larger-diameter vessels of lesions may not extend to the
superficial parenchyma and leptomeninges, and biopsy may not be
possible and ii) vessels of lesions in the potential biopsy field
may be excluded by sampling errors due to their focality (23).
The MRI manifestations of PACNS are highly varied,
with a sensitivity of 90–100% (3,5). Lesions
are mostly located in the subcortical and deep white matter, while
they are less common below the tentorium and occasionally occur in
the spinal cord (5%) (9). The
lesions in the present case series presented with slightly low
intensity on T1WI, slight hyperintensity on T2WI, hyperintensity on
FLAIR, isointensity or slight hyperintensity on DWI and
hyperintensity on ADC mapping. Restricted or normal ADC values were
found in the majority of acute and sub-acute phase PACNS lesions in
the present study. Lesions with restricted ADC values are thought
to be ischemic (6).
Infarcted lesions had cytotoxic oedema. The lesions
with normal ADC values may reflect persistence of cytotoxic oedema
and development of simultaneous vasogenic oedema (24).
PACNS mainly involves small and medium-sized
vessels, and most lesions do not present as a wedge shape and do
not conform to the typical distribution region of a vascular
territory; MRI detection has a fairly high sensitivity. DWI has
been recommended for the diagnosis of PACNS (3,25), and
is capable of detecting early or small-sized lesions, particularly
for identifying additional lesions in different vascular
distribution regions or in different stages (26). Cytotoxic oedema is thought to be one
characteristic of PACNS (27). DWI
is helpful in increasing the diagnostic accuracy (28). Occasionally, since microhaemorrhages
may occur, petechial hypointense lesions are seen on SWI, as in our
patients.
SWI is an advantageous sequence for diagnosing
PACNS. SWI is a technology with generates images by using magnetic
susceptibility, including phase images and magnitude images, which
are blended through reprocessing and made into SWI images. Vascular
imaging on SWI relies on magnetic susceptibility deviation in
oxygen saturation; it is free from interference by blood flow
velocity and is thus particularly advantageous for imaging of small
vessels around angiitis.
Lesions may be multiple, bilateral, unilateral,
symmetrical, asymmetrical and even tumour-mimicking, and may show
heterogeneous, cord-like and gyral enhancement patterns (4,28). MRI
contrast-enhanced scanning has a significant meaning for diagnosing
PACNS through diversified enhancement of the lesion. The vascular
walls of acute inflammatory lesions are thickened and enhanced,
which may be regarded as a direct sign of angiitis. Enhanced
scanning serves as a basis for judging old and new lesions.
With regard to differential diagnoses, PACNS
requires to be differentiated from the following diseases: i)
Cerebral infarction: This disease tends to appear in patients with
a medical history of hypertension, arteriosclerosis or diabetes
mellitus. DWI and ADC mapping are useful for diagnosing infarction.
ii) Multiple sclerosis: It mostly has an acute onset and a deferred
course of disease. Multiple sclerosis is generally present in the
white matter. iii) Infectious lesions. Onset may be acute or
sub-acute and the disease course is comparatively short. Common
signs of disease include fever, cephalalgia and meningeal
irritation. Cerebrospinal fluid examination may provide evidence
for intracranial infection. iv) Mitochondrial encephalomyopathy:
This disease mainly features epileptic seizures and increased
lactic acid, presenting as a disorder of energy metabolism and more
pronounced damage to the cortex.
For the treatment of PACNS, glucocorticoids are the
first choice for treating PACNS. In cases of resistant disease, CTX
may be added. The period of treatment is 1–2 years in general.
Within the cohort of the present study, 17 patients showed evident
improvement of their clinical symptoms and imaging performance. Two
patients had a relapse and progression of the symptoms during
ongoing steroid therapy but responded to subsequent combination
treatment with CTX. Furthermore, combination of steroids with CTX
produced clinical stabilization in 8 patients with severe PACNS.
Analysis of the cases of the present study as well as evidence from
other cases from the literature suggested that long-term remission
or cure is possible, particularly when therapy is combined with CTX
(29,30).
Acknowledgements
The authors' current research was supported by
grants from National Natural Science Foundation of China (grant no.
81271604) and Jiangsu Provincial Nature Science Foundation (grant
no. BL2012037).
References
|
1
|
Twilt M and Benseler SM: The spectrum of
CNS vasculitis in children and adults. Nat Rev Rheumatol. 8:97–107.
2011.PubMed/NCBI
|
|
2
|
Salvarani C, Brown RD Jr and Hunder GG:
Adult primary central nervous system vasculitis: An update. Curr
Opin Rheumatol. 24:46–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moore PM and Richardson B: Neurology of
the vasculitides and connective tissue diseases. J Neurol Neurosurg
Psychiatry. 65:10–22. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salvarani C, Brown RD Jr, Calamia KT,
Christianson TJ, Weigand SD, Miller DV, Giannini C, Meschia JF,
Huston J III and Hunder GG: Primary central nervous system
vasculitis: Analysis of 101 patients. Ann Neurol. 62:442–451. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neel A and Paganoux C: Primary angiitis of
the central nervous system. Clin Exp Rheumatol. 27:S95–S107.
2009.PubMed/NCBI
|
|
6
|
Kadkhodayan Y, Alreshaid A, Moran CJ,
Cross DT III, Powers WJ and Derdeyn CP: Primary angiitis of the
central nervous system at Conventional Angiography. Radiology.
233:878–882. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Birnbaum J and Hellmann DB: Primary
angiitis of the central nervous system. Arch Neurol. 66:704–709.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oon S, Roberts C, Gorelik A, Wicks I and
Brand C: Primary angiitis of the central nervous system: Experience
of a Victorian tertiary-referral hospital. Intern Med J.
43:685–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
White ML and Zhang Y: Primary angiitis of
the central nervous system: Apparent diffusion coefficient lesion
analysis. Clin Imaging. 34:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore PM: Diagnosis and management of
isolated angiitis of the central nervous system. Neurology.
39:167–173. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cravioto H and Feigin I: Noninfectious
granulomatous angiitis with a predilection for the nervous system.
Neurology. 9:599–609. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cupps TR, Moore PM and Fauci AS: Isolated
angiitis of the central nervous system. Prospective diagnostic and
therapeutic experience. Am J Med. 74:97–105. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Campi A, Benndorf G, Filippi M, Reganati
P, Martinelli V and Terreni MR: Primary angiitis of the central
nervous system: Serial MRI of brain and spinal cord.
Neuroradiology. 43:599–607. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelley RE: CNS vasculitis. Front Biosci.
9:946–955. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Néel A, Auffray-Calvier E, Guillon B,
Fontenoy AM, Loussouarn D, Pagnoux C and Hamidou MA: Challenging
the diagnosis of primary angiitis of the central nervous system: A
single-center retrospective study. J Rheumatology. 39:1026–1034.
2012. View Article : Google Scholar
|
|
16
|
Singh S, John S, Joseph TP and Soloman T:
Primary angiitis of the central nervous system: MRI features and
clinical presentation. Australas Radiol. 47:127–134. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alreshaid AA and Powers WJ: Prognosis of
patients with suspected primary CNS angiitis and negative brain
biopsy. Neurology. 61:831–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alrawi A, Trobe JD, Blaivas M and Musch
DC: Brain biopsy in primary angiitis of the central nervous system.
Neurology. 53:858–860. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nabika S, Kiya K, Satoh H, Mizoue T, Araki
H, Oshita J, Nishisaka T, Kurisu K and Sugiyama K: Primary angiitis
of the central nervous system mimicking dissemination from
brainstem neoplasm: A case report. Surg Neurol. 70:182–185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hammad TA and Hajj-Ali RA: Primary
angiitis of the central nervous system and reversible cerebral
vasoconstriction syndrome. Curr Atheroscler Rep. 15:3462013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duna GF and Calabrese LH: Limitation of
invasive modalities in the diagnosis of primary angiitis of the
central nervous system. J Rheumatol. 22:662–667. 1995.PubMed/NCBI
|
|
22
|
Parisi JE and Moore PM: The role biopsy in
vasculitis of the central nervous system. Semin Neurol. 14:341–348.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller DV, Salvarani C, Hunder GG, Brown
RD, Parisi JE, Christianson TJ and Giannini C: Biopsy findings in
primary angiitis of the central nervous system. Am J Surg Pathol.
33:35–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romero JM, Schaefer PW, Grant PE, Becerra
L and González RG: Diffusion MR imaging of acute ischemic stroke.
Neuroimaging Clin N Am. 12:35–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuh WT, Ueda T and Maley JE: Perfusion and
diffusion imaging: A potential tool for improved diagnosis of CNS
vasculitis. AJNR Am J Neuroradiol. 20:87–89. 1999.PubMed/NCBI
|
|
26
|
Yin Z, Li X, Fang Y, Luo B and Zhang A:
Primary angiitis of the central nervous system: Report of eight
cases from southern china. Eur J Neurol. 16:63–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carolei A and Sacco S: Central nervous
system vasculitis. Neurol Sci. 24 Suppl 1:S8–S10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moritani T, Hiwatashi A, Shrier DA, Wang
HZ, Numaguchi Y and Westesson PL: CNS vasculitis and vasculopathy:
Efficacy and usefulness of diffusion-weighted echoplanar MR
imaging. Clin Imaging. 28:261–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Molloy ES and Hajj-Ali RA: Primary
angiitis of the central nervous system. Curr Treat Options Neurol.
9:169–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho MG, Chai W, Vinters HV, Hathout G,
Mishra S, Yim C, Valdes-Sueiras M and Nishimura R: Unilateral
hemisphere primary angiitis of the central nervous system. J
Neurol. 258:1714–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|